Abstract
Umbilical cord (UC)‐derived mesenchymal stem cells (MSCs) show immunoregulatory properties on various immune cells and display therapeutic effects on various autoimmune diseases such as systemic lupus erythematosus (SLE). The aim of this study was to investigate the effect of the SLE environment on UC MSCs and to identify a potential serum biomarker to predict the therapeutic effect. UC MSCs were cocultured with peripheral blood mononuclear cells (PBMCs) from active lupus patients, and the proliferation, apoptosis and surface markers of UC MSCs were observed. UC MSC functional molecules were assessed by real‐time polymerase chain reaction, and the signaling pathways were analyzed by Western blot. The clinical effect of MSC transplantation (MSCT) for lupus patients was followed‐up, whereas baseline serum cytokines were analyzed by enzyme‐linked immunosorbent assay. The coculture of PBMC from lupus patients promoted MSC proliferation. Lupus PBMCs were more potent in stimulating UC MSCs to secrete vascular endothelial growth factor (VEGF) and CXCL‐12. Furthermore, lupus PBMCs activated Akt, IκB, and Stat5 signaling pathways in UC MSCs but did not affect Erk1/2 and Smad1/5/8 pathways. Moreover, our clinical study showed that higher baseline levels of IFN‐γ might predict a good response to MSCT in active lupus patients. Baseline IFN‐γ levels may predict clinical response to MSC therapy for active lupus patients, which will help to choose suitable patients for clinical transplantation. stem cells translational medicine 2017;6:1777–1785
Keywords: Indoleamine 2,3‐dioxygenase; Interferon γ; Mesenchymal stem cells; Systemic lupus erythematosus; Transplantation
Significance Statement.
Although mesenchymal stem cells (MSCs) play an important role in regulating autoimmune responses and have been efficiently applied in severe and refractory systemic lupus erythematosus (SLE), information about how MSCs interact with the microenvironment and initiate immunoregulation is limited. Using an in vitro coculture system, changes in MSC, when stimulated by the peripheral blood mononuclear cells of SLE patients, were demonstrated especially the modulatory factors produced by the MSCs. Importantly, high serum levels of IFN‐γ indicated a good clinical response to MSC transplantation when followed‐up for 1 year.
Introduction
Mesenchymal stem cells (MSCs) represent a heterogeneous progenitor cell population derived from various sources, including bone marrow, umbilical cord (UC), and adipose tissues. These cell populations are being extensively investigated for their regenerative, immunomodulatory, and tissue‐protective properties 1. In the past, we have shown that MSC transplantation (MSCT) had therapeutic effect on various autoimmune diseases, including systemic lupus erythematosus (SLE) 2, 3, Sjögren's syndrome 4, systemic sclerosis 5, and inflammatory bowel disease 6. The 1‐year follow‐up data showed that 60% of patients achieved complete or partial clinical remission after MSCT 7, 8. However, there was still a subset of patients who had no response to MSCs therapy and the reason for the nonresponse is unknown.
It is generally reported that the immunosuppressive function of MSCs is induced by proinflammatory cytokines. In vitro IFN‐γ can synergize with TNF‐α, IL‐1α, or IL‐1β to induce the ability of MSCs to inhibit T‐cell proliferation, and TNF‐α, IL‐1α, and IL‐1β act interchangeably. Other pro‐proinflammatory cytokines, such as GM‐CSF and IL‐6, have had no effect 9. Besides cytokines, some chemokines, such as CXCL1–2‐3/GRO, CCL2/MCP‐1, and CXCL8/IL‐8, determine the migratory capacity of human bone marrow‐derived MSCs. MSCs stimulated with hepatocellular carcinoma cell‐conditioned media showed more than 500 genes differentially expressed compared with unstimulated MSCs 10. However, animal model studies by Liu et al. revealed that high concentrations of IFN‐γ and TNF‐α were associated with a lack of MSCs‐mediated new bone formation, indicating that the recipient's local microenvironment may affect MSCs‐mediated bone formation 11. A recent study has shown that the combination of IFN‐γ and TNF‐α results in the synergist uniform polarization of MSCs toward a primarily Th1 phenotype, which likely has an important role in amplifying the immune response in the tumor microenvironment 12. Thus, whether the inflammatory cytokines and chemokines initiated MSCs immunosuppressive or immunoactive effect needs further investigation.
Our previous studies showed that, in SLE patients, peripheral blood CD8+ T cells produced high levels of IFN‐γ that induced MSCs to secrete large amount of indoleamine 2,3‐dioxygenase (IDO) via IFNGR1/JAK‐2/STAT signaling pathways for inhibiting T‐cell proliferation 13. However, whether serum levels of IFN‐γ in patients could predict MSCs therapeutic effect remains unknown. In the present study, we observed how MSCs changed in the lupus environment focusing on molecules that could potentially predict the clinical effect of MSCT in lupus patients.
Materials and Methods
Lupus Patients and Healthy Subjects
Fifty‐six active SLE patients and forty healthy subjects were included in this study. Informed consent was obtained from each subject for the collection of peripheral blood. Twenty‐six patients underwent UC‐derived MSCs (UC MSCs) transplantation as previously described 7, 8. Clinical study of UC MSCs transplantation for lupus patients was registered with ClinicalTrials.gov (identifier: NCT01741857). This study was approved by the Ethics Committee at The Affiliated Drum Tower Hospital of Nanjing University Medical School and was conducted in accordance with the 1989 Declaration of Helsinki.
