Abstract
Background
The relationship between marital status and prognosis of kidney cancer has not been explored in detail. In this study, we aimed to investigate the impact of marital status on survival outcomes in kidney cancer.
Methods
We used the Surveillance, Epidemiology and End Results (SEER) program to identify 112860 patients with kidney cancer diagnosed in 2004 through 2013. Kaplan-Meier methods and multivariable Cox regression models were used to analyze the influence of marital status on overall survival (OS) and cancer-specific survival (CSS).
Results
Married patients had better 5-year OS and CSS compared with patients who were divorced/separated, widowed, and single. After adjusting for known confounders, unmarried patients were at greater risk of overall and cancer-specific mortality, especially the widowed. Moreover, subgroup analysis showed that married still had better prognosis across different SEER stages, ages and sexes.
Conclusions
Our study revealed that marriage is associated with better outcomes of both OS and CSS in kidney cancer patients.
Keywords: kidney cancer, marital status, SEER database, survival analysis
INTRODUCTION
Marital status has been considered as an independent prognostic factor of survival in several types of cancer [1–4]. It is well known that social factors may influence the outcome of various diseases [5]. Marital status is regarded as an important social support factor and can greatly affect the patient’s emotions, life style, and financial status [6, 7]. Previous studies have shown that being married has a protective role in health and cancer survival compared with being unmarried [8, 9].
Kidney cancer accounts for nearly 2% of all cancers, and there were an estimated 338,000 newly diagnosed cases and 143,000 deaths due to kidney cancer in 2012 [10]. In addition, 70% of new cases occurred in developed countries. Incidence and mortality rates of kidney cancer have been increasing in most countries over the past 30 years [11]. It is well-established that cigarette smoking, obesity and hypertension are risk factors for renal cell cancer [12]. However, little is known regarding the association between marital status and survival of patients with kidney cancer. In this study, we used data from the Surveillance, Epidemiology, and End Results (SEER) program to explore the impact of marital status on survival outcomes of kidney cancer patients.
RESULTS
Patient characteristics
We investigated a total of 112860 kidney cancer patients in the SEER database from 2004 to 2013, including 71549 (63.4%) male and 41311 (36.6%) female. Most patients are white (82.3%). According to marital status, 71328 (63.2%) were married at diagnosis, and 41352 (36.8%) were unmarried, including 11896 (10.5%) divorced/separated, 13160 (11.7%) widowed, and 16467 (14.6%) single. Compared with unmarried patients, the married patients were more likely to be diagnosed at an earlier stage and receive treatment. The widowed group had the highest percentage of women, the most elderly patients, and the lowest proportion of stage I and localized patients compared with the other groups and more were less likely to receive treatment. The baseline clinicopathological features are shown in Table 1.
Table 1. Baseline clinicopathological features of kidney cancer patients in SEER database.
| Characteristic | Total | Married | Divorced/Separated | Widowed | Single | P value |
|---|---|---|---|---|---|---|
| 112860(100) | 71328(63.2) | 11896(10.5) | 13160(11.7) | 16476(14.6) | ||
