Abstract
Breast cancer‐related lymphedema (BCRL) is a debilitating late complication with a lack of treatment opportunities. Recent studies have suggested that mesenchymal stromal cells can alleviate lymphedema. Herein, we report the results from the first human pilot study with freshly isolated adipose‐derived regenerative cells (ADRC) for treating lymphedema with 6 months follow‐up. Ten BCRL patients were included. ADRC was injected directly into the axillary region, which was combined with a scar‐releasing fat graft procedure. Primary endpoints were change in arm volume. Secondary endpoints were change in patient reported outcome and safety. The study is registered with ClinicalTrials.gov (NCT02592213). During follow‐up, a small volume reduction was noted but was not significant. Five patients reduced their use of conservative management. Patient‐reported outcomes improved significantly over time. ADRCs were well tolerated and only minor transient adverse events related to liposuction were noted. In this pilot study, a single injection of ADRC improved lymphedema based on patient‐reported outcome measures, and there were no serious adverse events in the 6 months follow‐up period. In addition, half of the patients reduced their use of conservative management. ADRC therapy is a promising interventional therapy for alleviating lymphedema, but results need to be confirmed in randomized clinical trials. Stem Cells Translational Medicine 2017;6:1666–1672
Keywords: Lymphedema, Regenerative medicine, Adipose‐derived regenerative cells, Fat graft
Significance Statement.
Lymphedema is one of the most serious and debilitating late complications following breast cancer surgery with lackluster treatment opportunities. Cell therapy has shown promising results in preclinical models of lymphedema. Autologous adipose tissue is the most convenient source of cells for clinical therapy. In the present First in Human Pilot Study, it is shown that treatment with autologous adipose‐derived regenerative cells can alleviate symptoms and reduce the need for other treatment options during the 6 months follow‐up. The treatment proved safe without any noteworthy adverse events. Randomized controlled trials will be needed to verify the positive results.
Introduction
Lymphedema is one of the most common and severe complications after breast cancer treatment. Breast cancer‐related lymphedema (BCRL) occurs in 20%–30% of patients with lymph node involvement 1 and manifests itself with excess lymphatic fluid and swelling of subcutaneous tissues in the nearby arm due to obstruction and or destruction of lymphatic vessels following surgical treatment and/or radiation therapy. Lymphedema impacts the patients' lives both physically and psychologically. Physically, the swelling can result in fatigue, feeling of arm heaviness and tension, reduced range of arm motion, as well as difficulties in performing daily activities, including returning to work 2. Psychologically, the swelling can result in negative feelings with regard to body image. In addition, lymphedema is associated with increased risk of severe skin infections 3.
Standard of care for lymphedema today is conservative nonsurgical management, which includes a mix of compression garments on the affected arm(s), manual lymphatic drainage, exercises, as well as meticulous skin care 4. The nonsurgical management is only effective to a certain extent with excess volume reductions in the range of 20%–30% 5. Each treatment is time consuming, and treatment is lifelong because the underlying problem has not been addressed 6. Several microsurgical approaches are being used experimentally with promising initial results; however, the evidence for the efficacy is poor 7, 8.
The potential ability to treat diseases through regeneration has sparked the interest in cell therapy in almost all organ systems 9, including BCRL 10, 11. Two studies have so far examined the efficacy and safety of using bone marrow‐derived mesenchymal stromal cells to treat BCRL 10, 11. These results were promising with symptom relief and are supported by several preclinical studies using cell therapy to treat lymphedema 12. In recent years, several cell therapeutic protocols that utilize adipose‐derived stromal cells (also called stromal vascular fraction or adipose‐derived regenerative cells [ADRC]) have emerged. ADRCs are easily accessible through liposuction and are an abundant cell source 13. This pool of cells is, however, heterogeneous in nature and includes stem cells, endothelial cells, pericytes, progenitor cells, as well as hematopoietic cells. It has been proposed that these cells may work in synergistic fashion, especially through paracrine secretion of growth factors and cytokines 14, 15, and several studies, including our unpublished data, indicate that ADRC likely improves vascularity 16, 17.
