Abstract
Mesenchymal stromal cells (MSCs) from different sources have differential effects on lung injury. To compare the effects of murine MSCs from bone marrow (BM), adipose tissue (AD), and lung tissue (LUNG) on inflammatory and remodeling processes in experimental allergic asthma, female C57BL/6 mice were sensitized and challenged with ovalbumin (OVA) or saline (C). Twenty‐four hours after the last challenge, mice received either saline (50 µl, SAL), BM‐MSCs, AD‐MSCs, or LUNG‐MSCs (105 cells per mouse in 50 µl total volume) intratracheally. At 1 week, BM‐MSCs produced significantly greater reductions in resistive and viscoelastic pressures, bronchoconstriction index, collagen fiber content in lung parenchyma (but not airways), eosinophil infiltration, and levels of interleukin (IL)‐4, IL‐13, transforming growth factor (TGF)‐β, and vascular endothelial growth factor (VEGF) in lung homogenates compared to AD‐MSCs and LUNG‐MSCs. Only BM‐MSCs increased IL‐10 and interferon (IFN)‐γ in lung tissue. In parallel in vitro experiments, BM‐MSCs increased M2 macrophage polarization, whereas AD‐MSCs and LUNG‐MSCs had higher baseline levels of IL‐4, insulin‐like growth factor (IGF), and VEGF secretion. Exposure of MSCs to serum specimens obtained from asthmatic mice promoted reductions in secretion of these mediators, particularly in BM‐MSCs. Intratracheally administered BM‐MSCs, AD‐MSCs, and LUNG‐MSCs were differentially effective at reducing airway inflammation and remodeling and improving lung function in the current model of allergic asthma. In conclusion, intratracheal administration of MSCs from BM, AD, and LUNG were differentially effective at reducing airway inflammation and remodeling and improving lung function comparably reduced inflammation and fibrogenesis in this asthma model. However, altered lung mechanics and lung remodeling responded better to BM‐MSCs than to AD‐MSCs or LUNG‐MSCs. Moreover, each type of MSC was differentially affected in a surrogate in vitro model of the in vivo lung environment. Stem Cells Translational Medicine 2017;6:1557–1567
Keywords: Asthma, Mesenchymal stromal cells, Inflammation, Fibrosis, Macrophage
Significance Statement.
This study has demonstrated that murine mesenchymal stromal cells from bone marrow as well as adipose and lung tissues release different mediators, resulting in differential impact on pulmonary inflammation, fibrosis, and lung function when administered for treatment of experimental allergic asthma. This preclinical study aimed to collect data that could be used to support future clinical trials in patients with asthma.
Introduction
Asthma is a common, heterogeneous respiratory disease driven by a T‐helper (Th) 2 immune response to inhaled aeroallergens and characterized by airway hyper‐responsiveness (AHR) and eosinophilia. Chronic asthma is further associated with airway remodeling, including subepithelial fibrosis, collagen and elastic fiber deposition, goblet cell and smooth muscle cell hyperplasia and hypertrophy, and vascular proliferation/angiogenesis 1. Despite standard treatment with corticosteroids and long‐acting β‐agonists, no therapy can consistently reverse all these pathologic aspects of asthma. Therefore, new therapeutic options that attenuate remodeling and stimulate the repair process are required.
The reparative and immunoregulatory properties of mesenchymal stromal cells (MSCs) have made them attractive candidates for the treatment of asthma. In recent years, several studies have evaluated the effects of MSCs obtained from different sources, mainly bone marrow and adipose tissue, in several models of allergic airway inflammation 2, 3, 4, 5, 6, 7, 8, 9. In each case, MSC administration ameliorated the different experimental inflammatory markers. Several mechanisms for these MSC actions have been proposed, but, overall, the effects of these cells need further elucidation. Moreover, depending on their origin, MSCs may have differences in immunomodulatory, anti‐inflammatory, and regenerative activity, expansibility in culture, and specific phenotypes 10, 11, which may lead to different effects. Comparative studies of MSCs from different origins have been done in several models of experimental lung injury. However, to date, no study has compared the efficacy or potential mechanisms of action of MSCs from different sources in experimental allergic asthma.
The present study aimed to compare the extent to which MSCs from different sources (bone marrow, adipose tissue, or lung tissue) are able to decrease inflammation and remodeling and promote airway epithelial repair, leading to improvement in lung function, in experimental allergic asthma; and to investigate the possible mechanisms of action of MSCs from different sources. For this purpose, an in vivo study of MSC administration in a mouse model of ovalbumin‐stimulated allergic airway inflammation was paralleled by in vitro studies profiling release of cytokines and growth factors as well as effects of MSC coculture on macrophage polarization. Further experiments assessed the effects of exposing MSCs to the serum of asthmatic mice on cell behaviors in vitro.
Materials and Methods
This study was approved by the Ethics Committee of the Federal University of Rio de Janeiro Health Sciences Center (CEUA‐018/14). All animals received humane care under trained veterinarians and veterinary staff in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the U.S. National Academy of Sciences.
Animal Preparation and Experimental Allergic Asthma Protocol
One hundred and fifty C57BL/6 mice (weight 20–25 g, age 2 months) were used. MSCs were extracted and characterized from the same 22 male C57BL/6 animals. As bronchoalveolar lavage may affect lung morphological analysis and compromise lung function, 64 female C57BL/6 mice were used to evaluate lung mechanics and histology as well as levels of cytokines and growth factors in lung tissue (n = 6 per group), and another 64 female animals were used to analyze total and differential cell counts in bronchoalveolar lavage fluid (BALF) (n = 6 per group).
All animals were randomly assigned to two groups. In the OVA group, mice were immunized using an adjuvant‐free protocol by intraperitoneal injection of sterile ovalbumin (OVA, 10 μg of OVA in 100 μl saline) on seven alternate days (Supporting Information Fig. 1). Forty days after the start of sensitization, the animals were anesthetized with intravenous ketamine (25 mg/kg) and xylazine (2 mg/kg). Then, antigen challenge was performed in OVA mice by intratracheal administration of 20 μg of OVA in 20 μl saline. This procedure was performed three times with 3‐day intervals between applications 12. In the control group (C), sterile saline solution was administered using the same protocol. Both groups were subsequently randomized into four subgroups to receive sterile saline solution (0.9% NaCl, 50 µl, saline (SAL)) or MSCs derived from bone marrow, adipose tissue, and lung tissue (105 BM‐MSCs, AD‐MSCs, or LUNG‐MSCs per mouse at the third passage), intratracheally, 24 hours after the last challenge (Supporting Information Fig. 1). One week after MSC administration, all data were analyzed.
