Abstract
The ability to identify the precise time of ovulation is important for women who want to plan conception or practice contraception. Here, we review the current literature on various methods for detecting ovulation including a review of point‐of‐care device technology. We incorporate an examination of methods to detect ovulation that have been developed and practiced for decades and analyze the indications and limitations of each—transvaginal ultrasonography, urinary luteinizing hormone detection, serum progesterone and urinary pregnanediol 3‐glucuronide detection, urinary follicular stimulating hormone detection, basal body temperature monitoring, and cervical mucus and salivary ferning analysis. Some point‐of‐care ovulation detection devices have been developed and commercialized based on these methods, however previous research was limited by small sample size and an inconsistent standard reference to true ovulation.
Keywords: family planning, fertility window, ovulation detection
1. INTRODUCTION
From the beginning of adolescence to menopause, menstruation cycles affect female health, mood, and daily living quality for a span of approximately 35–40 years in the normal population. The main purpose underlying this sophisticated, naturally designed mechanism is to provide gametes and a well‐prepared environment for embryo implantation.
Inside the ovary, multiple follicles grow under the influence of follicular stimulating hormone (FSH), which is secreted by the pituitary gland. These growing follicles secrete estrogen, which subsequently inhibits FSH secretion in a negative feedback loop involving the pituitary gland, the hypothalamus, and inhibin B.1, 2, 3, 4 By making itself more sensitive to lowered FSH level while adjacent follicles experience atresia as a result of decreasing FSH, a dominant follicle is naturally selected and allowed to grow.5 This dominant follicle continues to secrete estrogen. The persistent high level of estrogen induces an abrupt release of luteinizing hormone from the pituitary gland,6 and this hormonal surge then triggers ovulation.7 After ovulation, the dominant follicle transforms into a corpus luteum, which secrets estrogen and progesterone and collapses, initiating menstruation.
Detection and monitoring of ovulation has long been practiced by women pursuing or avoiding pregnancy. The fertility window begins approximately 3–5 days (sperm lifespan) before ovulation and continues to a point approximately 1–2 days (oocyte lifespan) after ovulation.8 Identifying this window, rather than simply identifying or detecting ovulation, is vital for encouraging or discouraging contraception. For physicians or women who wish to know if a menstrual cycle is normal or to evaluate ovarian function, a test that retrospectively confirms ovulation should suffice, but for artificial reproductive techniques, the time of ovulation and the fertility window must be defined clearly.
By performing ultrasonography, the maximum growth of the dominant follicle and the subsequent decrease in size can be observed, so the time of ovulation, which lies in between, can be determined. Because this time can be clearly defined in this manner, it is recognized as the standard reference examination for ovulation detection and is used mainly in artificial reproductive techniques. Detection of the luteinizing hormone (LH) surge, whether in serum or in urine, is very sensitive and specific for ovulation and provides great accuracy for determining conception capacity. However, because sperm ejaculated before a woman's LH surge may survive long enough to fertilize the ovum, methods that simply determine this surge are not ideal for contraception. Before the LH surge, serum estrogen level rises and several changes occur in body fluid components, including cervical mucus and saliva. Observation of these differences may provide a better view of the fertility window. Progesterone is secreted by the corpus luteum only after ovulation. Detection of progesterone or its metabolites can retrospectively confirm the occurrence of ovulation. Because progesterone causes a rise in basal body temperature (BBT), a measure of this temperature may also be useful for determining ovulation. Because the oocyte dies shortly after ovulation, methods that correlate to progesterone and its effect identify fertility window closure.
Current methods to detect ovulation and their corresponding point‐of‐care (POC) devices are reviewed in this article. Physicians and those who are pursuing or avoiding pregnancy should know the indications and limitations of each method before employing them.
2. CONVENTIONAL METHODS
2.1. Ultrasonography
Transvaginal ultrasonography can clearly define ovulation time and is recognized as the standard reference examination for detecting ovulation. It is performed by experienced technicians, radiologists, or gynecologists. Being invasive, expensive, and inconvenient, this technique is not broadly used; it is used mainly in gynecological clinics and is often performed as a step in artificial reproductive techniques. Using serial ultrasonography examinations, the time of ovulation can be determined as the point between maximum follicular diameter and follicular collapse (Figure 1). Indications of ovulation include the following:
Disappearance or sudden decrease in follicle size.
Increased echogenicity inside the follicle, indicating corpus luteum formation.
Free fluid in pelvis (or pouch of Douglas).
Replacement of “triple‐line appearance” of endometrium by homogenous, hyperechoic “luteinized” endometrium.9, 10
Figure 1.