We therefore recently performed a case study of ADRC transplantation and fat grafting to alleviate lymphedema in one patient. Interestingly, both a patient‐reported and volumetric improvement was noted 18. To substantiate this promising result, we herein tested efficacy, safety, and feasibility of this procedure in the first pilot study performed. We here report the results from 10 patients with a 6‐month follow‐up period after ADRC transplantation and fat grafting for BCRL.
Materials and Methods
ADRC Transplantation and Fat Grafting
The procedures were performed under general anesthesia at the Department of Plastic Surgery, Odense University Hospital. Approximately 300 mL lipoaspirate from either thighs or abdomen with water‐jet‐assisted liposuction (body‐jet, Human med AG, Schwerin, Germany, http://www.humanmed.com/en) was obtained, and 30 mL was saved for lipotransfer to the axilla. After decantation, the lipoaspirate for lipotransfer was injected in a fan‐shaped pattern for loosening the scar tissue. The patient was transferred back to the ward awaiting cell injection to avoid occupying the operating theatre for 2 hours as the ADRC were being isolated. The remaining lipoaspirate was used for immediate ADRC isolation using the Celution 800/IV system (Cytori Therapeutics, San Diego, California, http://www.cytori.com/) according to the manufacturer's instructions. The ADRC was resuspended in 5 mL Lactated Ringer's solution, and 1 mL was saved for in vitro characterization. The remaining cells were injected in the axilla at eight standardized points around the scar into the subcutaneous plane.
Cell Characterization
Cells were counted with a NucleocounterNC‐100 (ChemoMetec, Denmark, https://chemometec.com/). ADRC surface marker analysis was performed as recommended internationally 15 and previously described 19. The following antibodies were used according to manufacturer's (BD Biosciences, Albertslund, Denmark, http://www.bdbiosciences.com/us/home) recommendations: anti‐CD235a (BV421, clone GA‐R2/HIR2), anti‐CD34 (PECF594, clone 581), anti‐CD45 (FITC, clone HI30), CD31 (Alexa Fluor 647, clone WM59), CD73 (APC, clone AD2), CD90 (APC, clone 5E10), and appropriate isotype (for single stains) or FMO control (for CD34 on multistain). Sample acquisition was performed on a BD LSRII flow cytometer and analyzed using the FACSDiva software v8.0.1 (BD Biosciences, Albertslund, Denmark, http://www.bdbiosciences.com/us/home) and FlowJo 10.0.8r1 (Flowjo, LLC, Ashland, Oregon, US https://www.flowjo.com/). Cell doublets were excluded from all analyses by sequential gating through forward and side scatter height/width plots.
The percentage of fibroblastoid colony‐forming units (CFU‐F) in each ADRC sample was determined by seeding cells at low density (4 densities in triplicate ranging from 28–280 live nucleated cells per cm2) in dulbecco's modified eagle medium (DMEM) with 1 g/L glucose, 10% fetal bovine serum (FBS), 1%PS, culturing for 14 days, and counting hematoxylin and eosin (HE)‐stained colonies comprising >50 cells. The differentiation potential of the adipose‐derived stem cells within the ADRC was tested using adipogenesis, osteogenesis, and chondrogenesis differentiation kits from StemPro (Thermo Fisher Scientific, Denmark, https://www.thermofisher.com/us/en/home.html) as recommended by the manufacturer.
Volumetric Outcome Assessment
Arm volume was calculated as previously described 20. Briefly, circumference measurements were made at five points on each arm: wrist, largest point on lower arm, elbow, middle of upper arm, and proximal on the upper arm. The length between each point was measured, and at each time point the same sites were measured. Based on these five measurements, the arm was divided in four segments and the volume of each segment was calculated based on the truncated cone formula.
Where V is the segment volume, h is the length of the segment, and C1 and C2 are the two circumference measurements at the two ends of the segment. Arm volume was calculated as the sum of the four segment volumes. Excess arm volume was calculated as the volume difference between the two arms.
Arm volume was also evaluated by DXA, which was performed preoperatively and 3 months and 6 months postoperatively. The DXA was performed as two whole body scans with the patient lying in a modified position enabling the arm to be free from the trunk. For analysis, a blinded assessor drew the region around the arm with the proximal end of the arm defined as just below the deltoid muscle, which is easily visualized on the DXA. The bone mineral content, fat mass, and lean mass were used to calculate a total arm volume based on known densities as has been previously described 21.
