Summary
Stem cells, with their therapeutic potential in tissue repair and regeneration, have been widely used in translational medicine. Recent evidence suggests that the beneficial effects are mediated largely by their paracrine actions rather than the engraftment and differentiation at the injured sites. Extracellular vesicles (EVs), actively released from cells, play important roles in cell‐to‐cell communication and display multiple functions in tissue regeneration. In the present report, we will briefly review the current knowledge related to the therapeutic potential of EVs, particularly stem cell or progenitor cell‐derived ones for promoting tissue repair and regeneration, and focus on the restorative properties of exosomes/microvesicles in cutaneous wound healing, bone regeneration, hindlimb ischemia, and vascular injury repair. Stem Cells Translational Medicine 2017;6:1753–1758
Significance Statement.
This perspective article summarizes recent findings concerning the therapeutic potential of extracellular vesicles in promoting tissue repair and regeneration, mainly focusing on the restorative properties of exosomes and microvesicles in cutaneous wound healing, bone regeneration, hindlimb ischemia, and vascular injury repair. The potential molecular mechanisms underlying extracellular vesicle‐mediated angiogenetic effects and osteogenic differentiation in recipient mesenchymal stem cells will be also reviewed.
Introduction
Stem cell technology has been proposed as a promising opportunity in translational medicine due to the therapeutic potential that cells possess in terms of tissue repair and regeneration, but the exact mechanisms underlying these beneficial properties are not fully understood. Recent studies have revealed that the main beneficial effects are not attributed to their engraftment and differentiation at the site of injury, but rather to their paracrine actions 1, 2, 3. Stem or progenitor cell‐sourced conditioned media (CM) are sufficient to promote the structural regeneration and functional recovery of bone defects and ischemic hindlimb in animal models 4, 5, while mesenchymal stem cell‐sourced conditioned media (MSC‐CM) could even outperform MSCs in bone regeneration 5. Subsequent works indicated the pro‐regenerative role for extracellular vesicles (EVs) isolated from CM, which were released by stem or progenitor cells, may at least in part contribute to the paracrine action 6, 7. And this new mechanism of cell‐to‐cell communication relies on EVs' mediated transfer of bio‐active lipids, proteins, and nucleic acids to recipient cells 8, 9, 10.
Extracellular vesicles are a heterogeneous family of spherical lipid‐bilayered vesicles released from the endosomal compartments or shed from the cell surface, including exosomes, microvesicles (MVs), and apoptotic bodies (Table 1, Fig. 1) 11, 12. Among the subtypes of EVs, exosomes and MVs are the primary focus for many researchers owing to their biological significance. In contrast to MVs, which are secreted by budding from the plasma membrane, exosomes are released into the extracellular milieu upon fusion of multivesicular bodies with the plasma membrane and exert diverse effects on recipient cells 11, 14, 15.
Table 1.
| Characteristic | Exosomes | Microvesicles | Apoptotic bodies |
|---|---|---|---|
| Cellular origin | Most cell types | Most cell types | All cell types |
| Intracellular origin | Multivesicular body | Plasma membrane | Not determined |
| Size | 30–150 nm | Up to 1,000 nm | 1,000–5,000 nm |
| Morphology | Cup/round shaped | Various shapes | Heterogeneous |
| Sucrose gradient | 1.13–1.19 g/mL | 1.04–1.07 g/mL | 1.16–1.28 g/ml |
| Markers | Tetraspanins, TSG101, Alix, flotillin, ESCRT componentsa | Nonspecific markers including Integrins, selectins, and CD40 | Elevated PS |
| Content | mRNA, miRNA, non‐coding RNAs, cytoplasmic and membrane proteins |
mRNA, miRNA, non‐coding RNAs, cytoplasmic and membrane proteins |
Nuclear fractions, cell organelles |
Most markers are not only specific to exosomes. Although exosomes and microvesicles are distinct types of vesicles, neither size, morphology, nor markers is a sufficient criterion to distinguish both types from each other.
Abbreviations: miRNA, microRNA; TSG101, tumor susceptibility gene 101; ESCRT, endosomal sorting complex required for transport; PS, phosphatidylserine.
Figure 1.