Extraction and Characterization of Mesenchymal Stromal Cells
Twenty‐two male C57BL/6 mice (weight 20–25 g, age 2 months) were anesthetized with intravenous ketamine (25 mg/kg) and xylazine (2 mg/kg) and used as donors. Bone marrow cells were obtained from femurs and tibias. After isolation, bone marrow‐derived cells were cultured (37°C, 5% T25 culture flasks; TPP, Schaffhausen, Switzerland, http://www.sigmaaldrich.com) with Dulbecco's Modified Eagle Medium (DMEM; Invitrogen, CA, https://www.thermofisher.com) containing 15 mM HEPES (Sigma, MO, http://www.sigmaaldrich.com), 15% inactivated fetal bovine serum (FBS) (Invitrogen, CA, https://www.thermofisher.com), 100 units per ml penicillin, and 100 mg/ml streptomycin antibiotic solution (Gibco, NM, https://www.thermofisher.com). MSCs from lung and adipose tissue (epididymal fat pad) were obtained as previously described 13. Tissues were collected, rinsed in PBS, transferred to a Petri dish, and cut into small pieces (approximately 0.2–0.8 cm2). The dissected pieces were washed with PBS, cut into smaller fragments, and subsequently digested with type I collagenase (1 mg/ml in DMEM/10 mM HEPES) for 30 minutes to 1 hour at 37°C. Whenever gross remnants persisted after collagenase digestion were allowed to settle for 1–3 minutes, and the supernatant was transferred to a new tube containing fresh medium and centrifuged at 400 g for 10 minutes at room temperature (RT). The pellets were resuspended in 3.5 ml D‐MEM containing 1% antibiotic‐antimycotic solution (Invitrogen, CA), seeded in six‐well dishes (3.5 ml per well), and incubated at 37°C in a humidified atmosphere containing 5% CO. On day 3 of culture, the medium was changed and nonadherent cells were removed. Adherent cells exhibited similar proliferation rates and, upon reaching 80% confluence, were passaged with 0.05% trypsin‐EDTA solution (Gibco, NM) and then maintained in DMEM with 10% FBS (complete medium).
Approximately 1 × 105 cells were characterized as MSCs at the third passage, according to the International Society of Cellular Therapy Consensus 14. Flow cytometry was performed using commercially available antibodies against CD45 (leukocytes), CD34 (hematopoietic precursors), CD29 and CD45 (nonhematopoietic precursors), and Sca‐1 (stem/progenitor cells) (BD Biosciences, http://www.bdbiosciences.com). The absence of CD34 and CD45 and the presence of CD29 and Sca‐1 were used to identify MSCs 15. The different MSCs populations were further characterized by their capacity to differentiate into osteoblasts and chondroblasts. Osteogenic differentiation was induced by culturing MSCs for up to 3 weeks in D‐MEM 10% FBS and 15 mM HEPES (Sigma, MO), supplemented with 10−8 M/l dexamethasone (Sigma, MO, www.sigmaaldrich.com), 5 μg/ml ascorbic acid 2‐phosphate (Sigma, MO, www.sigmaaldrich.com), and 10 mM/l β‐glycerolphosphate (Sigma MO, www.sigmaaldrich.com). To observe calcium deposition, cultures were stained with Alizarin Red S (Nuclear, SP, Brazil). To induce chondrogenic differentiation, MSCs were cultured in DMEM supplemented with 10 ng/ml TGF‐β1 (Sigma, MO, www.sigmaaldrich.com), 50 nM ascorbic acid 2‐phosphate (Sigma, MO), and 6.25 mg/ml insulin for 3 weeks. To confirm differentiation, cells were fixed with 4% paraformaldehyde in PBS for 1 hour at RT and stained with Alcian Blue pH 2.5.
Mechanical Parameters
One week after MSC administration, the animals were sedated (diazepam 1 mg/kg intraperitoneally), anesthetized (thiopental sodium 20 mg/kg intraperitoneally), tracheotomized, paralyzed (vecuronium bromide, 0.005 mg/kg intravenously), and ventilated using a constant flow ventilator (Samay VR15; Universidad de la Republica, Montevideo, Uruguay) with the following settings: frequency 100 breaths per minute, tidal volume (VT) 0.2 ml, and fraction of inspired oxygen 0.21. The anterior chest wall was surgically removed and a positive end‐expiratory pressure (PEEP) of 2 cm H2O applied. Airflow and tracheal pressure (Ptr) were measured 16. Lung mechanics were analyzed by the end‐inflation occlusion method 17. In an open chest preparation, Ptr reflects transpulmonary pressure (PL). Briefly, after end‐inspiratory occlusion, there is an initial fast drop in PL (ΔP1, L) from the preocclusion value down to an inflection point (Pi), followed by a slow pressure decay (ΔP2, L), until a plateau is reached. This plateau corresponds to the elastic recoil pressure of the lung (Pel). ΔP1, L selectively reflects the pressure used to overcome the airway resistance. ΔP2, L reproduces the pressure spent by stress relaxation, or viscoelastic properties of the lung, together with a small contribution of pendelluft. Static lung elastance (Est,L) was determined by dividing Pel by V T. Lung mechanics measurements were performed 10 times in each animal 16. All data were analyzed using ANADAT software (RHT‐InfoData, Inc., Montreal, Quebec, Canada).
Lung Histology
Laparotomy was performed immediately after determination of lung mechanics and heparin (1,000 IU) was injected into the vena cava. The trachea was clamped at end‐expiration (PEEP = 2 cmH2O), and the mice euthanized by exsanguination following transection of the abdominal aorta and vena cava. Lungs were then removed and flash‐frozen by immersion in liquid nitrogen. The left lung was fixed with Carnoy's solution and paraffin‐embedded 15. Sections (4 μm thick) were cut and stained with hematoxylin‐eosin (H&E).
Lung morphometry analysis was performed using an integrating eyepiece with a coherent system consisting of a grid with 100 points and 50 lines of known length coupled to a conventional light microscope (Olympus BX51, Olympus Latin America‐Inc., Brazil). The volume fraction of collapsed and normal pulmonary areas, magnitude of bronchoconstriction, and number of mononuclear (MN) and polymorphonuclear (PMN) cells in pulmonary tissue were determined by the point‐counting technique 18, 19 across 10 random, noncoincident microscopic fields per mouse 15, 20, 21.
Collagen fibers (Picrosirius‐polarization method) were quantified in airways and alveolar septa using ImagePro Plus 6.0 software 12, 22.