Patient‐Reported Outcome Assessment
Patients were asked to rate the feeling of heaviness in the lymphedema arm on a numerical rating scale ranging from 0 to 10, with 0 meaning no heaviness at all and 10 signifying the worst heaviness imaginable. Similarly, the patients were asked to rate the feeling of tension in the lymphedema arm. In addition, two questionnaires were used: the disabilities of the arm, shoulder and hand (DASH) outcome questionnaire 25 as well as the Lymphedema Quality‐of‐Life (LYMQOL) questionnaire 26 were filled out preoperatively and at each time point after treatment.
Safety Assessment
Safety was evaluated by a specific questionnaire given to the patients postoperatively, which was to be filled out prospectively during the first month following treatment. The questionnaire included questions regarding redness, swelling, itching, pain (which was not handled sufficiently with over the counter medicine), infection, other discomfort, and having seen their family doctor for any reason. Additionally, at the postoperative visits, adverse events were recorded by inspection of the injection and donor site and posing an open question, “Did you experience any other discomfort related to the operation since the last visit?”
Statistical Analysis
Statistical analysis was performed with GraphPad Prism 6.0 for Windows (GraphPad Software, La Jolla, California, www.graphpad.com). Subjective scores on visual analogue scale and questionnaire outcomes were analyzed by Friedman's test for multiple non‐parametric comparisons with Dunn's post‐hoc test for multiple comparisons. Volumetric changes were analyzed using One‐way analysis of variance (ANOVA) with Dunnett post‐hoc test for multiple comparisons. For subgroup analysis based on International Society of Lymphology (ISL) stage, a two‐way ANOVA was performed with Tukey's test for multiple comparisons. A two‐tailed p value of less than .05 was considered significant.
Results
Study Design and Patient Characteristics
This study was conducted as an open–label, single‐arm, single‐center feasibility and safety study in patients with breast cancer‐related lymphedema of the upper extremity (ClinicalTrials.gov NCT02592213). The study was approved by The Regional Committees on Health Research Ethics for Southern Denmark (3‐3013‐1572/1), who had oversight of the study. The study was registered with The Danish Data Protection Agency (2008‐58‐0035).
The aim was to include 10 patients for treatment, and 34 patients were screened for eligibility, of whom 11 were found to be eligible. The reason for the extra included patient was due to non‐protocolled treatment of the very first patient, who was therefore excluded from analysis (supplemental online Fig. 1). Inclusion period ranged from December 2015 to May 2016, and all patients were treated between January and May 2016.
Figure 1.
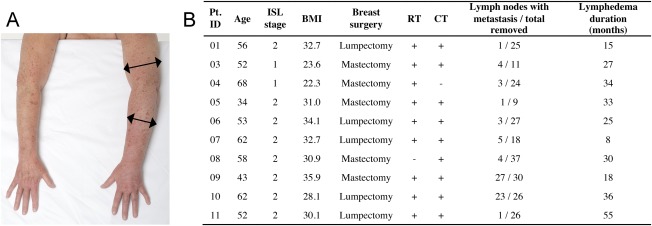
Patient overview. (A): Representative photo of breast cancer‐related lymphedema in the left arm compared with the healthy contralateral side. (B): Table showing baseline characteristics of included patients. Patient ID 02 was excluded due to non‐protocolled treatment as described in the manuscript. Abbreviations: +, yes; ‐, no; BMI, body mass index; CT, chemotherapy; ISL stage, International Society of Lymphology stage; Pt. ID, patient identification; RT, radiation therapy.