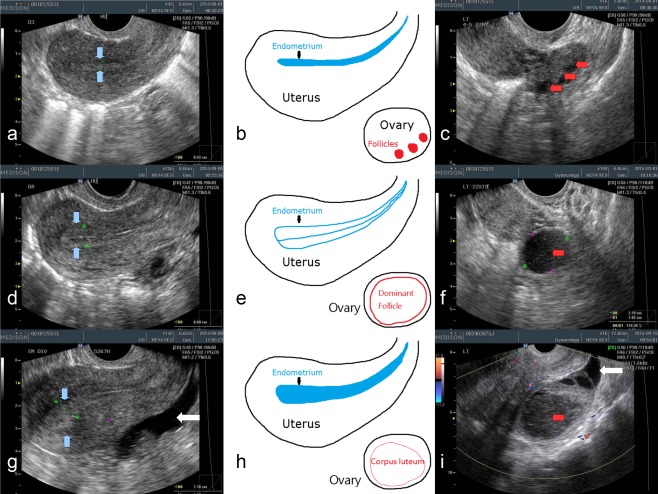
Transvaginal ultrasonography of ovary and endometrium in early follicular phase (a–c), late follicular (shortly before ovulation) phase (d–f), and post‐ovulatory luteal phase (g–i). In early follicular phase, the endometrium (between the blue arrows) just after menstruation appeared thin and homogenous (a). Multiple small follicles (red arrows) could be seen inside the ovary (c). In late follicular phase, the endometrium was thickened, and a typical “triple line” appearance could be seen (d). A dominant follicle, approximately 2 cm in diameter, is about to ovulate in late follicular phase (f). After ovulation, the endometrium becomes “luteinized,” with increased echogenicity (whitening) (g). The dominant follicle transforms into a corpus luteum (i). Note the increased echogenicity and inhomogenous content (with filament‐like structure) inside the corpus luteum compared to the dominant follicle in picture f. Free fluid can be seen in the pelvis (white arrows in pictures g and f)
2.2. Urinary luteinizing hormone
LH belongs to a group of glycoprotein hormones that share a common α subunit, and biological specificity for each hormone is conferred by their divergent β subunit. While serum estradiol (the most potent estrogen secreted by the dominant follicle) concentration reaches a threshold level (greater than 200 pg/ml for approximately 50 hr), a positive feedback mechanism works on the hypothalamus and anterior pituitary gland, which results in an abrupt secretion of LH into bloodstream. The onset of the LH surge precedes ovulation by 35–44 hr, and the peak serum level of LH precedes ovulation by 10–12 hr.11, 12 A study of 155 cycles from 35 women demonstrated that the onset of the LH surge primarily occurs between midnight and early morning (37% between 00:00 and 04:00, 48% between 04:00 and 08:00).13
Detection of LH in urine using an over‐the‐counter device is much more convenient and less invasive than measuring serum LH level by multiple venipuncture. Women wishing to accurately determine their fertility window are instructed to record several menstrual cycles to estimate the possible time of ovulation. Starting from the 10th to 11th day of a new cycle (day 1 is defined as the first day of menstrual bleeding), or 4 days before the estimated ovulation day, women can test their urinary LH once or twice daily. Highly sensitive urinary LH kits detect concentrations as low as 22 mIU/ml, while natural LH surge concentration in urine ranges from 20 to 100 mIU/ml.14 The mean time interval after a positive urinary LH test to follicular rupture detected by sonography was reported to be 20 ± 3 hr (95% CI 14–26),15 and in a study focused on infertile women, sensitivity, specificity, and accuracy of the urinary LH test to detect ovulation reached 1.00, 0.25, and 0.97, respectively.16 Its accuracy proven, the U.S. National Academy of Clinical Biochemistry Laboratory Medicine practical guidelines recommend using the urinary LH test, because a positive result predicts ovulation within 48 hr (Strength B, level II).17 Several studies18, 19, 20, 21, 22 describe methods to detect ovulation use urinary LH surge instead of ultrasonography because it is highly accurate, inexpensive, and less invasive.
Despite positive correlations used and mentioned in the literature, there are still some indications that LH surge may not signify true ovulation. In an observational study of 43 women in which urinary LH was recorded and analyzed daily, it was found that LH surges are not of one type, and they are extremely variable. The onset of urinary LH surge was categorized into rapid‐onset type (within one day, 42.9%) and gradual‐onset type (over 2–6 days, 57.1%). Configurations of LH surge can be categorized into three types: (a) spiking (41.9%); (b) biphasic (44.2%); and, (c) plateau (13.9%).23 Furthermore, two (4.3%) women demonstrated LH surge without ovulation. In infertile women, premature LH surge that did not trigger ovulation was detected in 46.8% of cycles.24 Also, a situation reported as “luteinized unruptured follicle syndrome” was reported to occur in 10.7% of menstrual cycles in normally fertile women. Women with this syndrome have a normal LH surge, functioning corpus luteum, and menstruation, but no oocyte is released.25, 26
Because a positive urinary LH test precedes ovulation, it is theoretically helpful for timed intercourse or intrauterine insemination because the clinical pregnancy rate after a single incident of intercourse is highest from a point 2 days before ovulation to the day of ovulation.27 In a study mainly focused on the psychological stress in women using a urinary LH kit, a trend toward increased self‐reported pregnancy rate can be seen, but this result did not reach statistical significance (the odds of pregnancy rate in the study group were 1.77 (95% CI: 0.9992, 3.1585) compared with the control group).28 A 2015 Cochrane review concluded that timed intercourse using urinary hormone monitoring was associated with an increased pregnancy rate (RR 1.36, 95% CI 1.06–1.73, 3 RCTs, n = 1,370).29 A promising unpublished study that may provide a better answer to this question is the Oxford Conception Study, which targets conception rate as its primary outcome.30
2.3. Serum progesterone and urinary pregnanediol 3‐glucuronide
After ovulation, the dominant follicle turns into a corpus luteum and begins to secrete progesterone. To confirm ovulation, serum progesterone or its metabolite in urine, can be measured. A single serum progesterone level >3 ng/ml in mid‐luteal phase has been used to retrospectively detect ovulation. A recent European study proposed a random serum progesterone ≥5 ng/ml for confirming ovulation with sensitivity and specificity at 89.6% and 98.4%, respectively.31 The same study group also demonstrated that levels of urinary pregnanediol 3‐glucuronide (PDG), a metabolite of progesterone, measured at levels over 5 μg/ml for three consecutive days could be used as positive confirmation of ovulation with a sensitivity of 92.2% and a specificity of 100%.32 Nevertheless, a convenient POC device to detect urinary PDG has not yet been developed.
2.4. Urinary follicular stimulating hormone
Li et al. reported that peak FSH level occurred within 1 day of ultrasonography‐detected follicular collapse in 97% of menstrual cycles. The threshold value of urinary FSH was not mentioned.33 No further study has been published regarding this method to detect ovulation.
2.5. Basal body temperature
In 1906, Theodoor Hendrik van de Velde noticed a biphasic change of BBT in women during menstruation. Monitoring of BBT has become one of the simplest and least invasive methods to detect ovulation. The rise of BBT results from the thermogenic effect of progesterone. During the follicular phase of the menstrual cycle, BBT keeps in the lower range, generally between 97.0 and 98.0°F, until approximately 1 day before ovulation, when BBT reaches its lowest point (nadir, or dip). After ovulation, the corpus luteum begins to secret progesterone. The BBT rises 0.5–1.0°F and plateaus throughout the luteal phase. In late luteal phase, when the corpus luteum regresses and serum progesterone level decreases, the BBT returns to the lower range within 1–2 days before, or just at, the onset of menstrual bleeding. This biphasic pattern of BBT retrospectively suggests ovulation (Figure 2).
