Abstract
The clinical application of the fetal membranes dates back to nearly a century. Their use has ranged from superficial skin dressings to surgical wound closure. The applications of the fetal membranes are constantly evolving, and key to this is the uncovering of multiple populations of stem and stem‐like cells, each with unique properties that can be exploited for regenerative medicine. In addition to pro‐angiogenic and immunomodulatory properties of the stem and stem‐like cells arising from the fetal membranes, the dehydrated and/or decellularized forms of the fetal membranes have been used to support the growth and function of other cells and tissues, including adipose‐derived mesenchymal stem cells. This concise review explores the biological origin of the fetal membranes, a history of their use in medicine, and recent developments in the use of fetal membranes and their derived stem and stem‐like cells in regenerative medicine. Stem Cells Translational Medicine 2017;6:1767–1776
Keywords: Cellular therapy, Fetal stem cells, Placental stem cells, Tissue‐specific stem cells, Tissue regeneration
Significance Statement.
The fetal membranes make up the amniotic sac, which surrounds the fetus during pregnancy. They have nearly a century long history in regenerative medicine. They have been used as biological bandages for skin grafts as well as for serious burns. A variety of stem cells can be isolated from the fetal membranes and their regenerative properties are closely associated to their biological function during pregnancy, which is to protect the fetus from the mother's immune system. We may be able to further exploit their use in regenerative medicine by improving our understanding of the role of fetal membranes during pregnancy.
Introduction
The interior of the amniotic sac is filled with amniotic fluid, which allows the fetus to move freely in the womb and absorbs physical forces of mechanical injury. The amniotic sac also participates in the metabolism of the fetus, allowing for nutrient transport and contributing to maternal‐fetal tolerance. After the delivery of a healthy baby, the fetal membranes are usually discarded as medical waste along with the rest of the placenta. However, the medical applications of fetal membranes have been established for nearly a century. The following concise review will describe the role of the fetal membranes during pregnancy, the stem cells and stem‐like cells isolated from the fetal membrane, as well as their applications in regenerative medicine.
Immunomodulation During Pregnancy
The placenta plays an important role in modulating the mother's immune system during pregnancy. The placental villi sprout from the chorionic plate are in contact with maternal blood, while branches of the fetal blood vessels carry fetal blood to the villi. During placentation, fetal derived extravillous trophoblasts from the placental villi infiltrate the decidua to remodel uterine spiral arteries in order to achieve an adequate blood supply to the developing fetus 1 (Fig. 1). The implantation site is richly populated by immune cells; ∼70% uterine natural killer cells, ∼10% T cells, and ∼20% myelomonocytic cells 1. Maternal and fetal immune cells interact directly with each other in the decidua. This interaction is thought to play an important role in fetomaternal tolerance 2. It is perhaps this evolutionary step in placentation that confers the multitude of beneficial effects associated with gestational tissues and their derived stem cells.
Figure 1.
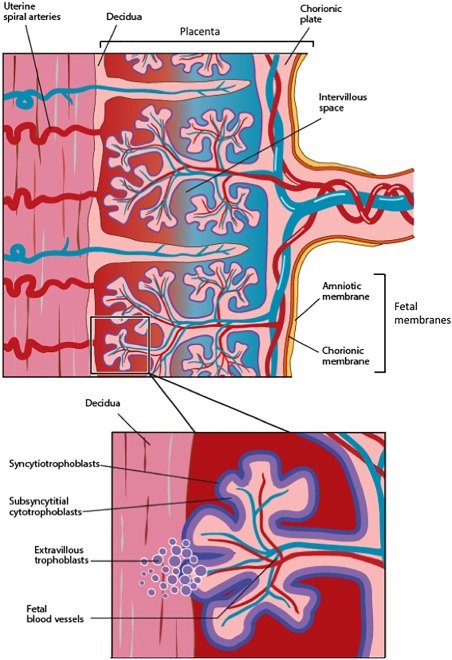
Simplified cross‐sectional diagram showing the fetal membranes (i.e., amniotic and chorionic membranes) in relation to the chorionic plate and decidua. The insert shows a representative placental villous tree surrounded by the syncytiotrophoblast layer and its underlying cytotrophoblasts. The extravillous trophoblasts invade the decidua and spiral arteries to remodel the uterine spiral blood vessels such that they become low resistance and thin‐walled, enabling a consistent blood supply to the developing fetus.
The switch from immune surveillance to immunotolerance is a crucial adaptation during pregnancy. In order to achieve a healthy pregnancy, the maternal immune system must remain quiescent to enable embryo growth. The initial implantation stage may be seen as an acute, aseptic inflammatory response by the mother's immune system 3. However, the embryo suppresses this and prevents rejection once it is implanted. This is partly achieved through atypical expression of major histocompatibility complex (MHC) by the trophoblasts and fetal membranes. The fetal membranes themselves express high levels of HLA‐G 4, which plays a critical role in maternal‐fetal tolerance 5. The trophoblast serves as an immunological barrier between the mother and the fetus. It is characterized by the lack of MHC class II antigens, 6, 7, 8 as well as classical MHC class I antigens—human leukocyte antigen (HLA)‐A and HLA‐B 9. This atypical expression of MHC molecules facilitates fetal tolerance by inhibiting the maternal immune response.
Class II antigens participate in the activation of CD4+ helper T (TH) lymphocytes and consecutively in antibody and cytotoxic T lymphocyte‐based immune responses 10. The absence of class II molecules protects the feto‐placental unit from allogeneic reactions against paternally derived MHC‐II antigens, and direct presentation of fetal derived peptides by MHC class II positive trophoblast cells to maternal helper T cells 10. Pregnancy complications such as spontaneous recurrent miscarriages arise in the absence of these maternal adaptations and when class II antigens are aberrantly expressed 11.
While human trophoblasts do not express the classical HLA‐A and HLA‐B molecules, they do express classical HLA‐C and non‐classical HLA‐E, ‐F, and ‐G class I molecules 10. HLA‐G differs from the other HLA class I molecules in terms of its low polymorphism, its unique promoter region and restricted tissue distribution. Another difference that separates HLA‐G from the rest of the HLA class Ia genes, is the alternative splicing of HLA‐G mRNA which produces alternative isoforms of the protein 12. HLA‐G can be expressed at the cell surface or as soluble isoforms. The soluble isoforms of HLA‐G have been identified at the feto‐maternal interface where they appear to modulate maternal‐fetal tolerance by inhibiting cytotoxic T cells activity, preventing CD4+ T cell proliferation 13, activating regulatory T cells 14 and inducing CD8+ T cell apoptosis via Fas/FasL 15, 16. The high expression of HLA‐G, and other immunomodulatory molecules such as indoleamine 2,3‐dioxygenase, may account for the immune privileged status and immunomodulatory properties of fetal membrane derived stem and stem‐like cells.
The Historical and Contemporary Use of Fetal Membranes
The fetal membranes and adjacent decidua provide a valuable source of cells with regenerative and immunosuppressive properties that may be useful for the treatment of a variety of inflammatory disorders 17. Fetal membranes have been used to treat severe burn injuries for almost a century 18. The fetal membranes comprise the amniotic and chorionic membranes, of which the amniotic membrane (AM) is the innermost layer and is in physical contact with the amniotic fluid. The AM is composed of a monolayer of cuboidal amniotic epithelial cells, which overlay a thick basement membrane, and sparse mesenchymal cells enclosed by an avascular matrix. The amniotic epithelial cells (hAECs) produce numerous cytokines/factors known to promote cell proliferation and differentiation, which appear to remain bound, at least partly, to the extracellular matrix even after de‐epithelialization and sterilization of the AM 19, 20.