Eligible patients had to fulfill the following criteria: diagnosed with upper extremity lymphedema due to previous breast cancer treatment with lymph node involvement, recurrence‐free for minimum 1 year, unilateral disease, lymphedema ISL stage I or II, circumference of arm a minimum of 2 cm larger than the healthy side, age between 18 and 70 years old, The American Society of Anesthesiologists Physical Status classification system (ASA) score 1 or 2, able to give written informed consent, and able to understand the Danish language. Additionally, they were excluded if they had a history of other cancer types, had diabetes mellitus, had psychiatric conditions that could interfere with participation, or used tobacco, which was not ceased in relation to the procedure (supplemental online Fig. 1). The ages of the 10 included patients ranged from 34–68 years (54.5 [12.3], median [interquartile range {IQR}]). Only two participants had ISL stage U lymphedema and the remaining participants had stage II (Fig. 1A and 1B). The majority of patients had received both radiation and chemotherapy for the primary breast cancer treatment, and the duration of lymphedema prior to inclusion in the trial was 28.5 (17.3; median [IQR]) months (Fig. 1B).
ADRC Isolation and Transplantation
For the ADRC treatment, all patients underwent liposuction under general anesthesia, during which approximately 300 mL of lipoaspirate (Fig. 2A) was harvested from either the abdomen or thighs depending on availability and preference of the patient. The ARDC isolation using the automated Celution system and cellular/biochemical characterization (Fig. 2B) has previously been described 19. In addition, a lipoaspirate was harvested for immediate grafting in the axilla, where fat was injected in a standard fan shaped pattern to release scar tissue (28.1, 7.8 mL [mean, SD]; Fig.2C). ADRCs (5.37 × 107, 1.08 × 107 cells [mean, SD]) were injected in the axilla 2 hours later (Fig. 2D).
Figure 2.
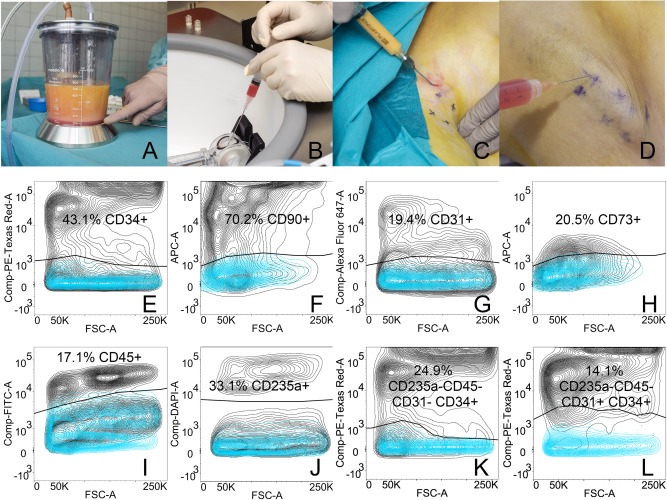
The procedure and cell characterization. (A): Approximately 300 mL of lipoaspirate was harvested for adipose‐derived regenerative cell (ADRC) isolation. (B): ADRC were isolated using the automated Celution IV system yielding a 5‐mL cell suspension in about 2 hours. (C): During surgery, 20–30 mL of lipoaspirate was injected in the axilla in a fan‐shaped pattern to loosen scar tissue. (D): Immediately following ADRC isolation, the cell suspension was injected subcutaneously in the axilla. Flow cytometric evaluation of ADRC surface markers was performed on fresh cells and analyzed for CD34 (E), CD90 (F), CD31 (G), CD73 (H), CD45 (I), CD235a (J). The stromal stem cell subpopulation was defined as CD235a‐CD45‐CD31‐CD34+ (K) and the endothelial progenitor cell subpopulation was defined as CD235a‐CD45‐CD31+CD34+ (L).
The characteristics of the isolated ADRC were comparable to those previously reported by us 19, including yield (ADRC cells/g fat tissue, 2.1 × 105, 4.2 × 104 [mean, SD]), cell size (10.8, 0.2 [mean, SD]), viability (83.4, 3.0 [mean, SD]), and percentage of fibroblastoid colony forming units (%CFU‐F, 0.4, 0.4 [mean, SD]). A large proportion of the ADRC expressed the surface markers CD34 (43.1, 14.5% [mean, SD]; Fig. 2E) and CD90 (70.2, 9.3% [mean, SD]; Fig. 2F), whereas CD31 and CD73 each defined smaller subpopulations (19.4%, 8.1% and 20.5%, 17.7%, respectively [mean, SD]; Fig. 2G and 2H). The amount of blood as determined by the hematopoietic and erythropoietic markers CD45 (17.1, 5.7% [mean, SD]; Fig. 2I) and CD235a (33.1, 16.0% [mean, SD]; Fig. 2J), respectively, was similar between samples. The fraction of stromal stem cells as defined phenotypically by the markers CD235a‐CD45‐CD31‐CD34+ encompassed 24.9 ± 8.4% (mean ± SD; Fig. 2K) of the parent ADRC, whereas the fraction of endothelial progenitor cells CD235a‐CD45‐CD31+CD34 + comprised 14.1 ± 6.1% (mean ± SD; Fig. 2L).
