Abstract
Stem cell sources for cell‐based therapeutics are often screened for infectious agents and genetic diseases prior to implantation; however, there are other risk factors that are often overlooked, which may ultimately lead to less efficacious clinical outcomes. One such risk factor is exposure of mesenchymal stem cells (MSCs) to cigarette smoke or nicotine. Recent data have shown that exposure to cigarette smoke or nicotine leads to decreased regenerative potential, namely decreased proliferation, decreased migration, and decreased differentiation potential of exposed MSCs. This review provides a brief introduction into MSCs and their respective niches and a summary regarding the interactions of cigarettes and nicotine with MSCs populations. Specifically, the effects of cigarette smoke and nicotine on the regenerative potential of MSCs (i.e., proliferation, migration, and differentiation) will be covered with an emphasis on considerations for the development of future cell‐based clinical trials and therapies. stem cells translational medicine 2017;6:1815–1821
Keywords: Nicotine, Tobacco products, Mesenchymal stem cells, Cell‐based and tissue‐based therapy, Electronic cigarettes
Significance Statement.
Recent translational approaches using mesenchymal stem cells (MSCs) have proven to be very effective for the treatment of a variety of injuries and diseases. However, compromising MSC function, through infectious or genetic diseases for example, can lead to ineffective clinical outcomes. Accordingly, these conditions are often included as exclusion criteria in patient recruitment for stem cell‐based therapies. However, environmental risk factors, such as cigarette smoking and nicotine use, can also compromise MSC function, leading to inefficacious outcomes. Recent data have demonstrated that cigarette smoking and nicotine exposure can negatively affect MSC regeneration potential (i.e., proliferation, migration, and differentiation). This review serves to provide evidence as to why cigarette smoking and nicotine usage deserve further consideration as exclusion criteria when designing future stem cell‐based trials and therapies.
Introduction
Mesenchymal stem cells (MSCs) are one of the most investigated sources of therapeutic stem cells for cell‐based translational approaches. MSCs have shown to be multipotent, able to secrete soluble factors that induce wound healing, display a lack of immune response after transplantation, and are nontumorigenic 1, 2. Complying with good manufacturing practices requires that, prior to transplantation, recipients and donors of these cells are screened for genetic and/or infectious agents that could alter MSC biological function and undermine treatment efficacy 3. However, there are other, often overlooked, risk factors that may similarly contribute to less efficacious clinical outcomes. One example is exposure to cigarette smoke or one of its primary components, nicotine. Both whole cigarette smoke extract (CSE) and nicotine have been shown to negatively impact the regenerative capacity of MSCs 4, 5, 6, 7, 8.
Cigarette smoking is the leading cause of preventable death worldwide. Despite initiatives to curb smoking in the U.S., 15.1% of U.S. adults actively smoke cigarettes 9. Cigarette smoke exposure increases the risk of various cancers 9 and systemic diseases 9 in smokers; however, smoking is a difficult habit to quit due to the addictive property of nicotine. Nicotine replacement therapies (NRT) have been developed to help smokers overcome their nicotine addiction through gradually decreasing nicotine dose delivery regimens. NRTs, which come in the form of gums, transdermal patches, and lozenges, are Food and Drug Administration (FDA) approved and, under proper use, are effective aids to help smokers overcome their nicotine addiction. The electronic cigarette (ecig) is a new device that has been marketed as a “safer alternative” to cigarette smoking. However, unlike the gum, patches, and lozenges, these devices have not been fully regulated by the FDA and are still believed to cause major health concerns due to the concentrated delivery of toxic chemicals like nicotine, among others 10, 11. Additionally, due to a lack of regulation, labeling and dosage of nicotine can vary widely among ecig products. Despite these concerns, ecig use continues to be on the rise. In the U.S. alone, ecig use has doubled amongst U.S. adults 12 and tripled amongst adolescents 9 within the last 5 years. This rapidly increasing user base, along with active smokers, necessitates that researchers and study coordinators are well versed on the effects of nicotine on MSCs and obtain detailed history on potential patient's nicotine usage.
Recent data have shown that exposure to whole cigarette smoke 8, 13 or even individual compounds such as nicotine 4, 5, 6, the predominant toxin found in ecig vapors 14 and the addictive component of tobacco 15, significantly inhibits the regenerative potential of MSCs. These cigarette and nicotine‐induced changes to MSC biological function have been argued by some to be the underlying causes of various smoking‐related diseases 16. Yet, despite these results, the majority of clinical trial recruitment sites omit or disregard tobacco and/or nicotine usage history as exclusion criteria for patients donating or receiving MSCs for therapeutic applications, especially for applications related to cigarette smoking diseases. This review will highlight how nicotine is distributed to the various tissues in the body, and the effects of nicotine or cigarette smoke exposure on MSC regenerative capacity (proliferation, migration, and differentiation). It is our hope that the evidence presented in this review will support efforts for a more thorough screening of MSC donors and recipients to determine whether the cells being used for research, development, and/or transplantation have been exposed to toxic levels of cigarette smoke or nicotine that could potentially render them ineffective.