| Sex | <0.001 | |||||
| Male | 71549(63.4) | 50344(70.6) | 6697(56.3) | 3973(30.2) | 10535(63.9) | |
| Female | 41311(36.6) | 20984(29.4) | 5199(43.7) | 9187(69.8) | 5941(36.1) | |
| Age | <0.001 | |||||
| <60 | 43627(38.7) | 27709(38.8) | 5307(44.6) | 847(6.4) | 9764(59.3) | |
| 60-70 | 31801(28.2) | 21745(30.5) | 3859(32.4) | 2347(17.8) | 3850(23.4) | |
| 70-80 | 24332(21.6) | 15603(21.9) | 2093(17.6) | 4607(35.0) | 2029(12.3) | |
| >80 | 13100(11.6) | 6271(8.8) | 637(5.4) | 5359(40.7) | 833(5.1) | |
| Race | <0.001 | |||||
| White | 92909(82.3) | 60549(84.9) | 9474(79.6) | 10975(83.4) | 11911(72.3) | |
| Black | 13076(11.6) | 6045(8.5) | 1920(16.1) | 1535(11.7) | 3576(21.7) | |
| American Indian/ Alaska Native |
952(0.8) | 470(0.7) | 125(1.1) | 117(0.9) | 240(1.5) | |
| Asian/Pacific Islander | 5324(4.7) | 3853(5.4) | 318(2.7) | 505(3.8) | 648(3.9) | |
| Unknown | 599(0.5) | 411(0.6) | 59(0.5) | 28(0.2) | 101(0.6) | |
| Grade | <0.001 | |||||
| High/Moderate | 51438(45.6) | 33725(47.3) | 5421(45.6) | 4745(36.1) | 7547(45.8) | |
| Poor/Undifferentiation | 29071(25.7) | 19216(26.9) | 3052(25.7) | 2604(19.8) | 4199(25.5) | |
| Unknown | 32351(28.7) | 18387(25.8) | 3423(28.8) | 5811(44.2) | 4730(28.7) | |
| TNM | <0.001 | |||||
| I | 63996(56.7) | 41329(57.9) | 6733(56.6) | 6552(49.8) | 9382(56.9) | |
| II | 9309(8.2) | 5943(8.3) | 986(8.3) | 870(6.6) | 1510(9.2) | |
| III | 14165(12.6) | 9441(13.2) | 1358(11.4) | 1511(11.5) | 1855(11.3) | |
| IV | 17801(15.8) | 10524(14.8) | 2044(17.2) | 2552(19.4) | 2681(16.3) | |
| Unknown | 7589(6.7) | 4091(5.7) | 775(6.5) | 1675(12.7) | 1048(6.4) | |
| SEER Stage | <0.001 | |||||
| Localized | 76192(67.5) | 48989(68.7) | 8025(67.5) | 7877(59.9) | 11301(68.6) | |
| Regioned | 16162(14.3) | 10653(14.9) | 1555(13.1) | 1810(13.8) | 2144(13.0) | |
| Distant | 16786(14.9) | 9912(13.9) | 1932(16.2) | 2394(18.2) | 2548(15.5) | |
| Unstage | 3720(3.3) | 1774(2.5) | 384(3.2) | 1079(8.2) | 483(2.9) | |
| Therapy | <0.001 | |||||
| Surgery, radiation or both | 94075(83.4) | 62033(87.0) | 9803(82.4) | 8581(65.2) | 13658(82.9) | |
| No surgery, radiation | 17823(15.8) | 8845(12.4) | 1980(16.6) | 4354(33.1) | 2644(16.0) | |
| Unknown | 962(0.9) | 450(0.6) | 113(0.9) | 225(1.7) | 174(1.1) |
Impact of marital status on overall survival in the SEER database
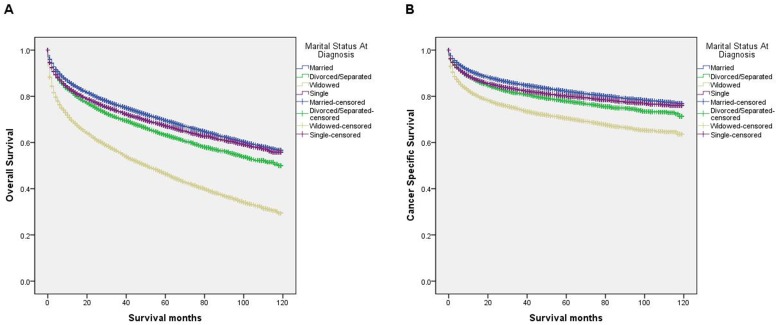
We performed a Kaplan-Meier analysis to reveal the difference in overall survival (OS) according to marital status (log-rank test p<0.001) (Figure 1A). The 5-year OS rate was 69.5% in the married group, 63.2% in the divorced/separated group, 46.4% in the widowed group, and 67.2% in the single group. In addition to marital status, the results of the Kaplan-Meier analysis indicated that sex, age, race, grade, TNM stage, SEER stage, and therapy were significantly associated with OS in these patients. Cox regression was also used to adjust the variables mentioned above in the multivariate analysis, and marital status was found to be an independent prognostic factor for overall survival (married, reference; divorced/separated, HR=1.25, 95% CI 1.21-1.30, P<0.001; widowed, HR=1.25, 95% CI 1.21-1.29, P<0.001; single, HR=1.18, 95% CI 1.14-1.22, P<0.001) (Table 2).
Figure 1. Survival curves in kidney cancer patients according to marital status.
(A) Overall survival: χ2 = 3122.46, P < 0.001. (B) Cancer specific survival:χ2 =1039.78, P < 0.001.
Table 2. Univariate and multivariate analysis for overall survival (OS) in kidney cancer patients.