The first report on the medical use of AM pertained to its application as a skin graft substitute dating back to 1910 21. More advanced studies on the possible clinical applications of AM did not begin to gather momentum until the second half of the 20th century where AM became one of the first biomaterials used in tissue engineering—as a biological scaffold to support cell growth and cell migration 22, 23. For the purposes of ocular surface reconstruction or wound treatment, in vitro culture of epithelial cells on AM have been used for graft preparation 24, 25. The utility of AM has also been extended to thoracic surgery (mainly for surgical closure) 26, 27, reconstruction of ocular surface damage 28, 29 and wound management, especially for severe burns 30, 31 and chronic skin ulcers 32, 33. It is therefore perhaps unsurprising that stem cells and stem‐like cells derived from these tissues retain their wound healing properties.
Fetal Membrane Derived Stem Cell Populations
A variety of stem and stem‐like cells can be isolated from term fetal membranes, displaying multi‐lineage properties, with abilities to modulate immune responses and release bioactive molecules. The chorion is made up of an inner chorionic mesodermal layer and an outer trophoblastic layer consisting of cytotrophoblast cells and syncytiotrophoblast of the villi. There are five minimum criteria for defining human amniotic and chorionic mesenchymal stem cells (MSC; hAMSC and hCMSC, respectively), namely:
fetal origin; ≤1% maternal contamination;
ability to develop fibroblast colony‐forming units;
surface antigen expression of CD90, CD73, and CD105 concurrent with the absence of CD45, CD34, CD14, and HLA‐DR;
multi‐lineage differentiation potential; and
plastic adherence 34
The existence of a high number of MSCs with osteogenic and adipogenic potential within the amniotic membrane was reported for the first time in 2004 by In't Anker and colleagues 35. However, fetal membrane derived MSCs appear to have similarly potent immunomodulatory properties as their adipose and bone marrow counterparts 34. In tissue culture conditions, hAMSC can support the hematopoiesis of CD34+ cells in the absence of exogenous cytokines. Cotransplantation of human cord blood‐derived hematopoietic stem cells with hAMSC induced an earlier and more complete recovery of hematopoiesis in mice 35; however, this paradigm has yet to be tested in humans. In addition to this, hAMSC have angiogenic abilities. They spontaneously form capillary‐like structures when cultured in semisolid medium, and this was improved by VEGF supplementation 36. The AM itself is able to support angiogenesis and tissue remodeling, and decrease inflammation by secreting factors including transforming growth factor‐β, basic fibroblast growth factor (FGF), epidermal growth factor (EGF), keratinocyte growth factor, and hepatocyte growth factor (HGF) 37. The hAMSC are able to retain a stable morphology for more than 20 passages with a phenotype that is similar to bone marrow and umbilical cord blood derived MSC (CD29, CD73, CD44 positive and CD14, CD34, CD45 negative) 38.
Transmission electron microscopy of hAMSC show physical characteristics common to both mesenchymal and epithelial cells, and this has been interpreted as a sign of multipotency. This trait is absent in hCMSC, which are more primitive and metabolically quiescent. hCMSC have a simpler cytoplasmic organization with stacks of rough endoplasmic reticulum cisternae, dispersed mitochondria and glycogen lakes. Unlike hAMSC, hCMSC lack assembled contractile filaments, prominence of endocytotic traffic and junctional communications 36. Notably, fetal membrane‐derived stem cells also appear to express numerous cell‐surface antigens and intracellular antigens similar to their better characterized counterparts, such as the BM‐derived MSC 39. Fetal membrane derived stem cells express typical mesenchymal markers and stage‐specific embryonic antigens (SSEA)‐3 and ‐4, but do not express hematopoietic‐, endothelial‐, and trophoblast‐ specific cell markers 36, 40. However, both hAMSC and hCMSC express low HLA‐ABC and no HLA‐DR, and their immunoprivileged status likely reflects their embryonic origin 40. Cultivars of MSC from amnions and chorions present as a homogeneous population of spindle‐shaped cells after the first passage. CD105+ hAMSC and hCMSC formed a homogeneous layer of fibroblastoid cells following magnetic bead separation, with the ability to differentiate into mesoderm‐type cells such as osteoblasts, adipocytes and chondrocytes 41.
The hAEC are another stem‐like cell population that has gained recent interest. They can be isolated from the amniotic epithelium, which arises from the epiblast prior to gastrulation, when cell fate is thought to be specified. In contrast, the chorion differentiates from extra‐embryonic trophectoderm. It has therefore been speculated that the hAEC may have escaped the specification process that accompanies gastrulation and as such, hAEC may retain some or all of the characteristics of the epiblast including pluripotency. This is reflected in their expression of surface markers and transcription factors characteristic of pluripotent stem cells such as SSEA‐3, SSEA‐4, TRA‐1‐60 and TRA‐1‐81, Oct4, and Nanog 42, 43. The hAEC are able to differentiate into cells of the ectoderm, endoderm and mesoderm lineage in vitro 42, with the unique ability to form acetylcholine and catecholamine releasing glial and neuronal progenitor‐like cells 44, 45, 46. Similar to MSC, hAEC are able to exert a broad range of immunomodulatory effects including suppression of T cell response, induction of Treg maturation and macrophage polarization 47, 48. However, the efficacy of hAEC from preterm donors have been found to be inferior to those isolated from term donors 49. This coincided with lower HLA‐G in the hAEC from preterm donors 49, further supporting that beneficial properties of fetal membrane derived stem cells are associated with their roles during pregnancy.
Yields and Growth Characteristics
The gestational tissues are a rich source of stem cells. For example, hAMSC and hCMSC are easily isolated through mechanical and sequential trypsin and collagenase digestion in significant numbers, ∼24 ± 10 million hAMSC and 21 ± 14 million hCMSC 50. Unlike MSC from bone marrow and adipose tissues, expansion of hAMSC and hCMSC in vitro is contentious where some report poor expansion beyond five passages 41, and others report a stable karyotype after 20 passages over a period of 120 days 51. Serial expansion of stem cells from gestational tissues, albeit with placental MSC, has also been previously shown to be associated with epigenetic alterations when the cells were passaged under serum‐free conditions 52. This may be a cause for concern since the dominant epigenetic changes observed were demethylation of genes associated with ageing and tumorigenesis. However, it should be noted that the reported epigenetic changes did not result in malignant transformation 52. In light of this, bulk manufacturing protocols for hAMSC and hCMSC with the ability to maintain vigorous growth and stable karyotypes will need to be developed if widespread clinical translation of their pro‐regenerative and pro‐reparative potential is to be realized. Given recent reports on the importance of MSC priming and impact of biomatrix stiffness on stem function 53, 54, 55, these factors should also be given due consideration in protocol development for bulk cutures.
Discrete morphological changes were observed with persistence of only some colonies arising from bulk cultures of hAMSC and hCMSC after 15 passages 50. This has led to the postulation that isolated fetal mesenchymal cells may contain progenitor cells that only expand under particular culture conditions, including low cell seeding and colony isolation 50. In contrast, yields of hAEC are 10 times or greater than that of hMSC and hCMSC 42, 56, 57. Monolayer cultures of hAEC are reactive with antibodies to low‐molecular weight cytokeratins, confirming their epithelial nature. However, initially vimentin‐negative hAEC can become vimentin‐positive during cell culture while remaining positive for cytokeratin 58. Furthermore, hAEC undergo replicative senescence where they enter a non‐dividing state with senescence after 6–10 passages and senescence has been reported to occur even earlier when plated at lower densities 58. This has been attributed to integrin‐dependent epidermal growth factor receptor (EGFR) activation, EGFR‐associated molecular complexes and cell‐to‐cell interactions, which are facilitated by higher density culture 58, 59. This is further supported by their maintenance of long telomeres after 5 passages 56.