Antibodies and Reagents
The following antibodies (to humans) were used in this study: fluorescein isothiocyanate (FITC)‐conjugated antihuman CD29, CD44, HLA‐DR, CD14, phycoerythrin (PE)‐conjugated antihuman CD105, CD166, CD45, CD34, and the respective isotype‐matched control antibodies (mouse IgG1 and mouse IgG2a) (all from BD Biosciences, BD Pharmingen, Fremont, CA, http://www.bdbiosciences.com). Recombinant human TGF‐β was from R&D Systems and recombinant human IFN‐α, IFN‐β, IFN‐γ, TNF‐α, IL‐6, IL‐1β, IL‐2, IL‐21, IL‐4, and IL‐10 were from PeproTech (Rocky Hill, NJ, https://www.peprotech.com/en-US).
Isolation and Culture of UC‐Derived MSCs
Thirty‐two samples of fresh UC were obtained from patients with active SLE and healthy mothers in local maternity hospitals after normal deliveries; 26 donor‐derived UC MSCs were used for transplantation in 26 SLE patients, and the other 6 donor‐derived UC MSCs were used for in vitro coculture experiments. UC MSCs for clinical use were prepared by Stem Cell Center of Jiangsu Province, a National Stem Cell Institute in China and member of the International Society for Cellular Therapy. All the UC donors were 20–30 years old, with no disease history and no family history of autoimmune disease. Before they delivered, they were screened for the normal range of blood routine test, normal hepatic and renal functions, and negative for hepatitis B surface antigen, hepatitis B core antibody, hepatitis C virus antibody, HIV antibodies I and II, cytomegalovirus immunoglobulin M, and syphilis antibody. The cords were rinsed with phosphate‐buffered saline (PBS), and cord blood was removed. The washed cords were cut into 1 mm2‐sized pieces and subsequently incubated at 37°C in humid air with 5% CO2 in Dulbecco's modified Eagle's medium with low glucose containing 10% fetal bovine serum. Nonadherent cells were removed by washing. After 10 days, fibroblast‐like cells appeared and were trypsinized and passaged into a new flask for further expansion. Cell surface markers were assessed by flow cytometric analysis. Moreover, the capacity of MSCs that differentiate along adipogenic and osteogenic lineages was also assayed in vitro. All the MSCs for in vivo and in vitro use were derived from passage 2 to passage 5.
Isolation and Culture of Peripheral Blood Mononuclear Cells
Peripheral blood mononuclear cells (PBMCs) were isolated from 56 active lupus patients and 40 healthy controls (HC) at the same time point, then were cocultured with UC MSCs at a ratio of 4:1 for 24 hours, 48 hours, and 72 hours, respectively. Then PBMCs were washed with PBS, and MSCs were trypsinized and harvested for further examination by flow cytometry or quantitative real‐time polymerase chain reaction (qRT‐PCR). For cell proliferation analysis, MSCs were cocultured with PBMC from SLE patients or HC for 5 days.
Flow Cytometry Analysis and Enzyme‐Linked Immunosorbent Assay
After cocultured with PBMCs for 72 hours, MSCs were harvested and resuspended in PBS. For the staining of surface antigens, cells were incubated with FITC‐, or PE‐conjugated monoclonal antibodies or their negative control antibodies as indicated for 30 minutes on ice. For the carboxyfluorescein succinimidyl ester (CFSE)‐labeling assay to assess cell proliferation, MSCs were incubated with 3 μmol/l of CFSE in PBS/0.5% bovine serum albumin at 37°C for 15 minutes. Cells were washed three times with fresh, ice‐cold complete 1640 medium and resuspended in complete 1640 medium for further culture. After the cells were cultured for 5 days as indicated, cells were harvested to examine the CFSE‐negative cells using flow cytometry. The apoptosis rate of MSCs was determined by Annexin V and 7‐AAD staining after 24 hours of stimulation in the absence or presence of different doses of IFN‐γ, or in the presence of PBMC from lupus patients or HC.
Human IL‐6, PGE2, IFN‐γ, IL‐10, IL‐6, IL‐17, TNF‐α, CXCL12, and VEGF enzyme‐linked immunosorbent assay (ELISA) kits were from eBioscience, San Diego, CA, http://www.ebioscience.com/. The human TGF‐β ELISA kit was from BioLegend (San Diego, CA, http://www.biolegend.com/). We detected the amounts of these cytokines according to the manufacturer's instructions.