Figure 2.
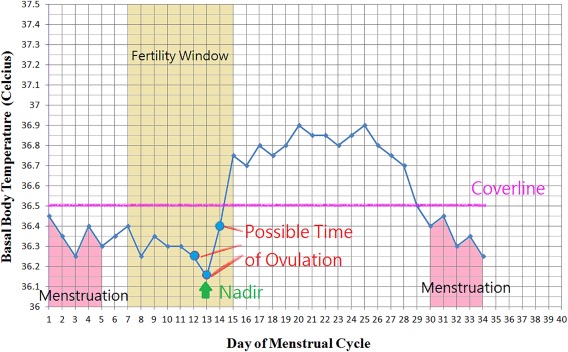
An illustration of BBT chart in degrees Celsius. This is a biphasic pattern in a normal ovulatory cycle. Ovulation can only be suggested after observing the rise and plateau of temperature above the purple “Coverline.” A drop in temperature occurs at the end of the luteal phase when progesterone decreases, which is followed by menstruation
Women interested in determining their fertility window are instructed to measure their oral, vaginal, or rectal temperature every day when they wake up and before any activity is initiated. Modern digital thermometers have recording abilities that make monitoring BBT more convenient. However, a traditional glass thermometer that is accurate to 1/10th of a degree is good enough for determining BBT. Women can record their BBT on specialized charts, online services, or applications on smart‐phone devices.
There are many ways to interpret BBT. In one approach, the “coverline” method, a horizontal coverline (which means a threshold temperature) is drawn on a BBT chart. When temperature above this coverline is recorded, ovulation is suggested. The coverline can be determined by adding 0.15°F to the highest temperature recorded during the first 10 days of a cycle, or by using previously recorded temperatures. Using the “three over six” rule,34 three temperatures are required to be 0.2°F above the highest point of the previous six temperatures with at least one of the higher temperatures being 0.4°F above the lower ones.
Factors influencing BBT include fever, alcohol, emotional or physical stress, sleep disturbance, change of room temperature, change of waking time, change of climate, and recent start or discontinuation of birth control pills or anti‐pyretics. A “GAP” technique was described in 2005 as a superior approach to the coverline method for women seeking contraception. By subtracting the BBT of a cohabiting male partner from the female partner's BBT, the temperature “gap” may be recorded. This gap is theoretically not influenced by environmental factors other than hormonal influences of the female. However, the study group was small (33 cycles), and the approach is not suitable for women who have not, or do not, live with their male partners.35
Interpretation of BBT is not always easy. Even for natural family planning experts, there has been poor agreement on the first day of BBT rise between different observers.36 It was reported that the time of ovulation determined by BBT coincided with the LH surge ± 1 day in only 17 of 77 cycles (22.1%).37 A review article published in 2005 concluded that monitoring BBT was no longer a good predictor of ovulation and, therefore, should not be recommended for couples seeking pregnancy.38 Nevertheless, BBT is still broadly used for contraception and for evaluation of ovulatory function, especially for couples who are reluctant or unable to pursue more formal and costly evaluation.
2.6. Cervical mucus
Cervical mucus is secreted by cervical and endocervical glands. The appearance of cervical mucus varies during different stages of the menstrual cycle. Outside of the periovulatory period, this mucus is mainly composed of high‐molecular‐weight glycoprotein (mucin), which forms a mesh‐like structure that provides a barrier to sperm and micro‐organisms. The mucus appears thick, scant, and viscous by inspection. During the periovulatory period, under the effect of estrogen, the production of acellular water increases and the production of mucin decreases. The mesh‐like structure consequently loosens and becomes highly receptive for sperm penetration.18, 39, 40 Women experience an increased amount of watery discharge resembling raw egg white during this period.
Observing cervical mucus is the least expensive method to detect ovulation. Women may simply observe mucus found externally at the vulva or collect vaginal mucus using their fingers. While uncomplicated, this approach has still been shown to be effective. In a small study involving 12 cycles recorded by 6 women, finding the most abundant fertile type of mucus correlated to ± 1 day of ovulation detected by ultrasonography.41 In a larger study involving 148 cycles recorded by 40 women, mucus sensation and characteristics yielded a 48.3% correlation to ultrasonography‐detected ovulation.42 In another large study, cervical mucus‐detected methodology for determining expected date of ovulation correlated to ± 1 day of ultrasonography‐detected ovulation in 160/215 cycles (74.4%).43 In a study involving 29 cycles recorded by 15 women, the sensitivity of detecting fertile mucus from the vulva and vagina compared to ± 1 day of ultrasonography‐detected ovulation were 75.9% and 75.9%, respectively. When the period was extended to −1 to +2 of ovulation, the sensitivities were 96.9% (vulva) and 89.6% (vagina), respectively.44
The combination of measuring BBT and observing cervical mucus for contraception is called the symptothermal method. Among women using this method for contraception in one study, the unintended pregnancy rate was 1.8%.45
2.7. Salivary ferning and analysis
The history of salivary ferning test can be traced back to 1969. Dr. Biel Cassal found that the arborization (or ferning) of saliva could be seen under a microscope during the periovulatory period. Increasing levels of estrogen and adrenocorticotropic hormone before ovulation stimulates the secretion of aldosterone, which regulates the electrolytes and fluid status in human body.46 Crystallization of NaCl produces the ferning appearance of saliva observed under a microscope.
A pocket microscope specifically designed for salivary ferning observation has been developed. A study conducted by Barbato et al. declared that salivary ferning correlated well to BBT and cervical mucus methods.46 It was later reported that salivary ferning can be detected in postmenopausal, pregnant, prepubertal women, and even in men. In women with regular menstruation, salivary ferning predicted ovulation with a sensitivity of only 53% correlated to ultrasonography and serum LH.48, 49 Guida et al. compared several methods to ultrasonography‐detected ovulation and found that positive salivary ferning test correlated to −1 to +1 days of actual ovulation in 42% of cases. However, a high percentage of uninterpretable patterns (58.7%) was reported.41 A recent study compared salivary ferning using a Geratherm ovu control microscope to a urinary LH test using EXACTO monitors and found a high correlation between these methods.50
To perform this test, women could use a small microscope with built‐in or removable slides. A drop of saliva may be applied to a slide, allowed to dry and inspected for ferning. The U.S. FDA announced that a positive test indicates that women may be near ovulation, but a negative test is unreliable for contraception.