Preclinical Applications
As mentioned previously, stem cells and stem‐like cells from the fetal membranes retain their immunomodulatory properties reflective of their biological function during pregnancy, which have been exploited across a broad range of experimental disease models. These are discussed in the following sections and summarized in Table 1.
Table 1.
Summary of preclinical applications of stem and stem‐like cells derived from fetal membranes
| Disease(s) | Cell type(s) | Reference(s) |
|---|---|---|
| Cardiovascular | ||
| Critical limb ischemia | hAMSC, hCMSC | 60 |
| hCMSC | 64 | |
| Myocardial infarction | hAMSC | 61, 62, 63 |
| hCMSC | 65 | |
| Stroke | hAEC | 66, 67 |
| Neurological | ||
| Traumatic brain injury | hAMSC | 69 |
| Spinal cord injury | hAMSC | 70 |
| hAEC | 74 | |
| Optic nerve crush injury | hCMSC | 71 |
| Encephalomyelitis | hAEC | 72 |
| Parkinson's Disease | hAEC | 73 |
| Diabetes | hAEC | 75 |
| hAMSC | 76, 77 | |
| hCMSC | 78 | |
| Gastrointestinal | ||
| Proctitis | hAMSC | 79 |
| Colitis | hAMSC | 80 |
| liver fibrosis | hCMSC | 81 |
| hAEC | 82, 83 | |
| Respiratory | ||
| Pulmonary fibrosis | hAEC | 47, 48, 106 |
| hAMSC, hCMSC | 85 | |
| Chronic obstructive pulmonary disease | hAEC | 89 |
| Asthma | hAEC | 90 |
| Bronchopulmonary dysplasia | hAEC | 91, 92 |
| hCMSC | 93 | |
| Cystic fibrosis | hAMSC | 94 |
| hAEC | 95 |
Abbreviations: hAEC, human amnion epithelial cells; hAMSC, human amnion mesenchymal stem cells; hCMSCs, human chorionic mesenchymal stem cells.
Cardiovascular Diseases
The angiogenic, cytoprotective, and immunosuppressive properties of hAMSC and hCMSC suggest that these cells, and their secreted soluble factors may be suited for applications for cardiovascular diseases. Their ability to secrete angiogenic factors such as HGF, IGF‐1, VEGF, and bFGF were concurrent with their ability to partially rescue experimental critical limb ischemia 60. The high concentration of PGE2 in hAMSC compared with hCMSC when cocultured with CD4+ T cells suggests that hAMSC may be a better cell source in such settings 60. These angiogenic and immunosuppressive properties have been exploited in experimental cardiac grafts in rats. Fibrin grafts containing spheroids of subamnion‐cord lining MSC embedded within failing rat hearts resulted in improved cardiac function and revascularization of the ischemic myocardium 61. Additionally, in vitro cardiomyogenic induction of hAMSCs has been reported to yield spontaneously beating cells with a 33% transdifferentiation efficiency. When transplanted into a rat model of myocardial infarction, they improved impaired left ventricular fractional shortening and significantly decreased the area of myocardial fibrosis 62. When hAMSC were transplanted into the infarcted myocardium of Wistar rats, cardiomyocyte transdifferentiation was observed in situ, with > 4‐week post‐transplant survival without immunosuppressants 62. This apparent tolerance was associated with HLA‐G expression, lack of MHC expression, and activation of FOXP3‐positive regulatory T cells. Notably, administration of IL‐10 or progesterone, which play an important role in feto‐maternal tolerance during pregnancy, significantly increased HLA‐G expression in hAMCs and increased cardiomyogenic transdifferentiation efficiency 62. When hAMSC were administered to immunocompromised mice following anterior descending artery ligation, hAMSC improved left ventricular function which correlated with increased myocardial viability and sustained engraftment 63.
The VCAM‐1+ subpopulation of hCMSC has been demonstrated to potent vasculo‐angiogenic abilities in vitro and in vivo. Conditioned medium from VCAM‐1+ hCMSC promoted proliferation and migration on endothelial cells compared with VCAM‐1−hCMSCs 64. Transplantation of VCAM‐1+ hCMSCs into the ischemic hind limb of BALB/c nude mice also significantly improved functional outcomes in comparison with VCAM‐1− hCMSC 64. This may therefore suggest that there are subpopulations within each type of fetal membrane derived stem cells with differential therapeutic potentials 64. In another study, unseparated hCMSC were administered weekly through intramyocardial injections to immunocompetent C57Bl6 mice following myocardial infarction. After 2 months, hCMSC‐treated mice had a significant increase in ejection fraction and a reduction in end‐systolic volume without a reduction in infarct size 65. hCMSC remained in the heart for up to 96 hours after the first injection; however, cell survival was reduced in subsequent injections. thereby suggesting that functional improvement was independent of permanent engraftment 65.
hAEC have been assessed for their potential to modulate inflammation associated with stroke. hAEC transplanted into a rat intracerebral hemorrhagic stroke model improved motor function, and reduced cerebral edema as well as activated microglia, with evidence of transplant survival in the lateral ventricular wall at 4 weeks 66. Interestingly, induction of epithelial‐to‐mesenchymal transition by priming with TGFβ‐1 enhanced the capacity of hAEC to support the ischemic myocardium 67. hAEC reportedly lost their cobblestone morphology and acquired a fibroblastic phenotype, with downregulation of E‐cadherin, upregulation of N‐cadherin, Akt phosphorylation, and intracellular periostin translocation 67. The mesenchymal‐hAEC displayed enhanced cell mobility and secreted gelatinase activity, concurrent with reduced surface presentation of CD105 and CD73, and transcriptional changes that mirrored the loss of epithelial characteristics 67. When injected intramyocardially into immunocompetent mice after myocardial infarction, global systolic function and longitudinal strain rate was improved in mice that received mesenchymal‐hAEC compared to unmanipulated hAEC 67. These findings indicate that cell priming prior to administration should be investigated as part of the clinical translation process.
Neurological Diseases
The ability of stem and stem‐like cells to differentiate into functioning neuronal progenitor‐like cells has led many to explore their utility in restoring neurological function hAMSC were reportedly able to reduce the viability and migratory ability of microglia, and this was associated with production of nitric oxide, which suppresses STAT5 phosphorylation in T cells and promotes immune cell apoptosis 68. In the setting of traumatic brain injury, transplantation of neuronal progenitors derived from hAMSC improved neurological function and brain histology, with elevated levels of neurotrophic factors (i.e., brain‐derived neurotrophic factor, nerve growth factor, neurotrophin 3, glial cell derived neurotrophic factor, and ciliary neurotrophic factor) 69. Expression of the same factors were observed in hAMSC following neural stem cell differentiation, suggesting that neurotrophic factors released by transplanted hAMSC derived neuronal progenitors may contribute to the improvements seen in experimental traumatic brain injury 69. In a rat model of spinal cord injury, hAMSC were injected into the contused dorsal spinal cord and transplanted hAMSC migrated into the spinal cord without differentiating into neuronal or glial cells 70. Compared with the control group, hAMSC transplantation significantly decreased the numbers of activated macrophages/ microglia and apoptotic cells 70. Transplantation of hAMSC transplantation also significantly increased the levels of brain‐derived neurotrophic factor and vascular endothelial growth factor in the injured spinal cord, thus promoting angiogenesis and axonal regeneration 70. Similarly, hCMSC administered to a rat model of optic nerve crush (ONC) injury increased axonal survival rates, growth‐associated protein‐43 (GAP‐43) and increased expression of hypoxia‐inducible factor 1α 71. Additionally, ERM‐like protein and SLIT‐ROBO Rho GTPase activating protein 2 (SRGAP2) were expressed in the optic nerves of the CP‐MSC‐injected rats with optical nerve crush injury.