| Variables | 5-year OS | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| Log rank Χ2 | P value | HR | 95% CI | P value | ||
| Sex | ||||||
| Male | 64.70% | 48.23 | <0.001 | Reference | ||
| Female | 67.50% | 0.89 | 0.87-0.91 | <0.001 | ||
| Age | ||||||
| <60 | 77.90% | 12093.60 | <0.001 | Reference | ||
| 60-70 | 68.70% | 1.38 | 1.34-1.42 | <0.001 | ||
| 70-80 | 58.60% | 1.84 | 1.78-1.89 | <0.001 | ||
| >80 | 32.20% | 2.61 | 2.52-2.71 | <0.001 | ||
| Race | ||||||
| White | 65.60% | 136.86 | <0.001 | Reference | ||
| Black | 65.00% | 1.07 | 1.04-1.11 | <0.001 | ||
| American Indian/Alaska Native | 63.40% | 0.97 | 0.87-1.09 | 0.587 | ||
| Asian/Pacific Islander | 68.50% | 0.87 | 0.82-0.91 | <0.001 | ||
| Unknown | 90.50% | 0.31 | 0.23-0.42 | <0.001 | ||
| Grade | ||||||
| High/Moderate | 83.10% | 16886.37 | <0.001 | Reference | ||
| Poor/Undifferentiation | 60.50% | 1.70 | 1.65-1.75 | <0.001 | ||
| Unknown | 42.00% | 1.65 | 1.60-1.71 | <0.001 | ||
| TNM | ||||||
| I | 83.10% | 59552.13 | <0.001 | Reference | ||
| II | 76.80% | 1.43 | 1.36-1.50 | <0.001 | ||
| III | 63.10% | 0.83 | 0.75-0.93 | 0.001 | ||
| IV | 10.30% | 2.04 | 1.81-2.30 | <0.001 | ||
| Unknown | 42.90% | 1.54 | 1.44-1.65 | <0.001 | ||
| SEER Stage | ||||||
| Localized | 81.60% | 61354.67 | <0.001 | Reference | ||
| Regioned | 58.90% | 2.73 | 2.46-3.03 | <0.001 | ||
| Distant | 9.40% | 4.27 | 3.79-4.81 | <0.001 | ||
| Unstage | 25.90% | 1.69 | 1.57-1.83 | <0.001 | ||
| Marital Status | ||||||
| Married | 69.50% | 3122.46 | <0.001 | Reference | ||
| Divorced/Separated | 63.20% | 1.25 | 1.21-1.30 | <0.001 | ||
| Widowed | 46.40% | 1.25 | 1.21-1.29 | <0.001 | ||
| Single | 67.20% | 1.18 | 1.14-1.22 | <0.001 | ||
| Therapy | ||||||
| Surgery, radiation or both | 75.00% | 39178.54 | <0.001 | Reference | ||
| No surgery, radiation | 17.60% | 2.49 | 2.41-2.56 | <0.001 | ||
| Unknown | 30.40% | 2.30 | 2.11-2.50 | <0.001 | ||
Impact of marital status on cancer-specific survival in the SEER database
Similarly, the survival curve showed that cancer-specific survival (CSS) among groups with different marital status was significant (log-rank test P<0.001) (Figure 1B). The 5-year CSS rates for the married group, the divorced/separated group, the widowed group and the single group were 82.1%, 77.8%, 70.4% and 80.1%, respectively. Among clinicopathological variables, sex, age, race, grade, TNM stage, SEER stage, therapy, and marital status were identified as risk factors for predicting CSS based on a Kaplan-Meier analysis. When a multivariate analysis with Cox regression was performed, marital status was confirmed to be an independent prognostic factor for kidney cancer prognosis (married, reference; divorced/separated, HR=1.20, 95% CI 1.15-1.26, P<0.001; widowed, HR=1.24, 95% CI 1.19-1.30, P<0.001; single, HR=1.14, 95% CI 1.09-1.19, P<0.001) (Table 3). In addition, age (≥ 60y), poor/undifferentiated, TNM stage, SEER stage, and no surgery and/or radiotherapy were associated with poorer CSS.
Table 3. Univariate and multivariate analysis for cancer specific survival (CSS) in kidney cancer patients.
