Abstract
The popularity of adipose‐derived cell therapy has increased over the last decade, and the number of studies published annually is growing. However, concerns regarding safety in the setting of previous malignancy or the use of allogeneic cells have been raised. We therefore aimed to systematically review all clinical studies using adipose‐derived cell therapy to identify reported adverse events with a special focus on risk of thromboembolic, immunological, and oncological safety concerns. Our systematic search resulted in 70 included studies involving more than 1,400 patients that were treated with adipose‐derived cell therapy. Safety assessment method was not described in 32 of the included studies. For studies involving systemic or cardiac administration, one case of pulmonary thromboembolism and cases of both myocardial and cerebral infarctions were described. In the setting of allogeneic cell therapy studies, where the production of specific antibodies toward donor cells was examined, it was noted that 19%–34% of patients develop antibodies, but the consequence of this is unknown. With regard to oncological safety, only one case of breast cancer recurrence was identified out of 121 patients. Adipose‐derived cell therapy has so far shown a favorable safety profile, but safety assessment description has, in general, been of poor quality, and only adverse events that are looked for will be found. We encourage future studies to maintain a strong focus on the safety profile of cell therapy, so its safeness can be confirmed. stem cells translational medicine 2017;6:1786–1794
Keywords: Adipose‐derived stromal cells, Stromal vascular fraction, Safety, Adverse events, Complications
Significance Statement.
This study reviewed the safety of adipose‐derived cell therapy. Thromboembolic complications were noted following systemic administration of cells. The treatment has so far shown to be safe in the setting of previous cancer. Donor‐specific antibodies are produced when using allogeneic cells, documenting that these cells are not immune privileged. The consequences of this needs further research. Future research should focus on higher quality of reporting of adverse events, as the present literature is of low quality.
Introduction
The field of regenerative medicine has been rapidly expanding over the last decade and especially cells derived from adipose tissue have received a lot of attention due to the ease of harvest and obtainable number of cells 1, 2. The cells from adipose tissue can be used for therapeutic purposes, either freshly isolated as the stromal vascular fraction (SVF, also called adipose‐derived regenerative cells [ADRC]), or culture‐expanded as the adipose‐derived stem cells (ASC). Adipose‐derived cell therapy has shown potential in almost every preclinical animal model 3, 4, 5, 6, 7 and the time is ripe for clinical translation of this potential.
The first results published from a clinical trial using adipose‐derived cell therapy, published in 2005, were for the treatment of Crohn's fistula [8]. Since then, a steady increase of publications and in treated conditions has occurred. However, as of yet there is still no clear evidence for the implementation of adipose‐derived cell therapy in the daily clinical routine.
The mechanisms of action of adipose‐derived cell therapy have been hypothesized to be through different pathways, such as paracrine secretion of growth factors, cytokines and microRNA promoting angiogenesis and modulating the immune response, as well as the ability of cells to differentiate into a variety of different cell types. However, very rarely can a beneficial effect be expected without the risk of adverse events. Consequently, safety concerns have been raised regarding the use of systemically administered cell therapy due to risk of thromboembolic complications 9, 10, the use of allogeneic cells and possible rejection 11, and in the setting of previous cancer therapy 12, 13, 14.
The aim of this systematic review was therefore to collect and review all reported adverse events related to adipose‐derived cell therapy with a focus on thromboembolic, immunological, and oncological safety concerns.
Materials and Methods
This systematic review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) statement 15. A systematic search was performed on PubMed using the following search string: “([adipose stem cell] OR [adipose stromal cell] or [adipose regenerative cell] or [stromal vascular fraction] or [processed lipoaspirate]) AND (trial or trials or pilot or ‘feasibility study’ or ‘safety study’).” A similar search was performed on EMBASE. All search results were imported to Covidence for further evaluation 16. Study selection was performed by two independent assessors (NMT and & MGJ). First, all studies were screened based on title and abstract. Secondly, full text versions of included studies were read for further evaluation. A hand search was also performed by skimming the references of included studies.
Inclusion criteria were human studies using adipose‐derived cells for treatment of any given disease published no later than 31st of December 2016. Exclusion criteria were non‐English language, reviews, case reports, or case series with fewer than five patients and animal or in vitro studies.
Data retrieved from included studies were year of publication, country of origin, disease treated, study design (randomized controlled trial, nonrandomized study, or case series/pilot study), primary aim (safety or efficacy), cell type used (freshly isolated or culture‐expanded as well as autologous or allogeneic), cell dosage, cell characterization (cell count/viability, surface marker analysis, and fibroblastoid colony forming units assay [CFU‐F]), number of participants, safety reporting described clearly in the Methods section (yes/no), and the reported adverse events including all‐cause mortality. The primary aim was set to safety if this was explicitly stated or was mentioned with equal weight as efficacy. The safety reporting was set to “yes” if anything pertaining to the evaluation of adverse events was noted in the Methods section, and it was set to “no” if nothing at all was described.
All studies with a comparison group were also evaluated for risk of bias using the Cochrane Collaboration tool where safety was set as outcome measure 17. In brief, seven aspects are evaluated for risk of bias and given an evaluation of either low risk, unclear, or high risk of bias. The seven aspects are random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other areas of bias.
Results
Description of Included Studies
In total, 907 unique studies were identified using our search string. In addition, 10 studies were found through hand search. After screening title and abstract, 97 studies remained. Full text evaluation excluded a further 27 studies leaving 70 studies to be included in the review with a total of 1,474 patients treated with adipose‐derived cell therapy (Fig. 1). Almost all organ system have been implicated in adipose‐derived cell therapy. The indication for treatment in the included studies was (in order count) soft tissue 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, gastrointestinal 8, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, musculoskeletal 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, rheumatological 59, 60, 61, 62, 63, ulcer/ischemic limb 64, 65, 66, 67, urogenital 68, 69, 70, 71, 72, cardiac 73, 74, 75, 76, neurological 77, 78, 79, 80, immunological 81, 82, 83, pulmonary 84, 85, and ophthalmological 86 (Fig. 2A). Studies were performed worldwide but Europe and Asia have so far published the majority of studies using adipose‐derived cell therapy (Fig. 2B).
Figure 1.