The immunomodulatory properties of the hAEC have also been reported in a mouse model of autoimmune encephalomyelitis where hAEC suppressed the development of encephalomyelitis and prevented disease relapse. T cell responses and production of the IL‐17A were reduced in hAEC‐treated animals. This coincided with a significant increase in the numbers of peripheral T regulatory cells and naïve CD4+ T cells as well as Th2 cells in the peripheral lymphoid organs and the central nervous system 72. The efficacy of hAEC in Parkinson's disease (PD) has also been evaluated. In a rat model of PD with 6‐hydroxydopamine lesions, a higher survival rate of dopaminergic neurons was reported, along with an increase in dopaminergic neurons in the substantia nigra and restrained stem cells growth 73. Transplantation of hAEC into a rat model of spinal cord injury significantly reduced mechanical allodynia 74, suggesting that hAEC transplantation may be therapeutic for spinal cord injury related neuropathic pain. Interestingly, the protective effects in the setting of experimental spinal cord injury was profound with hAEC than MSC from umbilical cord. hAEC transplantation also modulated activation of astrocytes and microglia in this model of spinal cord injury 74.
Together these reports indicate that stem cells derived from the human fetal membranes are likely to be beneficial for a spectrum of neurological injuries where inflammation is a predominant feature. Given that inflammation progresses in an ongoing continuum, it is likely that the timing of cell administration will be crucial in order to minimize cell death and preserve function. Rapid translation of these biomedical discoveries will require detailed design around these critical therapeutic windows.
Diabetes
A culture protocol was recently developed to grow pancreatic hormone releasing 3D spheroids of hAEC that released C‐peptide in hyperglycemic conditions 75. This may serve as a bridge to clinically useful applications of fetal membrane stem and stem‐like cells, which have been shown in experimental models of diabetes to show promise. Transplantation of hAMSC differentiated into pancreatic islet cells, into streptozotocin‐induced type I diabetic mice restored bodyweights and normalized hyperglycemia for 7 months 76. Encapsulation of hAMSC differentiated into insulin producing islet cells in polyurethane‐polyvinyl pyrrolidone macrocapsules have also previously been shown to protect the transplanted cells against immune rejection, while normalizing hyperglycemia in diabetic mice 77. Transplantation of hAMSC derived islet cells into the kidneys of mice with streptozotocin‐induced diabetes restored body weight, and normalized the blood glucose levels, which lasted for 210 days. Only human insulin and c‐peptide were detected in the blood of normalized mice after 2 months of transplantation, but little mouse insulin and C‐peptide. Removal of graft‐bearing kidneys from these mice resulted in causing hyperglycemia again 76.
Long‐term cultures of hCMSC have been successfully maintained while retaining their ability to differentiate into adipogenic, oesteogenic, chondrogenic, and neuronal lineages 78. hCMSC can also form islet‐like cell clusters (ILC) on stepwise exposure to serum‐free differentiation media. Gene transcriptional studies indicate that both undifferentiated hCMSC and ILC expressed insulin, glucagon, and somatostatin 78. However, protein expression of human insulin, glucagon, and somatostatin was only detected in differentiated cells. The differentiated ILC also showed abundance of pancreatic transcription factors ngn3 and isl1, and were able to secrete insulin in response to glucose 78. Transplantation of undifferentiated hCMSC or differentiated ILC in STZ‐induced diabetic mice restored normoglycemia 78.
These studies and others indicate that stem cells from the fetal membranes and adult tissues, such as MSCs derived from adipose tissue and bone marrow, are potentially capable of restoring normoglycemia. These reports are promising and certainly interesting to the scientific community. However, the likelihood for stem cell therapy to replace traditional management of diabetes must be weighed up against current established approaches to diabetes management as well as the emerging area of islet transplantation. Furthermore, long‐term biocompatibility and longevity of encapsulation devices remain underdetermined.
Gastrointestinal
The application of fetal membrane derived stem cells in experimental models of gastrointestinal disease has been varied. hAMSC transplantation has been evaluated in radiation proctitis, which is a serious, incurable and common side effect of therapeutic irradiation for intrapelvic cancer 79. Intravenous administration of hAMSC ameliorated experimental intestinal epithelial attrition associated with radiation injury. This was recapitulated using hAMSC conditioned media 79. In rats with severe colitis induced by 8% dextran sulfate sodium, hAMSC transplantation significantly ameliorated disease index, weight loss, colon shortening, and histological colitis 80. Furthermore, mRNA levels of tumor necrosis factor‐α, interleukin‐1β, and migration inhibitory factor MIF were significantly decreased in the rectums of hAMSC‐treated rats 80.
hCMSC has also been shown to promote liver regeneration. Recently, Hyun and colleagues showed that hCMSC suppressed pro‐fibrogenic Hedgehog signaling in a rat model of carbon tetrachloride induced liver fibrosis 81. Specifically, they showed that hCMSC released extracellular vesicles containing microRNA‐125b that target smo, a Hedgehog signaling ligand, and this was concurrent with the downregulation of Hh‐target genes 81. hCMSC were also reportedly able to suppress the expression of Hh and pro‐fibrotic genes in cocultured LX2 (human hepatic stellate cell) 81.
Similarly, hAEC have been exploited in a necroinflammatory carbon tetrachloride mouse model of liver fibrosis where intravenous hAEC administration reversed established liver fibrosis 82. This outcome was attributed to their polarization of macrophages from pro‐inflammatory M1 to pro‐regenerative M2 82, as well as directly effects on hepatic stellate cells 83. In the setting of thioacetamide‐induced chronic liver failure, intrasplenically transplanted hAEC showed therapeutic efficacy while preserving HLA‐G expression 84. These differentiated hAEC showed evidence of bile canaliculi, albumin secretion, indo‐cyanine green elimination, low‐density lipoprotein uptake, and inducible CYP3A4 and CYP2C9 enzyme function 84. These anti‐fibrotic and pro‐regenerative properties in liver disease have recently culminated in a first‐in‐man Phase 1b clinical trial for compensated liver cirrhosis (ACTRN12616000437460).
Respiratory Diseases
The anti‐fibrotic actions of placental‐derived stem cells including those derived from the fetal membranes have been compared in a mouse model of bleomycin‐induced lung fibrosis 85. This study showed that allogeneic and xenogeneic fetal membrane derived stem cells were similarly efficacious in reducing neutrophil infiltration and fibrosis associated with bleomycin instillation 85. These reports suggest that hAEC, hAMSC and hCMSC may be of use in clinical pulmonary fibrosis 47, 48, 86, 87, particularly given their ability to reverse established lung fibrosis 88. This approach may also be applied toward chronic obstructive pulmonary disease. In a rat model of cigarette smoke inhalation induced obstructive lung disease, intra‐tracheal instillation of hAEC delayed the progression of emphysema and alleviated lung damage by reducing systemic and pulmonary levels of pro‐inflammatory cytokine, IL‐8 89. A recent study in an ovalbumin induced chronic allergic airway disease model showed that hAEC were superior to bone marrow derived MSC in their ability to diminish ovalbumin induced fibrosis and airway hyper‐responsiveness 90. Similarly, hAEC reportedly exerted immunomodulatory effects in the developing lungs such that delivery of hAECs attenuated pulmonary inflammation in sheep fetuses that were challenged with lipopolysaccharide 91. In a murine model of hyperoxia induced neonatal lung injury, hAEC also reversed alveolar simplification 92. When media conditioned by hCMSC from preterm babies were cocultured with fetal rat lungs (E14.5–15.5), ex vivo lung growth was markedly accelerated after 72‐hour culture 93. Specifically, Di Bernardo and colleagues observed significant increases in lung surface area, terminal bud formation with a predominant feature of enhanced branching morphogenesis. These outcomes suggest that autologous hCMSC from premature babies may be beneficial for perinatal lung development. However, comparisons of hCMSC from term and preterm donors should be assessed, and their efficacy using in vivo models of perinatal lung injury are necessary to evaluate their potential clinical efficacy.