| Variables | 5-year CSS | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| Log rank Χ2 | P value | HR | 95% CI | P value | ||
| Sex | ||||||
| Male | 79.50% | 13.26 | <0.001 | Reference | ||
| Female | 81.00% | 1.01 | 0.98-1.04 | 0.524 | ||
| Age | ||||||
| <60 | 84.10% | 2813.62 | <0.001 | Reference | ||
| 60-70 | 81.20% | 1.07 | 1.03-1.11 | 0.001 | ||
| 70-80 | 79.00% | 1.14 | 1.09-1.19 | <0.001 | ||
| >80 | 63.30% | 1.52 | 1.45-1.60 | <0.001 | ||
| Race | ||||||
| White | 79.90% | 79.39 | <0.001 | Reference | ||
| Black | 81.60% | 0.99 | 0.95-1.04 | 0.736 | ||
| American Indian/Alaska Native | 75.90% | 1.00 | 0.87-1.16 | 0.970 | ||
| Asian/Pacific Islander | 78.90% | 0.98 | 0.92-1.05 | 0.583 | ||
| Unknown | 93.60% | 0.42 | 0.29-0.61 | <0.001 | ||
| Grade | ||||||
| High/Moderate | 93.90% | 12360.56 | <0.001 | Reference | ||
| Poor/Undifferentiation | 72.30% | 2.22 | 2.12-2.33 | <0.001 | ||
| Unknown | 62.90% | 2.12 | 2.02-2.23 | <0.001 | ||
| TNM | ||||||
| I | 96.20% | 65843.40 | <0.001 | Reference | ||
| II | 87.90% | 3.07 | 2.84-3.32 | <0.001 | ||
| III | 76.90% | 1.28 | 1.09-1.51 | 0.003 | ||
| IV | 17.20% | 3.75 | 3.16-4.46 | <0.001 | ||
| Unknown | 67.20% | 2.57 | 2.28-2.90 | <0.001 | ||
| SEER Stage | ||||||
| Localized | 94.90% | 67229.90 | <0.001 | Reference | ||
| Regioned | 73.20% | 4.55 | 3.91-5.30 | <0.001 | ||
| Distant | 15.90% | 8.24 | 7.00-9.73 | <0.001 | ||
| Unstage | 52.80% | 2.77 | 2.44-3.14 | <0.001 | ||
| Marital Status | ||||||
| Married | 82.10% | 1039.78 | <0.001 | Reference | ||
| Divorced/Separated | 77.80% | 1.20 | 1.15-1.26 | <0.001 | ||
| Widowed | 70.40% | 1.24 | 1.19-1.30 | <0.001 | ||
| Single | 80.10% | 1.14 | 1.09-1.19 | <0.001 | ||
| Therapy | ||||||
| Surgery, radiation or both | 86.00% | 21427.48 | <0.001 | Reference | ||
| No surgery, radiation | 42.00% | 2.09 | 2.00-2.18 | <0.001 | ||
| Unknown | 53.20% | 2.25 | 2.01-2.51 | <0.001 | ||
Subgroup analysis for evaluating the effect of marital status on CSS
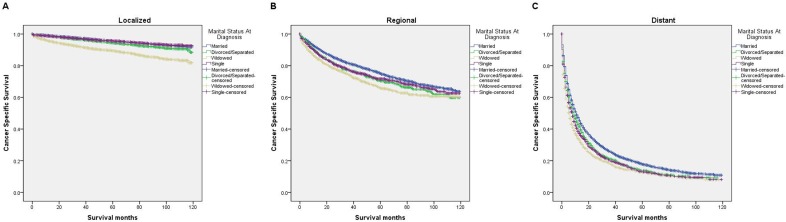
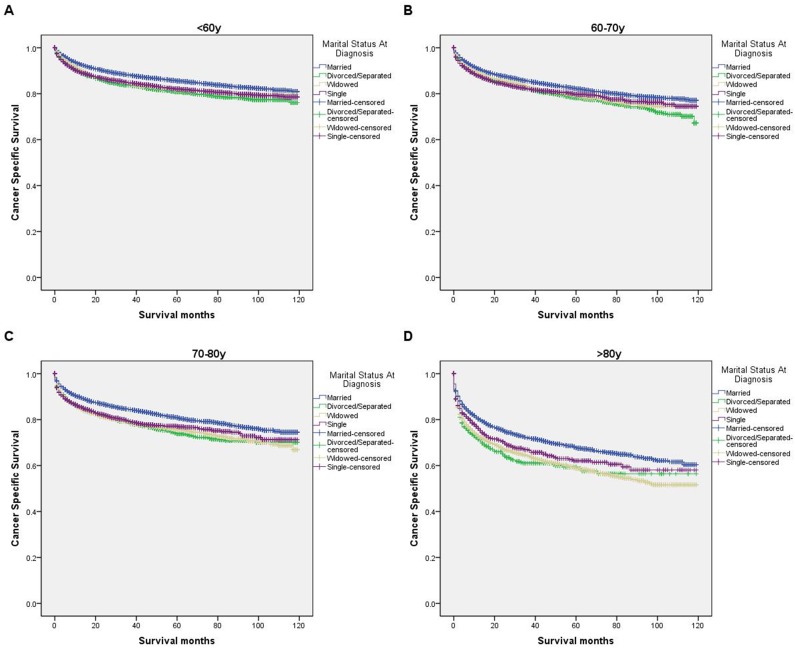
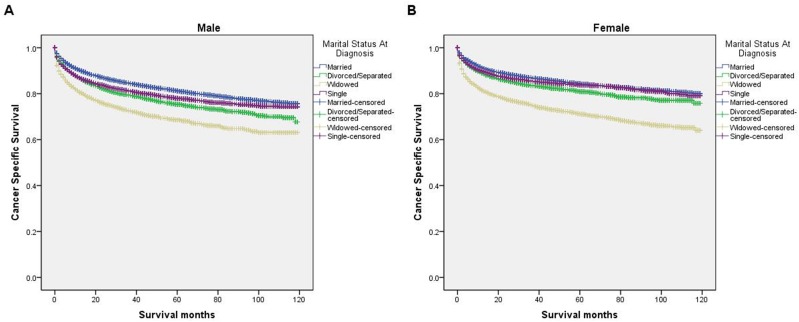
We further explored the effects of marital status on CSS according to SEER stage (Figure 2 and Table 4). Marital status was still an independent prognostic factor at each stage, both in the univariate and multivariate analysis (P<0.001). Moreover, we observed some interesting findings. The widowed patients had poorer CSS in localized, regional and distant stages compared with the other groups, and being widowed was associated with a higher risk of mortality compared with being married in the localized stage (HR=1.51, 95% CI 1.36-1.67, P<0.001) and the distant stage (HR=1.22, 95% CI 1.08-1.37, P<0.001). The survival difference between the single and married groups was not apparent in the localized stage (P=0.679). Furthermore, a previous study showed that prognosis of kidney cancer was associated with increasing age [13]. Therefore, we further analyzed the survival rates and hazard according to age (Figure 3 and Table 5). Married patients always had better survival in each age group, which were consistent with the above results. However, no survival discrepancy was found among divorced/separated, widowed and single patients. We also performed a further stratified analysis by sex. Unmarried patients were shown to have poorer outcomes both in males and females, which was consistent with the above results (Figure 4 and Table 6).
