Abstract
Mesenchymal stem cells (MSCs) have potent immunomodulatory functions and are a promising therapy for immune‐mediated inflammatory disorders. We previously demonstrated the efficacy of fresh, autologous, adipose‐derived MSCs (ASCs) to treat feline chronic gingivostomatitis (FCGS), a chronic oral mucosal inflammatory disease similar to human oral lichen planus. Here, we investigate the use of fresh allogeneic ASCs for treatment of FCGS in seven cats. Radiolabeled ASCs were also tracked systemically. Each cat received two intravenous injections of 20 million ASCs, 1 month apart. Oral inflammation, blood lymphocyte subsets, anti‐fetal bovine serum antibody levels, ASC crossmatching and serum proteins and cytokine concentrations were determined. Four of the 7 cats (57%) responded to treatment [complete clinical remission (n = 2) or substantial clinical improvement (n = 2)]. Three cats were nonresponders. Prior to therapy, most cats had increased circulating CD8+ T cells, decreased CD8lo cells, and a decreased CD4/CD8 ratio, however clinical resolution was not associated with normalization of these parameters. Nonresponders showed more severe systemic inflammation (neutrophilia, hyperglobulinemia and increased interferon gamma and tumor necrosis factor alpha concentration) prior to ASC therapy. Clinical remission took up to 20 months and no clinical relapse has occurred. A higher fraction of radiolabeled ASCs were identified in the oral cavity of FCGS affected cats than the control cat. The administration of fresh, allogenic ASCs appeared to have lower clinical efficacy with a delayed response as compared to the fresh, autologous ASCs. In addition, the mechanism(s) of action for autologous and allogenic ASCs may differ in this model of oral inflammation. Stem Cells Translational Medicine 2017;6:1710–1722
Keywords: Adipose‐derived stem cells, Fresh, Allogeneic, Cats, Gingivostomatitis, Oral Mucosa, Immunomodulation
Significance Statement.
This study is the first to demonstrate the safety and efficacy of fresh, allogeneic adipose derived stem cells systemic therapy for a naturally occurring, inflammatory disease in cats. We demonstrate that this therapy resulted in delayed clinical and histological resolution and immune modulation as compared to autologous therapy. We also demonstrated that the mechanism(s) of action for autologous and allogenic adipose‐derived MSCs (ASCs) may differ in this model of oral inflammation. Finally, we show that ASC in cats are initially engrafted to the lungs and that a higher fraction of cells were identified in the oral cavity of feline chronic gingivostomatitis affected cats than control.
Introduction
Stem cell‐based therapy and research have made exceptional progress in the last decade. Apart from their capacity to regenerate damaged tissues, mesenchymal stem cells (MSCs) possess unique immunomodulatory capabilities and have improved the outcome of clinical diseases with aberrant immune responses 1, 2, 3, 4. We recently published on the safety and efficacy of autologous adipose‐derived mesenchymal stem cells (ASCs) to treat naturally occurring feline chronic gingivostomatitis (FCGS). FCGS is a large animal model of immune‐mediated oral mucosal inflammatory diseases of humans including oral lichen planus (OLP), recurrent aphthous stomatitis, pemphigus, and pemphigoid 1. In both human and cats, these diseases result in painful mucosal lesions that markedly reduce quality of life and often require long‐term immunosuppressive therapy with significant associated risks and side effects. While the pathogenesis of these oral mucosal inflammatory diseases is complex and heterogeneous, the histological process consistently involves tissue infiltration primarily by activated, effector T and B cells, with a skew toward a Th1 phenotype 5, 6, 7.
Knowledge and clinical experience derived from veterinary medicine and specifically from naturally occurring diseases in client‐owned pets, such as FCGS, that mimic human diseases serve to inform the best practices for human clinical trials 8, 9. Naturally occurring diseases in dogs and cats that display the defining attributes of similar or identical diseases in humans hold promise for providing predictive and reliable proof of concept when evaluating new therapeutics 8. Importantly, naturally occurring diseases in companion animals reflect the complex genetic, environmental and physiologic variation present in outbred populations.
FCGS is a painful and debilitating severe oral mucosal inflammatory disease of cats that is estimated to affect 0.7%–12% of the general cat population 10, 11, 12, 13, 14, 15. Clinical signs are moderate to severe oral pain and discomfort, including inappetence, reduced grooming, weight loss and ptyalism 11, 12, 16. Approximately 70% of cats respond to the current standard of care for FCGS, which is full or near‐full mouth tooth extraction by either complete resolution (28.4%) or substantial improvement (39%) 11. The remaining ∼30% of cats do not respond to tooth extraction (refractory FGCS). These cats, like humans with oral inflammatory diseases including OLP, pemphigus, and pemphigoid, require life‐long therapy with antibiotics, corticosteroids, and pain medication As with the aforementioned human oral mucosal diseases, there is no cure and spontaneous disease resolution has not been reported in cats with FCGS 15. Due to the debilitating nature of FCGS, severely affected cats that do not respond to treatment are often euthanized. The etiology of FCGS remains elusive but is thought to be due to the host immune system responding inappropriately to chronic oral antigenic stimulation secondary to underlying oral disease or clinical/subclinical viral infections 16, 17, 18, 19.
MSCs are nonhematopoetic cells harvested from tissues such as adipose tissue, bone marrow, liver, placenta, and umbilical cord blood. These cells are plastic‐adherent, fibroblast‐like cells capable of high proliferation and tri‐lineage differentiation 20, 21. MSCs can inhibit T‐cell proliferation, alter B‐cell function, downregulate MHC II on antigen presenting cells and inhibit dendritic cell maturation and differentiation 22, 23, 24, 25. ASCs have been isolated and characterized in several species including humans 26, 27, 28, 29, 30, 31, 32.