In the context of cystic fibrosis, hAMSCs have been induced to CFTR expressing cells upon isolation and in coculture with CF airway epithelial cells. Freshly isolated hAMSCs initially display low levels of CFTR mRNA, which decreased with serial passaging. When hAMSCs were mixed with CFBE41o‐ respiratory epithelial cells and seeded onto permeable filters, 33%–50% of hAMSCs acquired CFTR expression on the apical membrane detectable by flow cytometry and confirmed by confocal microscopy 94. Similarly, hAEC have been reported to form 3D structures that express the CFTR gene and protein following long‐term culture in Small Airway Growth Medium (SAGM) 95. The distribution of CFTR was polarized on the membranes of hAEC cultured in SAGM, similar to that observed in polarized airway cells in vivo. Notably, hAEC expressing CFTR also possessed functional iodide/chloride ion channels 95. These studies indicate that there may be a potential to apply hAMSC and hAEC to cystic fibrosis; however, engraftment of CFTR‐expressing cells in sufficient numbers to overcome the genetic defect remains critical to success.
Fetal Membranes for Other Stem Cell Types
It is interesting to note that fetal membranes have also been used to support the growth of other stem cell types. Dehydrated fetal membranes consisting of both the amnion and chorion have been shown to support the proliferation and wound healing potential of adipose‐derived stem cells from diabetic patients 96. When implanted under the skin in a cutaneous ischemia model, the fetal membranes recruited circulating hematopoietic progenitor cells to sites of neovascularization 97. These findings have led some to postulate that dehydrated whole fetal membranes may be an alternative approach to regenerative medicine.
Human adipose‐derived mesenchymal stem cells (hADMSCs) seeded onto radiosterilized human amnion have been tested in their ability to aid cutaneous wound repair in the form a biological bandage. In a study by Sánchez‐Sánchez and colleagues, AM were used as a biological scaffold for the culture and maintenance of hADMSCs, where the membranes were found to induce hADMSC to secrete IL‐10 as well as IL‐1β, whose interplay is thought to be vital for a balanced restoration of all necessary tissues 98. Decellularized AM has also been found to be suitable as a scaffold for the delivery of hADMSC in tissue engineering and regenerative medicine applications 99. Sheets of cultured dental pulp MSCs prepared for periodontal tissue repair, using AM as a scaffold, retained their stem cell phenotype while promoting cell proliferation 100.
Other Applications
The fetal membranes have been used in other applications such as their utility as a feeder layer for human embryonic stem cell derivation and maintenance 101. Air‐dried and freeze‐dried amniotic membranes (AM) have been assessed for their feasibility as a growth substrate for chondrocyte expansion, and were found to improve chondrocyte proliferation, GAG expression and attachment than monolayer cultures 102. In their lyophilized state, decellularized AM showed potential as a novel barrier for guided bone regeneration in large bone defect where the membrane could afford protected space for osteogenesis while concurrently protecting against fibroblast invasion 103. Furthermore, an extract of AM has been postulated as an anti‐hemolytic and anti‐thrombotic due to its abundance in glycosaminoglycans (i.e., perlecan and hyaluronic acid) which inhibit coagulation, as well as as IL‐10 and MMP‐9 which inhibit platelet aggregation 104.
Conclusion
The fetal membranes have been used in regenerative medicine for nearly a century—long before the term “regenerative medicine” was coined 105. It is now clear that new uses for the fetal membranes, as well as the stem and stem‐like cells derived from the membranes, are being discovered. Fetal membrane derived stem and stem‐like cells are already being translated into early phase clinical trials. The publication of long‐term safety outcomes from these trials will significantly shape their broader application in years to come. Looking ahead, one has to also consider how best to design later phase clinical trials in order to ascertain the ideal therapeutic dose and dosing regimen, as well as route and timing of cell administration. It is also important to improve our current understanding on the biological role of fetal membrane derived stem and stem‐like cells in pregnancy and fetal wound healing responses. By understanding their microenvironment as they exert their potent pro‐regenerative effects, we can better develop cell priming protocols and design bioreactors that will retain their efficacy in a cost‐effective manner for large scale clinical use. It is also clear that the unique mechanical and biochemical properties of the fetal membranes lend themselves to bioengineering applications. Regardless of whether they are used as biological scaffolds for the propagation and maintenance of other cell types or used on their own to repair surgical defects, it is evident that fetal membranes may serve a multitude of clinical purposes. In summary, the field of regenerative medicine is becoming increasingly aware of the usefulness of the fetal membranes. While the use of fetal membranes in medical practice is not new, we are certainly seeing increased novel applications of these membranes and the cells derived from them.
Disclosure of Potential Conflicts of Interest
The author indicated no potential conflicts of interest.
References
- 1. Moffett‐King A. Natural killer cells and pregnancy. Nat Rev Immunol 2002;2:656–663. [DOI] [PubMed] [Google Scholar]
- 2. Alcayaga‐Miranda F, Varas‐Godoy M, Khoury M. Harnessing the angiogenic potential of stem cell‐derived exosomes for vascular regeneration. Stem Cells Int 2016;2016:3409169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Makrigiannakis A, Zoumakis E, Kalantaridou S et al. Corticotropin‐releasing hormone promotes blastocyst implantation and early maternal tolerance. Nat Immunol 2001;2:1018–1024. [DOI] [PubMed] [Google Scholar]
- 4. Houlihan JM, Biro PA, Harper HM et al. The human amnion is a site of MHC class Ib expression: Evidence for the expression of HLA‐E and HLA‐G. J Immunol 1995;154:5665–5674. [PubMed] [Google Scholar]
- 5. Schmidt CM, Orr HT. Maternal/fetal interactions: The role of the MHC class I molecule HLA‐G. Crit Rev Immunol 1993;13:207–224. [PubMed] [Google Scholar]
- 6. Feinman MA, Kliman HJ, Main EK. HLA antigen expression and induction by gamma‐interferon in cultured human trophoblasts. Am J Obstet Gynecol 1987;157:1429–1434. [DOI] [PubMed] [Google Scholar]
- 7. Hunt JS, Atherton RA, Pace JL. Differential responses of rat trophoblast cells and embryonic fibroblasts to cytokines that regulate proliferation and class I MHC antigen expression. J Immunol 1990;145:184–189. [PubMed] [Google Scholar]
- 8. Hunt JS, Andrews GK, Wood GW. Normal trophoblasts resist induction of class I HLA. J Immunol 1987;138:2481–2487. [PubMed] [Google Scholar]
- 9. King A, Boocock C, Sharkey AM et al. Evidence for the expression of HLAA‐C class I mRNA and protein by human first trimester trophoblast. J Immunol 1996;156:2068–2076. [PubMed] [Google Scholar]