Sex hormones were first measured in saliva by radioimmunoassay in 1978. Early publications focused on the correlation of salivary steroids to ovarian function,19, 20, 51, 52, 53, 54 but exact ovulation time was neither determined nor even estimated, partly because reliable methodology was not yet available. Based on the theory that increased circulating estrogens stimulate the breakdown of glycogen, Alagendran et al. reported that levels of sialic acid and glycosaminoglycans (CAG) increased during the ovulatory period.55 A change in urinary CAG level during the ovulatory phase was also demonstrated, as the ratio of urinary trypsin inhibitor/chondroitin sulphate peaked at menstrual cycle day 12.56
Recently, attention was given to examining salivary proteins for various diagnostic purposes. Protein concentration is at its highest during the ovulation phase. By single dimension SDS‐PAGE analysis, a 48 kDa protein was identified to exhibit predominantly during ovulatory phase.57 The author suggested that the protein level and the presence of 48 kDa band might be recognized as ovulation indicators. Further research is needed on this topic.
3. POC OVULATION DETECTION DEVICES
3.1. Urinary luteinizing hormone
Nowadays, many POC and over‐the‐counter ovulation detection devices are easily accessible for women planning conception or contraception. Most of these commercialized products determine ovulation by detecting urinary LH level. Several computerized fertility monitors have been used to detect urinary LH and estrone‐3‐glucoronide, which, if presented in urine, have also indicated ovulation.58 The manufacturer of the Clearblue easy fertility Monitor (CEFM) reported a significantly higher pregnancy rate during the first 2 cycles in women using its product compared to control groups (22.7% compared to 14.4%).59 (Figure 3a) Moreover, the same manufacturer developed an additional product, Clearblue DIGITAL Ovulation Test with Dual Hormone Indicator, which detects estrogen as well as LH. The manufacturer claims that this product can identify 4 or more fertile days with an accuracy rate of over 99%. Both monitors are digital immunoassays incorporating a disposable microspectrophotometer.66 Primarily used for contraceptive purposes, the Persona monitor, achieved a 94% correct‐use effectiveness.67, 68 (Figure 3a) The sensitivity of CEFM and Persona to accurately determine ovulation are 97% and 95.8%, respectively.61
Figure 3.
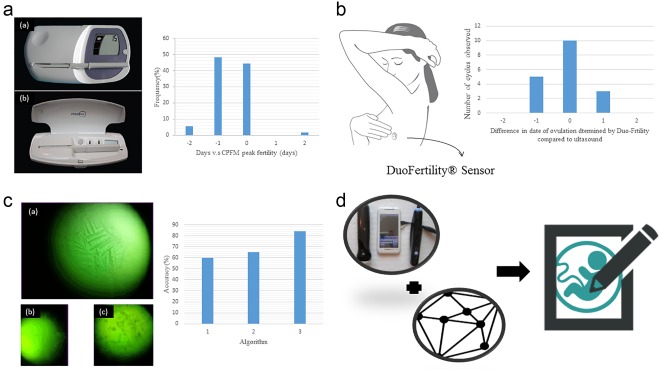
(a) The left graph is the photo of Clearblue Easy Fertility Monitor (a) and the Persona (b).60 The right bar chart is the Serum LH surge relative to CPFM peak fertility. The X‐axis −2 and 2 represents >1 day before or after CPFM peak fertility, respectively. Cycles with no CPFM peak fertility (n = 13), no serum LH surge day (n = 10), or neither (n = 1) are excluded from the table.61 (b) The left graph is the illustration of DuoFertility® Sensor worn by patients. The right bar chart is the correlation between ultrasound scans and DuoFertility® result.62 (c) The left graph is the result of ferning patterns by Knowhen Ovulation Monitoring System. (a) Fertile (b) preovulatory (c) postovulatory.63 The accuracy of three proposed algorithms. Algorithm 1 is binarizing + dark pixel density. Algorithm 2 is binarizing + dark pixel density + thinning. Algorithm 3 is binarizing + Hough transform + thinning + decision tree.64 (d) The illustration of possible combination of smartphone ultrasound device and imaging processing algorithms as an ovulation detection device65
3.2. Basal body temperature
In addition to urinary LH level detection, some commercialized POC devices adopt BBT monitoring methods to determine ovulation. OvuSense is a vaginal temperature sensor with a 99% claimed accuracy rate for ovulation date detection and an 89% accuracy rate for ovulation date prediction.69 In addition to measuring temperature intravaginally, some devices base their results on temperature measured elsewhere on the body. NaturalCycles, for example, which consists of thermometers and mobile application, is stated to misidentify only 0.05% of nonfertile days as fertile days.70 By measuring and recording temperature on a daily basis, the NaturalCycles application can detect ovulation and fertile days. Another product, DuoFertility® employs a small sensor worn under the arm that takes thousands of measurements throughout the day and night and reports temperature data to a server (Figure 3b). DuoFertility automatically recognizes ovulation through proprietary algorithms with 100% sensitivity.62
3.3. Salivary ferning and analysis
Some portable microscopes have been developed to detect ovulation via salivary ferning analysis. Among such devices, the manufacturers of the Knowhen Ovulation Monitoring System have shown a strong correlation between observed salivary ferning and ovulation among 22 patients (Log Odds ratio 7.64, p < .01, CI 4.26–11.02)63 (Figure 3c). Another device, Geratherm® ovu control, is reported to have a specificity of 78% and a sensitivity of 80%.71 Both of the abovementioned devices require patients to self‐evaluate salivary ferning. The agreement rate between saliva test carried out by patients and by laboratory staff is 89.4% with Geratherm® ovu control.71 To avoid potential self‐evaluation error WU, Hui‐Ching et al. introduced an automatic salivary ferning pattern recognition system for ovulation detection. They report an accuracy rate of 84% from 100 saliva samples.64
3.4. Smartphone‐based approaches
While promising, none of the above‐mentioned devices are robust. LH detection, detection, BBT monitoring, and salivary ferning analysis are easily influenced by other body conditions such as polycystic ovarian syndrome, abnormal increases in estrogen levels or fever.72 Some new technologies, including smartphone ultrasound apparatuses and novel software algorithms, may improve precision while keeping cost down. Considering the fact that transvaginal ultrasonography is the standard reference examination, smartphone ultrasound technology, which is now commercially available, may provide the most accurate at‐home ovulation detection results (Figure 3d). A fully developed imaging processing program may replace expertise in interpreting ultrasound scan results. Furthermore, imaging processing algorithms are available to improve the accuracy of salivary ferning pattern reading.64 Additionally, decision making algorithms can help detect ovulation by analyzing BBT patterns to increase the accuracy of ovulation detection by BBT analysis.72
3.5. Paper‐based approaches
Aside from smart‐phone based devices, paper‐based ovulation detection devices are full of potential. Paper has been successfully used for biological assays, such as ELISA73 and cell assays.74 Paper is economical, prevalent, disposable, and suitable for large‐scale manufacture.75 Paper‐based ovulation detection devices based on LH detection, such as One Step® Ovulation & Pregnancy Test Kit Strips, are currently available.