In compiling this review, we conducted all searches through the PubMed database (http://www.ncbi.nlm.nih.gov/pubmed). Search terms included the following: (mesenchymal stem cells OR stem cell) AND (nicotine OR cigarette smoking OR cigarettes OR electronic cigarettes OR cigarettes smoke extract OR ecig). Due to limited search results all species were included in this search. Background data were collected over all time frames; however, all articles studying the effects of nicotine on mesenchymal stem cells have been published within the last 10 years to ensure that this article reviews the current state of the field.
Route of Exposure and Interaction with MSCs
Exposure to cigarette smoke, which contains over 7,000 chemicals and 250 known toxins 8, can occur through several different methods. Inhalation of mainstream or “first‐hand” smoke is the predominant form of toxic exposure for active smokers and is generated from the filtered end of the cigarette 17. Smoke produced from the lit end of the cigarette is considered sidestream or “second‐hand” smoke and affects both active smokers and innocent bystanders 17. “Third‐hand” smoke is also a possible route of exposure occurring through direct contact with surfaces containing main‐ or sidestream smoke deposits 18. Exposure to ecig vapor can occur in similar fashions, except ecigs do not produce any “sidestream” smoke.
Internalization of smoking and vaping‐related toxins occurs primarily through the respiratory system (Fig. 1). For specific compounds like nicotine, the subsequent absorption into specific tissues is largely dependent on tissue pH 19. Nicotine is a weak base (pKa of 8.0) 19 and is more readily absorbed in slightly basic conditions where it is less “ionized” 19. The oral cavity, the initial site of smoke exposure, is a slightly acidic environment. Flue‐cured cigarette smoke is also slightly acidic (pH 5.5–6.0), thus the two conditions do not make for efficient absorption in the oral cavity 19. However, recent reports suggest that cigarette smoke may be more alkaline than originally thought 19, thereby improving oral nicotine absorption. Ecig liquids, on the other hand, are characterized by a slightly more basic pH 20. Therefore, nicotine delivered from these devices is believed to be more readily absorbed in the mouth. Nicotine has also been measured in smoker saliva, which traps ionized nicotine and maintains elevated levels of exposure in the mouth. In fact, salivary nicotine has been measured to be almost 87 times higher than in the blood plasma 21. Exposure to such concentrated doses of nicotine is dangerous to MSC subpopulations residing in the oral cavity. Periodontal ligament derived stem cells (PDLSCs), for example, reside in the periodontal ligament (PDL) and give rise to tooth supporting structures such as alveolar bone, PDL, and cementum 22. In addition to their regenerative capabilities, PDLSCs are easily isolated following natural tooth loss or routine dental procedures 22, 23 and have had recent success in clinical trials 24. Accordingly, PDLSCs are an ideal source of therapeutic stem cells. However, several recent reports have shown that nicotine exposure can negatively impact the regenerative potential of these cells. After the oral cavity, the smoke/vapor passes into the lungs, where the physiological pH (7.4) and large surface area of the alveoli facilitate rapid absorption of nicotine into the bloodstream for subsequent total body distribution via the circulatory system 19. Table 1 provides peak nicotine concentration measurements taken from the saliva and blood plasma of smokers after cigarette or ecig use. These values represent the typical concentrations that tissue restricted MSCs might experience during use.
Figure 1.
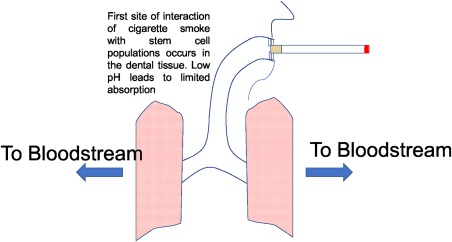
Routes of exposure for stem cells to cigarette smoke. Mainstream smoke is inhaled by the users, where some nicotine is deposited in the oral tissue, and continues onto the lungs, where it is readily absorbed and carried throughout the body via the bloodstream.
Table 1.
Peak mean nicotine concentrations measured in blood plasma and saliva after cigarette and ecig use
| Peak nicotine concentrations (μM) | |||||
|---|---|---|---|---|---|
|
Measurement location |
N |
Cigarette smoker Mean (SD) |
N |
Ecig vapor Mean (SD) |
Refs. |
| Blood plasma (Venous) | 24 | 0.180 (0.067) | 23 | 0.138 (0.047) | [20] |
| 6 | 0.147 (0.017) | N/A | N/A | [21] | |
| 10 | 0.115 (0.038) | N/A | N/A | [22] | |
| 10 | N.R. | 11 | 0.152 (0.072) | [23] | |
| N/A | N/A | 13 | 0.118 (0.014) | [24] | |
| N/A | N/A | 16 | 0.105 (0.110) | [25] | |
| Saliva | 12 | 14.478 (7.775) | N/A | N/A | [26] |
| N.R. | 8.605 (N.R.) | N.R. | 5.301 | [27] | |
| 36 | 1.276 (N.R.) | N/A | N/A | [28] | |
| 42 | 1.073 (N.R.) | N/A | N/A | [29] | |
| 122 | 0.398 (0.021) | N/A | N/A | [30] | |
The variation in mean nicotine values across studies is due to differences in study methods.