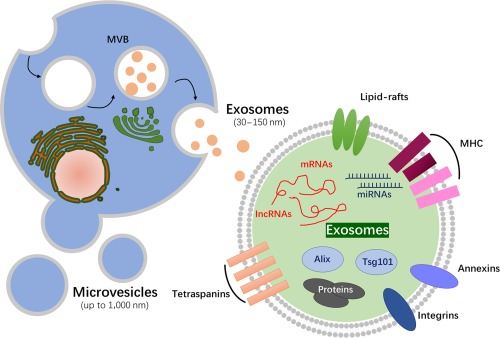
Schematic representation of the main two types of extracellular vesicles released by cells, either by fusion of MVB with the plasma membrane or by direct budding from the plasma membrane. A wide range of cargo is transported within exosomes, including mRNA, miRNA, proteins, etc. Abbreviations: IncRNA, long non‐coding RNA; MHC, major histocompatibility complex; miRNA, microRNA; MVB, multivesicular bodies.
Recent studies have indicated that exosomes and MVs harbor pro‐regenerative effects against multiple diseases in animal models, including myocardial infarction 16, 17, 18, hindlimb ischemia 19, and cutaneous wound 6, and EV‐based therapeutics have been reported to come into vogue in clinical application 20, 21. Compared with cell transplantation, EVs' mediated cell‐free therapies offer the advantages of being more stable and storable, having no risk of aneuploidy, and having a lower possibility of immune refection 22. Thus, a better understanding of the underlying mechanisms that exosomes and MVs mediate is required to fully harness their promising potential as therapeutic approaches in regeneration medicine.
In the present report, we will briefly review the latest reports regarding the therapeutic potential of EVs, particularly stem cell or progenitor cell‐derived ones, for promoting tissue repair and regeneration, and the potential molecular mechanisms underlying EV‐mediated angiogenetic effects will be also mentioned.
Stem or Progenitor Cells‐Derived Extracellular Vesicles Applied to Tissue Repair
Extracellular vesicles have been shown to facilitate regeneration of injured tissue in various animal models. In this report, we will focus on the restorative properties of exosomes/MVs in cutaneous wound healing, bone regeneration, hindlimb ischemia, and vascular injury repair.
Cutaneous Wound Healing
To understand the therapeutic effect of EVs on wound healing, we previously established a rat full‐thickness skin defect model and found that exosomes secreted from human induced pluripotent cell‐derived mesenchymal stem cells (hiPSC‐MSCs) could accelerate re‐epithelialization, reduce scar widths, and promote collagen maturity at wound sites 6. The highest vessel densities and numbers of mature vessels were identified in hiPSC‐MSC derived exosomes (hiPSC‐MSC‐Exos) group, and increased migration and proliferation of fibroblasts and human umbilical vein endothelial cells (HUVECs) were observed in the presence of hiPSC‐MSC‐Exos compared with MesenGro hMSC medium (StemRD, Burlingame, CA, http://www.stemrd.com) without hiPSC‐MSC‐Exos group, as well as the enhanced tube formation of HUVECs treated with hiPSC‐MSC‐Exos 6. The effect of MSCs‐derived exosomes on the migration and proliferation of normal and diabetic wound fibroblasts was confirmed by the Shabbir et al. in vitro study as well 23.
The underlying mechanisms of EVs mediated wound healing process are under investigation. MSC‐Exos were found to stimulate several signaling pathways involved in wound healing, including Akt, ERK, and STAT3, and induce the expression of cell cycle genes and growth factors as well (including HGF, IGF‐1, NGF, and SDF‐1) 23. Orderly integration of proliferation, migration, differentiation, and apoptosis of skin cells is critical for effective cutaneous wound healing. Excessive scar formation as a pathological response caused by myofibroblast aggregations should be prevented during cutaneous wound healing. It has been shown that MSC‐Exos' role is not static during the entire cutaneous tissue regeneration process and they exert distinct effects on skin cell proliferation at various cell densities 24. Zhang et al. 24 found that human umbilical cord mesenchymal stem cells‐derived exosomes (hucMSC‐Exos) promote activation of β‐catenin and skin stem cell proliferation in the early stages of cutaneous regeneration and restrict excessive cell expansion via transfer of the 14–3‐3ζ protein, inducing cytoplasmic retention of the YAP protein, and inhibiting Wnt/β‐catenin signaling. Fang et al. 25 found that umbilical cord mesenchymal stem cell‐derived exosomes (uMSC‐Exos) can promote wound healing and reduce scarring and in situ myofibroblast formation as well. Through high‐throughput RNA sequencing and functional analysis, uMSC‐Exo‐specific miRNAs (miR‐21, ‐23a, ‐125b, and ‐145) were demonstrated to suppress the differentiation of fibroblasts to myofibroblasts by inhibiting TGF‐β/SMAD2 signaling pathway.