Safety and Lymphedema Alleviation Using ADRC Transplantation and Fat Grafting
In total, nine minor adverse events in 4/10 patients were noted in the follow‐up period. Three patients noted bruising of the donor site following liposuction, accompanied by pain in two cases. Another patient noted itching of the axilla and donor site. All pain and itching resolved spontaneously within the first week after treatment. One patient complained of back pain 2 weeks after treatment, for which she sought a chiropractor. At 3 months follow‐up, one patient complained of reduced sensation at the donor site, which resolved spontaneously at 6 months follow‐up. One patient noted a slight irregularity in the skin surface at the donor site. All the above events are expected complications of liposuction and fat grafting and were therefore not attributed to the ADRC transplantation. There was no evidence of cancer recurrence during the follow‐up period.
Patients were evaluated 1, 3, and 6 months following ADRC transplantation. In general, the patients reported a decrease of their lymphedema symptoms over time (Fig. 3; see Materials and Methods for detailed assessment tools). The median (IQR, n = 10) score for heaviness of the arm at baseline was 5.5 (4.0), after 1 month 4.0 (2.5; p = .2498), after 3 months 3.5 (3.5; p = .0097), and after 6 months 2.5 (4.3; p = .0030; Fig. 3A). Likewise, we found a median score for arm tension at baseline to be 5.0(1.5), after 1 month 3.0 (3.0; p = .0281), after 3 months 3.0 (2.3; p = .0459), and after 6 months 2.5 (4.3; p = .0097; Fig. 3B). Finally the DASH questionnaire was also used, in which the median score at baseline was 21.3 (23.5), after 1 month 10.0 (14.5; p = .0097), after 3 months 10.4 (24.8; p = .0281), and after 6 months 12.9 (26.0; p = .0168; Fig. 3C). Moreover, five patients reduced their use of conservative treatment. A significant mood improvement was also observed at 3 and 6 months follow‐up with the LYMQOL questionnaire. However, only minor insignificant improvements were reported in the other LYMQOL subscales (supplemental online Table 1).
Figure 3.
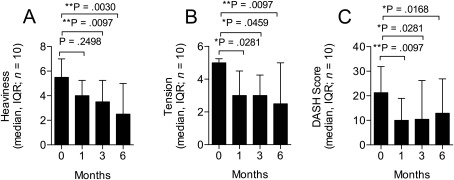
Patient reported outcome evaluation. Patient‐reported outcome was evaluated after 1, 3, and 6 months following treatment. There was a significant improvement in patient reported outcomes based on the degree of heaviness (A): as well as tension (B): in the arm measured on numerical rating scales (0–10) as well as the DASH questionnaire (C). Abbreviations: DASH, disabilities of the arm, shoulder and hand; IQR, interquartile range.
The change in excess arm volume was evaluated with two separate methods. As previously described 20, the volume of the arm can be estimated by five manual circumference measurements along the arm using the truncated cone formula (Fig. 4A). The arm volume can also be described by dual‐energy x‐ray absorptiometry (DXA; Fig. 4B). A subregional analysis of the arm will reveal the mass of bone, fat and lean mass, and, based on known densities of the tissue types, the volume of the arm can be calculated 21. For each method the difference in arm volume between the lymphedematous and healthy arm was defined as excess arm volume. Although a slight difference in absolute volume is present (Fig. 4A and 4B) between the results obtained from the two methods, they correlated significantly (r = 0.848, p < .0001, Pearson). A modest decrease in excess arm volume was observed after 1 month; however, after 3 and 6 months, the change was still decreased but failed to meet the significance level (Fig. 4A and 4B).
Figure 4.