qRT‐PCR
UC MSCs were cocultured with or without PBMCs from SLE patients or HC directly for 48 hours, and supernatants were collected after centrifugation. In some experiments, various cytokines, including IFN‐α (20 ng/ml), IFN‐β (20 ng/ml), IFN‐γ (20 ng/ml), TGF‐β1 (60 ng/ml), TNF‐α (100 ng/ml), IL‐6 (20 ng/ml), IL‐1β (80 ng/ml), IL‐21 (100 ng/ml), IL‐2 (200 IU/ml), IL‐4 (20 ng/ml), and IL‐10 (20 ng/ml), were used to stimulate UC MSCs in vitro for 48 hours. After being washed with PBS three times, complementary DNA (cDNA) from MSCs was synthesized from TRIzol‐isolated total RNA (TRIzol RNA Isolation Reagent, Thermo Fisher Scientific Inc, Waltham, MA) by use of the SuperScript III First‐Strand Synthesis SuperMix for qRT‐PCR (Takara, Dalian, China, http://www.clontech.com/). For real‐time PCR experiments, reactions containing the SYBR Premix EX Taq (Takara Dalian, China), ROX Reference Dye (503, Takara), cDNA, and gene primers were run on the StepOnePlus Real‐Time PCR Systems and analyzed with StepOne Software V2.1 (Applied Biosystems, New York, NY). Gene primers are listed in Table 1. The relative gene quantification was done by using the 2‐ΔΔCt method following normalization to glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) of unstimulated MSCs.
Table 1.
Primers for real‐time polymerase chain reaction
| Gene | Forward | Reverse |
|---|---|---|
| TGF‐β1 | 5′‐AGCGACTCGCCAGAGTGGTTA‐3′ | 5′‐GCAGTGTGTTATCCCTGCTGTCA‐3′ |
| COX2 | 5′‐TGACCAGAGCAGGCAGATGAA‐3′ | 5′‐CCACAGCATCGATGTCACCATAG‐3′ |
| COX1 | 5′‐TGGTGCTGGCATGGATAGTA‐3′ | 5′‐GGTTCTTGCTGTTCCTGCTC‐3′ |
| IL‐6 | 5′‐AAGCCAGAGCTGTGCAGATGAGTA‐3′ | 5′‐TGTCCTGCAGCCACTGGTTC‐3′ |
| IDO1 | 5′‐GAATGGCACACGCTATGGAA‐3′ | 5′‐CAGACTCTATGAGATCAGGCAGATG‐3′ |
| IDO2 | 5′‐GGCTCTTGGGAAACTCCTTC‐3′ | 5′‐TCAGGACATCACCAAAACCTT‐3′ |
| IL‐10 | 5′‐CTCATGGCTTTGTAGATGCCT‐3′ | 5′‐GCTGTCATCGATTTCTTCCC‐3′ |
| CXCL‐12 | 5′‐TGGGCTCCTACTGTAAGGGTT‐3′ | 5′‐TTGACCCGAAGCTAAAGTGG‐3′ |
| VEGF | 5′‐AGCTGCGCTGATAGACATCC‐3′ | 5′‐CTACCTCCACCATGCCAAGT‐3′ |
| GAPDH | 5′‐GCACCGTCAAGGCTGAGAAC‐3′ | 5′‐TGGTGAAGACGCCAGTGGA‐3′ |
Abbreviations: GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; VEGF, vascular endothelial growth factor.
Western Blot Analysis
We used antibodies recognizing human Akt, IκB, Stat‐5, Smad1/5/8, Erk1/2 and their phosphorylation forms, and GAPDH (1:1,000; Cell Signaling Technology, Beverly, MA, https://www.cellsignal.com) to examine the concentrations of proteins in MSCs lysates. The concentration of IDO1 protein in MSCs (1:400 dilution, Epitomics Technology, Burlingame, CA, http://www.epitomics.com/) was also determined.
Statistical Analysis
We used the t test for statistical analysis for parametric data and the Mann‐Whitney U test for non‐parametric data. One‐way analysis of variance was used when there were more than two groups, and then followed by Bonferroni test among different groups. We performed statistical analyses with SPSS16.0 software and GraphPad Prism 4.3 and considered a p value less than .05 as significant. Data are shown as mean ± standard error of mean.
Results
IFN‐γ Predict Clinical Response to MSCT in SLE
Allogeneic MSCT showed a clinical response rate of 50% to 60% in our single and multicenter clinical studies 7, 8. Based on the aforementioned data, we sought to examine the baseline cytokine levels between responders and nonresponders in our single‐center enrolled patients. Previously, we found that lupus patients displayed significantly higher levels of serum IFN‐γ than HC 13. Besides IFN‐γ, we also showed that serum TNF‐α significantly increased in active lupus patients compared with HCs (Fig. 1A), but there were no differences in serum IL‐6, IL‐17, IL‐10, and TGF‐β1 expressions between the two groups (Fig. 1B–1E).
Figure 1.