MicroRNAs (MiRNAs), as a class of endogenous small non‐coding RNAs, have been suggested to reduce inflammation by modulating target proteins in inflammatory signaling pathway 26. Li et al. 26 demonstrated that hucMSCs‐Exos‐derived miR‐181c bind to the Toll‐like receptor 4 (TLR4) mRNA 3′‐untranslational region and inhibit TLR4 expression, suppressing the inflammatory reaction of severe burn rats. Ti et al. 27 found exosomes secreted by lipopolysaccharide (LPS)‐preconditioned mesenchymal stromal cells (LPS pre‐MSC‐Exos) possessed the stronger anti‐inflammatory ability than untreated MSC‐derived exosomes. Further investigation indicated that LPS pre‐MSC‐Exos‐shuttled let‐7b facilitate the differentiation of macrophages to anti‐inflammatory M2 phenotype, but not M1 via TLR4/NF‐κB/STAT3/AKT signaling.
As indicated in our previous study 6, exosomes from hiPSC‐MSCs were found to exert beneficial effects on angiogenesis, which is a critical phase of the wound‐healing process. Exosomes treatment could enhance the proliferation, migration, and tube formation capability of vascular endothelial cells in vitro 23, 28, 29 and stimulate expression of angiogenesis‐related molecules (including fibroblast growth factor 1 (FGF‐1), angiogenin‐1 (ANG‐1), vascular endothelial growth factor receptor (VEGFA), etc.) of endothelial cells as well 28. Endothelial progenitor cell derived exosomes (EPC‐Exos) induced pro‐angiogenic effects on endothelial cells has been indicated to be mediated by Erk1/2 signaling during cutaneous wound healing process 30. HucMSCs‐Exos could promote wound healing in the rat model of skin deep second‐degree burn injury through parallel activation of Wnt/β‐catenin and AKT signaling 31 and promote angiogenesis to repair deep second‐degree burn injury skin by delivering Wnt4 and activating Wnt/β‐catenin signaling in endothelial cells 10.
Bone Regeneration
Mesenchymal stem cells are currently the most established promising cells for skeletal tissue engineering and regeneration 32, 33; however, the limited understanding of the mechanisms MSCs mediated hinders the clinical translation. The paracrine hypothesis has inspired an alternative approach in bone regeneration, which is based on EVs secreted by the stem or progenitor cells rather than cells themselves. Furuta et al. 34 established transverse femoral shaft fracture model of CD9‐/‐ mice whose bone healing capacity were obviously downregulated compared with wild‐type ones. Delayed fracture healing in CD9‐/‐ mice was rescued by the MSCs‐Exos and MSC‐CM injection into the fracture site, and the fracture healing process in wild‐type mice was enhanced as well.
The effective reconstruction of bone defects is still a challenging problem in orthopedic surgery. Bioactive materials offer an alternative solution to autologous and allogenic bone grafting in bone repair and regeneration. Considering the lack osteoinductive activity of tricalcium phosphate (β‐TCP), we previous investigated the osteogenic capacity of the hiPSC‐MSC‐Exos‐loaded β‐TCP in repairing critical‐sized calvarial defects in ovariectomized rats 35. In vitro, hiPSC‐MSC‐Exos promoted proliferation and osteoblastic differentiation of bone marrow MSCs origin from ovariectomized rats (rBMSCs‐OVX) in a concentration‐dependent manner. The expression levels of bone‐related proteins (OPN, RUNX‐2 and COL1) by rBMSCs‐OVX were remarkably upregulated after hiPSC‐MSC‐Exos treatment. In vivo, exosomes/β‐TCP incorporated scaffolds could significantly enhanced bone regeneration and new angiogenesis in the area of bone defects 35. We tried to interrogate the detailed mechanism by which hiPSC‐MSC‐Exos/TCP combination scaffolds enhance bone regeneration in critical‐sized calvarial bone defects, finding that the PI3K/Akt signaling pathway was the critical mediator during the exosome‐induced osteogenic responses of human BMSCs 36. Another study by Qin et al. using BMSC‐EVs‐loaded hydrogel for critical‐size calvarial bone defects repair has also shown the strong osteogenic capacity 37.