Enzyme‐Linked Immunosorbent Assay
In the right lung, levels of interleukin (IL)‐4, IL‐13, interferon (IFN)‐γ, vascular endothelial growth factor (VEGF) (PeproTech, Rocky Hill, NJ, https://www.peprotech.com), transforming growth factor (TGF)‐β, and IL‐10 (R&D, Minneapolis, MN, https://www.rndsystems.com) were quantified by enzyme‐linked immunosorbent assay (ELISA) as per manufacturer instructions. Results are expressed as pg/ml.
Bronchoalveolar Lavage Fluid
Additional female mice were used to analyze total and differential cell counts in BALF (n = 6 per group). For this purpose, a polyethylene cannula was inserted into the trachea and a total volume of 1.0 ml of PBS containing 10 mM EDTA was instilled and aspirated (only once). Samples were centrifuged at 300 g for 10 minutes. The supernatant was removed and the pellet resuspended in 0.25 ml of PBS. Total leukocyte counts in BALF were quantitated in Neubauer chambers under light microscopy after dilution of the samples in Türk solution (2% acetic acid). To obtain the percentage of each cell type in BALF, differential leukocyte counts were performed by counting 100 cells in cytocentrifuged smears stained by the May‐Grünwald‐Giemsa method 23.
In Vitro Analysis of Macrophage Phenotype
RAW 264.7 cells (a mouse peritoneal macrophage cell line) obtained from American Type Culture Collection (Rockville, MD, https://www.bioz.com) were maintained in coculture with the different MSCs (BM‐MSCs, AD‐MSCs, and LUNG‐MSCs) using DMEM–High Glucose, supplemented with 10% fetal bovine serum, 1,000 U/ml penicillin/streptomycin, and 2 mM L‐glutamine (Invitrogen, Life Technologies Grand Isle, NY, https://www.thermofisher.com). Cells were plated in 12‐well transwell plates [105 × RAW 264.7 (lower) and 105 × BM‐MSCs, AD‐MSCs and LUNG‐MSCs (upper)/well] for 48 hours. The supernatant was then removed and cells washed with 1 × PBS, harvested from the culture plates using 2.5% Trypsin/EDTA (Invitrogen Life Technologies Grand Isle, NY, https://www.thermofisher.com), and pelleted by centrifugation (600 × g for 5 minutes).
Quantitative real‐time reverse transcription (RT) polymerase chain reaction (PCR) was performed to measure mRNA expression of inducible nitric oxide synthase (iNOS) (M1 marker) and arginase (M2 marker). Cells were lysed for RNA extraction through the RNeasy Plus Mini Kit (Qiagen, Valencia, CA, https://www.qiagen.com) as per manufacturer instructions. The total RNA concentration was measured by spectrophotometry in a Nanodrop ND‐1000 system. First‐strand cDNA was synthesized from total RNA using an M‐MLV Reverse Transcriptase Kit (Invitrogen, https://www.thermofisher.com). Relative mRNA levels were measured with a SYBR Green detection system using ABI 7500 real‐time PCR (Applied Biosystems, Foster City, CA, https://www.thermofisher.com). All samples were measured in triplicate. The relative level of each gene was calculated as the ratio of the study gene to the control gene (acidic ribosomal phosphoprotein P0, 36β4) and given as the fold change relative to RAW cells incubated with regular medium. The following PCR primers were used: iNOS, forward 5CTTCAGGTATGCGGTATTGG‐3, reverse 5′ CATGGTGAACACGTTCTTGG‐5; arginase‐1, forward 5′ GCTCAGGTGAATCGG CCTTTT‐3′, reverse 5′ TGGCTTGCGAGA CGTAGAC‐3′; IL‐10, forward 5′ ATCCAAGACAACACTACTATA‐3′, reverse 5′ TAAATATCCTCAAAG TTCC‐3; and TGF‐β, forward 5′ GGCTACCATGCCAACTTCT‐3′, reverse 5′ TGTACAACCAGCATAAC CCGG‐3′.
Analysis of Multiple Soluble Factors After In Vitro Stimulation
To profile soluble mediators potentially released in vivo by the MSCs obtained from the different sources, MSCs were cultured for 48 hours in 12‐well tissue culture plates (Becton Dickinson, San Jose, CA) (105 cells per well) using DMEM–High Glucose, supplemented with 10% fetal bovine serum, 1,000 U/ml penicillin/streptomycin, 2 mM L‐glutamine (Invitrogen, Life Technologies Grand Isle, NY). On day 3, MSCs were exposed to 5% or 10% serum obtained from mice sensitized and challenged with ovalbumin (OVA‐serum, pool of 10 mice) 24. Twenty‐four hours after adding OVA‐serum, the medium of each well was collected and the levels of several cytokines, chemokines (IL‐4, IL‐13, and eotaxin) and growth factors [insulin‐like growth factor (IGF), VEGF, and platelet‐derived growth factor (PDGF)] were evaluated by ELISA as per manufacturer instructions (PeproTech, Rocky Hill, NJ) 25 and compared to levels produced by unexposed cells. Additionally, the levels of these mediators were measured in the OVA‐serum samples.
Statistical Analysis
Differences between groups were assessed using two‐way ANOVA followed by Tukey's test. One‐way ANOVA followed by Tukey's test was used to compare flow cytometry data. For nonparametric results, the Kruskal‐Wallis test followed by Dunn's test was used. Parametric data were expressed as mean ± SD, while nonparametric data were expressed as median (interquartile range). All tests were performed using the Prism 5.0 software package (GraphPad Software Inc., La Jolla, CA, http://www.graphpad.com), and statistical significance was established at p < .05.
Results
BM‐MSCs Led to Greater Improvement in Lung Mechanics Compared to AD‐MSCs and LUNG‐MSCs
OVA‐SAL animals presented a higher static lung elastance (Est,L) and resistive (ΔP1, L), and viscoelastic (ΔP2, L) pressures compared to C‐SAL (Fig. 1). Administration of each type of MSC was effective at reducing these parameters; however, these decrements were more pronounced after administration of BM‐MSCs than AD‐MSCs or LUNG‐MSCs. (Fig. 1).
Figure 1.