Figure 2. Survival curves in kidney cancer patients on CSS according to marital status in different SEER stages.
(A) Localized: χ2 = 554.76, P < 0.001; (B) regional: χ2 = 63.77, P < 0.001; (C) distant: χ2 = 198.85, P < 0.001.
Table 4. Univariate and multivariate analysis for evaluating marital status on CSS according to different SEER stage.
| Variables | 5-year CSS | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| Log rank Χ2 | P value | HR | 95% CI | P value | ||
| Localized | ||||||
| Marital status | 554.76 | <0.001 | ||||
| Married | 95.60% | Reference | ||||
| Divorced/Separated | 94.50% | 1.33 | 1.18-1.49 | <0.001 | ||
| Widowed | 89.50% | 1.51 | 1.36-1.67 | <0.001 | ||
| Single | 95.60% | 1.15 | 1.03-1.29 | 0.013 | ||
| Regional | ||||||
| Marital status | 63.77 | <0.001 | ||||
| Married | 75.00% | Reference | ||||
| Divorced/Separated | 70.50% | 1.11 | 0.99-1.25 | 0.072 | ||
| Widowed | 65.70% | 1.06 | 0.95-1.19 | 0.311 | ||
| Single | 71.60% | 1.11 | 1.00-1.23 | 0.050 | ||
| Distant | ||||||
| Marital status | 198.85 | <0.001 | ||||
| Married | 17.70% | Reference | ||||
| Divorced/Separated | 13.90% | 1.08 | 0.95-1.22 | 0.271 | ||
| Widowed | 12.60% | 1.22 | 1.08-1.37 | 0.001 | ||
| Single | 13.10% | 1.20 | 1.07-1.35 | 0.002 | ||
Figure 3. Survival curves in kidney cancer patients on CSS according to marital status in ages.
(A) <60y: χ2 = 116.50, P < 0.001; (B) 60-70y: χ2 = 54.83, P < 0.001; (C) 70-80y: χ2 = 100.93, P < 0.001 (D) >80y: χ2 = 101.93, P < 0.001.
Table 5. Univariate and multivariate analysis for evaluating marital status on CSS according to different age.
| Variables | 5-year CSS | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| Log rank Χ2 | P value | HR | 95% CI | P value | ||
| <60y | ||||||
| Marital status | 116.50 | <0.001 | ||||
| Married | 85.60% | Reference | ||||
| Divorced/Separated | 80.80% | 1.18 | 1.09-1.27 | <0.001 | ||
| Widowed | 81.80% | 1.22 | 1.03-1.46 | 0.025 | ||
| Single | 82.10% | 1.14 | 1.07-1.22 | <0.001 | ||
| 60-70y | ||||||
| Marital status | 54.83 | <0.001 | ||||
| Married | 82.20% | Reference | ||||
| Divorced/Separated | 78.30% | 1.17 | 1.08-1.27 | <0.001 | ||
| Widowed | 79.40% | 1.21 | 1.08-1.34 | 0.001 | ||
| Single | 79.80% | 1.13 | 1.03-1.23 | 0.006 | ||
| 70-80y | ||||||
| Marital status | 100.93 | <0.001 | ||||
| Married | 80.90% | Reference | ||||
| Divorced/Separated | 73.90% | 1.23 | 1.11-1.37 | <0.001 | ||
| Widowed | 75.70% | 1.24 | 1.14-1.35 | <0.001 | ||
| Single | 77.00% | 1.15 | 1.03-1.28 | 0.013 | ||
| >80y | ||||||
| Marital status | 101.93 | <0.001 | ||||
| Married | 67.70% | Reference | ||||
| Divorced/Separated | 59.30% | 1.25 | 1.08-1.45 | 0.003 | ||
| Widowed | 58.80% | 1.16 | 1.08-1.26 | <0.001 | ||
| Single | 62.10% | 1.07 | 0.93-1.23 | 0.328 | ||
Figure 4. Survival curves in kidney cancer patients on CSS according to marital status in different sexes.