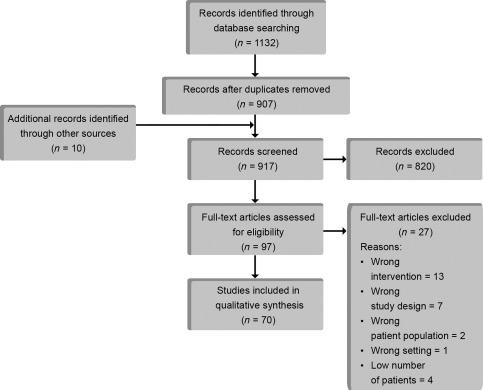
Flow chart of article selection process.
Figure 2.
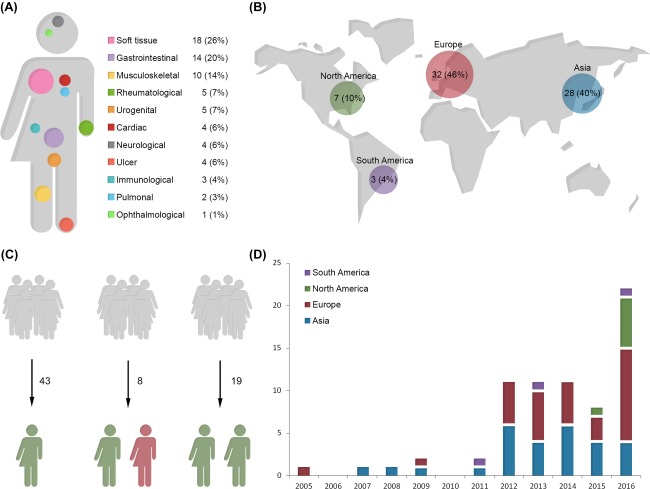
An overview of included studies. (A): Graphical overview of the range of indications adipose‐derived cell therapy has been used for. (B): Graphical overview of research activity bias on geographic location showing that the majority of included studies are from either Europe or Asia. (C): The majority of studies were of case series quality with no control group for comparison. Eight of studies included a nonrandom control group and 19 studies were randomized studies. (D): Since the first publication in 2005 there has been a steady rise in research output of clinical studies using adipose‐derived cell therapy.
The majority of studies 43 were small in scale and conducted as a series of cases without a comparison group. There were eight studies comparing adipose‐derived cell therapy with a nonrandom control group, and 19 studies were conducted as randomized controlled trials (Fig. 2C). The first clinical study using adipose‐derived cell therapy was published in 2005. Since then, a steady rise in publications has followed with an explosive growth annually since 2012 (Fig. 2D).
Overview
In the included studies ADRC treatment was given in 36 studies, whereas ASC was used in the 32 studies (two studies included both ADRC and ASC). The most frequent method to characterize cells was cell count with viability estimation. Cell dosage was not specified in fourteen studies in which the indication was soft tissue reconstruction 20, 22, 23, 25, 26, 28, 29, 31, 32, gastrointestinal 47, musculoskeletal 49, neurological 80, or urogenital disease 71, 72. In addition, 38 studies performed surface marker analysis of cells or at least had set release criteria for certain surface markers in studies using ASCs. CFU‐F was performed in only ten studies.
Almost any cell administration pathway was represented in the included studies. Remarkably, a total of 32 of 70 studies did not clearly describe any form of adverse event evaluation in the Methods section, including four studies in which safety was designated as the primary outcome 8, 50, 52, 73 (Supporting Information Table S1). The severity of adverse events depended on the underlying condition being treated, but no studies identified any adverse event as being related to the adipose‐derived cell therapy.
Thromboembolic Safety and Mortality
For studies administering cells systemically or intramyocardially, possible thromboembolic complications and all‐cause mortality were evaluated. Three RCT studies and one case series administered cells for cardiac indications with various methods.
Two double‐blinded RCT trials that were published together administered autologous ADRC intramyocardially with the difference between the two studies being cell dosage (40 or 80 × 106 cells vs. placebo) 76. Enrollment was terminated prematurely due to adverse events. In total, 31 patients were included and 17 received ADRC. Two patients treated with ADRC experienced cardiac death on day 2 and 291 after administration, and one experienced myocardial infarction at an unknown time point. In addition, a cerebrovascular event occurred in two patients in the ADRC group and one in the placebo group within 24 hours of injection. All neurological symptoms had complete or near‐complete recovery. The RCT study by Houtgraaf et al. administered ADRC intracoronarily for myocardial infarction with no mention of specific thromboembolic complications or deaths 74.
In an RCT study by Perin et al., who administered ADRC transendocardially, one out of 21 patients developed myocardial infarction immediately following injection and died 75. One patient out of six died in the control group. In a case series by Comella et al., which used a similar administration method for ADRC in 28 patients with chronic ischemic cardiomyopathy, three patients died after 1, 7, and 12 months 73.
Three RCT studies and two case series administered cells intravenously for various indications. One dose‐escalating RCT study by Álvaro‐Gracia et al., administered allogeneic ASC intravenously for the treatment of rheumatoid arthritis and observed a case of lacunar infarction in the low‐dose treatment group, eight days after cell administration 61. No further thromboembolic events occurred.
Vanikar et al. conducted a three‐armed randomized trial administering ASC together with hematopoietic stem cells versus hematopoietic stem cells alone versus no cell treatment to minimize rejection following renal transplantation 82. Herein the distribution of cardiovascular deaths was not significantly different across the groups of which 6/95 treated with ASCs died of either cardiovascular or cerebrovascular events compared with 9/190 in the two other groups combined. The all‐cause mortality in patients treated with ASCs was 7/95 compared with 20/190, which was not statistically significant. The remaining RCT and case series administering cells systemically or using near‐systemic administration did not describe cases of thromboembolic events 81, 83, 84. See Table 1 for overview of reported complications.
Table 1.