3.6. Cervicovaginal fluid
Besides, a new developed cotton‐based cervicovaginal fluid collecting device may offer another solution. Cheng et al. proposed a readily applicable cervicovaginal fluid collection device, which saves cervicovaginal fluid for later diagnosis. It is proven to successfully determine pathogen infection and to diagnose the presence of female genital cancer.60 This device may collect cervicovaginal fluid for determining ovulation by cerival mucus detection, which is the least expansive but effective method. Furthermore, coupling cervicovaginal fluid collecting device with thermometers makes a simple point‐of‐care ovulation detection applying symptothermal method.
4. SUMMARY AND PERSPECTIVES
Most of the currently available methods to detect ovulation were developed decades ago (Table 1). Research tests for the methods described here were limited to small sample sizes and subjects of limited cultural background (most studies were performed in European countries in less than 100 women with less than 1,000 cycles). Even fewer studies examined or included women having irregular menstruation, which is characterized by less predictable ovulation timing. Such women would particularly benefit from improved ovulation predictors. While differences in environmental hormones and dietary habits may considerably influence hormone status in women, re‐examination by large database study may change our view regarding these available methods.
Table 1.
Features of currently available methods to detect ovulation
| Cost | Accuracy | Accessibility | Invasion | Detect before ovulation | Features/disadvantage | |
|---|---|---|---|---|---|---|
| POC methods available | ||||||
| Urinary LH | Low cost of kits | High (97%) | High (OTC) | No | Yes | Repeated purchases of kits |
| Computerized monitor (urinary LH + E1‐3‐G) | Moderate cost of device | High (95.8–97%) | High (OTC) | No | Yes | Evidence to improve pregnancy rate Repeated purchases of sticks |
| Basal body temperature | Low cost of thermometer | Low (22.1%) | High | No | No |
Not easily interpreted Affected by environmental factors |
| Cervical mucus | No cost | Moderate (48–76%) | High | No | Yes | Unable to perform while vaginal infection |
| Salivary ferning | Low cost of kits | Moderate (42–53%) | High (OTC) | No | Yes | High percentage of unpredictable result |
| POC methods unavailable | ||||||
| Transvaginal ultrasound | High | High (standard reference examination) | Low (performed by physician) | Yes (introduce vaginal probe) | Yes | May be uncomfortable during exam |
| Serum progesterone | N/Aa | High (89.6%) | Low (need laboratory) | Yes (venipuncture) | No | Confirms ovulation |
| Urinary PDG | N/Aa | High (92.2%) | Low (need laboratory) | No | No | Confirms ovulation |
These two exams are not commonly performed. The cost may vary in different country.
E1‐3‐G = estrone‐3‐glucoronide; LH = leutinizing hormone; N/A = not applicable; OTC = over‐the‐counter; PDG = pregnanediol 3‐glucuronide.
An ideal method to detect ovulation should be (a) noninvasive, (b) inexpensive, (c) easily available and easy to use (as a POC method), (d) precise in determining ovulation, and (e) precise in determining the fertility window. None of the aforementioned methods fits all of these features. However, with modern technology, a combination of different methods may be incorporated into one small pocket machine for computerized analysis. A better understanding of physical and hormonal changes during ovulation and improvements in biotechnology may help develop additionally useful and accurate methods to detect ovulation.
CONFLICT OF INTERESTS
None of the authors have any conflict of interest to declare regarding the manuscript.
AUTHOR CONTRIBUTIONS
Design and conduct of the study: T.‐C.C. and C.‐M.C. Writing of the article: H.‐W. S., Y.‐C. Y., T.‐Y. W., T.‐C.C., and C.‐M.C. Final approval of the article: H.‐W. S., Y.‐C. Y., T.‐Y. W., T.‐C.C., and C.‐M.C. Literature search: H.‐W. S., Y.‐C. Y., and T.‐Y. W. Preparation of figures: H.‐W. S., Y.‐C. Y., and T.‐Y. W. Administrative, technical and logistical support: H.‐W. S., Y.‐C. Y., T.‐Y. W., T.‐C.C., and C.‐M.C.
ACKNOWLEDGMENTS
This work was supported in part by grants from Taiwan's Ministry of Science and Technology (MOST 104‐2628‐E‐007‐001‐MY3 & MOST 105‐2221‐E‐007‐053‐MY3 [C.M.C.]) and Chang Gung Memorial Hospital (CMRPG3D0691 [T.C.C.]).
Funding information Taiwan's Ministry of Science and Technology, Grant/Award Number: MOST 104‐2628‐E‐007‐001‐MY3 & MOST 105‐2221‐E‐007‐053‐MY3; Chang Gung Memorial Hospital, Grant/Award Number: CMRPG3D0691
Contributor Information
Ting‐Chang Chang, Email: tinchang@cgmh.org.tw, Email: tinchang.chang@gmail.com.
Chao‐Min Cheng, Email: chaomin@mx.nthu.edu.tw.