Abbreviations: N/A, not applicable; N.R., not reported or mentioned.
Once distributed within the tissues, nicotine is capable of interacting with resident stem cell populations through nicotinic receptors. Nicotinic acetylcholine receptors (nAChRs), believed to be the predominant mediators of nicotine's interactions with MSCs, are a prototypical ligand gated ion channel composed of five transmembrane subunits. In total, there are 16 different subunit varieties identified in humans so far: α1‐7, α9, α10, and β1‐4, γ, δ, and ɛ 25. Expression of nAChRs in MSCs has been well established. Gene expression analysis has confirmed the expression of α1‐α5, α7, α9, and ß2‐ß4 subunit mRNAs in human MSCs; however, further analysis revealed that MSCs only express α7, ß2, and ß4 subunit proteins 26. Nevertheless, the only functional nAChR identified in MSCs is the homopentameric α7 receptor 27. These receptors specifically gate for Ca2+ ions and, in the presence of receptor agonists like nicotine, increase intracellular calcium levels upon activation. Recently, Hoogduijn et al. showed that nicotine also leads to changes in intracellular pathways such as increased secondary messenger cAMP and phosphorylation of extracellular signal‐regulated protein kinases (ERKs) 27. This is of note, as ERKs are known to play a role in both proliferation and differentiation of stem cells 28.
Effect on Proliferation
The ability to self‐renew is one of the defining characteristics of stem cells and vital to their translational efficacy. To highlight the role that cigarette smoking may play on proliferation, Wahl et al. exposed human adipose‐derived MSCs in vitro to CSE at various concentrations. At levels of 5% and 10%, MSCs displayed no viability; however, under 1% showed no significant difference in cell viability. This study highlights that above specific thresholds cigarette smoking can be very toxic to MSC populations 8. These data, however, are only helpful in the empirical sense, as determining what concentrations specific compounds in extract are found in is unknown.
In vitro, human umbilical cord blood cells exposed to 0.5–1.5 mg/ml nicotine (3–9 mM) showed dose‐dependent decreases in proliferation and increases in apoptosis (p < .05 for all concentrations) 29. Significant decreases in proliferation have also been observed at concentrations as low as 0.1–10 µM (p < .05) 7, 30. Evaluating changes in proliferation in vivo can be challenging; however, decreased proliferation rates have also been observed in MSCs isolated from chronic smokers without in vitro exposure to any additional smoke or nicotine after isolation. These cells showed a 2.53‐fold decrease in proliferation compared with non‐smoker derived control cells. Decreased proliferation was still observed even after sub‐culturing cells 3–5 times, suggesting that the effects of nicotine exposure may be permanent or last for several generations of daughter cells 13. This result is very important for translational approaches as it highlights the need to gain a full history of nicotine usage for all potential stem cell donors.
Contrarily, several groups have reported an increase in cellular proliferation after nicotine exposure. Shen et al. showed that human MSCs exposed to 50–100 nM nicotine for 7 days showed significant increase in the cell number (p < .05); however, 1 µM nicotine significantly decreased cell concentration (p < .01) 5. Kim et al. report that 1 μM to 100 μM nicotine was not sufficient to alter proliferation in vitro, but that doses between 1 and 2 mM increased proliferation, and over 5 mM decreased proliferation of human alveolar bone marrow‐derived MSCs 4. Although the trend in this study is consistent with data presented by others the nicotine concentration used appears to be at minimum 1,000x higher compared with other studies.
There have been several theories to partially explain how nicotine alters proliferation of MSCs. One study suggests that the decrease in proliferation is dependent on the generation of reactive oxygen species. Culturing nicotine‐exposed cells with the antioxidant vitamin C, for example, increases the rate of proliferation compared with 1 µM nicotine‐exposed controls 5. Other groups suggest that the decrease in proliferation may be a result of changes in the cell cycle induced by nicotine. Nicotine has been shown to induce an increase in the ratio of G0/G1 phase cells 29. As G0 is considered the quiescent phase of the cell cycle, the increased ratio of cells in this phase may partially explain the observed decrease in proliferation induced by nicotine exposure 31.
Effect on Migration
In addition to their self‐renewing capabilities, MSCs also exhibit the ability to undergo cell migration. Much like their embryonic counterparts, which migrate extensively during early embryogenesis to achieve proper organogenesis 32, MSCs migrate toward sites of injury and promote postnatal wound healing through the release of growth factors and cytokines and through direct differentiation 33. This form of directed migration, termed “stem cell homing,” is unique to MSCs and allows populations of resident or transplanted MSCs to achieve targeted delivery to diseased areas 34.