Overview of safety analysis regarding thromboembolic safety and mortality, immunological as well as oncological safety
| Thromboembolic safety and mortality | ||||||
|---|---|---|---|---|---|---|
| Author | Study type | Administration route | Cell type | TE complications | Mortality | Follow‐up |
| Cardiac | ||||||
| Comella et al. [73] | Case series | Transendocardial | ADRC | 1/28 | 3/28 | 6 |
| Henry et al. [76] | RCT | Intramyocardial | ADRC | 3/17 (1/14) | 2/17 (0/14) | 12 |
| Houtgraaf et al. [74] | RCT | Intracoronary | ADRC | – | – | 6 |
| Perin et al. [75] | RCT | Transendocardial | ADRC | 1/21 (1/6) | 3/21 (2/6) | 36 |
| Immunological | ||||||
| Vanikar et al. [82] | RCT | Intravenous | alloASC | 6/95 (9/190) | 7/95 (20/190) | 6 |
| Fang et al. [81] | Case series | Intravenous | alloASC | 0/6 | 2/6 | 40 |
| Fang et al. [83] | Case series | Intravenous | alloASC | 0/7 | 0/7 | 8 |
| Pulmonary | ||||||
| Zheng et al. [84] | RCT | Intravenous | alloASC | 0/6 (0/6) | 1/6 (2/6) | 1 |
| Rheumatological | ||||||
| Álvares Garcia et al. [61] | RCT | Intravenous | alloASC | 1/46 (0/7) | 0/46 (0/7) | 6 |
| Immunological safety | ||||||
|---|---|---|---|---|---|---|
| Author | Study type | Administration route | Cell type | Complications | Biochemical reaction | Follow‐up |
| Gastrointestinal | ||||||
| Park et al. [45] | Case series | Wall of fistula | alloASC | 0/6 | CD4/CD8: N.s.i. | 6 |
| Panés et al. [44] | RCT | Wall of fistula | alloASC | N.d. | ASC/HLA‐I: 34% | 6 |
| Garcia‐Arranz et al. [39] | Case series | Wall of fistula | alloASC | 0/10 | Cytokine/US: N.s.i. | 12 |
| De la Portilla et al. [38] | Case series | Wall of fistula | alloASC | Fever: 1/24 | – | 4 |
| Immunological | ||||||
| Fang et al. [83] | Case series | Intravenous | alloASC | 0/7 | – | 8 |
| Fang et al. [81] | Case series | Intravenous | alloASC | 0/6 | – | 40 |
| Vanikar et al. [82] | RCT | Intravenous | alloASC | N.d. | – | 6 |
| Muskuloskeletal | ||||||
| Lee et al. [55] | Case series | Intratendinous | alloASC | 0/12 | CD4/CD8: N.s.i. | 12 |
| Ophthalmological | ||||||
| Oner et al. [86] | Case series | Subretinal | alloASC | 0/11 | – | 6 |
| Pulmonary | ||||||
| Zheng et al. [84] | RCT | Intravenous | alloASC | 0/6 (0/6) | – | 1 |
| Rheumatological | ||||||
| Álvaro‐Gracia et al. [61] | RCT | Intravenous | alloASC |
Fever: 9/46 (0/7) Infections: 20/46 (0/7) Rash: 2/46 (0/7) |
ASC/HLA‐I: 19%. | 6 |
| Oncological Safety | ||||||
|---|---|---|---|---|---|---|
| Author | Study type | Administration route | Cell type | Local recurrence | Metastasis | Follow‐up |
| Soft tissue, breast | ||||||
| Aronowitz et al. [19] | Case series | Subcutaneous | ADRC | 1/54 | 0/54 | 12 |
| Pérez‐Cano et al. [27] | Case series | Subcutaneous | ADRC | 0/67 | 1/67 | 12 |
| Urogenital | ||||||
| Choi et al. [72] | Case series | Transurethral | ADRC | 0/6 | 0/6 | 3 |
| Gotoh et al. [68] | Case series | Periurethral | ADRC | 0/9 | 0/9 | 6 |
| Haahr et al. [70] | Case series | Corpus cavernosum | ADRC | 0/17 | 0/17 | 6 |
Abbreviations: –, not described/not performed; CD4/CD8, CD4 to CD8 ratio; N.d., no difference in adverse events between groups. no immunological adverse events; N.s.i., no sign of immune rejection; alloASC, allogeneic ASC; ASC/HLA‐I, ASC‐specific anti‐HLA‐I antibodies; Cell type, ADRC were autologous in all cases; cytokine/US, cytokine and unspecific antibodies. Data shown as treatment group count/total (control count/total); mortality, all‐cause mortality; TE, thromboembolic.
The evaluation of thromboembolic complications and mortality included two subgroups of studies that used either autologous ADRC for cardiac indications, administered within the heart, or allogeneic ASC, administered intravenously for a variety of indications. It can be difficult to assess the mortality rate in studies without a control group, especially considering that the patient categories included were of poor prognosis to begin with. For the studies including controls there was no indication of an increased mortality for patients receiving cell therapy.
Thromboembolic complications were few, and the comparison across studies was difficult due to heterogeneous cell administration, as three different administration routes were applied in the four cardiac studies. It must also be taken into account that the complications noted were not necessarily due to the cell treatment, as the time frame between cell treatment and complications was not always clearly described.
Immunological Safety
Possible immunologic complications were noted for studies administering allogeneic cells (Table 1). This included four RCTs and seven case series.
Two RCTs evaluated the production of donor‐specific antibodies as a measure of immune reaction. In the study of Panés et al., 34% of patients without prior IgG HLA class I antibodies generated anti‐HLA class I antibodies during the study period 44. There were, however, no noted immune reactions or adverse events associated with the donor‐specific antibodies, and the presence of the antibodies was not associated with the therapeutic response. In another study using similar allogeneic ASC for intravenous administration, it was noted that 19% of patients developed donor‐specific antibodies 61. The most frequent adverse event was transient fever following cell administration (9/46 treated patients).
The remaining two RCTs did not evaluate the immune response to allogeneic cells biochemically. Vanikar et al. conducted a study in which allogeneic ASC were coinfused with allogeneic hematopoietic stem cell transplantation; they found no evidence of graft versus host disease 82. Zheng et al. described no side effects during allogeneic ASC infusion in the six treated patients 84. Two adverse events were noted the first day (1 diarrhea and 1 skin rash) , both of which resolved by the next day.
Two case series evaluated the possibility of immune reaction by measuring the ratio of CD4/CD8. In the study by Park et al., it was shown that the ratio of CD4/CD8 did not change 45. In the study by Lee et al., they found no immunological rejection responses in any of the subjects, based on the ratio of CD4‐positive to CD8‐positive T cells 55.