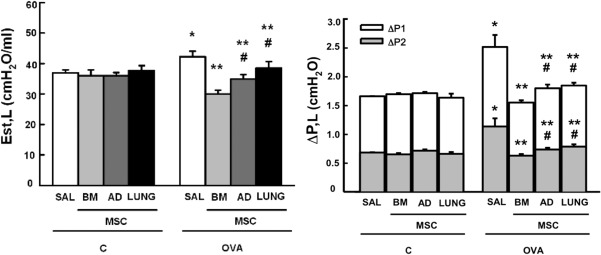
Lung mechanics. Static lung elastance (Est, L) and resistive (ΔP1, gray bar), viscoelastic (ΔP2, white bar), and total pressures (ΔPtot = ΔP1 + ΔP2). C: mice sensitized and challenged with saline; OVA: mice sensitized and challenged with ovalbumin; SAL: mice sensitized and challenged with ovalbumin and treated with saline; BM‐, AD‐, and LUNG‐MSCs: mice treated with MSCs derived from bone marrow, adipose, and lung tissues, respectively, 24 hours after the last challenge (105 cells per mice). *Significantly different from C‐SAL (p < .05). **Significantly different from OVA‐SAL (p < .05). #Significantly different from OVA‐BM‐MSCs (p < .05). Abbreviations: AD, adipose tissue; BM, bone marrow; LUNG, lung tissue; MSCs, mesenchymal stromal cells; OVA, ovalbumin; SAL, saline.
BM‐MSCs Were More Effective at Reducing Morphological Changes and Remodeling Than AD‐MSCs and LUNG‐MSCs
The fractional area of alveolar collapse, the number of total, mononuclear, and polymorphonuclear cells, as well as bronchoconstriction index were higher in OVA‐SAL compared to C‐SAL mice (Fig. 2 ). MSCs, regardless of source, similarly reduced the inflammatory cell infiltration in lung tissue and bronchoconstriction index, but BM‐MSCs were more effective at reducing alveolar collapse in lung parenchyma (Fig. 2, Supporting Information Fig. 2). Collagen fiber content in the airways and alveolar septa was significantly increased in the OVA‐SAL group as compared with C‐SAL (Fig. 3 ). Administration of MSCs, regardless of source, resulted in a decrease in collagen fiber content in alveolar septa, with a greater reduction observed with use of BM‐MSCs. Collagen fiber content in the airways was unaffected by MSC administration (Fig. 3, Supporting Information Fig. 2).
Figure 2.
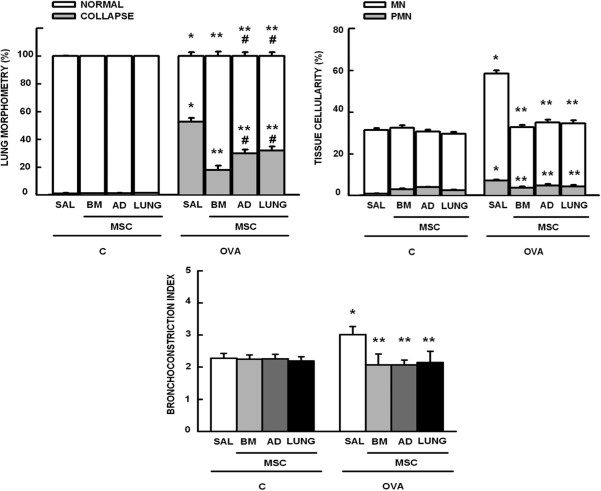
Morphometric parameters. Lung morphometry: fractional area of normal (white bar) and collapsed alveoli (gray bar); Tissue cellularity: fractional area of mononuclear (MN, white bar) and polymorphonuclear (PMN, gray bar) cells; and Bronchoconstriction index. Bars are means + SD of six animals per group. C: mice sensitized and challenged with saline; OVA: mice sensitized and challenged with ovalbumin; SAL: mice sensitized and challenged with ovalbumin and treated with saline; BM‐, AD‐, and LUNG‐MSCs: mice treated with MSCs derived from bone marrow, adipose, and lung tissues, respectively, 24 hours after the last challenge (105 cells per mice). *Significantly different from C‐SAL (p < .05). **Significantly different from OVA‐SAL (p < .05). #Significantly different from OVA‐BM‐MSCs (p < .05). Abbreviations: AD, adipose tissue; BM, bone marrow; LUNG, lung tissue; MSCs, mesenchymal stromal cells; OVA, ovalbumin; SAL, saline.
Figure 3.
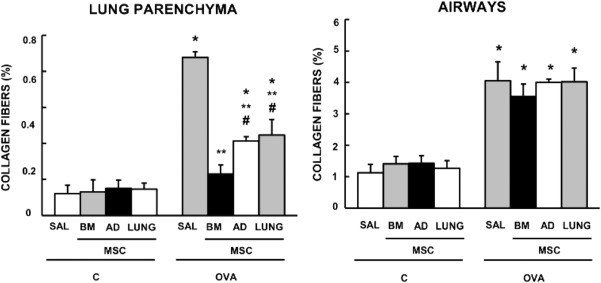
Collagen fiber content in lung parenchyma and airways. Bars are means + SD of six animals per group. C: mice sensitized and challenged with saline; OVA: mice sensitized and challenged with ovalbumin; SAL: mice sensitized and challenged with ovalbumin and treated with saline; BM‐, AD‐, and LUNG‐MSCs: mice treated with MSCs derived from bone marrow, adipose, and lung tissues, respectively, 24 hours after the last challenge (105 cells per mice). *Significantly different from C‐SAL (p < .05). **Significantly different from OVA‐SAL (p < .05). #Significantly different from OVA‐BM‐MSCs (p < .05). Abbreviations: AD, adipose tissue; BM, bone marrow; LUNG, lung tissue; MSCs, mesenchymal stromal cells; OVA, ovalbumin; SAL, saline.
BM‐MSCs Yielded Greater Reductions in Cytokine and Growth Factor Levels Compared to AD‐MSCs and LUNG‐MSCs
Levels of IL‐4, IL‐13, TGF‐β, and VEGF in lung tissue homogenates were greater in the OVA‐SAL than in the C‐SAL group. BM‐MSCs and AD‐MSCs were more effective at reducing IL‐4 than LUNG‐MSCs. BM‐MSCs were also associated with greater reductions in IL‐13 level and promoted more pronounced increases in IL‐10 and IFN‐γ compared to LUNG‐MSCs. MSC administration, independent of source, similarly reduced TGF‐β levels. Likewise, all MSCs reduced VEGF levels, but this reduction was more pronounced with BM‐MSCs compared to AD‐MSCs and LUNG‐MSCs (Fig. 4).
Figure 4.