Mesenchymal stem cells in the bone microenvironment possess multipotent, self‐renewing capacity, and they can differentiate into diverse cell types 38. When tissue repair is required, MSCs can be stimulated to proliferate and differentiate. Studies have shown that this osteogenic differentiation in recipient MSCs can be regulated by cargos extracellular vesicles shuttled (Table 2), while the effective osteogenic commitment is critical for bone regenerative therapies.
Table 2.
Extracellular vesicles mediated osteogenic differentiation of MSCs
| EVs origin | Factors | Mechanisms | Involved pathways | Osteogenic effects | Ref. |
|---|---|---|---|---|---|
| Senescent EC | miR‐31 | FZD3↓ | — | ↓ | 39 |
| Monocyte | — | — | — | ↑ | 40 |
| Mineralizing osteoblasts | miRNAs | Axin1↑, β‐catenin↑ |
Wnt/ β‐catenin |
↑ | 41 |
| HiPSC‐MSC | — | — | — | ↑ | 35 |
| HiPSC‐MSC | — | p‐AKT↑ | PI3K/AKT | ↑ | 36 |
| MSC | Fas |
miR29b↓→Nnmt1↑ → Notch1 Promoter Methylation |
Notch | ↑ | 42 |
| Osteogenically Induced‐MSC | — | — | — | ↑ | 43, 44 |
| BMSC | miR‐196a | — | — | ↑ | 37 |
Abbreviations: —, not mentioned; ↑, promote osteogenic differentiation; ↓, inhibit osteogenic differentiation; BMSC, bone marrow stromal/stem cell; EC, endothelial cell; EV, extracellular vesicle; hiPSC‐MSC, human‐induced pluripotent stem cell‐derived mesenchymal stem cells; MSC, mesenchymal stem cell.
Hindlimb Ischemia and Vascular Injury Repair
Peripheral vascular disease is characterized by a lack of proper blood perfusion to the extremities due to the narrowing or blocking of arterial vasculature, which can potentially progress to a severe condition called critical limb ischemia. As mentioned above, intramuscular administration of EPC‐derived CM can promote tissue revascularization and function recovery as effectively as EPC transplantation 4.
Subsequent study demonstrated that MVs intravenous administration which were isolated from EPC‐CM, significantly improved hindlimb blood perfusion after artery ligation surgery performed on severe combined immunodeficient mice, and much higher capillary density and more muscle fibers in ischemic limb were identified in MVs‐treated group. Further investigation suggested that the angiogenic activity of EPC‐MVs was mediated by MVs‐shuttled miR‐126 and miR‐296 [45]. HiPSC‐MSC‐Exos multiple intramuscular injections were also demonstrated to promote blood perfusion and ischemia limb functional recovery and increase the average microvessel density in ischemic muscle. Exosomes co‐culture can enhance the migration, proliferation, and the tube formation capacity of endothelial cells in vitro. And the expression level of angiogenesis‐related genes (HIF‐1α, VEGFA, PGF, TGFβ1, Angiogenin, KDR, and bFGFR) and proteins (VEGF, TGFβ1, and Angiogenin) were significantly upregulated in endothelial cells 19. Besides, hypoxia‐stimulated hucMSCs‐derived MVs intramuscular injections obviously increased the ratio of mean blood perfusion 2 weeks after treatment in the rat ischemic hindlimb model 46.
An early acceleration of re‐endothelialization after vascular injury is crucial for preserving endothelial integrity and preventing the development of vascular disease. It has been recently shown that transient engraftment of exogenously transplanted EPCs may promote the regeneration of injured vasculature through paracrine mechanisms 1. We have demonstrated that exosomes collected from human umbilical cord blood‐derived EPCs can promote vascular regeneration in rat models of balloon‐induced carotid artery injury by upregulating endothelial cells function. In vivo results indicated that EPC‐Exos exhibited statistically significant reduction of neointimal thickening at 14 and 21 days after injury and acceleration of re‐endothelialization as compared with phosphate buffered saline (PBS) treatment. EPC‐Exos treatment remarkably enhanced the proliferation and migration of human microvascular endothelial cells and promoted the expression of angiogenesis‐related genes (HIF‐1α, VEGFA, VEGFR2, eNOS, E‐selectin, IL‐8, ANG1, CXCL 16, PDGFA) in vitro 7.