The remaining case series did not use any biochemical assays to evaluate the possible immune reaction. Fang et al. was the first to use allogeneic ASC for the treatment of steroid resistant graft versus host disease, and they presented a case series of six patients 81. They noted no adverse events related to the ASC infusion. Fang et al. also conducted a study using allogeneic ASC obtained from haploidentical donors, and cells were infused intravenously for the treatment of chronic refractory immune thrombocytopenic purpura, also without any mention of adverse effects 83. De la Portilla described the use of an immunological assessment, but it was not mentioned in the Results section [38]. It was described that two patients were withdrawn from the study due to adverse events possibly due to treatment (pyrexia and perianal abscess); however, in the setting of treating perianal fistulas these events cannot necessarily be attributed to the allogeneic cells used. Oner et al. presented their results of a phase‐l trial administering subretinal allogeneic ASC for treatment of advanced stage retinitis pigmentosa 86. They included 11 patients and did not describe any form of immunological reaction.
In the present studies, only ASCs were administered in allogeneic fashion. Several different biochemical tests were applied in the studies of which the CD4/CD8, cytokine levels and unspecific IgM and IgG did not reveal any sign of activated immune response toward the foreign cells. Only the two studies testing for donor‐specific antibodies revealed that 19%–34% of patients developed these which suggest that the cells are not as immune privileged as once thought. The consequences of these reactions are unknown.
Oncological Safety
The oncological safety was evaluated for studies administering cells in the setting of previous malignancy (Table 1). This included five studies (all case series) with a follow‐up in the range of 3–12 months. Perez‐Cano et al. published a study including 67 patients where ADRC were injected into patients with previous breast cancer, where treatment was given as a cell‐assisted lipotransfer 27. Herein no cases of local recurrence were described, but one case of pelvic metastasis was observed without exact note of timing. Four other serious adverse events were noted; however, only one of these was described, which was donor site bleeding following liposuction. In another study, Aronowitz et al. found that of the 54 patients with previous breast cancer, one patient developed a recurrence after cell‐assisted lipotransfer with ADRC 11 months after treatment 19.
A study by Gotoh et al. gave a similar treatment with ADRC as a cell‐assisted lipotransfer for urinary incontinence, in which 9/11 patients were previously treated for prostate cancer 87. Here they found no evidence of recurrence during the 1‐year follow‐up. In a similar population group Haahr et al. injected ADRC intracavernosal to patients with erectile dysfunction due to previous prostate cancer surgery 70. The authors did not describe any case of recurrence in their 6‐month follow‐up study. Similarly, to treat urinary incontinence following prostatectomy, Choi et al. administered ADRC as a cell‐assisted lipotransfer to six patients 72. They described no cases of recurrence or any other side effects in their 3‐month follow‐up study.
All studies included in the evaluation of oncological safety used autologous ADRC for treatment. There was only one case of local breast cancer recurrence out of a total of 121 patients across two studies within the 12‐month follow‐up periods. The remaining three studies applied ADRC in 32 previous prostate cancer patients and observed no recurrences within the follow‐up period, ranging from 3 to 6 months. For determining long‐term oncological safety, follow‐up periods of several years are necessary to ensure that cell therapy is safe.
Bias
All studies with a comparison group were included in the analysis for bias looking specifically at the safety outcome (Table 2); 27 studies were eligible for inclusion. Only seven studies had proper randomization and allocation concealment. For the outcome safety, only five studies described proper blinding of patient, personnel and outcome assessor. Three of these studies were with ADRC and described proper blinding with the introduction of liposuction and subsequent sham ADRC injection 74, 75, 76.
Table 2.
Risk of bias analysis
| Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias | |
|---|---|---|---|---|---|---|---|
| Alvaro‐Garcia et al. 2016 [61] | |||||||
| Castillo‐Cardiel et al. 2015 [49] | |||||||
| Chang et al. 2013 [20] | |||||||
| Garcia‐Olmo et al. 2009 [41] | |||||||
| Gentile et al. 2012 [23] | |||||||
| Gentile et al. 2014 [22] | |||||||
| Guadalajara et al. 2012 [42] | |||||||
| Han et al. 2009 [66] | |||||||
| Henry et al. 2016 [76] | |||||||
| Herreros et al. 2012 [43] | |||||||
| Houtgraaf et al. 2012 [74] | |||||||
| Koh et al. 2012 [54] | |||||||
| Koh et al. 2012 [34] | |||||||
| Koh et al. 2014 [51] | |||||||
| Koh et al. 2016 [52] | |||||||
| Kølle et al. 2013 [24] | |||||||
| Li et al. 2013 [25] | |||||||
| Marino et al. 2013 [67] | |||||||
| Onesti et al. 2016 [60] | |||||||
| Panés et al. 2016 [44] | |||||||
| Peltoniemi et al. 2013[28] | |||||||
| Perin et al. 2014 [75] | |||||||
| Sterodimas et al. 2011 [29] | |||||||
| Tanikawa et al. 2013 [30] | |||||||
| Tissiani et al. 2016 [61] | |||||||
| Vanikar et al. 2014 [82] | |||||||
| Zheng et al. 2014 [84] | |||||||
| Low risk | Unclear | High risk | |||||
| Color codes | |||||||
All studies with a control group were included for the analysis. Green: Low risk of bias, Yellow: Unclear risk of bias, Red: High risk of bias.
Discussion
The clinical translation process of adipose‐derived cell products has begun, and it is crucial that implementation of cell therapy is based on the standard principles of evidence‐based medicine. Presently, cell therapy is widely considered as being equivalent to pharmaceutical drugs, which implies that cell therapies should adhere to the same standards for implementation as any newly developed drug, including the assessment of safety and adverse events.
This review includes more than 1,400 patients treated with adipose‐derived cell therapy with follow‐up ranging from less than a month to 3 years 75, 84. Very few adverse events have been reported that can be related directly to the cell therapy, Events were rather related to the harvesting of adipose tissue, trauma associated with injection, or the nature of the underlying condition being treated. Of all studies administering ASCs systemically, a case of pulmonary thromboembolism 73, as well as cases of myocardial and cerebral infarctions were described 75, 76. These are serious adverse events that can be fatal and since there is no clear clinical evidence for the efficacy of adipose‐derived cell therapy as of yet, future studies administering cells systemically should be cautious and monitor for these possible serious adverse events. The studies did not describe whether the cells were filtered before administration to ensure that the injected cells were single cell suspensions. Thromboembolic complication risk can be assumed to be higher when injecting clumped cells compared with single cell suspensions. In addition, the underlying condition must also be taken into account as the included patients had a poor prognosis.