Autologous ASCs have shown promise for cell‐based therapeutics in veterinary medicine 1. The benefits of autologous ASCs include that they are nonimmunogenic, safe in humans and animals, and have been used clinically for nearly a decade with no significant adverse reactions reported other than transient fever in humans, occasional transfusion reactions in cats and self‐resolving inflammatory flares in horses 31, 33, 34. The use of autologous ASCs have practical drawbacks including the time required for isolation and proliferation of sufficient cell number for treatment. In addition, a subset of cats are unable to receive autologous ASCs due to the deleterious effect of feline foamy virus on ASC expansion 26, 35. Allogeneic ASCs have the practical advantage of increased availability, higher quality control and standardization between treatments, decreased cost and reduced anesthetic events for the recipients 36. Systemic administration [i.e., intravenous (IV) injection] of allogeneic ASCs appears to be safe with no significant adverse events reported 34, 37. Currently, allogeneic ASCs are used in several human clinical trials 38, 39. However, unlike autologous ASCs, allogeneic ASCs may have low immunogenicity, and may provoke an innate immune response (instant blood‐mediated inflammatory reaction) and/or antibody development 40, 41, 42. Treatment of FCGS with autologous ASCs resulted in cure or substantial clinical improvement in 71% of cats, suggesting that FCGS may be a promising disease target for allogeneic ASC therapy.
The purpose of this study was to evaluate the clinical, histopathological, and immunologic effects resulting from systemic administration of fresh, allogeneic ASCs in a cohort of cats with refractory FCGS. We also investigated the immediate fate of systemically administered ASCs via scintigraphic tracking. We hypothesized that systemic allogeneic ASC therapy would be safe and result in systemic immunomodulation, reduction of oral inflammatory lesions, and improvement of clinical signs. We also hypothesized that radiolabeled ASCs would accumulate in the oral cavity of cats affected with gingivostomatitis. We found that ASCs administered systemically resulted in complete clinical remission or substantial clinical improvement in 57% of the cats (four out of seven). This improvement correlated with reduction of inflammatory lesions.
Materials and Methods
Study Population
This study was conducted with approval of the Institutional Animal Care and Use Committee, and the Clinical Trials Review Board, University of California—Davis (UCD). All owners signed an informed consent. Seven client‐owned cats with refractory FCGS were recruited to the study. Inclusion criteria included cats affected by FCGS with no other primary co‐morbidities. These cats did not respond to full‐mouth tooth extraction performed at least 6 months prior to enrollment. If corticosteroid or other immunosuppressive therapy had been prescribed, it was discontinued 2 weeks prior to and for the entire duration of the clinical trial. Full‐mouth dental radiographs were obtained to confirm the absence of retained root tips and to rule out any other underlying lesions. All cats were screened and tested negative for feline immune deficiency virus and feline leukemia virus infection.
Study Design
The study design is illustrated in Figure 1 (Fig. 1A, 1B). Cats that met the inclusion criteria had blood collected for a complete blood count, serum biochemistry profile, blood lymphocyte phenotyping, and serum cytokine analysis prior to treatment and at 3 and 6 months post treatment. In addition, the presence of anti‐bovine serum albumin (anti‐FBS) antibodies was evaluated prior to and at 6 months post ASC administration. Oral biopsies were collected prior to ASC administration (n = 7) and at 6 months post administration (n = 3). Clinical disease severity was evaluated using a stomatitis disease activity index (SDAI) scoring system 43. The SDAI scoring was performed at the time of study enrollment and at 6 months (Supporting Information Figure) 43. Briefly, the cats' owners completed a brief questionnaire and scored appetite, activity level, grooming behavior and perceived oral comfort on a scale of 0 to 3. Lesion severity was also scored by 2 veterinary dental specialists (BA, FV), experienced in FCGS evaluation, as 0 (no lesion), 1 (mild), 2 (moderate) or 3 (severe). The SDAI score for each cat was calculated at each time point (range = 0, no disease, to 20, severe disease). A final examination was performed at 6 months after the first ASC treatment. However, cats were followed up continually and data are presented from the final recheck as well. During the study period, the cats received only opioid analgesic management (i.e., buprenorphine or oxymorphone) without any immunosuppressive, antibiotic or nonsteroidal anti‐inflammatory medication.
Figure 1.
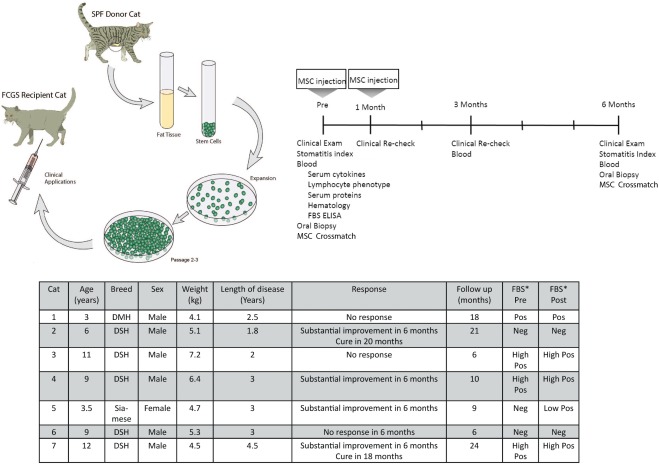
Study design layout and clinical outcome in cats receiving ASC therapy for FCGS. Images demonstrating the study design (A) and timeline (B) as well as the signalment and clinical data (C) for seven cats affected by FCGS enrolled in the clinical trial. Abbreviations: DMH, domestic medium hair; DSH, domestic short hair; FBS, fetal bovine serum; FCGS, feline chronic gingivostomatitis; SPF, specific pathogen free.