- 10. Makrigiannakis A, Karamouti M, Drakakis P et al. Fetomaternal immunotolerance. Am J Reprod Immunol 2008;60:482–496. [DOI] [PubMed] [Google Scholar]
- 11. Athanassakis I, Aifantis Y, Makrygiannakis A et al. Placental tissue from human miscarriages expresses Class II HLA‐DR antigens. Am J Reprod Immunol 1995;34:281–287. [DOI] [PubMed] [Google Scholar]
- 12. Hunt JS, Jadhav L, Chu W et al. Soluble HLA‐G circulates in maternal blood during pregnancy. Am J Obstet Gynecol 2000;183:682–688. [DOI] [PubMed] [Google Scholar]
- 13. Lila N, Rouas‐Freiss N, Dausset J et al. Soluble HLA‐G protein secreted by allo‐specific CD4+ T cells suppresses the allo‐proliferative response: A CD4+ T cell regulatory mechanism. Proc Natl Acad Sci USA 2001;98:12150–12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Le Rond S, Azema C, Krawice‐Radanne I et al. Evidence to support the role of HLA‐G5 in allograft acceptance through induction of immunosuppressive/regulatory T Cells. J Immunol 2006;176:3266–3276. [DOI] [PubMed] [Google Scholar]
- 15. Fournel S, Aguerre‐Girr M, Huc X et al. Cutting edge: Soluble HLA‐G1 triggers CD95/CD95 ligand‐mediated apoptosis in activated CD8+ cells by interacting with CD8. J Immunol 2000;164:6100–6104. [DOI] [PubMed] [Google Scholar]
- 16. Contini P, Ghio M, Poggi A et al. Soluble HLA‐A,‐B,‐C and ‐G molecules induce apoptosis in T and NK CD8+ cells and inhibit cytotoxic T cell activity through CD8 ligation. Eur J Immunol 2003;33:125–134. [DOI] [PubMed] [Google Scholar]
- 17. Ringdén O, Erkers T, Nava S et al. Fetal membrane cells for treatment of steroid‐refractory acute graft‐versus‐host disease. Stem Cells 2013;31:592–601. [DOI] [PubMed] [Google Scholar]
- 18. Kesting MR, Wolff K‐D, Hohlweg‐Majert B et al. The role of allogenic amniotic membrane in burn treatment. J Burn Care Res 2008;29:907–916. [DOI] [PubMed] [Google Scholar]
- 19. Russo A, Bonci P, Bonci P. The effects of different preservation processes on the total protein and growth factor content in a new biological product developed from human amniotic membrane. Cell Tissue Bank 2012;13:353–361. [DOI] [PubMed] [Google Scholar]
- 20. Wolbank S, Hildner F, Redl H et al. Impact of human amniotic membrane preparation on release of angiogenic factors. J Tissue Eng Regen Med 2009;3:651–654. [DOI] [PubMed] [Google Scholar]
- 21. Litwiniuk M, Grzela T. Amniotic membrane: New concepts for an old dressing. Wound Repair Regen 2014;22:451–456. [DOI] [PubMed] [Google Scholar]
- 22. Niknejad H, Peirovi H, Jorjani M et al. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater 2008;15:88–99. [DOI] [PubMed] [Google Scholar]
- 23. Riau AK, Beuerman RW, Lim LS et al. Preservation, sterilization and de‐epithelialization of human amniotic membrane for use in ocular surface reconstruction. Biomaterials 2010;31:216–225. [DOI] [PubMed] [Google Scholar]
- 24. Gheorghe A, Pop M, Burcea M et al. New clinical application of amniotic membrane transplant for ocular surface disease. J Med Life 2016;9:177–179. [PMC free article] [PubMed] [Google Scholar]
- 25. Sanluis‐Verdes A, Yebra‐Pimentel Vilar MT, García‐Barreiro JJ et al. Production of an acellular matrix from amniotic membrane for the synthesis of a human skin equivalent. Cell Tissue Bank 2015;16:411–423. [DOI] [PubMed] [Google Scholar]
- 26. Iravani K, Hashemi SB, Tehrani M et al. Amniotic membrane in reconstruction of larynx following chondrosarcoma resection: A case report. Am J Otolaryngol 2014;35:520–523. [DOI] [PubMed] [Google Scholar]
- 27. Muralidharan S, Gu J, Laub GW et al. A new biological membrane for pericardial closure. J Biomed Mater Res 1991;25:1201–1209. [DOI] [PubMed] [Google Scholar]
- 28. Cheung CSY, Ali A, Chew HF. Successful treatment of acute ocular‐involving toxic epidermal necrolysis using amniotic membrane suture fixated to custom designed symblepharon rings. Cornea 2016;35:578–581. [DOI] [PubMed] [Google Scholar]
- 29. Wang T, Liang C, Xu X et al. Total ocular surface amniotic membrane transplantation for paraquat‐induced ocular surface injury. Can J Ophthalmol 2015;50:461–465. [DOI] [PubMed] [Google Scholar]
- 30. Mohammadi AA, Johari HG, Eskandari S. Effect of amniotic membrane on graft take in extremity burns. Burns 2013;39:1137–1141. [DOI] [PubMed] [Google Scholar]
- 31. Mohammadi AA, Seyed Jafari SM, Kiasat M et al. Effect of fresh human amniotic membrane dressing on graft take in patients with chronic burn wounds compared with conventional methods. Burns 2013;39:349–353. [DOI] [PubMed] [Google Scholar]
- 32. Alsina‐Gibert M, Pedregosa‐Fauste S. Amniotic membrane transplantation in the treatment of chronic lower limb ulcers. Actas Dermosifiliogr 2012;103:608–613. [DOI] [PubMed] [Google Scholar]
- 33. Adly OA, Moghazy AM, Abbas AH et al. Assessment of amniotic and polyurethane membrane dressings in the treatment of burns. Burns 2010;36:703–710. [DOI] [PubMed] [Google Scholar]
- 34. Parolini O, Alviano F, Bagnara GP et al. Concise review: Isolation and characterization of cells from human term placenta: Outcome of the first international workshop on placenta derived stem Cells. Stem Cells 2008;26:300–311. [DOI] [PubMed] [Google Scholar]
- 35. In' t Anker PS, Scherjon SA, Kleijburg‐van der Keur C et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 2004;22:1338–1345. [DOI] [PubMed] [Google Scholar]
- 36. Karlsson H, Erkers T, Nava S et al. Stromal cells from term fetal membrane are highly suppressive in allogeneic settings in vitro. Clin Exp Immunol 2012;167:543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang Y, Adachi Y, Suzuki Y et al. Simultaneous injection of bone marrow cells and stromal cells into bone marrow accelerates hematopoiesis in vivo. Stem Cells 2004;22:1256–1262. [DOI] [PubMed] [Google Scholar]
- 38. Alviano F, Fossati V, Marchionni C et al. Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Dev Biol 2007;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koizumi N, Inatomi T, Sotozono C et al. Growth factor mRNA and protein in preserved human amniotic membrane. Cur Eye Res 2009;20:173–177. [PubMed] [Google Scholar]
- 40. Witkowska‐Zimny M, Wrobel E. Perinatal sources of mesenchymal stem cells: Wharton's jelly, amnion and chorion. Cell Mol Biol Lett 2011;16:493–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fukuchi Y, Nakajima H, Sugiyama D et al. Human placenta‐derived cells have mesenchymal stem/progenitor cell potential. Stem Cells 2004;22:649–658. [DOI] [PubMed] [Google Scholar]
- 42. Wolbank S, Peterbauer A, Fahrner M et al. Dose‐dependent immunomodulatory effect of human stem cells from amniotic membrane: A comparison with human mesenchymal stem cells from adipose tissue. Tissue Eng 2007;13:1173–1183. [DOI] [PubMed] [Google Scholar]
- 43. Bačenková D, Rosocha J, Tóthová T et al. Isolation and basic characterization of human term amnion and chorion mesenchymal stromal cells. Cytotherapy 2011;13:1047–1056. [DOI] [PubMed] [Google Scholar]
- 44. Miki T, Lehmann T, Cai H et al. Stem cell characteristics of amniotic epithelial cells. Stem Cells 2005;23:1549–1559. [DOI] [PubMed] [Google Scholar]
- 45. Kronsteiner B, Wolbank S, Peterbauer A et al. Human mesenchymal stem cells from adipose tissue and amnion influence T‐cells depending on stimulation method and presence of other immune cells. Stem Cells Dev 2011;20:2115–2126. [DOI] [PubMed] [Google Scholar]