Several studies used allogeneic ASC treatment and there was no clear evidence of a clinical immune response. However, for studies examining the presence and later production of donor‐specific antibodies, 19%–34% of patients developed these antibodies suggesting that indeed there is a cellular response occurring toward the allogeneic cells 44, 61. The consequence of this, if any, still remains unknown. In many instances it can be questioned whether the use of allogeneic ASCs has any value over autologous cells, as many of the treated conditions are not acute and life threatening, leaving room and time for the easy, simple isolation and culture‐expansion of ASCs. The use of ADRCs has the advantages of being completely autologous and requires much less advanced facilities as treatment can be offered as a same day procedure with everything needed being available in the operating theatre 88. On the other hand, the advantage of ASCs is the fact that an almost unlimited number of cells can be obtained and is also a more realistic option if one was to consider cell banking either as autologous or allogeneic treatment modalities, and already some studies have been published with funding from companies seeking to offer off‐the‐shelf allogeneic ASC therapy 44, 61.
Another concern is the use of cell therapy in areas with previous malignancy, as preclinical data have suggested that cell therapy can aggravate any remaining cancer cells 12, 13, 14. However, this has so far not been shown in the clinical setting, as we only could identify one case of recurrence following cell‐assisted lipotransfer among 121 breast cancer patients, which is well within what could be expected 89. It is vital in the setting of previous cancer treatment that safety evaluations are conducted thoroughly with sufficiently long follow‐up times, so these initial uplifting results can be confirmed.
During the last 5 years, there has been a marked increase in the number of adipose‐derived cell therapy clinical trials that have been published; however, at present most of them are at the case series level (Level IV evidence). In general, it is recognized that low quality studies increase risk of bias, which leads to an increasing chance of findings that do not represent reality 90. Therefore, it is important to transition toward well‐conducted randomized controlled trials with adequate blinding, which also includes the safety assessment.
A systematic review can only be as good as the available literature allows it to be, and this review was limited by the fact that so many studies did not clearly describe their method of assessing safety, and in the end, you will only find the adverse events that you are looking for. Another limitation of the review is the possibility of small overlap in some of the included studies, as case series were published over time from the same research groups with an increasing number of patients; this was deemed to be of such a small magnitude that it was insignificant.
While adipose‐derived cell therapy has shown great potential so far, there is very sparse clinical evidence to promote routine clinical implementation. There is a need for higher quality studies before rational conclusions can be made regarding the efficacy of adipose‐derived cell therapies. Future studies should place a larger emphasis on including a placebo/sham treatment for proper blinding of both patients and assessors. This is especially crucial when the primary outcome is subjective due to the placebo response 91.
Author Contributions
N.T., M.J., S.T., and J.S.: conception and design; N.T., S.P., and J.S.: financial support; S.P. and J.S.: administrative support; N.T., M.J., and S.T.: provision of study material or patients; N.T., M.J., S.T., and C.J.: collection and/or assembly of data, data analysis and interpretation; N.T., M.J., and S.T.: manuscript writing; N.T., M.J., S.T., C.J., and S.P.: final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Supporting information
Supporting Information Table 1.
Authored by a member of IFATS.
References
- 1. Stoltz J‐F, de Isla N, Li YP et al. Stem cells and regenerative medicine: Myth or reality of the 21th century. Stem Cells Int 2015;2015:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fraser JK, Wulur I, Alfonso Z et al. Fat tissue: An underappreciated source of stem cells for biotechnology. Trends Biotechnol 2006;24:150–154. [DOI] [PubMed] [Google Scholar]
- 3. Lin C‐S, Xin Z‐C, Wang Z et al. Stem cell therapy for erectile dysfunction: A critical review. Stem Cells Dev 2012;21:343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van der Spoel TIG, Jansen of Lorkeers SJ, Agostoni P et al. Human relevance of pre‐clinical studies in stem cell therapy: Systematic review and meta‐analysis of large animal models of ischaemic heart disease. Cardiovasc Res 2011;91:649–658. [DOI] [PubMed] [Google Scholar]
- 5. Toyserkani NM, Quaade ML, Sørensen JA. Cell‐assisted lipotransfer: A systematic review of its efficacy. Aesthetic Plast Surg 2016;40:309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Toyserkani NM, Christensen ML, Sheikh SP et al. Stem cells show promising results for lymphoedema treatment:‐A literature review. J Plast Surg Hand Surg 2015;75:117–123. [DOI] [PubMed] [Google Scholar]
- 7. Toyserkani NM, Christensen ML, Sheikh SP et al. Adipose‐derived stem cells: New treatment for wound healing? Ann Plast Surg 2014;49:65–71. [DOI] [PubMed] [Google Scholar]
- 8. García‐Olmo D, García‐Arranz M, Herreros D et al. A phase I clinical trial of the treatment of Crohn's fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum 2005;48:1416–1423. [DOI] [PubMed] [Google Scholar]
- 9. Furlani D, Ugurlucan M, Ong L et al. Is the intravascular administration of mesenchymal stem cells safe? Mesenchymal stem cells and intravital microscopy. Microvasc Res 2009;77:370–376. [DOI] [PubMed] [Google Scholar]
- 10. Tatsumi K, Ohashi K, Matsubara Y et al. Tissue factor triggers procoagulation in transplanted mesenchymal stem cells leading to thromboembolism. Biochem Biophys Res Commun 2013;431:203–209. [DOI] [PubMed] [Google Scholar]
- 11. Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat Biotechnol 2014;32:252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zimmerlin L, Donnenberg AD, Rubin JP et al. Regenerative therapy and cancer: In vitro and in vivo studies of the interaction between adipose‐derived stem cells and breast cancer cells from clinical isolates. Tissue Eng Part A 2011;17:93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martin‐Padura I, Gregato G, Marighetti P et al. The white adipose tissue used in lipotransfer procedures is a rich reservoir of CD34 + progenitors able to promote cancer progression. Cancer Res 2012;72:325–334. [DOI] [PubMed] [Google Scholar]
- 14. Rowan BG, Gimble JM, Sheng M et al. Human adipose tissue‐derived stromal/stem cells promote migration and early metastasis of triple negative breast cancer xenografts. PLoS One 2014;9:e89595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liberati A, Altman DG, Tetzlaff J et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Covidence systematic review software. Veritas Health Innovation. Melbourne, Australia. Available at www.covidence.org. Accessed January 22, 2017.