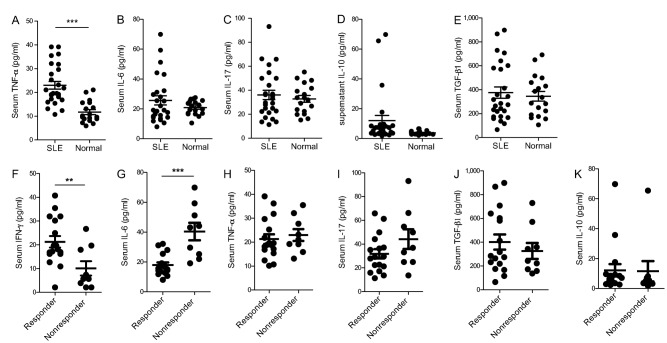
Higher baseline serum IFN‐γ indicated a good response to mesenchymal stem cells transplantation in lupus patients. Compared with healthy controls, lupus patients showed elevated serum TNF‐α (A), while serum IL‐6 (B), IL‐17 (C), IL‐10 (D), and TGF‐β1 (E) had no change. Lupus patients who received umbilical cord‐derived mesenchymal stem cells transplantation were divided into responder and nonresponder groups as indicated, and those responders showed significantly higher baseline levels of IFN‐γ (F), lower baseline IL‐6 (G), while TNF‐α (H), IL‐17 (I), TGF‐β1 (J), and IL‐10 (K) had no difference. **, p < .01; ***, p < .001. Abbreviation: SLE, systemic lupus erythematosus.
Twenty‐six patients underwent allogeneic UC‐derived MSCT, and at 1‐year follow‐up, 17 patients showed clinical response and the other 9 patients had no response according to our previous clinical assessment criteria 7 (Table 2). Baseline serum cytokines were analyzed and the data revealed that patients who had higher levels of baseline IFN‐γ and lower levels of baseline IL‐6 showed a good clinical response (Fig. 1F, 1G). Serum TNF‐α, IL‐17, TGF‐β1, and IL‐10 had no difference between responders and nonresponders among these patients (Fig. 1H–1K).
Table 2.
Patients' clinical response to MSCT
| Age/sex | Disease duration, months | Baseline SLEDAI | Baseline BILAG | Clinical manifestations | Clinical outcome after MSCT |
|---|---|---|---|---|---|
| 46/F | 40 | 17 | 12 | LN, A, C, V, H, ANA+, anti‐dsDNA+ | PCR |
| 37/F | 41 | 12 | 12 | A, LN, V, ANA+, anti‐dsDNA+, H | PCR |
| 21/F | 50 | 11 | 9 | V, LN, C, anti‐SM+ | NR |
| 28/F | 98 | 9 | 9 | V, A, alopecia, LN, C, ANA+, anti‐dsDNA+ | MCR |
| 26/F | 120 | 12 | 8 | V, A, LN, ANA+, anti‐dsDNA+ | NR |
| 23/F | 15 | 14 | 19 | V, A, F, LN, P, ANA+, anti‐dsDNA+ | NR |
| 20/F | 62 | 12 | 18 | A, F, LN, C, P, ANA+ | PCR |
| 43/F | 26 | 34 | 20 | C, V, LN, A, seizures, ANA+ | PCR |
| 36/F | 97 | 10 | 26 | C, V, A, LN, P, ANA+ | MCR |
| 39/F | 60 | 10 | 7 | LN, A, V, ANA+, anti‐SM+ | PCR |
| 22/F | 40 | 8 | 16 | LN, C, P, ANA+, anti‐dsDNA+ | NR |
| 20/F | 50 | 14 | 13 | A, severe thrombocytopenia, V, F, ANA+, anti‐dsDNA+, anti‐SM+ | NR |
| 17/F | 75 | 7 | 6 | Severe thrombocytopenia, LN, A, ANA+, anti‐dsDNA+ | NR |
| 21/F | 39 | 12 | 11 | LN, F, P, A, anti‐dsDNA+ | NR |
| 36/F | 60 | 10 | 7 | LN, V, P, A, ANA+, anti‐SM+ | MCR |
| 16/F | 49 | 11 | 15 | LN, A, V, ANA+ | NR |
| 35/F | 109 | 6 | 6 | LN, C, H, ANA+ | PCR |
| 44/F | 85 | 8 | 9 | A, LN, F, ANA+, anti‐dsDNA+ | PCR |
| 29/F | 86 | 10 | 5 | LN, A, P, F, ANA+, anti‐dsDNA+ | PCR |
| 54/F | 264 | 8 | 4 | LN, A, V, C, ANA+ | MCR |
| 36/F | 121 | 13 | 13 | LN, A, V, C | PCR |
| 40/F | 24 | 12 | 8 | F, V, LN, C, ANA+ | NR |
| 32/F | 156 | 14 | 12 | LN, A, C, P | MCR |
| 27/M | 48 | 12 | 7 | LN, F, A, P, ANA+, anti‐SM+ | MCR |
| 30/M | 102 | 10 | 7 | V, A, LN, ANA+, anti‐dsDNA+ | PCR |
| 31/F | 62 | 8 | 3 | LN, V, P, ANA+, anti‐dsDNA+ | MCR |
Abbreviations: A, arthralgia; ANA, antinuclear antibody; anti‐dsDNA, antidouble strand DNA antibody; BILAG, British Isles Lupus Activity Group assessment; C, cytopenia; F, febrile; H, hypocomplementemia; LN, lupus nephritis; MCR, major clinical response; MSCT, mesenchymal stem cells transplantation; NR, nonresponse; P, polyserositis; PCR, partial clinical response; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; V, vasculitis; VCR, vincristine.