EVs Mediated Angiogenesis
Angiogenesis, the process by which new blood vessels are formed, plays crucial roles in various physiological processes by restoring blood perfusion, delivering factors to injured sites, and ultimately promoting functional recovery and tissue regeneration. The mechanisms of tissue recovery following EVs treatment have been attributed in part to the ability of these vesicles to promote angiogenesis at the site of injury, including cutaneous wound healing 6, 10, bone regeneration 35, 36, ischemic hindlimb 19, 45, 46, and vascular injury repair 19. It has been shown that EVs are able to modify steps involved in angiogenesis, such as proliferation, migration, and tube formation of endothelial cells, and promote angiogenesis‐related gene expression and protein secretion 7, 19, 23, 28, 47, 48. In addition, exosomes not only promoted the generation of newly formed vessels 49 but also accelerated their maturation at wound sites 6. MiRNAs, as a class of short, single‐stranded, non‐coding RNAs that can be horizontal shuttled by EVs, have been implicated in the regulation of angiogenesis process, as well as the EVs‐encapsulated proteins (Table 3) 50.
Table 3.
Extracellular vesicles mediated angiogenic effects on endothelial effects
| EVs origin | Experimental model | Responsible factors | Targets/Mechanisms | Involved pathways | Reference |
|---|---|---|---|---|---|
| THP‐1/MVs | In vitro | miR‐150 | — | — | 51 |
| Endothelial cells/Exos | In vitro | miR‐214 | — | — | 52 |
| CD34+ PBMC/EVs | In vitro | miR‐126 | — | — | 53 |
| HBMMSC/Exos | In vitro | — | — | NFκB | [8] |
| EPC/MVs | In vitro | miRNAs | p‐AKT↑/p‐sNOS↑ | PI3K/Akt and eNOS | [9] |
| EDM‐preconditioned ASCs/MVs | In vitro | miR‐31 | FIH1↓ | — | 54 |
| HadMSC/Exos | In vitro | miR‐125a | DLL4↓ | Predicated to Notch | [49] |
| CMPC/MSC/Exos | In vitro | EMMPRIN, MMP‐9, VEGF | p‐ERK↑, p‐AKT↑ | Predicated to ERK and Akt | [55, 56] |
| Cardiomyocyte/Exos | Myocardial infarction | Hsp20 | VEGFR2(+)→p‐ERK/p‐AKT↑ | Predicated to ERK and Akt | [16] |
| CPCs/EVs | Myocardial infarction | miR‐132 | RasGAP‐p120↓ | — | [17] |
| EnMSCs/Exos | Myocardial infarction | miR‐21 | PTEN↓→p‐AKT↑→VEGF↑ | PTEN/Akt | [18] |
| BMMSC/Exos | Myocardial infarction | CXCR4 | IGF‐1↑→p‐AKT↑ | PI3K/Akt | 57 |
| EPC/MVs | Hindlimb ischemia | Predicated to miRNAs | — | — | [45] |
| EPC/MVs | Islets xenotransplantation | MiR‐126/miR‐296 | p‐AKT↑/p‐sNOS↑ | PI3K/Akt and eNOS | 58 |
| HucMSC/Exos | Cutaneous wound healing | Wnt4 | — | Wnt/β‐catenin | [10] |
| UCB‐EPCs/Exos | Cutaneous wound healing | — | p‐Erk1/2↑ | Erk1/2 | [30] |
Abbreviations: —, not mentioned; ASCs, adipose‐derived stem cells; CMPCs, cardiomyocyte progenitor cells; CPCs, cardiac progenitor cells; DLL4, delta‐like 4; EDM, endothelial differentiation medium; EMMPRIN, extracellular matrix metalloproteinase inducer; EnMSCs, endometrium‐derived mesenchymal stem cells; EPC, endothelial progenitor cells; EV, extracellular vesicle; HadMSC, human adipose‐derived MSCs; HBMMSC, human bone marrow mesenchymal stem cells; HucMSCs, human umbilical cord mesenchymal stem cells; MSC, mesenchymal stem cell; MV, microvesicle; PBMC, peripheral blood mononuclear cell; THP‐1, human acute monocytic leukemia cell line; UCB‐EPCs, human umbilical cord blood‐derived EPCs.
Thus, therapeutic stimulation of angiogenesis process by exogenously transplanted EVs represents a novel strategy against a large number of disease. Nevertheless, further work on the detailed mechanisms the EVs mediate is needed to achieve the clinical application of EVs mediated therapeutic angiogenesis, and the identification of key regulators of angiogenesis is of practical benefit as well.