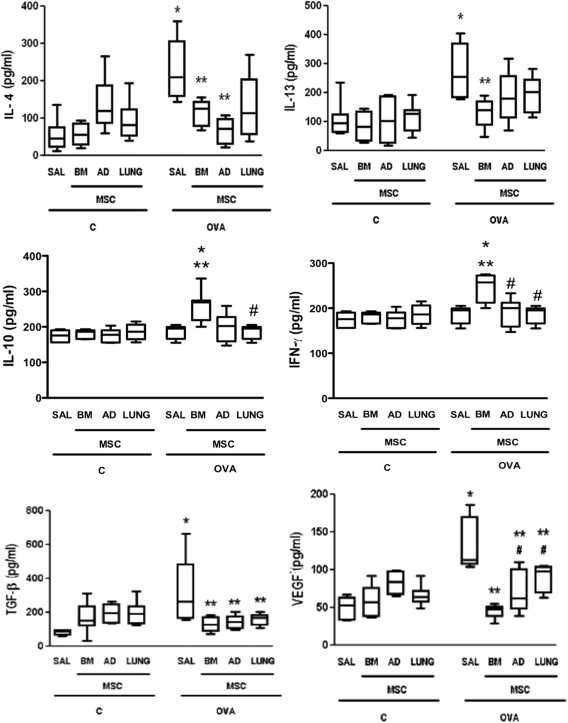
Levels of interleukin (IL)‐4, IL‐13, IL‐10, interferon (IFN)‐γ, transforming growth factor (TGF)‐β, and vascular endothelial growth factor in lung tissue. Boxes show the interquartile (25%–75%) range, whiskers encompass the range (minimum–maximum), and horizontal lines represent the median in six animals per group. C: mice sensitized and challenged with saline; OVA: mice sensitized and challenged with ovalbumin; SAL: mice sensitized and challenged with ovalbumin and treated with saline; BM, AD, and LUNG‐MSCs: mice treated with MSCs derived from bone marrow, adipose, and lung tissues, respectively, 24 hours after the last challenge (105 cells per mice). *Significantly different from C‐SAL (p < .05). **Significantly different from OVA‐SAL (p < .05). #Significantly different from OVA‐BM‐MSCs (p < .05). Abbreviations: AD, adipose tissue; BM, bone marrow; IL, interleukin; iNOS, inducible nitric oxide synthase; LUNG, lung tissue; MSCs, mesenchymal stromal cells; OVA, ovalbumin; SAL, saline; TGF, transforming growth factor.
BM‐MSCs Yielded Greater Reductions in BALF Cellularity Than AD‐MSCs and LUNG‐MSCs
The number of total cells, monocytes, neutrophils, and eosinophils in BALF was higher in the OVA‐SAL group compared to C‐SAL (Fig. 5). Administration of each type of MSCs led to statistically significant reductions in total cells, eosinophils, and neutrophils. Notably, reductions in total cells and eosinophils were statistically greater with BM‐MSC administration. No significant changes were observed in BALF monocyte counts regardless of the MSC source.
Figure 5.
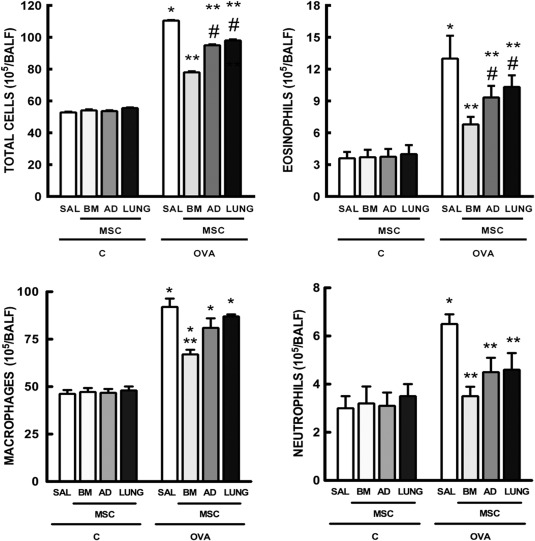
Number of total cells, eosinophils, macrophages, and neutrophils in bronchoalveolar lavage fluid. Bars are means + SD of six animals per group. C: mice sensitized and challenged with saline; OVA: mice sensitized and challenged with ovalbumin; SAL: mice sensitized and challenged with ovalbumin and treated with saline; BM, AD, and LUNG‐MSCs: mice treated with MSCs derived from bone marrow, adipose, and lung tissues, respectively, 24 hours after the last challenge (105 cells per mice). *Significantly different from C‐SAL (p < .05). **Significantly different from OVA‐SAL (p < .05). #Significantly different from OVA‐BM‐MSCs (p < .05). Abbreviations: AD, adipose tissue; BM, bone marrow; LUNG, lung tissue; MSCs, mesenchymal stromal cells; OVA, ovalbumin; SAL, saline.
BM‐MSCs Alone Induced M2 Macrophage Polarization
iNOS mRNA expression was similarly reduced after coculture of macrophages (RAW 264.7 cells) with MSCs obtained from BM, AD, and LUNG. Only coculture with BM‐MSCs was able to increase arginase, IL‐10, and TGF‐β mRNA expressions, demonstrating M2 macrophage polarization (Fig. 6).
Figure 6.
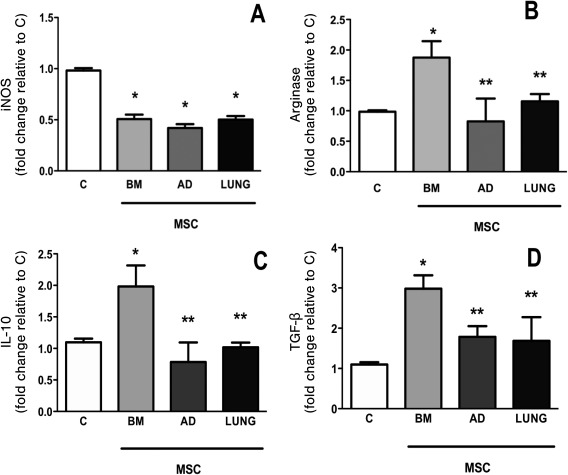
In vitro assay. RAW 264.7, a macrophage cell line, was cocultured with different MSCs for 24 hours. Relative gene expression of iNOS (A), arginase (B), IL‐10 (C), and (D) TGF‐β was calculated as a ratio of the average gene expression levels compared with the reference gene (36B4) and expressed as fold changes relative to C group (noncontacting cell cocultures, 105 RAW 264.7/well). Bars are means + SD of five wells per condition. BM‐, AD‐, and LUNG‐MSCs: macrophages were co‐cultured in transwell plates with MSCs derived from bone marrow, adipose, and lung tissues (105 MSCs per well). *Significantly different from C (p < .05). **Significantly different from BM‐MSCs (p < .05). Abbreviations: AD, adipose tissue; BM, bone marrow; IL, interleukin; iNOS, inducible nitric oxide synthase; LUNG, lung tissue; MSCs, mesenchymal stromal cells; OVA, ovalbumin; SAL, saline; TGF, transforming growth factor.