Lupus PBMCs Promote the Proliferation of UC MSCs
Flow cytometric analysis revealed that cultured UC MSCs were positive for CD29, CD105, CD166, and CD44, but negative for CD34, CD45, CD14, CD164, and HLA‐DR. Moreover, in vitro differentiation of UC MSCs into adipocytes and osteoblasts was also assessed (Supporting Information Fig. 1). We previously found that when cocultured with UC MSCs, lupus patients' peripheral CD4+CD25+Foxp3+ regulatory T cells markedly increased and CD4+IL17+ Th17 cells significantly decreased, which were mediated by TGF‐β and PGE2, respectively 14. However, how MSCs changed after cocultured with lupus PBMCs was unknown. Here, we found that when cocultured with PBMCs from active lupus patients or HCs, there was no change in MSCs surface CD34, CD29, and CD105 expression (Fig. 2A). When cocultured for 24 hours, SLE PBMCs‐stimulated UC MSCs showed a little higher level of apoptosis labeled by Annexin V and 7‐AAD, but with no significant difference compared with HC PBMCs‐stimulated or ‐unstimulated UC MSCs (Fig. 2B). Furthermore, when cocultured for 5 days, SLE PBMCs‐stimulated UC MSCs showed a significantly higher rate of proliferation (Fig. 2C).
Figure 2.
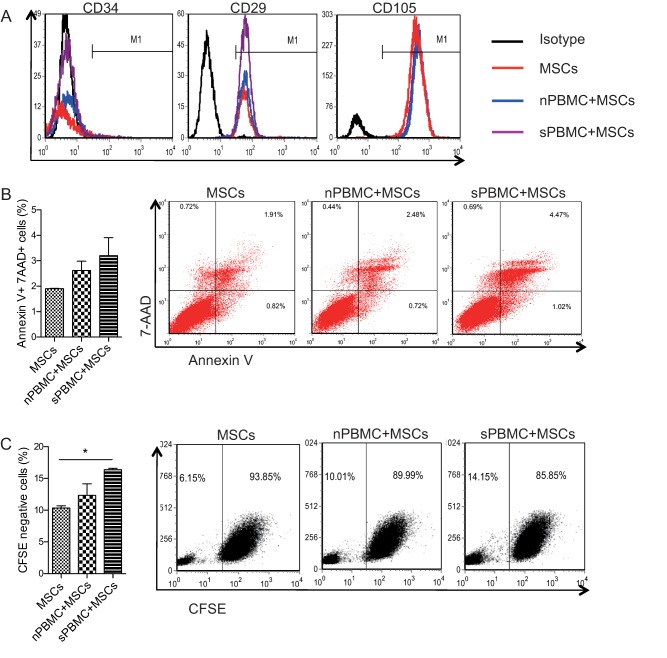
The effect of lupus PBMCs stimulation on the surface markers, cell apoptosis, and proliferation of MSCs. PBMCs were isolated from active lupus patients as well as healthy controls, then cocultured with umbilical cord‐derived mesenchymal stem cells (UC MSCs) for 3 days, and the cell surface expressions of CD34, CD29, and CD105 were analyzed (A). UC MSCs were cocultured with lupus or healthy PBMCs for 24 hours, and the apoptosis of MSCs were assessed (B). CFSE‐labeled UC MSCs were cocultured with PBMCs for 5 days, and the proliferation of MSCs were determined by assessing CFSE negative cells (C). *, p < .05. Abbreviations: CFSE, carboxyfluorescein succinimidyl ester; MSCs, mesenchymal stem cells; PBMCs, peripheral blood mononuclear cells; nPBMC, normal control peripheral blood mononuclear cells; sPBMC, systemic lupus erythematosus patient peripheral blood mononuclear cells.
Lupus PBMCs Activate Akt, IκB, and Stat5 Pathways in UC MSCs
Except for TGF‐β1, IDO, and HGF that were previously reported 13, we found that SLE PBMCs‐stimulated and HC PBMCs‐stimulated UC MSCs secreted an equivalent high amount of IL‐6 (Fig. 3A), but SLE PBMCs‐stimulated UC MSCs produced higher levels of VEGF and CXCL12 compared with HC PBMCs‐stimulated UC MSCs (Fig. 3B, 3C, Supporting Information Fig. 2), while IL‐10 had no change among different groups (Fig. 3D). Both CXCL12 and VEGF in supernatants also significantly increased when UC MSCs were cocultured with SLE patient‐derived PBMC compared with that cocultured with HC‐derived PBMC (Fig. 3E, 3F). These data demonstrated that UC MSCs were activated when stimulated by SLE PBMCs. So next, we examined different signaling pathways, and the results revealed that the Akt, IκB, and Stat5 signaling pathways significantly activated when stimulated by SLE PBMCs for 24 hours (Fig. 3G), while Smad1/5/8 and Erk1/2 pathways had no change (Fig. 3H). The activation of Stat5 and IκB pathways was mediated by inflammatory cytokines IFN‐γ and TNF‐α, respectively, because the addition of IFN‐γ and TNF‐α markedly activated Stat5 and IκB signaling pathways in culture (Fig. 3I).
Figure 3.