ASC Isolation and Expansion
Adipose tissue was obtained from three specific pathogen free (SPF) cats that were euthanized for reasons unrelated to this study. ASC isolation and expansion was performed at the Regenerative Medicine Laboratory at the William T Pritchard Veterinary Medical Teaching Hospital, UCD, exactly as previously described 1, 26. Only fresh, expanded, early passage (P2 or P3) cells were used for treatment.
ASC Phenotyping
Surface protein expression for ASC activation, identity, and purity was determined using flow cytometry as described previously 17. All antibodies were purchased from the Leukocyte Antigen Biology Laboratory, UCD, unless otherwise indicated. Antibodies included MHC II (42.3; activation), CD18 (FE3.9F2; purity), CD90 (CA1.4G8; identity), and CD105 (SN6; eBioscience; identity). For unconjugated antibodies, a mouse IgG‐phycoerythrin (PE) antibody (Jackson ImmunoResearch Labs, West Grove, PA) was used for secondary labeling. Canine CD8a (CA9.JD3), rat immunoglobulin G‐allophycocyanin (IgG‐APC) (eBR2a; eBioscience, San Diego, CA), and mouse IgG‐APC (MCA928; AbD Serotec, Raleigh, NC) were used as isotype controls. Samples were run on a flow cytometer (Cytomics FC500; Beckman Coulter, Brea, CA). Flow cytometry data were analyzed using FlowJo flow cytometry software (Tree Star, Inc.; Ashland, OR).
PBMC Proliferation Assay
To assess ASC potency, each ASC line was tested for its ability to inhibit lymphocyte proliferation using a mixed leukocyte reaction (MLR). Peripheral blood mononuclear cell (PBMC) isolation and MLRs were carried out exactly as described 1. In brief, PBMCs were activated with 5 microgram concanavalin A (Con‐A; Sigma‐Aldrich), stained with a viability dye (Fixable Viability Dye eFlour780; eBioscience, San Diego, CA), proliferation was measured via BrdU incorporation (BrdU Flow Kit; BD Biosciences; San Jose, CA), and analyzed on a flow cytometer (Cytomics FC500). Flow cytometry data were analyzed using FlowJo flow cytometry software (Tree Star, Inc.).
ASC Treatment
All cats received 2 IV transfusions of 20 × 106 (∼5 million ASCs/kg) fresh allogeneic ASCs as previously described for autologous MSC therapy 1. Cryopreserved ASCs were revived for 72 hours in culture prior to administration. Fresh ASCs were prepared and transferred to glass vials. ASCs were administered over a period of 20–30 minutes by dividing the total dose into four separate aliquots (∼5 million cells at a time) to prevent ASC adherence to syringe plastic. All cats were hospitalized for 24 hours to monitor for adverse reactions.
Histology and Immunohistochemistry
Oral biopsies were fixed en bloc in 10% neutral buffered formalin, embedded in paraffin, sectioned, mounted, and stained with H&E according to standard laboratory protocols. Immunohistochemistry was performed on 4‐µm thick, formalin‐fixed, paraffin‐embedded tissue sections, as previously described, with antigen retrieval performed in Dako Target Retrieval Solution (S1699) for 30 minutes at 95°C 1. Primary antibodies and dilutions were: rat anti‐CD3 (clone 3–12, diluted 1:10, Leukocyte Antigen Biology Lab, UCD); and rabbit anti‐CD20 (NeoMarker RB‐9013‐P1; 1:300; Thermo Fisher Scientific, Pittsburgh). Sections were then incubated with a Streptavidin‐HRP label (anti‐rabbit link, or anti‐rat link; Biocare Medical's 4+ Detection System GR608, or GR607 and HP604, respectively). Detection was visualized with Vector NovaRED for peroxidase (SK‐4800), per manufacturer's instructions. Sections were counterstained in Mayer's hematoxylin. Nonspecific background was evaluated with duplicate sections that received diluent in place of the primary antibody. Biopsies were interpreted by a board‐certified veterinary pathologist (NV).
Hematology and Serum Biochemical Profiles
Complete blood counts (Bayer ADVIA 120; Bayer Diagnostics, Tarrytown, NY, http://healthcare.bayer.com) and biochemical profiles (Cobas c501; Roche Diagnostics International, Risch, Switzerland, http://www.roche-diagnostics.ch) were run, as previously described, to monitor for any adverse responses to ASC therapy 1.
Cytokine ELISAs
Serum was isolated from whole blood and stored at −80°C until analyzed. Enzyme‐linked immunosorbent assays (ELISAs) for interferon gamma (IFN‐γ, Feline IFN‐gamma Duoset ELISA, R&D Systems, Minneapolis, MN), tumor necrosis factor alpha (TNF‐α, Feline TNF‐α DuoSet, R&D), and interleukin 6 (IL‐6, Feline IL‐6 Duoset, R&D) were run on stored serum (0, 3, and 6 months post ASC administration) per manufacturer's instructions 1. All ELISA samples were read on a Synergy HT Multi‐Mode microplate reader with Gen5 software (Biotek, Winooski, VT).