Conclusion
Extracellular vesicles released from stem/progenitor cells have been shown to possess pro‐regenerative capacity in animal models, and several signaling pathways have been demonstrated to be involved in EVs mediated tissue regeneration (Fig. 2). Extracellular vesicles‐based therapeutics have also been reported in clinical trials (e.g., a phase I anti‐colon cancer clinical trial [NCT01294072] was conducted) 21. However, several challenges prevent the development of EVs into approved clinical application. One major challenge is the large‐scale production of a homogeneous and pure specific EVs subpopulation. Overall, EVs comprise a wide variety of vesicles ranging from 30 to 1,000 nm in diameter, while the different vesicles overlap in their size distribution. Actually, no received standards have been established to classify the different types of vesicles, and the method of EVs subtypes isolation is still controversial.
Figure 2.
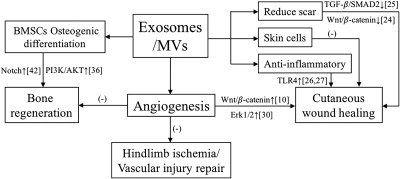
Schematic representation of signaling pathways that were involved in exosomes/MVs mediated pro‐regenerative capacity in cutaneous wound healing, bone regeneration, hindlimb ischemia, and vascular injury repair. The mechanisms of tissue recovery following EVs treatment have been attributed in part to the ability of exosomes/MVs to promote angiogenesis at the site of injury. Exosomes/MVs can promote bone regeneration through modulating the osteogenic differentiation of recipient BMSCs. Exosomes/EVs treatment can promote the cutaneous wound healing process, while excessive scar formation can be prevented in later stages. Exosomes/MVs‐shuttled miRNAs have been demonstrated to reduce inflammation by modulating target proteins in inflammatory signaling pathway during wound healing. Abbreviations: ‐, the specific signaling pathway has not been identified; BMSC, bone marrow mesenchymal stem cell; EV, extracellular vesicle; miRNA, microRNA; MV, microvesicle.
Another concern is that the exact mechanisms of EVs mediated pro‐regenerative effects remain elusive, and more thorough investigations of EVs and their components are needed. The majority of recent studies on EVs function mainly focused on miRNA, and protein content and effects, exosomal lipids, and other non‐coding RNAs have also been shown to exert important functions. The detailed mechanisms of how miRNAs and other cargos are selected into extracellular milieu via exosomes/MVs must be better understood. Taken together, more research on EVs will be necessary to fully harness the potential of exosomes as therapeutics for future clinical application.
Author Contributions
B.C., Q.L., B.Z., and Y. W.: manuscript writing, final approval of the manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (Grant No. 81472152 and 81572223).
References
- 1. Zhang M, Malik AB, Rehman J. Endothelial progenitor cells and vascular repair. Curr Opin Hematol 2014;21:224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Han C, Sun X, Liu L et al. Exosomes and their therapeutic potentials of stem cells. Stem Cells Int 2016;2016:7653489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lai RC, Yeo RW, Lim SK. Mesenchymal stem cell exosomes. Semin Cell Dev Biol 2015;40:82–88. [DOI] [PubMed] [Google Scholar]
- 4. Di Santo S, Yang Z, Wyler von Ballmoos M et al. Novel cell‐free strategy for therapeutic angiogenesis: In vitro generated conditioned medium can replace progenitor cell transplantation. PLoS One 2009;4:e5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Osugi M, Katagiri W, Yoshimi R et al. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng Part A 2012;18:1479–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang J, Guan J, Niu X et al. Exosomes released from human induced pluripotent stem cells‐derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med 2015;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li X, Chen C, Wei L et al. Exosomes derived from endothelial progenitor cells attenuate vascular repair and accelerate reendothelialization by enhancing endothelial function. Cytotherapy 2016;18:253–262. [DOI] [PubMed] [Google Scholar]
- 8. Anderson JD, Johansson HJ, Graham CS et al. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor‐kappaB signaling. Stem Cells 2016;34:601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deregibus MC, Cantaluppi V, Calogero R et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 2007;110:2440–2448. [DOI] [PubMed] [Google Scholar]
- 10. Zhang B, Wu X, Zhang X et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/beta‐catenin pathway. Stem Cells Transl Med 2015;4:513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van der Pol E, Böing AN, Harrison P et al. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev 2012;64:676–705. [DOI] [PubMed] [Google Scholar]