The migratory potential of MSCs, however, can be affected by cigarette smoking. Zhou et al. were the first to report that cigarette smoking inhibited the targeted migration of transplanted MSCs to the uterus in an in vivo rat model 35. An in vitro wound closure assay using MSCs derived from human smoker PDL yielded similar results 13. On average, smoker PDLSCs migrated 12% slower than those isolated from nonsmokers (p < .05) resulting in decreased wound closure potential after 24 hours 13. The authors of this study mention that the isolated cells were cultured in non‐cigarette smoke media for several weeks prior to analysis. Therefore, the results also suggest that the effects of cigarette smoke exposure on MSCs could be irreversible, even after long periods of recovery, for example, cessation.
But just like proliferation potential, the effect of cigarette smoke exposure on MSC migration is dose‐dependent. The migratory potential of human adipose‐derived MSCs (hAMSCs), for example, was unaffected in vitro by CSE concentrations of less than 1%, but severely affected by concentrations greater than 5% 8. In a separate study, the in vitro exposure to 100% CSE induced the epithelial to mesenchymal transition of breast epithelial cells and, thus, promoted MSC migration and invasion (i.e., metastasis) 36. Given the results from the first set of CSE exposures, one would expect that the use of 100% CSE would be extremely detrimental to cell migration. However, the compared studies used different cigarettes and methods for the collection of CSE, and thus the percent extracts are not identical.
Individual cigarette smoking compounds, like nicotine, can also affect MSC migration. Schraufstatter et al. showed that 1 µM nicotine significantly increases the spontaneous migration of human, bone marrow‐derived MSCs (hBMMSCs) in chemokine free cultures by more than 40% 26. In chemokine‐supplemented cultures, however, the addition of 1 µM nicotine significantly decreased MSC migration 26. These effects were reversed by alpha bungarotoxin pretreatments, suggesting that nicotine's effects are likely mediated by α7 nAChRs. Ng et al. have also shown that hBMMSCs treated with 1 µM of nicotine experienced a 60% reduction (p < .05) in both migration distance and speed when compared with nontreated controls 7. MSC derived from human PDL suffered a similar fate, but with reductions of only 38% (p < .05) 7. Moreover, nicotine also downregulated protein tyrosine kinase‐2 (PTK2) gene expression in both MSC populations (p < .01) and significantly upregulated PTK2‐targeting microRNA miR‐1305 expression in PDLSCs. These results are interesting because they allude to another possible mechanism behind the effects of nicotine on MSC migration.
Effect on Differentiation
MSCs are routinely considered for many regenerative medicine applications because of their ability to form a variety of cell types 37. Transplanted cells, however, must be screened to avoid infectious or genetic diseases that can interfere with MSC function. Often overlooked is the presence of external factors such as cigarette smoking that can affect MSC differentiation potential and render cells ineffective for transplantation. The following sections aim to summarize how cigarette smoking and nicotine exposure affect the three main lineages of MSC differentiation.
Adipogenic Differentiation
Wahl et al. demonstrated that in vitro exposure to 0.5% CSE did not significantly affect the adipogenic differentiation potential of hAMSCs after 21 days as evident by similar adipogenic marker expression (i.e., PPARγ, ADIPOQ, and LEP) and Oil Red O staining between treated and nontreated hAMSCs 8. On the other hand, Ng et al. demonstrated that MSCs derived from cigarette smoker PDLs experience increased lipid production compared with nonsmokers even after several weeks of in vitro culture with nonexposed medias 13.
Given such contrasting results and limited availability of references, it is difficult to determine the effect smoking has on MSC adipogenic differentiation. The former study models typical smoking exposure conditions in vitro with extract; however, additional analysis with regard to the measurement and concentration of smoking‐related toxins was not performed. Therefore, it is impossible to determine if 0.5% CSE falls within the physiological ranges of toxic smoke exposure to reflect in vivo conditions. The results from the latter study, however, are much more indicative of in vivo conditions since the MSCs were extracted from actual smoking and non‐smoking donors. Even so, the claim that smoking promotes the adipogenic differentiation potential of MSCs cannot be made due to a lack of exposure normalization between donors and small sample size of this study. Therefore, the effect of cigarette smoking on the adipogenic differentiation of MSCs remains inconclusive.