- 17. Higgins JPT, Altman DG. Assessing risk of bias in included studies. In Cochrane Handbook of Systematic Reviews of Interventions. In: Julian PTH, Sally G, eds. Cochrane B. Series, The Cochrane Collaboration, 2008:187–241. [Google Scholar]
- 18. Amirkhani MA, Shoae‐Hassani A, Soleimani M et al. Rejuvenation of facial skin and improvement in the dermal architecture by transplantation of autologous stromal vascular fraction: A clinical study. Bioimpacts 2016;6:149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aronowitz JA, Lockhart RA, Hakakian CS et al. Clinical safety of stromal vascular fraction separation at the point of care. Ann Plast Surg 2015;75:666–671. [DOI] [PubMed] [Google Scholar]
- 20. Chang Q, Li J, Dong Z, Liu L et al. Quantitative volumetric analysis of progressive hemifacial atrophy corrected using stromal vascular fraction‐supplemented autologous fat grafts. Dermatol Surg 2013;39:1465–1473. [DOI] [PubMed] [Google Scholar]
- 21. Doi K, Tanaka S, Iida H et al. Stromal vascular fraction isolated from lipo‐aspirates using an automated processing system: Bench and bed analysis. J Tissue Eng Regen Med 2013;7:864–870. [DOI] [PubMed] [Google Scholar]
- 22. Gentile P, De Angelis B, Pasin M et al. Adipose‐derived stromal vascular fraction cells and platelet‐rich plasma: Basic and clinical evaluation for cell‐based therapies in patients with scars on the face. J Craniofac Surg 2014;25:267–272. [DOI] [PubMed] [Google Scholar]
- 23. Gentile P, Orlandi A, Scioli MG et al. A comparative translational study: The combined use of enhanced stromal vascular fraction and platelet‐rich plasma improves fat grafting maintenance in breast reconstruction. Stem Cells Transl Med 2012;1:341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kølle S‐FT, Fischer‐Nielsen A, Mathiasen AB et al. Enrichment of autologous fat grafts with ex‐vivo expanded adipose tissue‐derived stem cells for graft survival: A randomised placebo‐controlled trial. Lancet 2013;382:1113–1120. [DOI] [PubMed] [Google Scholar]
- 25. Li J, Gao J, Cha P et al. Supplementing fat grafts with adipose stromal cells for cosmetic facial contouring. Dermatol Surg 2013;39:449–456. [DOI] [PubMed] [Google Scholar]
- 26. Lee SK, Kim D‐W, Dhong E‐S et al. Facial soft tissue augmentation using autologous fat mixed with stromal vascular fraction. Arch Plast Surg 2012;39:534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pérez‐Cano R, Vranckx JJ, Lasso JM et al. Prospective trial of adipose‐derived regenerative cell (ADRC)‐enriched fat grafting for partial mastectomy defects: The RESTORE‐2 trial. Eur J Surg Oncol 2012;38:382–389. [DOI] [PubMed] [Google Scholar]
- 28. Peltoniemi HH, Salmi A, Miettinen S et al. Stem cell enrichment does not warrant a higher graft survival in lipofilling of the breast: A prospective comparative study. J Plast Reconstr Aesthet Surg 2013;66:1494–1503. [DOI] [PubMed] [Google Scholar]
- 29. Sterodimas A, de Faria J, Nicaretta B et al. Autologous fat transplantation versus adipose‐derived stem cell‐enriched lipografts: A study. Aesthetic Surg J 2011;31:682–693. [DOI] [PubMed] [Google Scholar]
- 30. Tanikawa DYS, Aguena M, Bueno DF et al. Fat grafts supplemented with adipose‐derived stromal cells in the rehabilitation of patients with craniofacial microsomia. Plast Reconstr Surg 2013;132:141–152. [DOI] [PubMed] [Google Scholar]
- 31. Tissiani LAL, Alonso N. A prospective and controlled clinical trial on stromal vascular fraction enriched fat grafts in secondary breast reconstruction. Stem Cells Int 2016;2016:2636454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yoshimura K, Sato K, Aoi N et al. Cell‐assisted lipotransfer for cosmetic breast augmentation: Supportive use of adipose‐derived stem/stromal cells. Aesthetic Plast Surg 2008;32:48–55; discussion 56–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang L, Luo X, Lu Y et al. Is the resorption of grafted fat reduced in cell‐assisted lipotransfer for breast augmentation? Ann Plast Surg 2014;75:128–134. [DOI] [PubMed] [Google Scholar]
- 34. Koh KS, Oh TS, Kim H et al. Clinical application of human adipose tissue‐derived mesenchymal stem cells in progressive hemifacial atrophy (Parry‐Romberg disease) with microfat grafting techniques using 3‐dimensional computed tomography and 3‐dimensional camera. Ann Plast Surg 2012;69:331–337. [DOI] [PubMed] [Google Scholar]
- 35. Rigotti G, Charles‐de‐ Sá L, Gontijo‐de‐Amorim NF et al. Expanded stem cells, stromal‐vascular fraction, and platelet‐rich plasma enriched fat: Comparing results of different facial rejuvenation approaches in a clinical trial. Aesthetic Surg J 2016;36:261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cho YB, Park KJ, Yoon SN et al. Long‐term results of adipose‐derived stem cell therapy for the treatment of Crohn's fistula. Stem Cells Transl Med 2015;4:532–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cho YB, Lee WY, Park KJ et al. Autologous adipose tissue‐derived stem cells for the treatment of Crohn's fistula: a phase I clinical study. Cell Transplant 2013;22:279–285. [DOI] [PubMed] [Google Scholar]
- 38. de la Portilla F, Alba F, García‐Olmo D et al. Expanded allogeneic adipose‐derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn's disease: Results from a multicenter phase I/IIa clinical trial. Int J Colorectal Dis 2013;28:313–323. [DOI] [PubMed] [Google Scholar]
- 39. García‐Arranz M, Dolores Herreros M, González‐Gómez C et al. Treatment of Crohn's‐related rectovaginal fistula with allogeneic expanded‐adipose derived stem cells: A phase I‐IIa clinical trial. Stem Cells Transl Med 2016;5:1441–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Garcia‐Olmo D, Herreros D, Pascual I et al. Expanded adipose‐derived stem cells for the treatment of complex perianal fistula: A phase II clinical trial. Dis Colon Rectum 2009;52:79–86. [DOI] [PubMed] [Google Scholar]
- 41. Guadalajara H, Herreros D, De‐La‐Quintana P et al. Long‐term follow‐up of patients undergoing adipose‐derived adult stem cell administration to treat complex perianal fistulas. Int J Colorectal Dis 2012;27:595–600. [DOI] [PubMed] [Google Scholar]