(A) Male: χ2 = 465.71, P < 0.001; (B) female: χ2 = 741.71, P < 0.001.
Table 6. Univariate and multivariate analysis for evaluating marital status on CSS according to sex.
| Variables | 5-year CSS | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| Log rank Χ2 | P value | HR | 95% CI | P value | ||
| Male | ||||||
| Marital status | 465.71 | <0.001 | ||||
| Married | 81.20% | Reference | ||||
| Divorced/Separated | 75.40% | 1.22 | 1.15-1.29 | <0.001 | ||
| Widowed | 68.60% | 1.28 | 1.20-1.38 | <0.001 | ||
| Single | 77.90% | 1.17 | 1.11-1.23 | <0.001 | ||
| Female | ||||||
| Marital status | 741.71 | <0.001 | ||||
| Married | 84.30% | Reference | ||||
| Divorced/Separated | 80.90% | 1.16 | 1.07-1.25 | <0.001 | ||
| Widowed | 71.20% | 1.14 | 1.07-1.22 | <0.001 | ||
| Single | 83.80% | 1.05 | 0.97-1.14 | 0.218 | ||
DISCUSSION
In this study, we explored the influence of marriage on OS and CSS in kidney cancer patients based on the SEER program. Our study revealed that married patients had better survival outcomes than unmarried patients, including divorced or separated, widowed, and single patients. In addition, the widowed were at a higher risk of mortality compared with other unmarried groups. Both univariate and multivariate analysis indicated that marital status was an independent prognostic factor for predicting OS and CSS of kidney cancer. Moreover, the survival discrepancy between married and unmarried still existed across different sexes, ages and SEER stages.
The results of our study showed that married patients can enjoy a survival advantage compared with unmarried patients, in agreement with previous studies on other types of cancer [1–4]. However, the mechanisms that drive this correlation are not yet clearly understood. We propose the following underlying mechanisms: Psychologically, depression is frequently observed in cancer patients [14]. Meta-analyses have shown that the depression in cancer patients can increase cancer mortality by 19% to 39% [15, 16]. A recent study showed that depression was significantly associated with a comparatively higher incidence of hospital admissions, emergency department visits, and health system utilization [17, 18]. Married patients have a lower risk of psychological distress, anxiety and depression, as their spouses can help share their emotional burden. Compared with married patients, unmarried patients showed not only greater levels of psychological distress but also lower levels of the fighting spirit and higher levels of helplessness [19]. Cancer survivors in the United States reported medication use for anxiety and depression at rates nearly two times those reported by the general public, and unmarried are more likely to use medication [20]. In addition, psychological stress may cause poorer adherence to treatment [21].
Physiologically, marriage may have direct influences on cardiovascular, endocrine, immune, neurosensory, and other physiological functions [22]. Endocrine hormones, such as cortisol and catecholamine, can promote tumor growth and metastasis [23]. Married individuals had lower cortisol levels than their never married and previously married counterparts. Cortisol could be regarded as one candidate mechanism accounting for the association of marital status and health [24]. Additionally, the hypothalamus-pituitary-adrenal axis may also have an effect on the immune system, leading to poorer survival [25, 26].
Another widely accepted explanation of why married patients have better prognosis of cancer and other diseases is that married patients have better social-economic support. It has been reported that married persons are more likely to have better access to medical care and more financial support than unmarried persons [8]. In addition, married couples may have wider health insurance coverage [27]. Thus, spouses might contribute to earlier disease detection [8]. These factors could partly explain why married patients corresponded to a higher percentage of earlier stage cancer in our study. Married patients also have better adherence with prescribed treatments. We found that unmarried, especially widowed, had a lower percentage of surgery and radiotherapy, which could be attributed to the survival difference between the married and unmarried patients. In addition, lifestyles, such as diet habit and behaviors, may also be influenced by marriage [28]. For example, it is well accepted that smoking increases the risk of kidney cancer. Studies show that living without a spouse increases daily smoking rates [29]. Moreover, marriage is associated with an increased probability of cessation for men [30].
Nevertheless, some limitations should be noted. First, the SEER database only provides marital status at the time of diagnosis, which may change after diagnosis. In addition, the quality of the marriage could not be evaluated in the follow-up. SEER also does not record cohabitation status, patients cohabitating with their partners may be categorized as unmarried in the SEER database. Second, we had no direct information on socioeconomic factors, such as educational information, income status, insurance status, and lifestyle (such as smoking and alcohol use), which are not recorded in the SEER database. Third, the SEER database is unable to provide other important survival factors, such as chemotherapy, details of surgery, and other types of therapy. Lastly, as a retrospective study, we could not avoid various forms of bias.