Effects of MSC Stimulation with Serum Obtained from OVA Mice
Unstimulated AD‐MSCs and LUNG‐MSCs produced higher levels of IL‐4, and IGF compared to BM‐MSCs. BM‐MSCs, AD‐MSCs, and LUNG‐MSCs stimulated with 5% and 10% of OVA‐serum similarly decreased IL‐4. AD‐MSCs stimulated with 5% OVA‐serum and LUNG‐MSCs stimulated with 10% OVA‐serum decreased IGF levels. VEGF levels were increased with AD‐MSCs before and after OVA‐serum stimulation. Eotaxin levels were not increased without stimulation, but were augmented by OVA‐serum stimulation, regardless of MSC source (Fig. 7).
Figure 7.
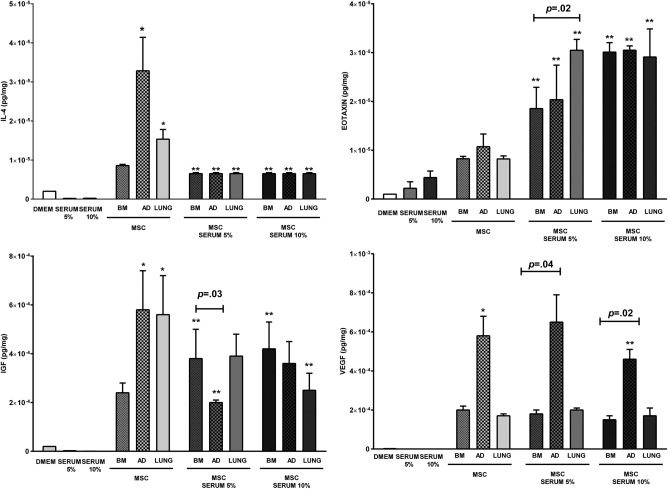
Levels of interleukin (IL)‐4, eotaxin, IGF, and vascular endothelial growth factor in the coculture medium after BM‐, AD‐, and LUNG‐MSC stimulation with serum obtained from OVA mice. DMEM: Dulbecco's Modified Eagle's Medium; SERUM 5%: serum obtained from OVA mice at a concentration of 5%; SERUM 10%: serum obtained from OVA mice at a concentration of 10%; BM‐, AD‐, and LUNG‐: MSCs from bone marrow, adipose, and lung tissues (105 cells per well), respectively, unstimulated or stimulated with 5% or 10% serum obtained from OVA mice during 24 hours. *Significantly different from BM‐MSCs (p < .05). **Significantly different from unstimulated MSCs (p < .05). Abbreviations: IGF, insulin‐like growth factor; MSCs, mesenchymal stromal cells.
Discussion
The main findings of the present study were that BM‐MSCs, AD‐MSCs, and LUNG‐MSCs reduced lung inflammation as well as lung mechanics and histology. However, these effects were more pronounced with BM‐MSCs than with either AD‐MSCs or LUNG‐MSCs. Even though administration of each type of MSC comparably reduced collagen fiber content in alveolar septa, none were unable to reduce the amount of collagen fibers in the airway; in vitro analyses showed reductions in the M1 (inflammatory and antimicrobial) macrophage phenotype (independent of MSC source), while only BM‐MSCs increased M2 polarization (wound repair and inflammation resolution). BM‐MSCs, AD‐MSCs, and LUNG‐MSCs differentially released selected mediators and were differentially affected by exposure to inflammatory serum.
Although all MSCs share similar general properties, cells from different sources can exhibit significant differences in anti‐inflammatory or regenerative potency depending on the particular injury 26. Furthermore, different MSCs may exhibit specific phenotypic, gene expression, and immunomodulatory profiles, and may thus present different anti‐inflammatory or regenerative effects 27, even when proliferation and differentiation capacities are similar 28, 29. BM‐MSCs are well characterized and have been used frequently in experimental asthma models 2, 3, 4, 30, 31, but the numbers obtained are limited 23. Several alternative MSCs sources from different tissues (spleen, amniotic fluid, umbilical cord, placenta, adipose, lung) 32 have been suggested. AD‐MSCs are abundant and may also be able to reduce inflammation in experimental allergic asthma 5, 6. LUNG‐MSCs, like all MSCs, are immunoprivileged, do not express MHC II, and can inhibit T cell‐based allorecognition 8. Furthermore, compared to BM‐MSCs, LUNG‐MSCs have higher expression of several basement membrane proteins and growth factors and have been reported to have longer persistence in injured lung tissue following systemic administration 33.
MSCs from different sources may reduce, direct or indirectly, the number of immune cells in in vitro and in vivo experimental models of asthma 2, 3, 4, 6, 9, 30, 34, 35. However, no head‐to‐head comparison of MSCs from different sources in the same study has been conducted to date. We found that intratracheal administration of MSCs obtained from each source following sensitization and challenge were all effective at reducing the inflammatory processes characteristic of allergic asthma, as demonstrated by a decrease in IL‐4 and IL‐13 levels in lung tissue and eosinophil infiltration in BALF. However, these reductions were more pronounced with BM‐MSCs compared to AD‐MSCs and LUNG‐MSCs. In addition, anti‐inflammatory mediators such as IFN‐γ and IL‐10 were higher in BM‐MSCs than AD‐MSCs and LUNG‐MSCs. Furthermore, TGF‐β and VEGF levels were reduced in lung tissue regardless of MSC source. These growth factors can promote airway remodeling in asthma, and thus may be a mechanism by which MSC administration reduces collagen fiber deposition in alveolar septa, with subsequent effects on lung mechanics. MSCs did not decrease collagen fiber content in the airway, which may be attributed to the fact that the type III collagen fibers present in alveolar septa are relatively easily to break as compared with the type I collagen fibers of the airway.
In this study, MSCs were administered intratracheally, which allows a reduction in the number of cells, delivers them to the desired site without the need to traverse vessel walls, and minimizes the risk of colonization of other organs 36. Macrophages can be activated by various extracellular signals to polarize toward either the M1 or the M2 phenotype. The mechanisms of macrophage activation in the presence of allergic reaction are still not completely understood 28. Emerging data suggest that BM‐MSCs have the ability to modulate cells of the macrophage/monocyte/dendritic cell lineage, including their differentiation, maturation, and activation 37. A recent study reported that BM‐MSCs attenuated lung inflammation through macrophage phagocytosis in experimental asthma, with subsequent acquisition of a suppressive M2 phenotype 27. Our study showed that MSCs were able to reduce a marker of the M1 phenotype (iNOS), while only BM‐MSCs promoted an increase in the M2 phenotype markers arginase, IL‐10, and TGF‐β. Further studies are required to evaluate whether different MSCs may phagocytose macrophages differentially.