- 42. Herreros MD, Garcia‐Arranz M, Guadalajara H et al. Autologous expanded adipose‐derived stem cells for the treatment of complex cryptoglandular perianal fistulas: A phase III randomized clinical trial (FATT 1: Fistula Advanced Therapy Trial 1) and long‐term evaluation. Dis Colon Rectum 2012;55:762–772. [DOI] [PubMed] [Google Scholar]
- 43. Mizushima T, Takahashi H, Takeyama H et al. A clinical trial of autologous adipose‐derived regenerative cell transplantation for a postoperative enterocutaneous fistula. Surg Today 2016;46:835–842. [DOI] [PubMed] [Google Scholar]
- 44. Panés J, García‐Olmo D, Van Assche G et al. Expanded allogeneic adipose‐derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn's disease: A phase 3 randomised, double‐blind controlled trial. Lancet 2016;388:1281–1290. [DOI] [PubMed] [Google Scholar]
- 45. Park KJ, Ryoo S‐B, Kim JS et al. Allogeneic adipose‐derived stem cells for the treatment of perianal fistula in Crohn's disease: A pilot clinical trial. Colorectal Dis 2016;18:468–476. [DOI] [PubMed] [Google Scholar]
- 46. Sanz‐Baro R, García‐Arranz M, Guadalajara H et al. First‐in‐human case study: Pregnancy in women with Crohn's perianal fistula treated with adipose‐derived stem cells: A safety study. Stem Cells Transl Med 2015;4:598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Garcia‐Olmo D, Guadalajara H, Rubio‐Perez I et al. Recurrent anal fistulae: Limited surgery supported by stem cells. World J Gastroenterol 2015;21:3330–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee WY, Park KJ, Cho YB et al. Autologous adipose tissue‐derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for Crohn's fistula. Stem Cells 2013;31:2575–2581. [DOI] [PubMed] [Google Scholar]
- 49. Castillo‐Cardiel G, López‐Echaury AC, Saucedo‐Ortiz JA et al. Bone regeneration in mandibular fractures after the application of autologous mesenchymal stem cells, a randomized clinical trial. Dent Traumatol 2016;33:38–44. [DOI] [PubMed] [Google Scholar]
- 50. Fodor PB, Paulseth SG. Adipose derived stromal cell (ADSC) injections for pain management of osteoarthritis in the human knee joint. Aesthetic Surg J 2016;36:229–236. [DOI] [PubMed] [Google Scholar]
- 51. Koh Y‐G, Kwon O‐R, Kim Y‐S et al. Comparative outcomes of open‐wedge high tibial osteotomy with platelet‐rich plasma alone or in combination with mesenchymal stem cell treatment: A prospective study. Arthroscopy 2014;30:1453–1460. [DOI] [PubMed] [Google Scholar]
- 52. Koh Y‐G, Kwon O‐R, Kim Y‐S et al. Adipose‐derived mesenchymal stem cells with microfracture versus microfracture alone: 2‐year follow‐up of a prospective randomized trial. Arthroscopy 2016;32:97–109. [DOI] [PubMed] [Google Scholar]
- 53. Jo CH, Lee YG, Shin WH et al. Intra‐articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof‐of‐concept clinical trial. Stem Cells 2014;32:1254–1266. [DOI] [PubMed] [Google Scholar]
- 54. Koh Y‐G, Choi Y‐J. Infrapatellar fat pad‐derived mesenchymal stem cell therapy for knee osteoarthritis. Knee 2012;19:902–907. [DOI] [PubMed] [Google Scholar]
- 55. Lee SY, Kim W, Lim C, Chung SG. Treatment of lateral epicondylosis by using allogeneic adipose‐derived mesenchymal stem cells: A pilot study. Stem Cells 2015;33:2995–3005. [DOI] [PubMed] [Google Scholar]
- 56. Pers Y‐M, Rackwitz L, Ferreira R et al. Adipose mesenchymal stromal cell‐based therapy for severe osteoarthritis of the knee: A phase i dose‐escalation trial. Stem Cells Transl Med 2016;5:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sándor GK, Numminen J, Wolff J et al. Adipose stem cells used to reconstruct 13 cases with cranio‐maxillofacial hard‐tissue defects. Stem Cells Transl Med 2014;3:530–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Saxer F, Scherberich A, Todorov A et al. Implantation of stromal vascular fraction progenitors at bone fracture sites: From a rat model to a first‐in‐man study. Stem Cells 2016;34:2956–2966. [DOI] [PubMed] [Google Scholar]
- 59. Scuderi N, Ceccarelli S, Onesti MG et al. Human adipose‐derived stromal cells for cell‐based therapies in the treatment of systemic sclerosis. Cell Transplant 2013;22:779–795. [DOI] [PubMed] [Google Scholar]
- 60. Onesti MG, Fioramonti P, Carella S et al. Improvement of mouth functional disability in systemic sclerosis patients over one year in a trial of fat transplantation versus adipose‐derived stromal cells. Stem Cells Int 2016;2016:2416192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Álvaro‐Gracia JM, Jover JA, García‐ Vicuña R et al. Intravenous administration of expanded allogeneic adipose‐derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): Results of a multicentre, dose escalation, randomised, single‐blind, placebo‐controlled phase Ib/IIa clinical trial. Ann Rheum Dis 2017;76:196–202. [DOI] [PubMed] [Google Scholar]
- 62. Granel B, Daumas A, Jouve E et al. Safety, tolerability and potential efficacy of injection of autologous adipose‐derived stromal vascular fraction in the fingers of patients with systemic sclerosis: An open‐label phase I trial. Ann Rheum Dis 2015;74:2175–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Guillaume‐Jugnot P, Daumas A, Magalon J et al. Autologous adipose‐derived stromal vascular fraction in patients with systemic sclerosis: 12‐month follow‐up. Rheumatology 2016;55:301–306. [DOI] [PubMed] [Google Scholar]
- 64. Bura A, Planat‐Benard V, Bourin P et al. Phase I trial: The use of autologous cultured adipose‐derived stroma/stem cells to treat patients with non‐revascularizable critical limb ischemia. Cytotherapy 2014;16:245–257. [DOI] [PubMed] [Google Scholar]
- 65. Lee HC, An SG, Lee HW et al. Safety and effect of adipose tissue‐derived stem cell implantation in patients with critical limb ischemia: A pilot study. Circ J 2012;76:1750–1760. [DOI] [PubMed] [Google Scholar]
- 66. Han S‐K, Kim H‐R, Kim W‐K. The treatment of diabetic foot ulcers with uncultured, processed lipoaspirate cells: A pilot study. Wound Repair Regen 2010;18:342–348. [DOI] [PubMed] [Google Scholar]