Changes of MSCs functional molecules and signaling pathways after stimulated by lupus peripheral blood mononuclear cells (PBMCs). Umbilical cord‐derived mesenchymal stem cells (UC MSCs) were cocultured with PBMCs from active lupus patients as well as healthy controls, and 48 hours later, the functional molecules including IL‐6 (A), CXCL12 (B), VEGF (C), and IL‐10 (D) from UC MSCs were assessed by real‐time polymerase chain reaction. The supernatant levels of CXCL12 (E) and VEGF (F) were analyzed by enzyme‐linked immunosorbent assay. Signaling pathways including Akt, IκB, Stat 5 (G), Smad1/5/8 and Erk1/2 (H) were assessed by Western blot. The changes of Akt, IκB, Stat 5 signaling pathways stimulated by recombinant IFN‐γ and TNF‐α were also assessed (I). *, p < .05; **, p < .01. Abbreviations: GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; MSCs, mesenchymal stem cells; nPBMC, normal control peripheral blood mononuclear cells; sPBMC, SLE patient peripheral blood mononuclear cells; VEGF, vascular endothelial growth factor.
UC MSCs Produce Functional Molecules After Stimulation With Inflammatory Cytokines
It is confirmed that Th1, Th2, Treg, and Th17 cells all participate in the pathogenesis of SLE 15, and type 1 interferon also plays an important role in lupus 16. Previously, we also showed significant elevated IFN‐γ 13 and T follicular helper cells (Tfh) in active lupus patients 17, the latter of which was mainly a result of IL‐21 stimulation. So next, we used different cytokines, including IFN‐α (20 ng/ml), IFN‐β (20 ng/ml), IFN‐γ (20 ng/ml), TGF‐β1 (60 ng/ml), TNF‐α (100 ng/ml), IL‐6 (20 ng/ml), IL‐1β (80 ng/ml), IL‐21 (100 ng/ml), IL‐2 (200 IU/ml), IL‐4 (20 ng/ml), and IL‐10 (20 ng/ml), to stimulate UC MSCs in vitro for 48 hours, to detect IDO1, IDO2, TGF‐β1, IL‐6, COX1, and COX2 expressions by real‐time PCR. In addition to the previously reported elevated IDO1 that was induced by IFN‐γ 13, we also showed that IFN‐γ induced more than a 40‐fold increase of IDO2, an 8‐fold increase of COX‐1, and a 15‐fold increase of IL‐6 in MSCs, that TNF‐α induced a 6‐fold increase of COX2 and a 30‐fold increase of IL‐6, that IFN‐α induced a 2‐fold increase of TGF‐β1, and that IL‐1β induced a 20‐fold increase of IL‐6 (Fig. 4A–4E).
Figure 4.
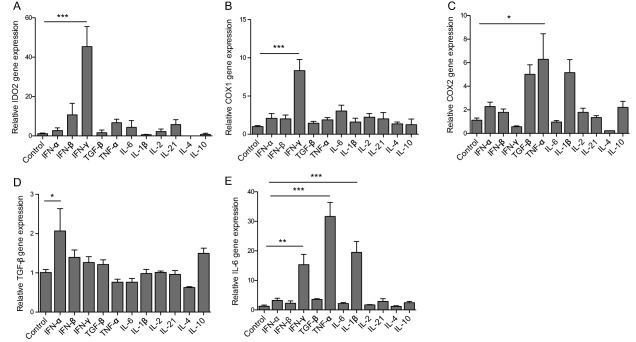
Functional factor expressions of umbilical cord‐derived mesenchymal stem cells (UC MSCs) after stimulated by different cytokines. UC MSCs were cultured with or without different cytokines in vitro for 48 hours, then the functional factors including IDO2 (A), COX1 (B), COX2 (C), TGF‐β (D), and IL‐6 (E) were determined by real time polymerase chain reaction. *, p < .05; **, p < .01; ***, p < .001.
IFN‐γ Dose Dependently Induced IDO Expressions
Of all the cytokines, IFN‐γ is still the most important, inducing significantly high levels of IDO1 as well as IDO2. In vitro, we used different doses of IFN‐γ (0, 10, 20, 50, and 100 ng/ml) to stimulate UC MSCs for 48 hours and found that IFN‐γ dose dependently induced IDO1 as well as IDO2 expressions (Fig. 5A, 5B). In vitro, 100 ng/ml IFN‐γ inhibited UC MSCs proliferation after 5 days stimulation but had no significant difference (Fig. 5C). IFN‐γ stimulation had no effect on T‐cell apoptosis after 24 hours of stimulation (Fig. 5D). Moreover, in the coculture of lupus PBMC and UC MSC, we found that treatment with anti‐IFN‐γ antibody significantly inhibited the expression of IDO1 and IDO2 in UC MSCs (Fig. 5E, 5F), further indicating that the induction of IDO expression by the lupus patients' PBMCs was mediated by IFN‐γ.
Figure 5.

Recombinant human IFN‐γ dose dependently enhance indoleamine 2,3‐dioxygenase expression. In vitro, IFN‐γ stimulated umbilical cord‐derived mesenchymal stem cells (UC MSCs) to produce IDO1 (A) as well as IDO2 (B), both had dose‐dependent manners. High dose IFN‐γ slightly inhibited UC MSCs proliferation (C), but did not affect MSCs apoptosis (D). The addition of anti‐IFN‐γ significantly inhibited the production of IDO1 (E) as well as IDO2 (F) by MSCs induced by lupus patients' peripheral blood mononuclear cell. *, p < .05; **, p < .01; ***, p < .001. Abbreviations: CFSE, carboxyfluorescein succinimidyl ester; GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; sPBMC, SLE patient peripheral blood mononuclear cells.