Chondrogenic Differentiation
Wahl and Ng also investigated the effects of cigarette smoking on MSC chondrogenic differentiation. Wahl et al. demonstrated that chondrogenic induced hAMSCs exposed to 0.5% CSE caused an initial upregulation in aggrecan (ACAN) (∼6‐fold, p < .0001) and chondrogenic transcription factor SOX9 (∼3‐fold, p < .01) gene expression after 7 days of induction compared with non‐treated controls in vitro 8. Expression of both markers decreased and was comparable to non‐treated control levels by 21 days 8. Collagen type II alpha I gene expression was consistently below nonexposed control levels and Alcian Blue staining was slightly decreased in CSE‐treated hAMSCs after 21 days; however, no statistical difference was observed 8. Similar Alcian Blue results were observed by Ng et al.'s group in MSCs isolated from smoker PDLSCs after 14 days of differentiation 13. The consistency of these results suggest that cigarette smoking is capable of undermining MSC chondrogenic differentiation; but, as previously mentioned, additional experiments with greater sample size and quantifiable levels of smoke exposure must be conducted in order to establish a more representative outcome.
Nicotine has also been shown to affect MSC chondrogenic differentiation potential. Rat BMMSCs treated with 25, 50, and 100 µM nicotine experienced dose‐dependent decreases in ACAN and COL2A1 gene expression (p < .01 across all concentrations) and Alcian Blue staining (15, 51, and 95% reduction; p < .01) after 4‐weeks of chondrogenic induction compared with nonexposed MSCs in vitro 38. It should be noted that the nicotine concentrations used in this study are at least 2.5 times higher than the most extreme concentrations experienced by smokers (around 10 µM in saliva) 39, 40 and therefore nonrepresentative of actual use. More physiological concentrations were investigated by Ying et al. in a separate in vitro study using human BMMSCs. Ultimately, only the 10 µM nicotine inhibited Alcian Blue and sulfated glycosaminoglycan staining (p < .05) after 14 days, whereas 0.1 and 1 µM (physiological concentrations of blood and saliva, respectively), had minimal effect 41. 10μM nicotine also downregulated COL‐1 and COL‐X (p < .05) gene expression, but showed minimal effect on ACAN and COL‐2 expression. Less concentrated nicotine doses resulted in similar outcomes, except for the expression of COL‐2, which was upregulated (p < .05) throughout the 21‐day differentiation protocol 41. hAMSCs show yet a different response to in vitro nicotine with 100 ng/ml (0.61 µM) causing a twofold increase (p < .05) in ACAN gene expression, but no change in COL‐1 or COL‐X after 14 days of chondrogenic induction 42.
Although it is difficult to compare this results due to differences in differentiation protocol and cell source, it is easy to see that physiological doses of nicotine can negatively impact MSC chondrogenic differentiation potential. The inability to effectively produce aggrecan, an integral extracellular matrix proteoglycan, or collagen for example, could render MSCs ineffective for current stem cell‐based therapies aimed at treating cartilage repair in debilitating diseases like osteoarthritis.
Osteogenic Differentiation
The osteogenic differentiation potential of MSCs is well known and has been extensively studied. Accordingly, MSCs have been routinely considered in therapies for metabolic bone diseases 43, 44, 45, 46, and fracture fixation 47, 48. Cigarette smoking, however, can deteriorate bone health and inhibit normal and reparative bone formation. Compared with non‐smokers, cigarette smokers are more likely to experience osteoporosis 49, 50, 51 and delayed healing times following skeletal fracture 52, 53, 54. These delays are partly due to the inefficient osteogenic differentiation of MSCs 8, 13.
MSCs derived from smoker PDL have been shown to exhibit an overall reduction in calcium deposition and alkaline phosphatase production compared with nonsmokers after 14 days of osteogenic differentiation in vitro 13. In the same study, smoker MSCs also experienced a significant upregulation in the expression of RUNX2‐targeting microRNA miR‐1305‐a correlation that hints at a possible mechanism for smoking induced effects (13). 0.5% CSE studies, on the other hand, have been shown to have a non‐significant effect on hBMMSC calcium deposition after 20 days of in vitro culture 8. Although CSE did upregulate RUNX2 (2.5‐fold) and osteocalcin (2‐fold) gene expression (p < .001) after 14 days induction 8.
Nicotine exposure has also been associated with inefficient skeletal healing 55. Nicotine is known to affect the osteogenic differentiation of MSCs in vitro 6, 7 and therefore likely contributes to these outcomes. Specifically, 1 µM nicotine exposure significantly decreases inherent RUNX2, COL1A1, COL1A2, ALPL, and OCN gene expression in both hBMMSCs and hPDLSCs (p < .05) and significantly upregulates the expression of RUNX2‐targeting miR‐1305 by more than 120‐fold (p < .001) after just 3 days of exposure 7. Decreased Alizarin Red S and alkaline phosphatase staining confirmed these results 7. Zhou et al. observed similar results, and, in addition, demonstrated that the nicotinic effects were dose‐dependent and mediated through a7 nAChRs 6.
It should be noted that the results from Ng et al.'s 1 µM nicotine in vitro exposures showed similar trends with those of the smoker‐derived MSC experiments. Even though cigarette smoke contains over 7,000 chemicals, the similarity of outcomes from the two studies suggest that nicotine is one of the more potent inducers of the effects seen in cigarette smoke exposures. Accordingly, 1 µM nicotine in vitro exposure studies could be used a model for an indication of in vivo exposure outcomes.