Anti‐Bovine Serum Albumin (BSA) ELISA
The anti‐BSA ELISA was performed as previously described 1. Briefly, 96‐well ELISA plates (Thermo Fisher Scientific) were coated with 1 µg BSA (Fraction V, Thermo Fisher Scientific) overnight at 4°C. Feline serum samples were diluted 1:5,000 along with negative and positive assay controls. Following sample incubation, diluted rabbit anti‐feline IgG H&L‐HRP (Southern Biotech, Birmingham, AL) was added to each well. TMB Peroxidase Substrate (KPL, Gaithersburg, MD) was added and samples were read on a microplate reader with Gen5 software (Biotek). Data is presented as a fold increase of color relative to the negative control for each patient.
Lymphocyte Phenotyping
100 μl aliquots of whole blood (EDTA) were labeled with 25 μl mouse antibodies directed against anti‐feline CD4 (clone FE1.7B12), CD8α (clone FE1.10E9), or CD21 (clone CA2.1D6) (Leukocyte Antigen Biology Laboratory, UCD) as previously described 1. Each antibody was detected using a PE‐conjugated donkey anti‐mouse IgG H+L F(ab')2 (Jackson Immunotech, West Grove, PA). All samples were read on a Beckman Coulter FC500 Flow Cytometer with, and analyzed using FlowJo software (Treestar).
MSC Crossmatch
To determine if cats developed antibodies to allogenic ASCs after administration, we modified a flow cytometric MSC crossmatch procedure that we developed for use in horses based on a procedure developed to identify transplant associated anti‐MSC alloantibodies. This assay was performed as previously described 41 with the following changes. Paired pre‐ and post‐injection (6 months) sera from 5/7 cats was available for the crossmatch. Serum was heat‐inactivated (56°C, 30 minutes), diluted 1:1 with 12.5% pooled feline serum (collected from healthy cats, heat‐inactivated, aliquoted, and maintained at −80°C) in phosphate buffered saline, and 100 µl of diluted sera was added to donor or irrelevant ASCs (4–6 × 105 cells) that had been thawed and blocked 41. After primary incubation and washing, all samples were incubated with feline IgG (1:500, FITC labeled, goat polyclonal antibody; Southern Biotech). Samples were read on an FC500 flow cytometer (Beckman Coulter) and data was further analyzed using FlowJo software (TreeStar).
For each cat, serum antibody binding to the irrelevant ASC line (ASCs the cat had not received) was evaluated first to determine the amount of background binding. The percent of positive binding to irrelevant ASCs was subtracted from the percent of positive binding to the donor ASCs to determine the donor cell‐specific antibody binding at a given time point. The percentage of donor ASC‐specific antibody binding of the pre‐treatment serum was subtracted from the post‐treatment serum to determine net change of antibody binding.
ASC Labeling and Tracking
ASCs were radiolabeled and administered to three additional FCGS affected cats and one healthy SPF cat. The ASCs were labeled with Technetium (Tc)‐hexamethylpropyleneamineoxime (HMPAO) as previously described 44. Briefly, ∼ 25 × 106 ASCs were labeled with 15 mCi (555MBq) of 99mTc ‐HMPAO. 20 million labeled ASCs were used for injection and 5 million ASCs were kept to measure viability, labeling efficiency and label persistence at 6 hours as previously described 44. Cell viability was assessed using the trypan blue exclusion test. Radiolabeled ASCs were injected via a cephalic vein catheter. The treatment was divided into four parts and was slowly injected every 5 minutes. A gamma camera (IS2 Medical Systems, Ottawa, Canada) equipped with a low energy all‐purpose collimator and peaked at 140 keV was used for the imaging. Right lateral, left lateral, and ventral images of the whole body of the cats were acquired, with a 1‐minute static acquisition, immediately after injection (T0), at 1 hour (T1), 6 hours (T6), and 24 hours (T24) after injection. Finally, the distribution of the radiolabeled ASCs was assessed subjectively through the whole body. The radioactive signal was quantified on the right lateral view as a ratio of the radioactivity in the region of the head and the radioactivity in the whole body of the patient. Regions of interest were drawn using the free hand region of interest tool (DICOM viewing software, OsiriX, Pixmeo, Geneva, Switzerland). The head/whole body ratio was measured at all time points available. Due to lower signal to noise ratio, the measurements at 24 hours were corrected for background noise.
Statistical Analyses
Data are reported as individual animals in the responder and nonresponder group, denoted by unique symbols (means indicated). Significance for all data analysis was set at p < .05. Statistical significance between 2 groups was determined by nonparametric Mann–Whitney‐Wilcoxon t test (GraphPad InStat version 3.06 for Windows, La Jolla, CA). Normal distribution of animals was not achieved due to low sample size in the responder and nonresponder groups. For study parameters containing animals having low to unmeasurable quantities, basic descriptive statistics were used.
Results
ASCs Derived from Healthy SPF Donor Cats Are Potent, have a Typical ASC Identity and do not Express MHC II
Feline ASCs were uniformly positive for CD105 and CD90 (Fig. 2A, 2B, identity), and negative for MHCII (activation marker; Fig. 2C) and CD18 (purity; Fig. 2D). All three donor ASCs suppressed T‐cell proliferation when stimulated with ConA in co‐culture with allogeneic PBMCs (potency; Fig. 2E) as measured by BrdU incorporation. There was no difference in lymphocyte suppressive ability between the three SPF allogenic ASC lines used in this study and the autologous ASC lines used in the previous study 1.
Figure 2.