- 67. Marino G, Moraci M, Armenia E et al. Therapy with autologous adipose‐derived regenerative cells for the care of chronic ulcer of lower limbs in patients with peripheral arterial disease. J Surg Res 2013;185:36–44. [DOI] [PubMed] [Google Scholar]
- 68. Gotoh M, Yamamoto T, Kato M et al. Regenerative treatment of male stress urinary incontinence by periurethral injection of autologous adipose‐derived regenerative cells: 1‐year outcomes in 11 patients. Int J Urol 2014;21:294–300. [DOI] [PubMed] [Google Scholar]
- 69. Kuismanen K, Sartoneva R, Haimi S et al. Autologous adipose stem cells in treatment of female stress urinary incontinence: Results of a pilot study. Stem Cells Transl Med 2014;3:936–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Haahr MK, Jensen CH, Toyserkani NM et al. Safety and potential effect of a single intracavernous injection of autologous adipose‐derived regenerative cells in patients with erectile dysfunction following radical prostatectomy: An open‐label phase I clinical trial. EBio Med 2016;5:204–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lander EB, Berman MH, See JR. Stromal vascular fraction combined with shock wave for the treatment of Peyronie's disease. Plast Reconstr Surgery 2016;4:e631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Choi JY, Kim T‐H, Yang JD et al. Adipose‐derived regenerative cell injection therapy for postprostatectomy incontinence: A phase i clinical study. Yonsei Med J 2016;57:1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Comella K, Parcero J, Bansal H et al. Effects of the intramyocardial implantation of stromal vascular fraction in patients with chronic ischemic cardiomyopathy. J Transl Med 2016;14:158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Houtgraaf JH, den Dekker WK, van Dalen BM et al. First experience in humans using adipose tissue‐derived regenerative cells in the treatment of patients with ST‐segment elevation myocardial infarction. J Am Coll Cardiol 2012;59:539–540. [DOI] [PubMed] [Google Scholar]
- 75. Perin EC, Sanz‐Ruiz R, Sánchez PL et al. Adipose‐derived regenerative cells in patients with ischemic cardiomyopathy: The PRECISE Trial. Am Heart J 2014;168:88–95.e2. [DOI] [PubMed] [Google Scholar]
- 76. Henry TD, Pepine CJ, Lambert CR et al. The Athena trials: Autologous adipose‐derived regenerative cells for refractory chronic myocardial ischemia with left ventricular dysfunction. Catheter Cardiovasc Interv 2016, [DOI] [PubMed] [Google Scholar]
- 77. Ra JC, Shin IS, Kim SH et al. Safety of intravenous infusion of human adipose tissue‐derived mesenchymal stem cells in animals and humans. Stem Cells Dev 2011;20:1297–1308. [DOI] [PubMed] [Google Scholar]
- 78. Staff NP, Madigan NN, Morris J et al. Safety of intrathecal autologous adipose‐derived mesenchymal stromal cells in patients with ALS. Neurology 2016;87:2230–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hur JW, Cho T‐H, Park D‐H et al. Intrathecal transplantation of autologous adipose‐derived mesenchymal stem cells for treating spinal cord injury: A human trial. J Spinal Cord Med 2016;39:655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Calcagni M, Zimmermann S, Scaglioni MF et al. The novel treatment of SVF‐enriched fat grafting for painful end‐neuromas of superficial radial nerve. Microsurgery 2016. [DOI] [PubMed] [Google Scholar]
- 81. Fang B, Song Y, Liao L et al. Favorable response to human adipose tissue‐derived mesenchymal stem cells in steroid‐refractory acute graft‐versus‐host disease. Transplant Proc 2007;39:3358–3362. [DOI] [PubMed] [Google Scholar]
- 82. Vanikar AV, Trivedi HL, Kumar A et al. Co‐infusion of donor adipose tissue‐derived mesenchymal and hematopoietic stem cells helps safe minimization of immunosuppression in renal transplantation ‐ single center experience. Ren Fail 2014;36:1376–1384. [DOI] [PubMed] [Google Scholar]
- 83. Fang B, Mai L, Li N et al. Favorable response of chronic refractory immune thrombocytopenic purpura to mesenchymal stem cells. Stem Cells Dev 2012;21:497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zheng G, Huang L, Tong H et al. Treatment of acute respiratory distress syndrome with allogeneic adipose‐derived mesenchymal stem cells: A randomized, placebo‐controlled pilot study. Respir Res 2014;15:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tzouvelekis A, Paspaliaris V, Koliakos G et al. A prospective, non‐randomized, no placebo‐controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells‐stromal vascular fraction in idiopathic pulmonary fibrosis. J Transl Med 2013;11:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Oner A, Gonen ZB, Sinim N et al. Subretinal adipose tissue‐derived mesenchymal stem cell implantation in advanced stage retinitis pigmentosa: A phase I clinical safety study. Stem Cell Res Ther 2016;7:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gotoh M, Yamamoto T, Kato M et al. Regenerative treatment of male stress urinary incontinence by periurethral injection of autologous adipose‐derived regenerative cells: 1‐year outcomes in 11 patients. Int J Urol 2014;21:294–300. [DOI] [PubMed] [Google Scholar]
- 88. Gimble JM, Bunnell BA, Chiu ES et al. Concise review: Adipose‐derived stromal vascular fraction cells and stem cells: Let's not get lost in translation. Stem Cells 2011;29:749–754. [DOI] [PubMed] [Google Scholar]
- 89. Waked K, Colle J, Doornaert M et al. Systematic review: The oncological safety of adipose fat transfer after breast cancer surgery. Breast 2016;31:128–136. [DOI] [PubMed] [Google Scholar]
- 90. Ioannidis JPA. Why most published research findings are false. PLoS Med 2005;2:e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wartolowska KA, Feakins BG, Collins GS et al. The magnitude and temporal changes of response in the placebo arm of surgical randomized controlled trials: A systematic review and meta‐analysis. Trials 2016;17:589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table 1.