Discussion
SLE is one of the most complicated autoimmune diseases. It can involve almost all organs or systems and presents with protean clinical manifestations 18. On the basis of data from the Chinese SLE Treatment and Research group (CSTAR) registry, 56.1% of patients presented with hematological disorders, 47.4% of patients presented with nephropathy, and 4.8% of patients had neurologic involvement 19. The pathogenesis of SLE differed among patients. Some patients showed elevated serum B cells and some patients showed activated dendritic cells 20. We previously showed a CD8+T cells‐IFNγ‐IDO axis that mediated T‐cell proliferation and induced immunoregulation in patients. However, not all the patients showed elevated serum IFN‐γ and increased CD8+T cells 13, so the correlation between the patient's environment and clinical treatment effect by MSCT is of pivotal importance.
Previously, we showed that PBMC from active SLE patients stimulated MSCs to produce more levels of TGF‐β and IDO, the two important immunoregulatory factors. In the present study, we further showed that two chemokines, CXCL12 and VEGF, were significantly produced by MSCs when stimulated by PBMC from SLE patients compared with PBMC from HCs. These results showed that, in the lupus microenvironment, MSCs upregulated the migration‐related molecules CXCL12 and VEGF, which further explained our previous conclusion that allogeneic MSCs could efficiently migrate to the kidney in MRL/lpr lupus mice but not in normal C57BL/6 mice 21. We believe that MSCs could act systemically and locally in the recipient; however, the correlation between MSCs migration and immunoregulation is unclear.
The signaling pathways analysis showed that when cocultured with lupus patients, PBMC, the Akt, IκB, and Stat5 pathways were activated, while the Smad1/5/8 and Erk1/2 pathways had no change. The activation of the Stat5 pathway may be stimulated by elevated IFN‐γ produced by patients, CD8+T cells 13, which then mediate inhibition on T cell proliferation, while the activation of Akt and IκB may participate in the apoptosis and proliferation of MSCs. We previously showed that TNF‐α activated the NF‐κB pathway and inhibited the phosphorylation of Smad1/5/8 and BMP‐2‐induced osteoblastic differentiation ability for lupus bone marrow‐derived MSCs, which is the main cause for the pathology of osteoporosis in SLE patients 22. In the present study, we did not detect the change in Smad1/5/8 pathways, which may be because we used normal UC‐derived MSCs, which showed a normal ability of osteoblastic differentiation.
As mentioned above, the microenvironment in human SLE is very complicated, so we used different cytokines to simulate MSCs in vitro, simply to know how MSCs changed after different stimulations. The used cytokines were Th1, Th2, Treg, Th17, and Tfh cells, among the T cell subsets that have been reported to participate in the pathogenesis of SLE 23. Among all the cytokines, IFN‐γ is the most important one to stimulate IDO1 and IDO2 expressions, but not dose‐dependently affect MSCs apoptosis, and slightly inhibit MSCs proliferation at a high dose of 100 ng/ml, suggesting a pivotal role of IFN‐γ in initiating MSCs function. Besides IFN‐γ, TNF‐α can induce COX2 as well as IL‐6 production in MSCs, the two factors that contrarily affect Th17 cells regulation 14, 24. IFN‐α stimulated MSCs producing 2‐fold of TGF‐β1, which may participate in the Treg differentiation.
The most important result of this study is that we showed the correlation between baseline cytokine levels and the clinical efficacy of MSCT. Higher baseline serum IFN‐γ predicted a good response to MSCT. So in our clinical treatment, we are prone to choose SLE patients who had elevated serum IFN‐γ levels. However, this conclusion still needs more enrolled patients to confirm.
Conclusion
We preliminarily showed that IFN‐γ might predict clinical efficacy of allogeneic MSCT in SLE patients.
Author Contributions
D.D.W.: conception and design, financial support, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; S.Y.W.: conception and design, financial support, provision of study material and patients, data analysis and interpretation, final approval of manuscript; S.S.H., X.R.Y., Z.Y.Z., and X.B.F.: provision of study material, data analysis and interpretation, final approval of manuscript; L.W.L. and L.Y.S.: conception and design, financial support, data analysis, financial support, and final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Supporting information
Supporting Information Figure 1.
Supporting Information Figure 2.
Supporting Information Figure 3.
Acknowledgments
The study was supported by the Major International (Regional) Joint Research Project (81120108021 to Dr. Sun), National Natural Science Foundation of China (81273304 to Dr. Sun, 81401347 to Dr. D.D. Wang, 81471533 to Dr. S.Y. Wang), Jiangsu Province Major Research and Development Program (BE 2015602 to Dr. Sun), Jiangsu Provincial Natural Science Foundation (BK20140098 to Dr. D.D. Wang) and Jiangsu Provincial Health Department Foundation (Q201411 to Dr. D.D. Wang).