- 46. Sakuragawa N, Misawa H, Ohsugi K et al. Evidence for active acetylcholine metabolism in human amniotic epithelial cells: Applicable to intracerebral allografting for neurologic disease. Neurosci Lett 1997;232:53–56. [DOI] [PubMed] [Google Scholar]
- 47. Elwan MA, Sakuragawa N. Evidence for synthesis and release of catecholamines by human amniotic epithelial cells. Neuroreport 1997;8:3435–3438. [DOI] [PubMed] [Google Scholar]
- 48. Kakishita K, Elwan MA, Nakao N et al. Human amniotic epithelial cells produce dopamine and survive after implantation into the striatum of a rat model of Parkinson's disease: A potential source of donor for transplantation therapy. Exp Neurol 2000;165:27–34. [DOI] [PubMed] [Google Scholar]
- 49. Tan JL, Chan ST, Lo CY et al. Amnion cell mediated immune modulation following bleomycin challenge: Controlling the regulatory T cell response. Stem Cell Res Ther 2015;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tan JL, Chan ST, Wallace EM et al. Human amnion epithelial cells mediate lung repair by directly modulating macrophage recruitment and polarization. Cell Transplant 2013;23:319–328. [DOI] [PubMed] [Google Scholar]
- 51. Lim R, Chan ST, Tan JL et al. Preterm human amnion epithelial cells have limited reparative potential. Placenta 2013;34:486–492. [DOI] [PubMed] [Google Scholar]
- 52. Soncini M, Vertua E, Gibelli L et al. Isolation and characterization of mesenchymal cells from human fetal membranes. J Tissue Eng Regen Med 2007;1:296–305. [DOI] [PubMed] [Google Scholar]
- 53. Katsiani E, Garas A, Skentou C et al. Chorionic villi derived mesenchymal like stem cells and expression of embryonic stem cells markers during long‐term culturing. Cell Tissue Bank 2016;17:517–529. [DOI] [PubMed] [Google Scholar]
- 54. Zhu Y, Song X, Wang J et al. Placental mesenchymal stem cells of fetal origin deposit epigenetic alterations during long‐term culture under serum‐free condition. Expert Opin Biol Ther 2015;15:163–180. [DOI] [PubMed] [Google Scholar]
- 55. Muscari C, Giordano E, Bonafè F et al. Priming adult stem cells by hypoxic pretreatments for applications in regenerative medicine. J Bio Sci 2013;20:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Qiu Y, Guo J, Mao R et al. TLR3 preconditioning enhances the therapeutic efficacy of umbilical cord mesenchymal stem cells in TNBS‐induced colitis via the TLR3‐Jagged‐1‐Notch‐1 pathway. Mucosal Immunol 2016;10:727–742. [DOI] [PubMed] [Google Scholar]
- 57. Han NR, Yun JI, Park YH et al. Generation of priming mesenchymal stem cells with enhanced potential to differentiate into specific cell lineages using extracellular matrix proteins. Biochem Biophys Res Commun 2013;436:413–417. [DOI] [PubMed] [Google Scholar]
- 58. Murphy S, Rosli S, Acharya R et al. Amnion epithelial cell isolation and characterization for clinical use. Curr Protoc Stem Cell Biol 2010;Chapter 1:Unit 1E.6. [DOI] [PubMed] [Google Scholar]
- 59. Murphy SV, Kidyoor A, Reid T et al. Isolation, cryopreservation and culture of human amnion epithelial cells for clinical applications. J Vis Exp 2014;94:e52085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Miki T, Strom SC. Amnion‐derived pluripotent/multipotent stem cells. Stem Cell Rev 2006;2:133–141. [DOI] [PubMed] [Google Scholar]
- 61. Schlaepfer DD, Hunter T. Signal transduction from the extracellular matrix. A role for the focal adhesion protein‐tyrosine kinase FAK. Cell Struct Funct 1996;21:445–450. [DOI] [PubMed] [Google Scholar]
- 62. Yamahara K, Harada K, Ohshima M et al. Comparison of angiogenic, cytoprotective, and immunosuppressive properties of human amnion‐ and chorion‐derived mesenchymal stem cells. PLoS One 2014;9:e88319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Martinez EC, Vu D‐T, Wang J et al. Grafts enriched with subamnion‐cord‐lining mesenchymal stem cell angiogenic spheroids induce post‐ischemic myocardial revascularization and preserve cardiac function in failing rat hearts. Stem Cells Dev 2013;22:3087–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tsuji H, Miyoshi S, Ikegami Y et al. Xenografted human amniotic membrane‐derived mesenchymal stem cells are immunologically tolerated and transdifferentiated into cardiomyocytes. Circ Res 2010;106:1613–1623. [DOI] [PubMed] [Google Scholar]
- 65. Kim PJ, Mahmoudi M, Ge X et al. Direct evaluation of myocardial viability and stem cell engraftment demonstrates salvage of the injured myocardium. Circ Res 2015;116:e40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Du W, Li X, Chi Y et al. VCAM‐1+ placenta chorionic villi‐derived mesenchymal stem cells display potent pro‐angiogenic activity. Stem Cell Res Ther 2016;7:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Passipieri JA, Kasai‐Brunswick TH, Suhett G et al. Improvement of cardiac function by placenta‐derived mesenchymal stem cells does not require permanent engraftment and is independent of the insulin signaling pathway. Stem Cell Res Ther 2014;5:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dong W, Chen H, Yang X et al. Treatment of intracerebral haemorrhage in rats with intraventricular transplantation of human amniotic epithelial cells. Cell Biol Int 2010;34:573–577. [DOI] [PubMed] [Google Scholar]
- 69. Roy R, Kukucka M, Messroghli D et al. Epithelial‐to‐mesenchymal transition enhances the cardioprotective capacity of human amniotic epithelial cells. Cell Transplant 2015;24:985–1002. [DOI] [PubMed] [Google Scholar]
- 70. Yan K, Zhang R, Chen L et al. Nitric oxide‐mediated immunosuppressive effect of human amniotic membrane‐derived mesenchymal stem cells on the viability and migration of microglia. Brain Res 2014;1590:1–9. [DOI] [PubMed] [Google Scholar]
- 71. Yan Z‐J, Zhang P, Hu Y‐Q et al. Neural stem‐like cells derived from human amnion tissue are effective in treating traumatic brain injury in rat. Neurochem Res 2013;38:1022–1033. [DOI] [PubMed] [Google Scholar]
- 72. Zhou H‐L, Zhang X‐J, Zhang M‐Y et al. Transplantation of human amniotic mesenchymal stem cells promotes functional recovery in a rat model of traumatic spinal cord injury. Neurochem Res 2016;41:2708–2718. [DOI] [PubMed] [Google Scholar]
- 73. Chung S, Rho S, Kim G et al. Human umbilical cord blood mononuclear cells and chorionic plate‐derived mesenchymal stem cells promote axon survival in a rat model of optic nerve crush injury. Int J Mol Med 2016;37:1170–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. McDonald CA, Payne NL, Sun G et al. Immunosuppressive potential of human amnion epithelial cells in the treatment of experimental autoimmune encephalomyelitis. J Neuroinflammation 2015;12:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kakishita K, Nakao N, Sakuragawa N et al. Implantation of human amniotic epithelial cells prevents the degeneration of nigral dopamine neurons in rats with 6‐hydroxydopamine lesions. Brain Res 2003;980:48–56. [DOI] [PubMed] [Google Scholar]
- 76. Roh D‐H, Seo M‐S, Choi H‐S et al. Transplantation of human umbilical cord blood or amniotic epithelial stem cells alleviates mechanical allodynia after spinal cord injury in rats. Cell Transplant 2013;22:1577–1590. [DOI] [PubMed] [Google Scholar]