- 12. Rani S, Ryan AE, Griffin MD et al. Mesenchymal stem cell‐derived extracellular vesicles: Toward cell‐free therapeutic applications. Mol Ther 2015;23:812–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 2009;9:581–593. [DOI] [PubMed] [Google Scholar]
- 14. Edgar JR. Q&A: What are exosomes, exactly? BMC Biol 2016;14:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raposo G, Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J Cell Biol 2013;200:373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang X, Wang X, Zhu H et al. Hsp20 functions as a novel cardiokine in promoting angiogenesis via activation of VEGFR2. PLoS One 2012;7:e32765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barile L, Lionetti V, Cervio E et al. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res 2014;103:530–541. [DOI] [PubMed] [Google Scholar]
- 18. Wang K, Jiang Z, Webster KA et al. Enhanced cardioprotection by human endometrium mesenchymal stem cells driven by exosomal microRNA‐21. Stem Cells Transl Med 2017;6:209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hu GW, Li Q, Niu X et al. Exosomes secreted by human‐induced pluripotent stem cell‐derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res Ther 2015;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kordelas L, Rebmann V, Ludwig AK et al. MSC‐derived exosomes: A novel tool to treat therapy‐refractory graft‐versus‐host disease. Leukemia 2014;28:970–973. [DOI] [PubMed] [Google Scholar]
- 21. Lener T, Gimona M, Aigner L et al. Applying extracellular vesicles based therapeutics in clinical trials ‐ An ISEV position paper. J Extracell Vesicles 2015;4:30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Basu J, Ludlow JW. Exosomes for repair, regeneration and rejuvenation. Expert Opin Biol Ther 2016;16:489–506. [DOI] [PubMed] [Google Scholar]
- 23. Shabbir A, Cox A, Rodriguez‐Menocal L et al. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev 2015;24:1635–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang B, Shi Y, Gong A et al. HucMSC exosome‐delivered 14‐3‐3ζ orchestrates self‐control of the Wnt response via modulation of YAP during cutaneous regeneration. Stem Cells 2016;34:2485–2500. [DOI] [PubMed] [Google Scholar]
- 25. Fang S, Xu C, Zhang Y et al. Umbilical cord‐derived mesenchymal stem cell‐derived exosomal microRNAs suppress myofibroblast differentiation by inhibiting the transforming growth factor‐β/SMAD2 pathway during wound healing. Stem Cells Transl Med 2016;5:1425–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li X, Liu L, Yang J et al. Exosome derived from human umbilical cord mesenchymal stem cell mediates MiR‐181c attenuating burn‐induced excessive inflammation. EBioMedicine 2016;8:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ti D, Hao H, Tong C et al. LPS‐preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome‐shuttled let‐7b. J Transl Med 2015;13:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li X, Jiang C, Zhao J. Human endothelial progenitor cells‐derived exosomes accelerate cutaneous wound healing in diabetic rats by promoting endothelial function. J Diabetes Complications 2016;30:986–992. [DOI] [PubMed] [Google Scholar]
- 29. Yuan H, Guan J, Zhang J et al. Exosomes secreted by human urine‐derived stem cells accelerate skin wound healing by promoting angiogenesis in rat . Cell Biol Int 2016. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 30. Zhang J, Chen C, Hu B et al. Exosomes derived from human endothelial progenitor cells accelerate cutaneous wound healing by promoting angiogenesis through Erk1/2 signaling. Int J Biol Sci 2016;12:1472–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang B, Wang M, Gong A et al. HucMSC‐exosome mediated‐Wnt4 signaling is required for cutaneous wound healing. Stem Cells 2015;33:2158–2168. [DOI] [PubMed] [Google Scholar]
- 32. Sheyn D, Ben‐David S, Shapiro G et al. Human induced pluripotent stem cells differentiate into functional mesenchymal stem cells and repair bone defects. Stem Cells Transl Med 2016;5:1447–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Littman N, Abo A. Proceedings: Using stem cell therapies to reestablish osteogenic capability for bone regeneration. Stem Cells Transl Med 2015;4:1247–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Furuta T, Miyaki S, Ishitobi H et al. Mesenchymal stem cell‐derived exosomes promote fracture healing in a mouse model. Stem Cells Transl Med 2016;5:1620–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Qi X, Zhang J, Yuan H et al. Exosomes secreted by human‐induced pluripotent stem cell‐derived mesenchymal stem cells repair critical‐sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int J Biol Sci 2016;12:836–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang J, Liu X, Li H et al. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res Ther 2016;7:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Qin Y, Wang L, Gao Z et al. Bone marrow stromal/stem cell‐derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci Rep 2016;6:21961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pittenger MF, Mackay AM, Beck SC et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–147. [DOI] [PubMed] [Google Scholar]