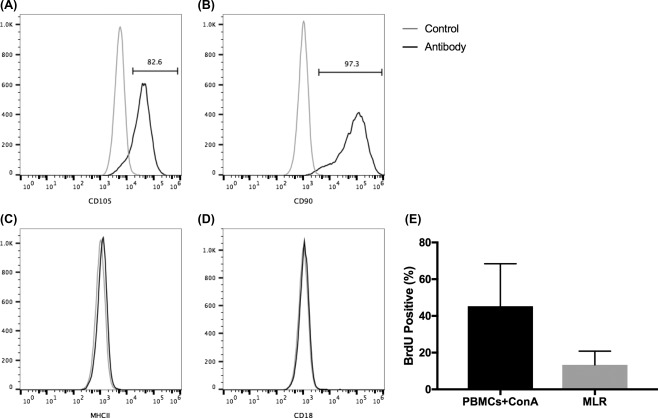
In vitro feline ASC phenotype and function. Feline adipose‐derived mesenchymal stem cells (ASCs) expressed surface markers consistent with an mesenchymal stem cells phenotype: CD105+ (A) and CD90+ (B), both markers of identity; MHC II‐ (C; not activated) and CD18‐(D; purity). All three ASC donor lines suppressed proliferation of activated PBMCs in MLR (E). Abbreviations: BrdU, 5‐bromo‐29‐deoxyuridine; ConA, concanavalin A; MLR, mixed leukocyte reactions; PBMC, peripheral blood mononuclear cell.
Allogenic ASC Treatment Induced Marked but Delayed Clinical Improvement in Cats with FCGS
Seven cats were enrolled (six males, one female), all cats completed the study and their signalment is presented in Figure 1C. All cats had full‐mouth tooth extractions and were considered refractory to any therapeutic intervention. All had chronic severe mucosal inflammation in the caudal oral cavity and at various other locations within the oral cavity with disease duration of 1.8–4.5 years (mean 2.8 years). We did not observe immediate or delayed adverse events in any of the cats. No changes were noted in the complete blood counts or in the serum biochemical profiles. At 6 months (the formal end of the study), 4/7 cats (57%) responded to treatment with substantial clinical improvement. Two of these 4 cats exhibited progressive improvement and complete cure at 18–20 months after ASC treatment (Figs. 1C, 3A, 3B). Three cats had either minimal or no clinical response.
Figure 3.
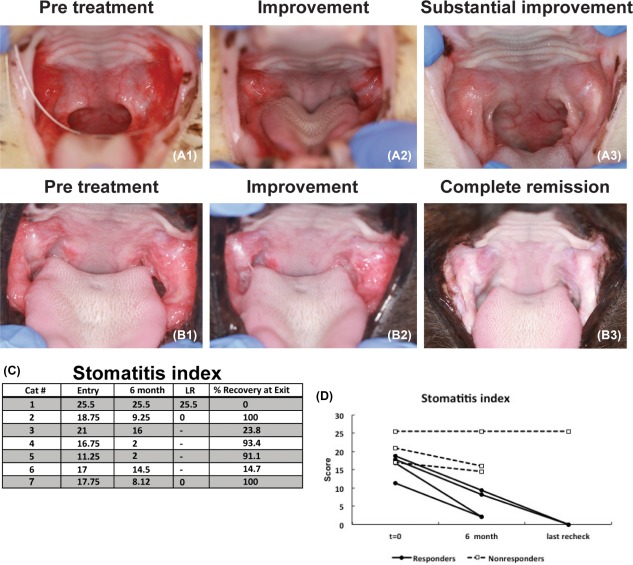
Clinical assessments of disease severity by means of clinical images and stomatitis disease activity index (SDAI) over time. Representative pretreatment images for two different cats (A1, B1) are characterized by severe proliferative and ulcerative inflammation at the caudal oral cavity. For two cats, a clinical response was observed within 3 months (A2) with substantial improvement at 6 months (A3). In two cats, a delayed response was observed and resulted in improvement at 6 months (B2) and complete resolution in 18 months (B3); SDAI table (C) and graph (D) demonstrate the score at entry and exit examination as well as the last recheck available. Note that there were four responder cats and a delayed positive response was observed in two out of the four responder cats. Abbreviation: LR, last recheck.
Clinical assessment of disease severity, by means of the SDAI, confirmed our clinical observations (Fig. 3C, 3D). In general, the improvement of clinical signs corresponded with improvement of the oral mucosal lesions. The responder cats began eating more, gaining weight, resuming grooming behavior and resuming sociability. The owners reported a return to pre‐FCGS activity levels in the responder cats. The three cats that did not respond to treatment had static SDAI and the owners reported the same activity levels as with historic immunosuppressive therapy. Two nonresponder cats were euthanized 6–12 months after exiting the trial due to the lack of improvement and continued inflammation.
Histopathologic Features Correlated with Clinical Findings in Responder and Nonresponder Cats
Oral mucosal biopsies were obtained from all cats prior to study enrollment. Post ASC treatment, oral biopsies were available from one cat that achieved complete clinical remission, one cat that exhibited substantial improvement and one cat that did not respond to treatment. In the two cats that showed improvement in SADI clinical scores, a profound reduction of inflammation was observed on histopathological examination (Fig. 4). In all pretreatment biopsies, the epithelium and subepithelial stroma were expanded by a mixed inflammatory infiltrate composed of lymphocytes, plasma cells, and neutrophils, with occasional Mott cells, mast cells and histiocytes. Ulceration of the surface epithelium was frequently observed. Remnant surface epithelium was hyperplastic with multiple rete pegs extending deep into the subjacent stroma. Immunohistochemistry revealed that CD3+ T cells were present within the epithelium and subepithelial stroma, while CD20+ B cells were restricted to the subepithelial stroma (Fig. 4).
Figure 4.