LITERATURE CITED
- 1. Mais V, Keyser RR, Cetel NS, Rivier J, Vale W, Yen SSC. The dependency of folliculogenesis and corpus luteum function on pulsatile gonadotropin secretion in cycling women using a gonadotropin‐releasing hormone antagonist as a probe. J Clin Endocrinol Metab. 1986;62(6):1250–1255. [DOI] [PubMed] [Google Scholar]
- 2. Chappel SC, Resko JA, Norman RL, Spies HG. Studies in Rhesus monkeys on the site where estrogen inhibits gonadotropins: delivery of 17β‐estradiol to the hypothalamus and pituitary gland*. J Clin Endocrinol Metab. 1981;52(1):1–8. [DOI] [PubMed] [Google Scholar]
- 3. Buckler HM, Healy DL, Burger HG. Purified FSH stimulates production of inhibin by the human ovary. J Endocrinol. 1989;122(1):279–285. [DOI] [PubMed] [Google Scholar]
- 4. Chikazawa K, Araki S, Tamada T. Morphological and endocrinological studies on follicular development during the human menstrual cycle. J Clin Endocrinol Metab. 1986;62(2):305–313. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization Task Force Investigators . Temporal relationships between ovulation and defined changes in the concentration of plasma estradiol‐17β, luteinizing hormone, follicle‐stimulating hormone, and progesterone. Am J Obstet Gynecol. 1980;138(4):383–390. [PubMed] [Google Scholar]
- 6. Yoshimura Y, Wallach EE. Studies of the mechanism(s) of mammalian ovulation. Fertil Steril. 1987;47(1):22–34. [PubMed] [Google Scholar]
- 7. Gould JE, Overstreet JW, Hanson FW. Assessment of human sperm function after recovery from the female reproductive tract. Biol Reprod. 1984;31(5):888–894. [DOI] [PubMed] [Google Scholar]
- 8. Ecochard R, Marret H, Rabilloud M, et al. Sensitivity and specificity of ultrasound indices of ovulation in spontaneous cycles. Eur J Obstet Gynecol Reprod Biol. 2000;91(1):59–64. [DOI] [PubMed] [Google Scholar]
- 9. Matijevic R, Grgic O. Predictive values of ultrasound monitoring of the menstrual cycle. Curr Opin Obstet Gynecol. 2005;17(4):405–410. [DOI] [PubMed] [Google Scholar]
- 10. Young JR, Jaffe RB. Strength‐duration characteristics of estrogen effects on gonadotropin response to gonadotropin‐releasing hormone in women. II. Effects of varying concentrations of estradiol. J Clin Endocrinol Metab. 1976;42(3):432–442. [DOI] [PubMed] [Google Scholar]
- 11. Hoff JD, Quigley ME, Yen SS. Hormonal dynamics at midcycle: a reevaluation. J Clin Endocrinol Metab. 1983;57(4):792–796. [DOI] [PubMed] [Google Scholar]
- 12. Cahill DJ, Wardle PG, Harlow CR, Hull MGR. Onset of the preovulatory luteinizing hormone surge: diurnal timing and critical follicular prerequisites. Fertil Steril. 1998;70(1):56–59. [DOI] [PubMed] [Google Scholar]
- 13. Eichner SF, Timpe EM. Urinary‐based ovulation and pregnancy: point‐of‐care testing. Ann Pharmacother. 2003;38(2):325–331. [DOI] [PubMed] [Google Scholar]
- 14. Miller PB, Soules MR. The usefulness of a urinary LH kit for ovulation prediction during menstrual cycles of normal women. Obstet Gynecol. 1996;87(1):13–17. [DOI] [PubMed] [Google Scholar]
- 15. Guermandi E, Vegetti W, Bianchi MM, Uglietti A, Ragni G, Crosignani P. Reliability of ovulation tests in infertile women. Obstet Gynecol. 2001;97(1):92–96. [DOI] [PubMed] [Google Scholar]
- 16. Nichols JH. The national academy of clinical biochemistry laboratory medicine practice guidelines. Point Care. 2007;6(4):213–214. [Google Scholar]
- 17. Park S, Goldsmith L, Skurnick J, Wojtczuk A, Weiss G. Characteristics of the urinary luteinizing hormone surge in young ovulatory women. Fertil Steril. 2007;88(3):684–690. [DOI] [PubMed] [Google Scholar]
- 18. Ceric F, Silva D, Vigil P. Ultrastructure of the human periovulatory cervical mucus. J Electron Microsc (Tokyo). 2005;54(5):479–484. [DOI] [PubMed] [Google Scholar]
- 19. Ellison PT. Salivary steroids and natural variation in human ovarian function. Ann N Y Acad Sci. 1994;709(1):287–298. [DOI] [PubMed] [Google Scholar]
- 20. Lu YC, Bentley GR, Gann PH, Hodges KR, Chatterton RT. Salivary estradiol and progesterone levels in conception and nonconception cycles in women: evaluation of a new assay for salivary estradiol. Fertil Steril. 1999;71(5):863–868. [DOI] [PubMed] [Google Scholar]
- 21. Fehring RJ. Accuracy of the peak day of cervical mucus as a biological marker of fertility. Contraception. 2002;66(4):231–235. [DOI] [PubMed] [Google Scholar]
- 22. Berglund Scherwitzl E, Lindén Hirschberg A, Scherwitzl R. Identification and wwprediction of the fertile window using NaturalCycles. Eur J Contracept Reprod Health Care. 2015;20(5):403–408. [DOI] [PubMed] [Google Scholar]
- 23. Krotz S, McKenzie LJ, Cisneros P, Buster J, Amato P, Carson S. Prevalence of premature urinary luteinizing hormone surges in women with regular menstrual cycles and its effect on implantation of frozen‐thawed embryos. Fertil Steril. 2005;83(6):1742–1744. [DOI] [PubMed] [Google Scholar]
- 24. Killick S, Elstein M. Pharmacologic production of luteinized unruptured follicles by prostaglandin synthetase inhibitors. Fertil Steril. 1987;47(5):773–777. [DOI] [PubMed] [Google Scholar]
- 25. Qublan H, Amarin Z, Nawasreh M, et al. Luteinized unruptured follicle syndrome: incidence and recurrence rate in infertile women with unexplained infertility undergoing intrauterine insemination. Hum Reprod. 2006;21(8):2110–2113. [DOI] [PubMed] [Google Scholar]