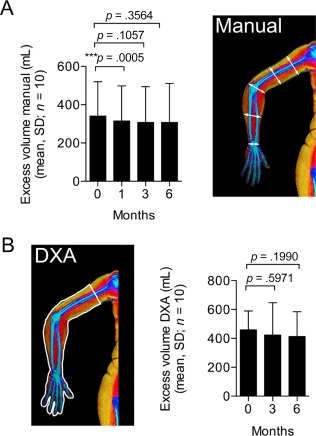
Volumentric outcome evaluation. (A): Volume was calculated manually by circumference measurements at five points along the arm and measurement of the distance between each point. A transient significant reduction in arm volume was seen after 1 month but results were insignificant after 3 and 6 months. (B): Volume was also assessed by DXA after 3 and 6 months after treatment. A subregion was drawn around the arm and based on known densities of bone, fat, and lean tissue types, the volume was calculated. Similar to the circumference measurements, volume change over time assessed by DXA also showed a small non‐significant decrease at the 3 and 6 month time points. Abbreviation: DXA, dual‐energy x‐ray absorptiometry.
We performed subgroup analysis based on ISL stage I (n = 2) and II (n = 8). Visual interpretation showed a trend towards greater response for ISL stage I patients (supplemental online Fig 2). Two‐way ANOVA testing revealed no significant differences between the groups but for feeling of tension (p = .0583), DASH questionnaire (p = .0971), as well as volume (p = .0947). The p value neared significance despite the low number of patients. Interestingly for volume, there was a significant decrease in volume after 6 months for ISL stage I (p = .0253) but not ISL stage II patients.
Discussion
In this clinical trial, we show that a single treatment of cell‐assisted lipotransfer using ADRCs can alleviate lymphedema symptoms and moreover reduce the need for conservative treatment in 50% of patients. The data from the numerical rating scales and the DASH questionnaire were significant, whereas only minimal trends were seen for the LYMQOL questionnaire, which concerns quality‐of‐life perception. Four patients had previous cellulitis/erysipelas infection in their lymphedema arm, and there were no episodes of infection in the arm following treatment, a finding which has also been documented for microsurgical procedures 22.
Although, our qualitative measures all point towards the conclusion that ADRC‐assisted lipotransfer is a promising future intervention for lymphedema patients, we did not find any significant reduction in excess volume as measured by two quantitative techniques. The latter may be explained by the use of adjunct conservative management in our patients, because Hou et al. recently reported that patients without prior conservative treatment experience a substantial volume reduction 3 and 12 months after bone marrow‐derived stromal cell treatment as compared to combined treatment that includes conservative management 10. Likewise, Maldonado et al. have shown that bone marrow‐derived stromal cell therapy alone was as effective as compression therapy with a 200‐mL volume reduction in the lymphedema arm 11. In this regard, it seems limiting for our assessment of volumes (manual and DXA) that our patients wore their compression garment up until the point of examination, leaving no window for “stress testing” of the lymphatic drainage of the arm. Currently, we are developing a new quantitative measure based on lymphoscintigraphy that enables us to determine changes in lymphatic drainage when using conservative treatment in parallel.
Importantly, we did not observe any adverse events within a 6‐month time frame. While this is an important result, a long‐term evaluation of the safety is necessary, especially regarding the risk of cancer recurrence, in light of ADRC injection into an area with previous malignancy 23. Yet hereto, the safety profiles of using ADRC transplantation and fat grafting are in agreement with other recent studies using bone marrow‐derived mesenchymal stromal cells for lymphedema 10, 11. Still, the field needs to document long‐term (years) safety of cellular therapy in these patients.
The study was not without limitations. First, the trial was unblinded. It is possible that the added visits and the patients' own expectations of stem cell therapy may have caused a placebo effect. The trial was also one‐armed, and the lack of a control group is a weakness. However, lymphedema is a condition that very rarely improves and, if anything, only worsens, so it could be speculated that any positive change might be attributed to the given treatment. Another potential weakness lies in the patient population, as most patients were late ISL stage II, in which chronic changes are dominant. ISL stage I patients might have a higher chance of successful treatment based on our very preliminary subgroup analysis, which is in agreement with microsurgical procedures, where it is generally accepted that the chance for successful treatment is greatest for early‐stage lymphedema without chronic changes 24. It is important to increase the chance of treatment success through the optimization of the selection criteria for patients. We aimed to harvest of 300 mL lipoaspirate for cell isolation, as we estimated that it could be harvested from almost any patient, so that the treatment was made as uniform as possible. Further studies are needed to determine an optimal dose. Additionally, it will be interesting to examine in the future if either ADRC or fat grafting can stand alone as treatment, which could possibly simplify the procedure.