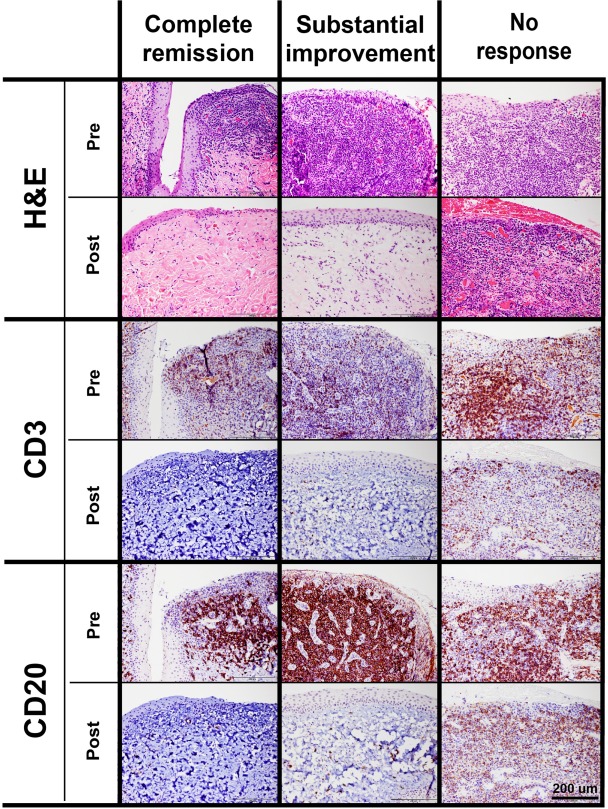
Histology of all pretreatment biopsies demonstrated that the epithelium and subepithelial stroma were expanded by a mixed inflammatory infiltrate composed of lymphocytes, plasma cells, and neutrophils. The surface epithelium was frequently ulcerated and hyperplastic with multiple rete pegs extending into the subjacent stroma. Immunohistochemistry demonstrated that CD3+ T cells were present mostly within the epithelium and subepithelial stroma, while CD20+ B cells were mostly restricted to the subepithleial stroma. A return to normal tissue architecture was observed in biopsies of cats with complete clinical remission and substantial clinical improvement. However, occasional lymphocytes were observed within subepithelial stroma with no evidence of epithelial hyperplasia, ulceration, or inflammation. The post treatment biopsies from the cats with no clinical improvement were similar to the pretreatment biopsies.
Upon completion of the study, a complete return to normal tissue architecture was observed in the biopsies of cats with complete clinical remission or substantial clinical improvement. Occasional lymphocytes were observed within subepithelial stroma with no evidence of epithelial hyperplasia, ulceration, or inflammation. The very low number of CD3+ T cells and CD20+ B cells was confirmed by immunohistochemistry (Fig. 4, left and middle columns). The biopsies from the cat with no clinical improvement were consistent with severe lymphoplasmacytic and neutrophilic ulcerative stomatitis prior to and after treatment. The immunohistochemical labeling revealed high numbers of CD3+ T cells intraepithelially and within the subepithelial stroma. An equally high density of CD20+ B cells was observed in the subepithelial stroma (Fig. 4, right column).
Allogenic ASC Administration did not Predictably Alter Blood Lymphocyte Subsets
Our previous work demonstrated that cats with FCGS have increased percentages of blood CD8+ T cells with a resultant decreased CD4/CD8 T cell ratio 1. The cats in this study generally recapitulated this phenotype (Fig. 5). Prior to ASC administration, most cats had normal percentages of blood CD4+ T cells (5/7 cats, Fig. 5A), increased percentages of CD8+ T cells (5/7 cats; Fig. 5B) and a decreased CD4/CD8 ratio (6/7 cats; Fig. 5C). All seven cats had a decreased percentage of CD8lo cells, as we previously described (Fig. 5D) 1.
Figure 5.
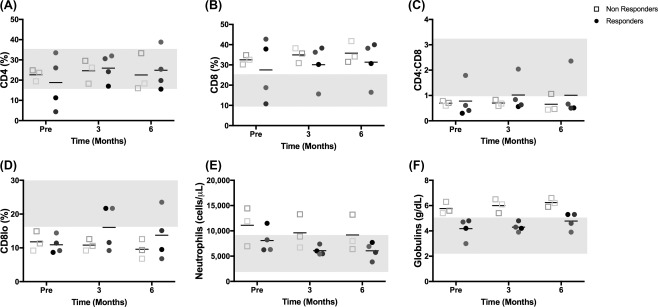
Circulating inflammatory cells and serum immunoglobulins. Flow cytometric analysis of whole blood for percent CD4+ T cells (A), percent CD8+ T cells (B), calculated ratio of CD4/CD8 T cells (C), and percent CD8lo cells (D). Neutrophil number (E, from a complete blood count) and serum globulin concentration (F, from serum biochemistry panel). Plain gray bars represent either the range of values of control cats (A–D), which were used for parameters that did not have standard reference intervals, or the standard reference intervals obtained from University of California, Davis, School of Veterinary Medicine reference intervals (E, F). Abbreviation: Pre, before treatment.
In our previous study using autologous ASC therapy, disease resolution was associated with decreased blood CD8+ T cells, normalization of the CD4/CD8 ratio and increased numbers of CD8lo T cells. In this study, at 6 months post allogeneic ASC administration, immune cell subsets (percent CD4+ T cells, percent CD8+ T cells, CD4/CD8 ratio) did not notably change over time, with or without disease cure. Two of the four cats (50%) that responded to ASC therapy showed an increase in the percentage of CD8lo T cells (moved to within the reference range) with therapy while all the other cats maintained a low percentage of CD8lo T cells (Fig. 5D). This is in contrast with autologous ASC administration for cats with FGCS where increased CD8lo T cell percentage was 100% predictive of response to therapy.