- 77. Okere B, Alviano F, Costa R et al. In vitro differentiation of human amniotic epithelial cells into insulin‐producing 3D spheroids. Int J Immunopathol Pharmacol 2015;28:390–402. [DOI] [PubMed] [Google Scholar]
- 78. Kim J, Park S, Kang HM et al. Human insulin secreted from insulinogenic xenograft restores normoglycemia in type 1 diabetic mice without immunosuppression. Cell Transplant 2012;21:2131–2147. [DOI] [PubMed] [Google Scholar]
- 79. Kadam SS, Sudhakar M, Nair PD et al. Reversal of experimental diabetes in mice by transplantation of neo‐islets generated from human amnion‐derived mesenchymal stromal cells using immuno‐isolatory macrocapsules. Cytotherapy 2010;12:982–991. [DOI] [PubMed] [Google Scholar]
- 80. Kadam S, Muthyala S, Nair P et al. Human placenta‐derived mesenchymal stem cells and islet‐like cell clusters generated from these cells as a novel source for stem cell therapy in diabetes. Rev Diabet Stud 2010;7:168–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ono M, Ohnishi S, Honda M et al. Effects of human amnion‐derived mesenchymal stromal cell transplantation in rats with radiation proctitis. Cytotherapy 2015;17:1545–1559. [DOI] [PubMed] [Google Scholar]
- 82. Onishi R, Ohnishi S, Higashi R et al. Human amnion‐derived mesenchymal stem cell transplantation ameliorates dextran sulfate sodium‐induced severe colitis in rats. Cell Transplant 2015;24:2601–2614. [DOI] [PubMed] [Google Scholar]
- 83. Hyun J, Wang S, Kim J et al. MicroRNA125b‐mediated Hedgehog signaling influences liver regeneration by chorionic plate‐derived mesenchymal stem cells. Sci Rep 2015;5:14135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Manuelpillai U, Lourensz D, Vaghjiani V et al. Human amniotic epithelial cell transplantation induces markers of alternative macrophage activation and reduces established hepatic fibrosis. PLoS One 2012;7:e38631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hodge A, Lourensz D, Vaghjiani V et al. Soluble factors derived from human amniotic epithelial cells suppress collagen production in human hepatic stellate cells. Cytotherapy 2014;16:1132–1144. [DOI] [PubMed] [Google Scholar]
- 86. Lin JS, Zhou L, Sagayaraj A et al. Hepatic differentiation of human amniotic epithelial cells and in vivo therapeutic effect on animal model of cirrhosis. J Gastroenterol Hepatol 2015;30:1673–1682. [DOI] [PubMed] [Google Scholar]
- 87. Cargnoni A, Gibelli L, Tosini A et al. Transplantation of allogeneic and xenogeneic placenta‐derived cells reduces bleomycin‐induced lung fibrosis. Cell Transplant 2009;18:405–422. [DOI] [PubMed] [Google Scholar]
- 88. Murphy S, Lim R, Dickinson H et al. Human amnion epithelial cells prevent bleomycin‐induced lung injury and preserve lung function. Cell Transplant 2010;20:909–923. [DOI] [PubMed] [Google Scholar]
- 89. Moodley Y, Ilancheran S, Samuel C et al. Human amnion epithelial cell transplantation abrogates lung fibrosis and augments repair. Am J Respir Crit Care Med 2010;182:643–651. [DOI] [PubMed] [Google Scholar]
- 90. Vosdoganes P, Wallace EM, Chan ST et al. Human amnion epithelial cells repair established lung injury. Cell Transplant 2012;22:1337–1349. [DOI] [PubMed] [Google Scholar]
- 91. Geng L, Chen Z, Ren H et al. Effects of an early intervention using human amniotic epithelial cells in a COPD rat model. Pathol Res Pract 2016;212:1027–1033. [DOI] [PubMed] [Google Scholar]
- 92. Royce SG, Tominaga AM, Shen M et al. Serelaxin improves the therapeutic efficacy of RXFP1‐expressing human amnion epithelial cells in experimental allergic airways disease. Clin Sci 2016;130:2151–2165. [DOI] [PubMed] [Google Scholar]
- 93. Vosdoganes P, Hodges RJ, Lim R et al. Human amnion epithelial cells as a treatment for inflammation‐induced fetal lung injury in sheep. Am J Obstet Gynecol 2011;205:156.e26–33. [DOI] [PubMed] [Google Scholar]
- 94. Vosdoganes P, Lim R, Koulaeva E et al. Human amnion epithelial cells modulate hyperoxia‐induced neonatal lung injury in mice. Cytotherapy 2013;15:1021–1029. [DOI] [PubMed] [Google Scholar]
- 95. Di Bernardo J, Maiden MM, Jiang G et al. Paracrine regulation of fetal lung morphogenesis using human placenta‐derived mesenchymal stromal cells. J Surg Res 2014;190:255–263. [DOI] [PubMed] [Google Scholar]
- 96. Paracchini V, Carbone A, Colombo F et al. Amniotic mesenchymal stem cells: A new source for hepatocyte‐like cells and induction of CFTR expression by coculture with cystic fibrosis airway epithelial cells. J Biomed Biotechnol 2012;2012:575471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Murphy SV, Lim R, Heraud P et al. Human amnion epithelial cells induced to express functional cystic fibrosis transmembrane conductance regulator. PLoS One 2012;7:e46533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Massee M, Chinn K, Lim JJ et al. Type I and II diabetic adipose‐derived stem cells respond in vitro to dehydrated human amnion/chorion membrane allograft treatment by increasing proliferation, migration, and altering cytokine secretion. Adv Wound Care (New Rochelle) 2016;5:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Maan ZN, Rennert RC, Koob TJ et al. Cell recruitment by amnion chorion grafts promotes neovascularization. J Surg Res 2015;193:953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sánchez‐Sánchez R, Brena‐Molina A, Martínez‐López V et al. Generation of two biological wound dressings as a potential delivery system of human adipose‐derived mesenchymal stem cells. ASAIO J 2015;61:718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Gholipourmalekabadi M, Sameni M, Radenkovic D et al. Decellularized human amniotic membrane: How viable is it as a delivery system for human adipose tissue‐derived stromal cells? Cell Prolif 2016;49:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Honjo K‐I, Yamamoto T, Adachi T et al. Evaluation of a dental pulp‐derived cell sheet cultured on amniotic membrane substrate. Biomed Mater Eng 2015;25:203–212. [DOI] [PubMed] [Google Scholar]
- 103. Ávila‐González D, Vega‐Hernández E, Regalado‐Hernández JC et al. Human amniotic epithelial cells as feeder layer to derive and maintain human embryonic stem cells from poor‐quality embryos. Stem Cell Res Ther 2015;15:322–324. [DOI] [PubMed] [Google Scholar]
- 104. Krishnamurithy G, Shilpa PN, Ahmad RE et al. Human amniotic membrane as a chondrocyte carrier vehicle/substrate: In vitro study. J Biomed Mater Res A 2011;99:500–506. [DOI] [PubMed] [Google Scholar]
- 105. Li W, Ma G, Brazile B et al. Investigating the potential of amnion‐based scaffolds as a barrier membrane for guided bone regeneration. Langmuir 2015;31:8642–8653. [DOI] [PubMed] [Google Scholar]
- 106. Niknejad H, Yazdanpanah G, Kakavand M. Extract of fetal membrane would inhibit thrombosis and hemolysis. Med Hypotheses 2015;85:197–202. [DOI] [PubMed] [Google Scholar]
- 107. Kaiser LR. The future of multihospital systems. Top Health Care Financ 1992;18:32–45. [PubMed] [Google Scholar]
- 108. Murphy SV, Shiyun SC, Tan JL et al. Human amnion epithelial cells do not abrogate pulmonary fibrosis in mice with impaired macrophage function. Cell Transplant 2012;21:1477–1492. [DOI] [PubMed] [Google Scholar]