The underlying mechanism of the attenuation in inflammation and remodeling observed in this experimental model of allergic asthma might be related to release of different soluble factors by each MSC type. However, whether the inflammatory environment in asthma might differentially affect release of soluble mediators and other MSC actions is not well understood. Fohonjy et al. demonstrated that chronic inflammatory processes, like those that occur in asthma, affect lung‐resident MSC differentiation. The functional and differentiation programs of such cells are likely altered by their transformation into myofibroblasts, which participate in remodeling rather than in repair through anti‐inflammatory actions 29. In the present study, which sought to mimic in vitro the in vivo inflammatory microenvironment of asthma, MSCs from different sources were stimulated with 5% or 10% serum obtained from mice sensitized and challenge with ovalbumin (OVA‐serum). In baseline conditions, AD‐MSCs exhibited higher levels of eotaxin and VEGF, while both AD‐MSCs and LUNG‐MSCs were associated with increased IL‐4 and IGF levels compared to BM‐MSCs. Furthermore, after OVA‐serum sensitization, MSC stimulation led to similar reductions in IL‐4 level in the culture medium (CM). Conversely, BM‐MSCs were associated with higher levels of IGF, a relevant growth factor involved in epithelial repair that is essential in the pathophysiology of asthma. Nevertheless, AD‐MSCs and LUNG‐MSCs were more responsive when added to an asthmatic tissue microenvironment, presenting higher levels of eotaxin (which plays an important role in the recruitment of eosinophils to injured areas, thus contributing to perpetuation and chronicity of the inflammatory process) and VEGF, which is both indirectly (through TGF‐β induction) and directly related to fibrogenesis and angiogenesis in asthma. In this context, our findings support the hypothesis that each type of MSC can be differentially modified by the in vivo inflammatory microenvironment 38. In short, the behavior presented by BM‐MSCs, compared to AD‐MSCs and LUNG‐MSCs, after OVA‐serum stimulation may explain the differences in amelioration of airway inflammation and remodeling in our experimental model of allergic asthma. Interestingly, considering that in vitro studies are commonly used as a framework for in vivo studies where a much more complicated microenvironment is present, not all data from in vitro experiments correlated with in vivo data.
Conclusion
Intratracheally administered BM‐MSCs, AD‐MSCs, and LUNG‐MSCs were differentially effective at reducing airway inflammation and remodeling and improving lung function in the current model of allergic asthma. Moreover, each type of MSC was differentially affected in a surrogate in vitro model of the in vivo lung environment. Further studies will help elucidate which of the many anti‐inflammatory actions of different MSC populations are most relevant and which can be potentially altered so as to increase MSC potency for potential use in clinical asthma.
Authors Contributions
S.C.A., M.A.A., D.G.X., P.C.O., M.M.M., D.J.W., B.L.D., M.M.M., and P.R.M.R.: conceived and designed the experiments; S.C.A., M.A.A., and D.G.X.: performed isolation and culture of different MSCs; S.C.A., M.A.A., D.G.X., F.F.C., V.C.V., E.B., A.F.A., and L.D.T.: performed the experiments; S.C.A., M.A.A., D.G.X., F.F.C., V.C.B., J.Z.K., A.F.A., and L.D.T.: analyzed the data; S.C.A., M.M.M., D.J.W., B.L.D., M.M.M., and P.R.M.R.: wrote this manuscript. All authors approved the final version of the manuscript.
Disclosure of Potential Conflicts of Interest
Priscilla C. Olsen is an employee of Federal University of Rio de Janeiro and has research funding from FAPERJ. Daniel J. Weiss has Research funding from a: Athersys Inc.; Biotek Inc.; United Therapeutics Inc. The other authors indicated no potential conflicts of interest.
Supporting information
Supporting Information
Supporting Information
Acknowledgments
The authors would like to express their gratitude to Mr. Andre Benedito da Silva and Mrs. Ana Lucia Neves da Silva for their help for technical assistance during the experiments and to Mrs. Moira Elizabeth Schottler and Mr. Filippe Vasconcellos for their assistance in editing the manuscript. This study was supported by the Brazilian Council for Scientific and Technological Development (CNPq), the Rio de Janeiro State Research Foundation (FAPERJ), the Department of Science and Technology (DECIT)/Brazilian Ministry of Health, the Coordination for the Improvement of Higher Education Personnel (CAPES), and National Institute of Science and Technology for Regenerative Medicine.
References
- 1. Al‐Muhsen S, Johnson JR, Hamid Q. Remodeling in asthma. J Allergy Clin Immunol 2011;128:451–462; quiz 463–454. [DOI] [PubMed] [Google Scholar]
- 2. Cruz FF, Borg ZD, Goodwin M et al. Systemic administration of human bone marrow‐derived mesenchymal stromal cell extracellular vesicles ameliorates aspergillus hyphal extract‐induced allergic airway inflammation in immunocompetent mice. Stem Cell Transl Med 2015;4:1302–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fu QL, Chow YY, Sun SJ et al. Mesenchymal stem cells derived from human induced pluripotent stem cells modulate T‐cell phenotypes in allergic rhinitis. Allergy 2012;67:1215–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lathrop MJ, Brooks EM, Bonenfant NR et al. Mesenchymal stromal cells mediate Aspergillus hyphal extract‐induced allergic airway inflammation by inhibition of the th17 signaling pathway. Stem Cells Transl Med 2014;3:194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jang HJ, Cho KS, Park HY et al. Adipose tissue‐derived stem cells for cell therapy of airway allergic diseases in mouse. Acta Histochem 2011;113:501–507. [DOI] [PubMed] [Google Scholar]
- 6. Cho KS, Roh HJ. Immunomodulatory effects of adipose‐derived stem cells in airway allergic diseases. Curr Stem Cell Res Ther 2010;5:111–115. [DOI] [PubMed] [Google Scholar]
- 7. Bentley JK, Popova AP, Bozyk PD et al. Ovalbumin sensitization and challenge increases the number of lung cells possessing a mesenchymal stromal cell phenotype. Respir Res 2010;11:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jarvinen L, Badri L, Wettlaufer S et al. Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator. J Immunol 2008;181:4389–4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chiu CJ, Ling TY, Chiang BL. Lung‐derived SSEA‐1(+) stem/progenitor cells inhibit allergic airway inflammation in mice. Allergy 2015;70:374–383. [DOI] [PubMed] [Google Scholar]