Cats that Responded to Allogeneic ASC Therapy had Less Severe Systemic Inflammation
Cats with FCGS often have systemic evidence of inflammation including blood neutrophilia, polyclonal hypergammaglobulinemia and increased concentrations of pro‐inflammatory serum cytokines. In the current study, neutrophilia was present in 3/7 cats (Fig. 5E) and a hypergammaglobulinemia was present in 2/7 cats (Fig. 5F) prior to ASC administration. Interestingly, cats that did not respond to allogeneic ASC therapy had higher initial neutrophil counts (Fig. 5E) and globulin concentrations (Fig. 5F, p = .057) compared to responder cats. Similarly, prior to ASC administration, all three cats that did not respond to therapy had higher concentrations of serum IFNγ compared to cats that responded to therapy (Fig. 6A, p = .057) and 2/3 cats that did not respond had detectable systemic TNFα concentrations (Fig. 6B) whereas none of the responder cats had detectable TNFα. These data suggest that allogenic ASC therapy was less effective in cats with more severe systemic inflammation (neutrophilia, hyperglobulinemia, and increased serum IFNγ and TNFα) in this small cohort of cats.
Figure 6.
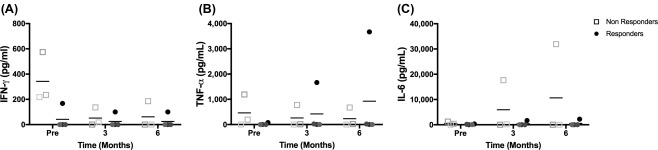
Circulating cytokines in FCGS affected cats before and after ASC therapy. Enzyme‐linked immunosorbent assay results for serum concentrations of TNF‐α (A), IFN‐γ (B), and IL‐6 (C) over time. Note that nonresponder cats tended to have higher concentration of IFNγ prior to treatment. Abbreviations: IFN, interferon; IL, interleukin; Pre, before treatment; TNF, tumor necrosis factor.
Allogenic ASC Infusion and Clinical Response was not Associated with Alterations in Serum Biomarkers
The pro‐inflammatory serum cytokines TNFα, IFNγ, and IL6 did not show any consistent change after ASC infusion (Fig. 6). Only one responder cat spiked with both IFNγ and TNFα after ASC infusion. All nonresponder cats showed decreased IFNγ levels 3 months after ASC infusion however this was not associated with a clinical response (Fig. 6A). Similar to our previous study, serum IL6 was essentially undetectable at baseline in all cats (Fig. 6C). Over the course of therapy, only one nonresponder cat had measurable IL6. These findings are in direct contrast to what was observed in cats that responded to autologous ASC therapy. In our previous study, there was a peak increase in serum IL6 in all responder cats at 3 months that plateaued through the 6 month time point. The absence of serum IL6 alterations combined with the inconsistent changes in CD8lo cells suggest that the mechanism(s) of action of allogeneic ASC therapy in cats with FCGS may differ from how autologous ASCs work.
Cats did not Develop Significant Anti‐FBS Antibodies After Allogenic ASC Administration
Feline ASCs were cultured in FBS, and, as such, it was important to determine (a) if cats developed antibodies to BSA, the primary protein in FBS, and (b) if the development of antibodies was associated with treatment failure. Most cats (4/7) had varying amounts of anti‐BSA antibody prior to ASC administration (three cats were negative, no correlation with age; most cats are highly vaccinated with vaccine products produced with FBS). Anti‐BSA titers did not change after cell administration (stayed high or stayed low) in 6/7 of the cats (86%). One cat changed from a negative titer to a low positive titer. This cat responded with substantial clinical improvement to allogenic ASC therapy. As such, the development of low anti‐BSA titers did not correlate with response (or absence of a response) to ASC therapy (Fig. 1C).
Most Cats did not Develop any Anti‐ASC Antibodies After Allogeneic ASC Administration
Serum (pre and 6 months post) was available from five of the seven cats for detection of anti‐ASC antibodies. Of these five cats, serum from only one cat (20%) demonstrated a specific increase in antibody binding to the donor ASCs (compared to serum binding to irrelevant control ASCs; Supporting Information Table 1). This same cat responded well to ASC therapy (substantial improvement) and also developed a mild increase in titer to BSA via ELISA.
After IV Administration, Most ASCs were Trapped in the Lung; ASCs Adhered to the Cephalic Vein and Trafficked to the Oral Cavity in Cats with FCGS
Immediately after injection, most of the radioactive signal was localized within the lungs (Fig. 7A, 7B, arrow). A low level of radioactivity was present diffusely throughout the whole body including the area of the oral cavity (Fig. 7A, 7B, arrowhead). Subjectively, FCGS‐affected cats had a slightly higher uptake in the area of the oral cavity (Fig. 7B, arrowhead) when compared with the healthy SPF cat (Fig. 7A). Moderate to marked radioactive uptake was also identified in the urinary tract. In all three FCGS cats, immediate, focal uptake was identified in the cephalic vein (Fig. 7B) whereas this was not present in the healthy control cat. The ratio head/whole body for all four cats is presented in Figure 7C. The ratio tends to decrease over time. The values measured in the FCGS‐affected cats were higher than in the control cat.
Figure 7.