- 26. Baird DT, Cameron S, Evers JLH, et al. Emergency contraception. Widely available and effective but disappointing as a public health intervention: a review. Human Reproduction 2015;30(4):751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tiplady S, Jones G, Campbell M, Johnson S, Ledger W. Home ovulation tests and stress in women trying to conceive: a randomized controlled trial. Hum Reprod. 2012;28(1):138–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Manders M, McLindon L, Schulze B, Beckmann MM, Kremer JA, Farquhar C. Timed intercourse for couples trying to conceive. Cochrane Library 2015;173:CD011345. [DOI] [PubMed] [Google Scholar]
- 29. Pyper C, Bromhall L, Dummett S, Altman DG, Brownbill P, Murphy M. The Oxford Conception Study design and recruitment experience. Paediatr Perinat Epidemiol. 2006;20:51–59. [DOI] [PubMed] [Google Scholar]
- 30. Branch CM, Collins PO, Collins WP. Ovulation prediction: changes in the concentrations of urinary estrone‐3‐glucuronide, estradiol‐17β‐glucuronide and estriol‐16α‐glucuronide during conceptional cycles. J Steroid Biochem. 1982;16(2):345–347. [DOI] [PubMed] [Google Scholar]
- 31. Ecochard R, Leiva R, Bouchard T, et al. Use of urinary pregnanediol 3‐glucuronide to confirm ovulation. Steroids. 2013;78(10):1035–1040. [DOI] [PubMed] [Google Scholar]
- 32. Li H, Chen J, Overstreet JW, Nakajima ST, Lasley BL. Urinary follicle‐stimulating hormone peak as a biomarker for estimating the day of ovulation. Fertil Steril. 2002;77(5):961–966. [DOI] [PubMed] [Google Scholar]
- 33. Marshall J. A field trial of the basal‐body‐temperature method of regulating births. Lancet. 1968;292(7558):8–10. [DOI] [PubMed] [Google Scholar]
- 34. Dunlop AL, Schultz R, Frank E. Interpretation of the BBT chart: using the “Gap” technique compared to the Coverline technique. Contraception. 2005;71(3):188–192. [DOI] [PubMed] [Google Scholar]
- 35. Kambic R, Gray RH. Interobserver variation in estimation of day of conception intercourse using selected natural family planning charts. Fertil Steril. 1989;51(3):430–434. [DOI] [PubMed] [Google Scholar]
- 36. Bauman JE. Basal body temperature: unreliable method of ovulation detection. Fertil Steril. 1981;36(6):729–733. [DOI] [PubMed] [Google Scholar]
- 37. Barron ML, Fehring RJ. Basal body temperature assessment: is it useful to couples seeking pregnancy? MCN Am J Matern Child Nurs. 2005;30(5):290–296. [DOI] [PubMed] [Google Scholar]
- 38. Menárguez M, Pastor LM, Odeblad E. Morphological characterization of different human cervical mucus types using light and scanning electron microscopy. Hum Reprod. 2003;18(9):1782–1789. [DOI] [PubMed] [Google Scholar]
- 39. Brunelli R, Papi M, Arcovito G, et al. Globular structure of human ovulatory cervical mucus. FASEB J. 2007;21(14):3872–3876. [DOI] [PubMed] [Google Scholar]
- 40. Depares J, Ryder RE, Walker SM, Scanlon MF, Norman CM. Ovarian ultrasonography highlights precision of symptoms of ovulation as markers of ovulation. Br Med J (Clinical Research ed.). 1986;292(6535):1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guida M, Tommaselli GA, Palomba S, et al. Efficacy of methods for determining ovulation in a natural family planning program. Fertil Steril. 1999;72(5):900–904. [DOI] [PubMed] [Google Scholar]
- 42. Ecochard R, Boehringer H, Rabilloud M, Marret H. Chronological aspects of ultrasonic, hormonal, and other indirect indices of ovulation. BJOG Int J Obstet Gynaecol. 2001;108(8):822–829. [DOI] [PubMed] [Google Scholar]
- 43. Alliende ME, Cabezón C, Figueroa H, Kottmann C. Cervicovaginal fluid changes to detect ovulation accurately. Am J Obstet Gynecol. 2005;193(1):71–75. [DOI] [PubMed] [Google Scholar]
- 44. Frank‐Herrmann P, Heil J, Gnoth C, et al. The effectiveness of a fertility awareness based method to avoid pregnancy in relation to a couple's sexual behaviour during the fertile time: a prospective longitudinal study. Hum Reprod. 2007;22(5):1310–1319. [DOI] [PubMed] [Google Scholar]
- 45. Fernando RS, Regas J, Betz G. Physiological mechanisms associated with ovulation prediction using the CUE® Ovulation Predictor. Hum Reprod. 1988;3(4):413–417. [DOI] [PubMed] [Google Scholar]
- 46. Barbato M, Pandolfi A, Guida M. A new diagnostic aid for natural family planning. Adv Contracept. 1993;9(4):335–340. [DOI] [PubMed] [Google Scholar]
- 47. Berardono B, Melani D, Ranaldi F, Giachetti E, Vanni P. Is the salivary “ferning” a reliable index of the fertile period? Acta Eur Fertil. 1992;24(2):61–65. [PubMed] [Google Scholar]
- 48. Braat DD, Smeenk JM, Manger AP, Thomas CM, Veersema S, Merkus JM. Saliva test as ovulation predictor. Lancet. 1998;352(9136):1283–1284. [DOI] [PubMed] [Google Scholar]
- 49. Günther V, Bauer I, Hedderich J, et al. Changes of salivary estrogen levels for detecting the fertile period. Eur J Obstet Gynecol Reprod Biol. 2015;194:38–42. [DOI] [PubMed] [Google Scholar]
- 50. Walker R, Hughes I, Riad‐Fahmy D, Read G. Assessment of ovarian function by salivary progesterone. Lancet. 1978;312(8089):585. [DOI] [PubMed] [Google Scholar]