Effect on Paracrine Signaling
In many cell‐based therapies, although improvement in conditions are observed, engrafted cells may not be observed 56. It is hypothesized that the improvements that are observed are a result of paracrine signaling by the transplanted stem cells. These factors promote angiogenesis, and decrease inflammation among other processes. In hAMSC's, which were exposed to 0.5% CSE for 48 hours in vitro, investigators studied the release profile of 36 different cytokines secreted by MSCs. Of those studied IL‐6 and IL‐8 showed significantly (p < .05) lowered amounts secreted by those exposed to CSE. Both factors play roles in inflammatory response and angiogenesis and their decreased secretion may lead to delayed wound healing 8.
In addition to their role in tissue regeneration, naïve MSC's also perform a crucial role in the niche acting as players in the supporting role of hematopoietic stem cells (HSC's) which give rise to the cellular components of blood. Within the bone marrow niche HSC's colocalize next to MSC's that secrete factors such as Fibroblast Activation Protein. In models with MSC's removed anemia and bone marrow hypocellularity ensue 57. Cigarette smoking has been shown to directly alter MSC's in the HSC niche. In MSC's that were isolated from mice that had been exposed to cigarette smoke for 9 months, aberrant gene expression changes were noted which span across several pathways of MSCs that are known to regulate HSC function which may lead to a loss of niche functionality 58.
Conclusion
Together with the concern over the increasing use of highly concentrated nicotine e‐liquids in ecigs, the results herein warrant further investigation into the biological effects of cigarette and nicotine use on MSC populations. Specifically, further investigation is needed in regards to the permanence of induced effects (i.e., reversible or irreversible after quitting) in areas such as tissue regeneration and paracrine potential—two of the most exploited characteristics of MSCs in translational approaches. Building this understanding will undoubtedly help to improve the efficacy of stem‐based therapeutic applications especially in patients who have been exposed, or continue to be exposed, to cigarette smoke and other forms of nicotine delivery.
As this review has illustrated, cigarette smoking and nicotine use has the potential to undermine the regenerative capacity of numerous MSC populations. Stem cell‐based therapeutic developers and study coordinators should be cognizant of these detrimental effects as they could potentially impact the results of pre‐developmental research or, even worse, outcomes of stem‐based interventions in candidate patients. Accordingly, stem cell donors and recipients should be extensively screened prior to study initiations in order to identify the extent of toxic exposure. Gathering information regarding daily consumption, concentration of e‐liquid nicotine, and frequency or length of use will help distinguish between dangerous and safe levels of exposure in prospective patients. By incorporating this information with other inclusion/exclusion criteria, researchers can further hone in on the most ideal cells/candidates for research or transplantation.
Author Contributions
J.G. and C.C.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; H.C.: conception and design, financial support, final approval of manuscript.
Disclousre of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1. Chamberlain G, Fox J, Ashton B et al. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007;25:2739–2749. [DOI] [PubMed] [Google Scholar]
- 2. Madrigal M, Rao KS, Riordan NH. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J Transl Med 2014;12:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sensebe L, Gadelorge M, Fleury‐Cappellesso S. Production of mesenchymal stromal/stem cells according to good manufacturing practices: A review. Stem Cell Res Ther 2013;4:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim BS, Kim SJ, Kim HJ et al. Effects of nicotine on proliferation and osteoblast differentiation in human alveolar bone marrow‐derived mesenchymal stem cells. Life Sci 2012;90:109–115. [DOI] [PubMed] [Google Scholar]
- 5. Shen Y, Liu HX, Ying XZ et al. Dose‐dependent effects of nicotine on proliferation and differentiation of human bone marrow stromal cells and the antagonistic action of vitamin C. J Cell Biochem 2013;114:1720–1728. [DOI] [PubMed] [Google Scholar]
- 6. Zhou Z, Li B, Dong Z, Liu F et al. Nicotine deteriorates the osteogenic differentiation of periodontal ligament stem cells through alpha7 nicotinic acetylcholine receptor regulating Wnt pathway. PLoS One 2013;8:e83102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ng TK, Carballosa CM, Pelaez D et al. Nicotine alters MicroRNA expression and hinders human adult stem cell regenerative potential. Stem Cells Dev 2013;22:781–790. [DOI] [PubMed] [Google Scholar]
- 8. Wahl EA, Schenck TL, Machens HG et al. Acute stimulation of mesenchymal stem cells with cigarette smoke extract affects their migration, differentiation, and paracrine potential. Sci Rep 2016;6:22957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S Department of Health and Human Services. The Health Consequences of Smoking‐50 Years of Progress: A Report of the Surgeon General. In: U.S. Department of Health and Human Services CfDCaP, National Center for Chronic Disease Prevention and Health Promotion, Atlanta, GA, 2014.