Despite these potential limitations, our study revealed that married kidney cancer patients had survival advantages, while unmarried patients were at higher risk of overall and cancer-specific mortality. We supposed that psychological, physiological and social-economic factors may contribute more to survival outcomes among married patients. Physicians should realize this significant survival difference according to marital status. More social support services and medical interventions should be provided for unmarried patients. Furthermore, future studies should focus on the mechanisms among marital functioning, physiology, and health and on genetic and other variable explanations for marriage-related health outcomes. Additional studies of objective social behavior after changes in marital status are also warranted.
MATERIALS AND METHODS
Data source and patient selection
This study used data from the SEER database released in March 2017, which covered approximately 28% of the United States population. By using SEER-stat software (version 8.3.4), we searched the database for patients diagnosed between 2004 and 2013 with kidney cancer. Patients aged 18 or older at the time of diagnosis with kidney cancer (International Classification of Diseases for Oncology, Third Edition [ICD-O-3], code C64.9) were included for analysis. Patients were excluded if they had unknown marital status, unknown cause of death or unknown survival time. A total of 112860 patients were included in the cohort. This study was based on public data from the SEER database; we obtained permission to access research data files with the reference number 14920-Nov2015. Because this study did not include the use of human subjects or personal identifying information, this study did not require informed consent.
Study variables
Variables extracted from the SEER database included marital status, sex, age at diagnosis, race, histological grade, TNM stage, SEER stage, and selection of therapy (surgery/radiotherapy). Marital status is coded as married, divorced or separated, widowed, and single. Age at diagnosis was divided into four groups: <60 years, 60-70 years, 70-80 years, and ≥80 years. Race was classified as white, black, American Indian/Alaska Native, Asian/Pacific Islander, or unknown. Histological grade was classified as well/moderately differentiated, poorly differentiated/undifferentiated, or unknown. SEER stage was categorized as localized, regional, distant, or unknown. Selection of therapy was divided into three groups: surgery or radiation or both, no surgery or radiation, and unknown.
Outcomes
The primary outcomes were OS and CSS. OS was calculated from the date of diagnosis to the date of death from any cause. Patients who were alive on the date of last contact or at the follow-up cut-off date were censored. CSS was defined as the time from diagnosis to the date of death due to kidney cancer. Death caused by kidney cancer was considered an event. Patients who died from other causes or who were still alive at the time of the last follow-up were treated as censored. The follow-up cut-off date was December 31, 2013, according to the SEER database.
Statistical analysis
The association between marital status and clinicopathological features was assessed by the chi-square (χ2) test. Survival curves were generated by the Kaplan-Meier method; differences between the curves were analyzed by using the Log-rank test. Multivariable Cox proportional hazards regression models were used to assess potential risk factors for survival outcomes. All statistical analyses were performed using SPSS for Windows, version 19 (SPSS Inc., Chicago, IL, USA). All P values were 2-sided, and P<0.05 was considered statistical significance.
Acknowledgments
We thank the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries for providing high quality open resources for researchers. This work was supported by grants from Zhejiang Provincial Natural Science Foundation of China (LY12H07003).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Li Q, Gan L, Liang L, Li X, Cai S. The influence of marital status on stage at diagnosis and survival of patients with colorectal cancer. Oncotarget. 2015;6:7339–47. doi: 10.18632/oncotarget.3129. https://doi.org/10.18632/oncotarget.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi RL, Qu N, Lu ZW, Liao T, Gao Y, Ji QH. The impact of marital status at diagnosis on cancer survival in patients with differentiated thyroid cancer. Cancer Med. 2016;5:2145–54. doi: 10.1002/cam4.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li M, Dai CY, Wang YN, Chen T, Wang L, Yang P, Xie D, Mao R, Chen C. Marital status is an independent prognostic factor for tracheal cancer patients: an analysis of the SEER database. Oncotarget. 2016;7:77152–62. doi: 10.18632/oncotarget.12809. https://doi.org/10.18632/oncotarget.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aizer AA, Chen MH, McCarthy EP, Mendu ML, Koo S, Wilhite TJ, Graham PL, Choueiri TK, Hoffman KE, Martin NE, Hu JC, Nguyen PL. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31:3869–76. doi: 10.1200/JCO.2013.49.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Preda A, Voigt K. The social determinants of health: why should we care. Am J Bioeth. 2015;15:25–36. doi: 10.1080/15265161.2014.998374. [DOI] [PubMed] [Google Scholar]