- 10. Ostanin AA, Petrovskii YL, Shevela EY et al. Multiplex analysis of cytokines, chemokines, growth factors, MMP‐9 and TIMP‐1 produced by human bone marrow, adipose tissue, and placental mesenchymal stromal cells. Bull Exp Biol Med 2011;151:133–141. [DOI] [PubMed] [Google Scholar]
- 11. Ricciardi M, Malpeli G, Bifari F et al. Comparison of epithelial differentiation and immune regulatory properties of mesenchymal stromal cells derived from human lung and bone marrow. PLoS One 2012;7:e35639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xisto DG, Farias LL, Ferreira HC et al. Lung parenchyma remodeling in a murine model of chronic allergic inflammation. Am J Respir Crit Care Med 2005;171:829–837. [DOI] [PubMed] [Google Scholar]
- 13. da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post‐natal organs and tissues. J Cell Sci 2006;119(Pt 11):2204–2213. [DOI] [PubMed] [Google Scholar]
- 14. Dominici M, Le Blanc K, Mueller I et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 15. Rocco PR, Negri EM, Kurtz PM et al. Lung tissue mechanics and extracellular matrix remodeling in acute lung injury. Am J Respir Crit Care Med 2001;164:1067–1071. [DOI] [PubMed] [Google Scholar]
- 16. Burburan SM, Xisto DG, Ferreira HC et al. Lung mechanics and histology during sevoflurane anesthesia in a model of chronic allergic asthma. Anesth Analg 2007;104:631–637. [DOI] [PubMed] [Google Scholar]
- 17. Bates JH, Ludwig MS, Sly PD et al. Interrupter resistance elucidated by alveolar pressure measurement in open‐chest normal dogs. J Appl Physiol (1985) 1988;65:408–414. [DOI] [PubMed] [Google Scholar]
- 18. ER W. Morphometry : Stereological theory and practical methods In: Gil J, ed. Models of Lung Disease‐Microscopy and Structural Methods. New York: Marcel Dekker, 1990:199–247. [Google Scholar]
- 19. Hsia CC, Hyde DM, Ochs M et al. An official research policy statement of the American Thoracic Society/European Respiratory Society: Standards for quantitative assessment of lung structure. Am J Respir Crit Care Med 2010;181:394–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Antunes MA, Abreu SC, Damaceno‐Rodrigues NR et al. Different strains of mice present distinct lung tissue mechanics and extracellular matrix composition in a model of chronic allergic asthma. Respir Physiol Neurobiol 2009;165:202–207. [DOI] [PubMed] [Google Scholar]
- 21. da Silva AL, Martini SV, Abreu SC et al. DNA nanoparticle‐mediated thymulin gene therapy prevents airway remodeling in experimental allergic asthma. J Control Release 2014;180:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Antunes MA, Abreu SC, Silva AL et al. Sex‐specific lung remodeling and inflammation changes in experimental allergic asthma. J Appl Physiol (1985) 2010;109:855–863. [DOI] [PubMed] [Google Scholar]
- 23. Abreu SC, Antunes MA, Mendonca L et al. Effects of bone marrow mononuclear cells from healthy or ovalbumin‐induced lung inflammation donors on recipient allergic asthma mice. Stem Cell Res Ther 2014;5:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bustos ML, Huleihel L, Kapetanaki MG et al. Aging mesenchymal stem cells fail to protect because of impaired migration and antiinflammatory response. Am J Respir Crit Care Med 2014;189:787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nemeth K, Keane‐Myers A, Brown JM et al. Bone marrow stromal cells use TGF‐beta to suppress allergic responses in a mouse model of ragweed‐induced asthma. Proc Natl Acad Sci USA 2010;107:5652–5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Elahi KC, Klein G, Avci‐Adali M et al. Human mesenchymal stromal cells from different sources diverge in their expression of cell surface proteins and display distinct differentiation patterns. Stem Cells Int 2016;2016:5646384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Braza F, Dirou S, Forest V et al. Mesenchymal stem cells induce suppressive macrophages through phagocytosis in a mouse model of asthma. Stem Cells 2016;34:1836–1845. [DOI] [PubMed] [Google Scholar]
- 28. Bagheri M, Dong Y, Ono M. Molecular diversity of macrophages in allergic reaction: Comparison between the allergenic modes; Th1‐ and ‐Th2‐derived immune conditions. Iran J Allergy Asthma Immunol 2015;14:261–272. [PubMed] [Google Scholar]
- 29. Foronjy RF, Majka SM. The potential for resident lung mesenchymal stem cells to promote functional tissue regeneration: Understanding microenvironmental cues. Cells 2012;1:874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Firinci F, Karaman M, Baran Y et al. Mesenchymal stem cells ameliorate the histopathological changes in a murine model of chronic asthma. Int Immunopharmacol 2011;11:1120–1126. [DOI] [PubMed] [Google Scholar]
- 31. Goodwin M, Sueblinvong V, Eisenhauer P et al. Bone marrow‐derived mesenchymal stromal cells inhibit Th2‐mediated allergic airways inflammation in mice. Stem Cells 2011;29:1137–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marquez‐Curtis LA, Janowska‐Wieczorek A, McGann LE et al. Mesenchymal stromal cells derived from various tissues: Biological, clinical and cryopreservation aspects. Cryobiology 2015;71:181–197. [DOI] [PubMed] [Google Scholar]
- 33. Hoffman AM, Paxson JA, Mazan MR et al. Lung‐derived mesenchymal stromal cell post‐transplantation survival, persistence, paracrine expression, and repair of elastase‐injured lung. Stem Cells Dev 2011;20:1779–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marinas‐Pardo L, Mirones I, Amor‐Carro O et al. Mesenchymal stem cells regulate airway contractile tissue remodeling in murine experimental asthma. Allergy 2014;69:730–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martinez‐Gonzalez I, Cruz MJ, Moreno R et al. Human mesenchymal stem cells resolve airway inflammation, hyperreactivity, and histopathology in a mouse model of occupational asthma. Stem Cells Dev 2014;23:2352–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Urbanek K, De Angelis A, Spaziano G et al. Intratracheal administration of mesenchymal stem cells modulates tachykinin system, suppresses airway remodeling and reduces airway hyperresponsiveness in an animal model. PLoS One 2016;11:e0158746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brown JM, Nemeth K, Kushnir‐Sukhov NM et al. Bone marrow stromal cells inhibit mast cell function via a COX2‐dependent mechanism. Clin Exp Allergy 2011;41:526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bonfield TL, Koloze M, Lennon DP et al. Human mesenchymal stem cells suppress chronic airway inflammation in the murine ovalbumin asthma model. Am J Physiol Lung Cell Mol Physiol 2010;299:L760–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