References
- 1. Tyndall A. Mesenchymal stem cell treatments in rheumatology: A glass half full? Nat Rev Rheumatol 2014;10:117–124. [DOI] [PubMed] [Google Scholar]
- 2. Sun L, Wang D, Liang J et al. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum 2010;62:2467–2475. [DOI] [PubMed] [Google Scholar]
- 3. Liang J, Zhang H, Hua B et al. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: A pilot clinical study. Ann Rheum Dis 2010;69:1423–1429. [DOI] [PubMed] [Google Scholar]
- 4. Akiyama K, Chen C, Wang D et al. Mesenchymal stem cell induced immunoregulation involves FAS‐ligand‐/FAS‐mediated T cell apoptosis. Cell Stem Cell 2012;10:544–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang D, Zhang H, Cao M et al. Efficacy of allogeneic mesenchymal stem cell transplantation in patients with drug‐resistant polymyositis and dermatomyositis. Ann Rheum Dis 2011;70:1285–1288. [DOI] [PubMed] [Google Scholar]
- 6. Liang J, Zhang H, Wang D et al. Allogeneic mesenchymal stem cell transplantation in seven patients with refractory inflammatory bowel disease. Gut 2012;61:468–469. [DOI] [PubMed] [Google Scholar]
- 7. Wang D, Li J, Zhang Y et al. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: A multicenter clinical study. Arthritis Res Ther 2014;16:R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang D, Zhang H, Liang J et al. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years experience. Cell Transplant 2013;22:2267–2277. [DOI] [PubMed] [Google Scholar]
- 9. Ren G, Zhang L, Zhao X et al. Mesenchymal stem cell‐mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008;2:141–150. [DOI] [PubMed] [Google Scholar]
- 10. Bayo J, Real A, Fiore EJ et al. IL‐8, GRO and MCP‐1 produced by hepatocellular carcinoma microenvironment determine the migratory capacity of human bone marrow‐derived mesenchymal stromal cells without affecting tumor aggressiveness. Oncotarget 2016. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu Y, Wang L, Kikuiri T et al. Mesenchymal stem cell‐based tissue regeneration is governed by recipient T lymphocytes via IFN‐γ and TNF‐α. Nat Med 2011;17:1594–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jin P, Zhao Y, Liu H et al. Interferon‐γ and tumor necrosis factor‐α polarize bone marrow stromal cells uniformly to a Th1 phenotype. Sci Rep 2016;6:26345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang D, Feng X, Lu L et al. A CD8 T cell‐IDO axis is required for mesenchymal stem cell suppression of human SLE. Arthritis Rheumatol 2014;66:2234–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang D, Huang S, Yuan X et al. The regulation of the Treg/Th17 balance by mesenchymal stem cells in human systemic lupus erythematosus. Cell Mol Immunol 2015;14:423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Talaat RM, Mohamed SF, Bassyouni IH et al. Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus erythematosus (SLE) patients: Correlation with disease activity. Cytokine 2015;72:146–153. [DOI] [PubMed] [Google Scholar]
- 16. Connelly KL, Kandane‐Rathnayake R, Hoi A et al. Association of MIF, but not type I interferon‐induced chemokines, with increased disease activity in Asian patients with systemic lupus erythematosus. Sci Rep 2016;6:29909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feng X, Wang D, Chen J et al. Inhibition of aberrant circulating tfh cell proportions by corticosteroids in patients with systemic lupus erythematosus. PLoS One 2012;7:e51982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. D'Cruz DP, Khamashta MA, Hughes GR. Systemic lupus erythematosus. Lancet 2007;369:587–596. [DOI] [PubMed] [Google Scholar]
- 19. Li M, Zhang W, Leng X et al. Chinese SLE Treatment and Research group (CSTAR) registry: I. Major clinical characteristics of Chinese patients with systemic lupus erythematosus. Lupus 2013;22:1192–1199. [DOI] [PubMed] [Google Scholar]
- 20. Menon M, Blair PA, Isenberg DA et al. A regulatory feedback between plasmacytoid dendritic cells and regulatory B cells is aberrant in systemic lupus erythematosus. Immunity 2016;44:683–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gu ZF, Akiyama K, Ma X et al. Transplantation of umbilical cord mesenchymal stem cells alleviates lupus nephritis in MRL/lpr mice. Lupus 2010;19:1502–1514. [DOI] [PubMed] [Google Scholar]
- 22. Tang Y, Xie H, Chen J et al. Activated NF‐κB in bone marrow mesenchymal stem cells from systemic lupus erythematosus patients inhibits osteogenic differentiation through downregulating Smad signaling. Stem Cell Dev 2013;22:678–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rother N, van der Vlag J. Disturbed T cell signaling and altered Th17 and regulatory T cell subsets in the pathogenesis of systemic lupus erythematosus. Front Immunol 2015;6:610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang Y, Xing F, Ye S et al. Jagged‐1 signaling suppresses the IL‐6 and TGF‐β treatment‐induced Th17 cell differentiation via the reduction of RORγt/IL‐17A/IL‐17F/IL‐23a/IL‐12rb1. Sci Rep 2015;5:8234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure 1.
Supporting Information Figure 2.
Supporting Information Figure 3.