- 6.Byers TE, Wolf HJ, Bauer KR, Bolick-Aldrich S, Chen VW, Finch JL, Fulton JP, Schymura MJ, Shen T, Van Heest S, Yin X. The impact of socioeconomic status on survival after cancer in the United States: findings from the National Program of Cancer Registries Patterns of Care Study. Cancer. 2008;113:582–91. doi: 10.1002/cncr.23567. [DOI] [PubMed] [Google Scholar]
- 7.Goodwin JS, Hunt WC, Key CR, Samet JM. The effect of marital status on stage, treatment, and survival of cancer patients. JAMA. 1987;258:3125–30. [PubMed] [Google Scholar]
- 8.Rendall MS, Weden MM, Favreault MM, Waldron H. The protective effect of marriage for survival: a review and update. Demography. 2011;48:481–506. doi: 10.1007/s13524-011-0032-5. [DOI] [PubMed] [Google Scholar]
- 9.Kissane DW. Marriage is as protective as chemotherapy in cancer care. J Clin Oncol. 2013;31:3852–3. doi: 10.1200/JCO.2013.51.5080. [DOI] [PubMed] [Google Scholar]
- 10.Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015;67:519–30. doi: 10.1016/j.eururo.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7:245–57. doi: 10.1038/nrurol.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capitanio U, Montorsi F. Renal cancer. Lancet. 2017;387:894–906. doi: 10.1016/S0140-6736(15)00046-X. [DOI] [PubMed] [Google Scholar]
- 13.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Five-year survival after surgical treatment for kidney cancer: a population-based competing risk analysis. Cancer. 2007;109:1763–8. doi: 10.1002/cncr.22600. [DOI] [PubMed] [Google Scholar]
- 14.Chochinov HM. Depression in cancer patients. Lancet Oncol. 2001;2:499–505. doi: 10.1016/S1470-2045(01)00456-9. [DOI] [PubMed] [Google Scholar]
- 15.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115:5349–61. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- 16.Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med. 2010;40:1797–810. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mausbach BT, Irwin SA. Depression and healthcare service utilization in patients with cancer. Psychooncology. 2016;26:1133–9. doi: 10.1002/pon.4133. https://doi.org/10.1002/pon.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gunjur A. The added effect of depression on patients with cancer. Lancet Oncol. 2016;17:e225. doi: 10.1016/S1470-2045(16)30111-5. [DOI] [PubMed] [Google Scholar]
- 19.Goldzweig G, Andritsch E, Hubert A, Brenner B, Walach N, Perry S, Baider L. Psychological distress among male patients and male spouses: what do oncologists need to know. Ann Oncol. 2010;21:877–83. doi: 10.1093/annonc/mdp398. [DOI] [PubMed] [Google Scholar]
- 20.Hawkins NA, Soman A, Buchanan LN, Leadbetter S, Rodriguez JL. Use of medications for treating anxiety and depression in cancer survivors in the United States. J Clin Oncol. 2017;35:78–85. doi: 10.1200/JCO.2016.67.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–7. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 22.Kiecolt-Glaser JK, Newton TL. Marriage and health: his and hers. Psychol Bull. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- 23.Moreno-Smith M, Lutgendorf SK, Sood AK. Impact of stress on cancer metastasis. Future Oncol. 2010;6:1863–81. doi: 10.2217/fon.10.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chin B, Murphy ML, Janicki-Deverts D, Cohen S. Marital status as a predictor of diurnal salivary cortisol levels and slopes in a community sample of healthy adults. Psychoneuroendocrinology. 2017;78:68–75. doi: 10.1016/j.psyneuen.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5:617–25. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- 26.Dhabhar FS. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol Res. 2014;58:193–210. doi: 10.1007/s12026-014-8517-0. [DOI] [PubMed] [Google Scholar]
- 27.Peters HE, Simon K, Taber JR. Marital disruption and health insurance. Demography. 2014;51:1397–421. doi: 10.1007/s13524-014-0317-6. [DOI] [PubMed] [Google Scholar]
- 28.Umberson D. Gender, marital status and the social control of health behavior. Soc Sci Med. 1992;34:907–17. doi: 10.1016/0277-9536(92)90259-s. [DOI] [PubMed] [Google Scholar]
- 29.Pennanen M, Broms U, Korhonen T, Haukkala A, Partonen T, Tuulio-Henriksson A, Laatikainen T, Patja K, Kaprio J. Smoking, nicotine dependence and nicotine intake by socio-economic status and marital status. Addict Behav. 2014;39:1145–51. doi: 10.1016/j.addbeh.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Broms U, Silventoinen K, Lahelma E, Koskenvuo M, Kaprio J. Smoking cessation by socioeconomic status and marital status: the contribution of smoking behavior and family background. Nicotine Tob Res. 2004;6:447–55. doi: 10.1080/14622200410001696637. [DOI] [PubMed] [Google Scholar]