Conclusion
The principal findings in this study are that autologous ADRC combined with a scar‐releasing fat graft was safe during the 6 months follow‐up period and can alleviate symptoms of breast cancer‐related lymphedema, minimizing the need for conservative treatment. As the effect was primarily noted on patient‐reported outcomes, it is important to confirm the benefit of this treatment modality in a properly blinded randomized controlled trial, in which sensitive methods to quantify excess volumes are included also included and patients are followed for several years to ensure long‐term safety. Finally, future studies may also seek to test if the observed beneficial effect is caused by the ADRC or the fat graft alone, or a combination.
Supplemental Material
Three items were included as supplementary material. First is a figure illustrating the study flow chart. The second is a table that summarizes all results during the study. Third is a figure illustrating the subgroup analysis of the patient‐reported outcomes of feeling of heaviness and tension in the arm grouped by ISL stage.
Author Contributions
N.T. and C.J.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; S.P.: conception and design, revision/enhancement of manuscript for intellectual content, final approval of manuscript; J.S.: conception and design, collection and/or assembly of data, revision/enhancement of manuscript for intellectual content, final approval of manuscript; D.A.: collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Supporting information
Supporting Information Figure 1.
Supporting Information Figure 3.
Supporting Information Table 2.
Acknowledgments
This study was funded by Axel Muusfeldt fonden, Frimodt‐Heineke fonden, and participating departments. The study was part of a Ph.D. project, which was partly funded by stipends from Odense University Hospital and University of Southern Denmark. We are grateful for the help of lab technician Tonja Lyngse Jørgensen for her assistance during cell isolation and cell characterization.
Authored by a member of IFATS.
References
- 1. DiSipio T, Rye S, Newman B et al. Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta‐analysis. Lancet Oncol 2013;14:500–515. [DOI] [PubMed] [Google Scholar]
- 2. Taghian NR, Miller CL, Jammallo LS et al. Lymphedema following breast cancer treatment and impact on quality of life: A review. Crit Rev Oncol Hematol 2014;92:227–234. [DOI] [PubMed] [Google Scholar]
- 3. Dupuy A, Benchikhi H, Roujeau JC et al. Risk factors for erysipelas of the leg (cellulitis): Case‐control study. BMJ 1999;318:1591–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2013 Consensus Document of the International Society of Lymphology. Lymphology 2013;46:1–11. [PubMed] [Google Scholar]
- 5. Dayes IS, Whelan TJ, Julian JA et al. Randomized trial of decongestive lymphatic therapy for the treatment of lymphedema in women with breast cancer. J Clin Oncol 2013;31:3758–3763. [DOI] [PubMed] [Google Scholar]
- 6. Lasinski BB, McKillip Thrift K, Squire D et al. A systematic review of the evidence for complete decongestive therapy in the treatment of lymphedema from 2004 to 2011. PM R 2012;4:580–601. [DOI] [PubMed] [Google Scholar]
- 7. Scaglioni MF, Arvanitakis M, Chen YC et al. Comprehensive review of vascularized lymph node transfers for lymphedema: Outcomes and complications. Microsurgery; 2016. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 8. Penha TR, Ijsbrandy C, Hendrix NA et al. Microsurgical techniques for the treatment of breast cancer‐related lymphedema: A systematic review. J Reconstr Microsurg 2013;29:99–106. [DOI] [PubMed] [Google Scholar]
- 9. Minteer DM, Marra KG, Rubin JP. Adipose stem cells: Biology, safety, regulation, and regenerative potential. Clin Plast Surg 2015;42:169–179. [DOI] [PubMed] [Google Scholar]