- 10. Goniewicz ML, Hajek P, McRobbie H. Nicotine content of electronic cigarettes, its release in vapour and its consistency across batches: Regulatory implications. Addiction 2014;109:500–507. [DOI] [PubMed] [Google Scholar]
- 11. Goniewicz ML, Knysak J, Gawron M et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 2014;23:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. King BA, Alam S, Promoff G et al. Awareness and ever‐use of electronic cigarettes among U.S. adults, 2010–2011. Nicotine Tob Res 2013;15:1623–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ng TK, Huang L, Cao D et al. Cigarette smoking hinders human periodontal ligament‐derived stem cell proliferation, migration and differentiation potentials. Sci Rep 2015;5:7828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hahn J, Monakhova YB, Hengen J et al. Electronic cigarettes: Overview of chemical composition and exposure estimation. Tob Induc Dis 2014;12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362:2295–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huertas A, Testa U, Riccioni R et al. Bone marrow‐derived progenitors are greatly reduced in patients with severe COPD and low‐BMI. Respir Physiol Neurobiol 2010;170:23–31. [DOI] [PubMed] [Google Scholar]
- 17. Behera SN, Xian H, Balasubramanian R. Human health risk associated with exposure to toxic elements in mainstream and sidestream cigarette smoke. Sci Total Environ 2014;472:947–956. [DOI] [PubMed] [Google Scholar]
- 18. Acuff L, Fristoe K, Hamblen J et al. Third‐hand smoke: Old smoke, new concerns. J Commun Health 2016;41:680–687. [DOI] [PubMed] [Google Scholar]
- 19. Benowitz NL, Hukkanen J, Jacob P, 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol 2009:29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stepanov I, Fujioka N. Bringing attention to e‐cigarette pH as an important element for research and regulation. Tob Control 2015;24:413–414. [DOI] [PubMed] [Google Scholar]
- 21. Lindell G, Lunell E, Graffner H. Transdermally administered nicotine accumulates in gastric juice. Eur J Clin Pharmacol 1996;51:315–318. [DOI] [PubMed] [Google Scholar]
- 22. Liu J, Yu F, Sun Y, Jiang B et al. Concise reviews: Characteristics and potential applications of human dental tissue‐derived mesenchymal stem cells. Stem Cells 2015;33:627–638. [DOI] [PubMed] [Google Scholar]
- 23. Huang CY, Pelaez D, Dominguez‐Bendala J et al. Plasticity of stem cells derived from adult periodontal ligament. Regen Med 2009;4:809–821. [DOI] [PubMed] [Google Scholar]
- 24. Chen FM, Gao LN, Tian BM et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: A randomized clinical trial. Stem Cell Res Ther 2016;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Albuquerque EX, Pereira EF, Alkondon M et al. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol Rev 2009;89:73–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schraufstatter IU, DiScipio RG, Khaldoyanidi SK. Alpha 7 subunit of nAChR regulates migration of human mesenchymal stem cells. J Stem Cells 2009;4:203–215. [PMC free article] [PubMed] [Google Scholar]
- 27. Hoogduijn MJ, Cheng A, Genever PG. Functional nicotinic and muscarinic receptors on mesenchymal stem cells. Stem Cells Dev 2009;18:103–112. [DOI] [PubMed] [Google Scholar]
- 28. Michailovici I, Harrington HA, Azogui HH et al. Nuclear to cytoplasmic shuttling of ERK promotes differentiation of muscle stem/progenitor cells. Development 2014;141:2611–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zeng HL, Qin YL, Chen HZ et al. Effects of nicotine on proliferation and survival in human umbilical cord mesenchymal stem cells. J Biochem Mol Toxicol 2014;28:181–189. [DOI] [PubMed] [Google Scholar]
- 30. Kim DH, Liu J, Bhat S et al. Peroxisome proliferator‐activated receptor delta agonist attenuates nicotine suppression effect on human mesenchymal stem cell‐derived osteogenesis and involves increased expression of heme oxygenase‐1. J Bone Miner Metab 2013;31:44–52. [DOI] [PubMed] [Google Scholar]
- 31. Matson JP, Cook JG. Cell cycle proliferation decisions: The impact of single cell analyses. FEBS J; 2016;284:362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kurosaka S, Kashina A. Cell biology of embryonic migration. Birth Defects Res C Embryo Today 2008;84:102–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nuschke A. Activity of mesenchymal stem cells in therapies for chronic skin wound healing. Organogenesis 2014;10:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. De Becker A, Riet IV. Homing and migration of mesenchymal stromal cells: How to improve the efficacy of cell therapy? World J Stem Cells 2016;8:73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhou Y, Gan Y, Taylor HS. Cigarette smoke inhibits recruitment of bone‐marrow‐derived stem cells to the uterus. Reprod Toxicol 2011;31:123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Di Cello F, Flowers VL, Li H et al. Cigarette smoke induces epithelial to mesenchymal transition and increases the metastatic ability of breast cancer cells. Mol Cancer 2013;12:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pittenger MF, Mackay AM, Beck SC et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–147. [DOI] [PubMed] [Google Scholar]