- 51. Walker RF, Read GF, Fahmy DR. Salivary progesterone and testosterone concentrations for investigating gonadal function proceedings. J Endocrinol 1979;81(2):164P. [PubMed] [Google Scholar]
- 52. Walker RF, Read GF, Riad‐Fahmy D. Radioimmunoassay of progesterone in saliva: application to the assessment of ovarian function. Clin Chem. 1979;25(12):2030–2033. [PubMed] [Google Scholar]
- 53. Gröschl M, Rauh M, Schmid P, Dörr HG. Relationship between salivary progesterone, 17‐hydroxyprogesterone, and cortisol levels throughout the normal menstrual cycle of healthy postmenarcheal girls. Fertil Steril. 2001;76(3):615–617. [DOI] [PubMed] [Google Scholar]
- 54. Alagendran S, Archunan G, Prabhu SV, Orozco BEA, Guzman RG. Biochemical evaluation in human saliva with special reference to ovulation detection. Indian J Dent Res. 2010;21(2):165. [DOI] [PubMed] [Google Scholar]
- 55. De Muro P, Capobianco G, Formato M, et al. Glycosaminoglycan and transforming growth factor β1 changes in human plasma and urine during the menstrual cycle, in vitro fertilization treatment, and pregnancy. Fertil Steril. 2009;92(1):320–327. [DOI] [PubMed] [Google Scholar]
- 56. Alagendran S, Saibaba G, Muthukumar S, Rajkumar R, Guzman RG, Archunan G. Characterization of salivary protein during ovulatory phase of menstrual cycle through MALDI‐TOF/MS. Indian J Dent Res. 2013;24(2):157 [DOI] [PubMed] [Google Scholar]
- 57. Martinez AR, van Hooff MH, Schoute E, van der Meer M, Broekmans FJ, Hompes PG. The reliability, acceptability and applications of basal body temperature (BBT) records in the diagnosis and treatment of infertility. Eur J Obstet Gynecol Reprod Biol. 1992;47(2):121–127. [DOI] [PubMed] [Google Scholar]
- 58. Robinson JE, Wakelin M, Ellis JE. Increased pregnancy rate with use of the Clearblue Easy Fertility Monitor. Fertil Steril. 2007;87(2):329–334. [DOI] [PubMed] [Google Scholar]
- 59. Bonnar J, Flynn A, Freundl G, Kirkman R, Royston R, Snowden R. Personal hormone monitoring for contraception. Br J Fam Plann. 1999;24(4):128–134. [PubMed] [Google Scholar]
- 60. Wojtczak J, Bonadonna P. Pocket mobile smartphone system for the point‐of‐care submandibular ultrasonography. Am J Emerg Med. 2013;31(3):573–577. [DOI] [PubMed] [Google Scholar]
- 61. Leiva R, Bouchard T, Boehringer H, Abulla S, Ecochard R. Random serum progesterone threshold to confirm ovulation. Steroids. 2015;101:125–129. [DOI] [PubMed] [Google Scholar]
- 62. Papaioannou S, Aslam M, Al Wattar BH, Milnes RC, Knowles TG. User's acceptability of OvuSense: A novel vaginal temperature sensor for prediction of the fertile period. J Obstet Gynaecol. 2013;33(7):705–709. [DOI] [PubMed] [Google Scholar]
- 63. Salmassi A, Schmutzler AG, Püngel F, Schubert M, Alkatout I, Mettler L. Ovulation detection in saliva, is it possible. Gynecol Obstet Invest. 2013;76(3):171–176. [DOI] [PubMed] [Google Scholar]
- 64. Park JY, Kricka LJ. Prospects for the commercialization of chemiluminescence‐based point‐of‐care and on‐site testing devices. Anal Bioanal Chem. 2014;406(23):5631–5637. [DOI] [PubMed] [Google Scholar]
- 65. Chen Y, Kuo Z, Cheng C. Paper—a potential platform in pharmaceutical development. Trends Biotechnol. 2015;33(1):4–9. [DOI] [PubMed] [Google Scholar]
- 66. Eissa HM, Ahmed AM, Elsehely EA. Implementation of smart ovulation detection device. Recent Adv Biomed Chem Eng Mater Sci. 2014;82–86. [Google Scholar]
- 67. Guida M, Bramante S, Acunzo G, Pellicano M, Cirillo D, Nappi C. Diagnosis of fertility with a personal hormonal evaluation test. Minerva Ginecol. 2003;55(2):167–173. [PubMed] [Google Scholar]
- 68. Behre HM, Kuhlage J, Gaβner C, et al. Prediction of ovulation by urinary hormone measurements with the home use ClearPlan® Fertility Monitor: comparison with transvaginal ultrasound scans and serum hormone measurements. Hum Reprod. 2000;15(12):2478–2482. [DOI] [PubMed] [Google Scholar]
- 69. Melnick H, Goudas VT. The detection of a salivary ferning pattern using the knowhen ovulation monitoring system as an indication of ovulation. J Women's Health Care. 2015;4:235. [Google Scholar]
- 70. Rollason JC, Outtrim JG, Mathur RS. A pilot study comparing the DuoFertility® monitor with ultrasound in infertile women. Int J Womens Health. 2014;6:657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wu HC, Lin CY, Huang SH, Tseng MH. An intelligent saliva recognition system for women's ovulation detection. In: Asian Conference on Intelligent Information and Database Systems. Bali, Indonesia: Springer International Publishing; 2015:614‐623. [Google Scholar]
- 72. Bouchard TP, Genuis SJ. Personal fertility monitors for contraception. Can Med Assoc J. 2011;183(1):73–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Derda R, Tang SK, Laromaine A, et al. Multizone paper platform for 3D cell cultures. PLoS One. 2011;6(5):e18940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cheng JY, Feng MJ, Wu CC, Wang J, Chang TC, Cheng CM. Development of a sampling collection device with diagnostic procedures. Anal Chem. 2016;88(15):7591–7596. [DOI] [PubMed] [Google Scholar]
- 75. Cheng CM, Martinez AW, Gong J, et al. Paper‐based ELISA. Angew Chem Int Ed Engl. 2010;49(28):4771–4774. [DOI] [PubMed] [Google Scholar]