- 39. Weilner S, Schraml E, Wieser M et al. Secreted microvesicular miR‐31 inhibits osteogenic differentiation of mesenchymal stem cells. Aging Cell 2016;15:744–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ekström K, Omar O, Granéli C et al. Monocyte exosomes stimulate the osteogenic gene expression of mesenchymal stem cells. PLoS One 2013;8:e75227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cui Y, Luan J, Li H et al. Exosomes derived from mineralizing osteoblasts promote ST2 cell osteogenic differentiation by alteration of microRNA expression. FEBS Lett 2016;590:185–192. [DOI] [PubMed] [Google Scholar]
- 42. Liu S, Liu D, Chen C et al. MSC transplantation improves osteopenia via epigenetic regulation of notch signaling in lupus. Cell Metab 2015;22:606–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Martins M, Ribeiro D, Martins A et al. Extracellular vesicles derived from osteogenically induced human bone marrow mesenchymal stem cells can modulate lineage commitment. Stem Cell Reports 2016;6:284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Narayanan R, Huang CC, Ravindran S. Hijacking the cellular mail: Exosome mediated differentiation of mesenchymal stem cells. Stem Cells Int 2016;2016:3808674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ranghino A, Cantaluppi V, Grange C et al. Endothelial progenitor cell‐derived microvesicles improve neovascularization in a murine model of hindlimb ischemia. Int J Immunopathol Pharmacol 2012;25:75–85. [DOI] [PubMed] [Google Scholar]
- 46. Zhang HC, Liu XB, Huang S et al. Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells Dev 2012;21:3289–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jiang ZZ, Liu YM, Niu X et al. Exosomes secreted by human urine‐derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res Ther 2016;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Montemurro T, Viganò M, Ragni E et al. Angiogenic and anti‐inflammatory properties of mesenchymal stem cells from cord blood: Soluble factors and extracellular vesicles for cell regeneration. Eur J Cell Biol 2016;95:228–238. [DOI] [PubMed] [Google Scholar]
- 49. Liang X, Zhang L, Wang S et al. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR‐125a. J Cell Sci 2016;129:2182–2189. [DOI] [PubMed] [Google Scholar]
- 50. Eirin A, Riester SM, Zhu XY et al. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue‐derived mesenchymal stem cells. Gene 2014;551:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li J, Zhang Y, Liu Y et al. Microvesicle‐mediated transfer of microRNA‐150 from monocytes to endothelial cells promotes angiogenesis. J Biol Chem 2013;288:23586–23596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. van Balkom BW, de Jong OG, Smits M et al. Endothelial cells require miR‐214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood 2013;121:3997–4006, s1–S15. [DOI] [PubMed] [Google Scholar]
- 53. Mocharla P, Briand S, Giannotti G et al. AngiomiR‐126 expression and secretion from circulating CD34(+) and CD14(+) PBMCs: Role for proangiogenic effects and alterations in type 2 diabetics. Blood 2013;121:226–236. [DOI] [PubMed] [Google Scholar]
- 54. Kang T, Jones TM, Naddell C et al. Adipose‐derived stem cells induce angiogenesis via microvesicle transport of miRNA‐31. Stem Cells Transl Med 2016;5:440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vrijsen KR, Maring JA, Chamuleau SA et al. Exosomes from cardiomyocyte progenitor cells and mesenchymal stem cells stimulate angiogenesis via EMMPRIN. Adv Healthc Mater 2016;5:2555–2565. [DOI] [PubMed] [Google Scholar]
- 56. Vrijsen KR, Sluijter JP, Schuchardt MW et al. Cardiomyocyte progenitor cell‐derived exosomes stimulate migration of endothelial cells. J Cell Mol Med 2010;14:1064–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kang K, Ma R, Cai W et al. Exosomes secreted from CXCR4 overexpressing mesenchymal stem cells promote cardioprotection via Akt signaling pathway following myocardial infarction. Stem Cells Int 2015;2015:659890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cantaluppi V, Biancone L, Figliolini F et al. Microvesicles derived from endothelial progenitor cells enhance neoangiogenesis of human pancreatic islets. Cell Transplant 2012;21:1305–1320. [DOI] [PubMed] [Google Scholar]