- 38. Deng Y, Li TQ, Yan YE et al. Effect of nicotine on chondrogenic differentiation of rat bone marrow mesenchymal stem cells in alginate bead culture. Biomed Mater Eng 2012;22:81–87. [DOI] [PubMed] [Google Scholar]
- 39. Papaseit E, Farre M, Graziano S et al. Monitoring nicotine intake from e‐cigarettes: Measurement of parent drug and metabolites in oral fluid and plasma. Clin Chem Lab Med 2017;55:415–423. [DOI] [PubMed] [Google Scholar]
- 40. Robson N, Bond AJ, Wolff K. Salivary nicotine and cotinine concentrations in unstimulated and stimulated saliva. Afr J Pharm Pharmacol 2010;4:61–65. [Google Scholar]
- 41. Ying X, Zhang W, Cheng S et al. Nicotine‐induced chondrogenic differentiation of human bone marrow stromal cells in vitro. Knee Surg Sports Traumatol Arthrosc 2012;20:2329–2336. [DOI] [PubMed] [Google Scholar]
- 42. Roux C, Pisani DF, Yahia HB et al. Chondrogenic potential of stem cells derived from adipose tissue: A powerful pharmacological tool. Biochem Biophys Res Commun 2013;440:786–791. [DOI] [PubMed] [Google Scholar]
- 43. Gotherstrom C, Westgren M, Shaw SW et al. Pre‐ and postnatal transplantation of fetal mesenchymal stem cells in osteogenesis imperfecta: A two‐center experience. Stem Cells Transl Med 2014;3:255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Otsuru S, Gordon PL, Shimono K et al. Transplanted bone marrow mononuclear cells and MSCs impart clinical benefit to children with osteogenesis imperfecta through different mechanisms. Blood 2012;120:1933–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Taketani T, Kanai R, Abe M et al. Therapy‐related Ph+ leukemia after both bone marrow and mesenchymal stem cell transplantation for hypophosphatasia. Pediatr Int 2013;55:e52–55. [DOI] [PubMed] [Google Scholar]
- 46. Taketani T, Oyama C, Mihara A et al. Ex vivo expanded allogeneic mesenchymal stem cells with bone marrow transplantation improved osteogenesis in infants with severe hypophosphatasia. Cell Transplant 2015;24:1931–1943. [DOI] [PubMed] [Google Scholar]
- 47. Huang S, Xu L, Zhang Y et al. Systemic and local administration of allogeneic bone marrow‐derived mesenchymal stem cells promotes fracture healing in rats. Cell Transplant 2015;24:2643–2655. [DOI] [PubMed] [Google Scholar]
- 48. Murena L, Canton G, Vulcano E et al. Treatment of humeral shaft aseptic nonunions in elderly patients with opposite structural allograft, BMP‐7, and mesenchymal stem cells. Orthopedics 2014;37:e201–206. [DOI] [PubMed] [Google Scholar]
- 49. Ayo‐Yusuf OA, Olutola BG. Epidemiological association between osteoporosis and combined smoking and use of snuff among South African women. Niger J Clin Pract 2014;17:174–177. [DOI] [PubMed] [Google Scholar]
- 50. Brook JS, Balka EB, Zhang C. The smoking patterns of women in their forties: Their relationship to later osteoporosis. Psychol Rep 2012;110351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kim KH, Lee CM, Park SM et al. Secondhand smoke exposure and osteoporosis in never‐smoking postmenopausal women: The Fourth Korea National Health and Nutrition Examination Survey. Osteoporos Int 2013;24:523–532. [DOI] [PubMed] [Google Scholar]
- 52. Pearson RG, Clement RG, Edwards KL et al. Do smokers have greater risk of delayed and non‐union after fracture, osteotomy and arthrodesis? A systematic review with meta‐analysis. BMJ Open 2016;6:e010303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Scolaro JA, Schenker ML, Yannascoli S et al. Cigarette smoking increases complications following fracture: A systematic review. J Bone Joint Surg Am 2014;96:674–681. [DOI] [PubMed] [Google Scholar]
- 54. Patel RA, Wilson RF, Patel PA et al. The effect of smoking on bone healing: A systematic review. Bone Joint Res 2013;2:102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Donigan JA, Fredericks DC, Nepola JV et al. The effect of transdermal nicotine on fracture healing in a rabbit model. J Orthop Trauma 2012;26:724–727. [DOI] [PubMed] [Google Scholar]
- 56. Liang X, Ding Y, Zhang Y et al. Paracrine mechanisms of mesenchymal stem cell‐based therapy: Current status and perspectives. Cell Transplant 2014;23:1045–1059. [DOI] [PubMed] [Google Scholar]
- 57. Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature 2014;505:327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Siggins RW, Hossain F, Rehman T et al. Cigarette smoke alters the hematopoietic stem cell niche. Med Sci 2014;2:37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]