- 10. Hou C, Wu X, Jin X. Autologous bone marrow stromal cells transplantation for the treatment of secondary arm lymphedema: A prospective controlled study in patients with breast cancer related lymphedema. Jpn J Clin Oncol 2008;38:670–674. [DOI] [PubMed] [Google Scholar]
- 11. Maldonado GE, Pérez CA, Covarrubias EE et al. Autologous stem cells for the treatment of post‐mastectomy lymphedema: A pilot study. Cytotherapy 2011;13:1249–1255. [DOI] [PubMed] [Google Scholar]
- 12. Toyserkani NM, Christensen ML, Sheikh SP et al. Stem cells show promising results for lymphoedema treatment–A literature review. J Plast Surg Hand Surg 2015;49:65–71. [DOI] [PubMed] [Google Scholar]
- 13. Fraser JK, Wulur I, Alfonso Z et al. Fat tissue: An underappreciated source of stem cells for biotechnology. Trends Biotechnol 2006;24:150–154. [DOI] [PubMed] [Google Scholar]
- 14. Yoshimura K, Suga H, Eto H. Adipose‐derived stem/progenitor cells: Roles in adipose tissue remodeling and potential use for soft tissue augmentation. Regen Med 2009;4:265–273. [DOI] [PubMed] [Google Scholar]
- 15. Bourin P, Bunnell BA, Casteilla L et al. Stromal cells from the adipose tissue‐derived stromal vascular fraction and culture expanded adipose tissue‐derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013;15:641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Premaratne GU, Ma LP, Fujita M et al. Stromal vascular fraction transplantation as an alternative therapy for ischemic heart failure: Anti‐inflammatory role. J Cardiothorac Surg 2011;6:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harada Y, Yamamoto Y, Tsujimoto S et al. Transplantation of freshly isolated adipose tissue‐derived regenerative cells enhances angiogenesis in a murine model of hind limb ischemia. Biomed Res 2013;34:23–29. [DOI] [PubMed] [Google Scholar]
- 18. Toyserkani NM, Jensen CH, Sheikh SP et al. Cell‐assisted lipotransfer using autologous adipose‐derived stromal cells for alleviation of breast cancer‐related lymphedema. Stem Cells Translational Medicine 2016;5:857–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haahr MK, Jensen CH, Toyserkani NM et al. Safety and potential effect of a single intracavernous injection of autologous adipose‐derived regenerative cells in patients with erectile dysfunction following radical prostatectomy: An open‐labelphase I clinical trial. EBioMedicine 2016;5:204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brorson H, Höijer P. Standardised measurements used to order compression garments can be used to calculate arm volumes to evaluate lymphoedema treatment. J Plast Surg Hand Surg 2012;46:410–415. [DOI] [PubMed] [Google Scholar]
- 21. Gjorup C, Zerahn B, Hendel HW. Assessment of volume measurement of breast cancer‐related lymphedema by three methods: Circumference measurement, water displacement, and dual energy X‐ray absorptiometry. Lymphat Res Biol 2010;8:111–119. [DOI] [PubMed] [Google Scholar]
- 22. Mihara M, Hara H, Furniss D et al. Lymphaticovenular anastomosis to prevent cellulitis associated with lymphoedema. Br J Surg 2014;101:1391–1396. [DOI] [PubMed] [Google Scholar]
- 23. Gennari R, Griguolo G, Dieci MV et al. Fat grafting for breast cancer patients: From basic science to clinical studies. Eur J Surg Oncol 2016;42:1088–1102. [DOI] [PubMed] [Google Scholar]
- 24. Allen RJ Jr, Cheng MH. Lymphedema surgery: Patient selection and an overview of surgical techniques. J Surg Oncol 2016;113:923–931. [DOI] [PubMed] [Google Scholar]
- 25. Park JE, Jang HJ, Seo KS. Quality of life, upper extremity function and the effect of lymphedema treatment in breast cancer related lymphedema patients. Ann Rehabil Med 2012;36:240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keeley V, Crooks S, Locke J et al. A quality of life measure for limb lymphoedema (LYMQOL). J Lymphoedema 2010;5:27–37. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure 1.
Supporting Information Figure 3.
Supporting Information Table 2.