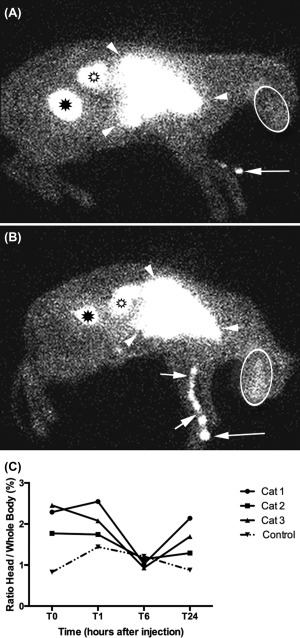
ASC tracking in FCGS affected cats vs. a control cat. Right lateral scintigraphic images obtained 1 hour post radiolabeled mesenchymal stem cells (MSC) injection in a control cat (A) and an feline chronic gingivostomatitis (FCGS) affected cat (B). Most of the radioactive signal was present within the lungs, likely due to trapping of the MSCs in the capillary bed. Marked radioactive uptake is also present in the kidneys and bladder, likely due to the presence of dissociated label. Additionally, the affected cat appears to have higher radioactive uptake in the region of the oral cavity as compared to the control cat. Focal radioactive uptake is also present in the area of the injected cephalic vein in the FCGS affected cat. The only uptake associated with the thoracic limb in the control cat is within the catheter and injection port. Line graph (C) demonstrates the ratio head/whole body at the different time points for all four cats.
Discussion
This is the first study to investigate the clinical safety and efficacy of allogeneic ASCs for the treatment of a severe oral mucosal inflammatory disease in a naturally occurring animal model. We found that systemically administered, fresh, allogeneic ASCs were safe and could achieve cure, or a substantial reduction in the inflammation associated with FCGS, resulting in regeneration of the oral mucosa and improved clinical signs. However, although patient numbers are low, the efficacy of allogeneic ASCs may be lower as compared to autologous ASC therapy for the treatment of FCGS [57% (4/7) cats in current study, compared to 71% (5/7 cats) in the autologous study] 1. This potential reduction in efficacy for allogeneic ASCs was particularly noted in cats with the most severe inflammation, as measured by blood neutrophil number, serum globulin concentration and serum IFNγ concentration. The clinical response to allogeneic therapy (cure by ∼12–20 months) also appears to be delayed compared to autologous ASC treatment (cure in ∼3–9 months). A clinical response to autologous ASC therapy was also correlated with elevations in serum IL6 and increased percentages of blood CD8lo lymphocyte subsets whereas allogeneic ASC therapy was not associated with detectable alterations in these biomarkers.
In this study, we demonstrated that the IV infusion of a relatively high dose (20 million cells/cat, equivalent to ∼5 million cells/kg) of allogeneic ASCs was safe and well tolerated in cats with severe oral inflammatory disease. Unlike the transfusion reactions noted in two cats that received autologous treatment in our previous study, we did not observe any transfusion reactions, changes in bloodwork or delayed adverse events associated with allogeneic ASC administration. These data are compatible with other studies that have safely infused allogenic ASCs in different disease settings in cats 31, 36.
However, while no side effects ware noted in this cohort of cats, the efficacy of allogeneic ASCs for FCGS is less clear. This study recapitulated our autologous study in all measurable ways including study location and clinicians (UCD School of Veterinary Medicine, Regenerative Medicine Lab, Drs. Arzi and Verstraete), patient enrollment criteria, patient signalment (predominantly male cats, average age was 7.5–9 years, average duration of disease was 2.7–2.8 years), cell dose, and route (IV), number (2) and timing (4 weeks apart) of ASC administration. Similar to the three SPF donor ASC cell lines that we used in this study, all autologous ASCs had ASC identity, as defined by CD90 and CD105 expression, were potent, as defined by the ability to suppress activated T cell proliferation, were pure (as defined by the absence of leukocytes) and did not express MHC II. At presentation, the average neutrophil number (9,500 vs. 9,800) and globulin concentration (average 5.3 vs. 4.8) in enrolled cats for both studies were comparable. Thus, the reason for discrepancies between the results in the autologous ASC study and the current study using allogeneic ASC therapy remains elusive.
We investigated the possibility that the development of antibodies against the ASCs may have resulted in a decreased life span and, hence, reduced efficacy, of allogeneic ASCs as compared to our previous autologous ASC study. We were able to detect specific antibody development in one out of the five cats tested. This alloantibody formation in cats parallels and confirms what has been noted in many species including horses, pigs, macaques, rats and humans 38, 41, 45, 46, 47, 48, 49. Nonetheless, the significance of antibody development, in terms of clinical outcome, is hotly debated as many of these animals and humans show clinical improvement in spite of antibody development. Similarly, the cat in our study that developed a measurable anti‐ASC antibody reaction was one of the cats who responded favorably to treatment. However, antibody production is consistent with the memory functions of the cellular and humoral arms of the adaptive immune response 40, 50, 51 and it is possible that the innate immune system, which is responsible for the more immediate confrontation and removal of foreign antigens, may have been activated by the administration of the allogeneic ASCs 40, 52, 53, including T cell mediated rejection of the allogenic cells. The innate immune system has a key role in the initiation of the adaptive immune response to allogenic antigens and its activity is largely nonspecific 40. We did not include any measures of innate immune cell response to the ASCs in our study (e.g., pro‐thrombotic ASC markers or activation of complement) 42. It appears that the fate of allogenic ASCs in cats, as in other species, is not yet fully predictable and it may be that an undetermined number of ASCs are cleared or destroyed resulting in reduced/delayed efficacy and diminished immunomodulatory potential.
In our current study, the pretreatment percentages of blood CD8+ T cells and the resultant decreased CD4/CD8 T cell ratio mirrored findings in our autologous study 1. However, unlike the results of the autologous clinical trial, at 6 months post allogenic ASC therapy, immune cell subsets (percent CD8+ T cells, CD4/CD8 ratio) did not notably change regardless of the clinical outcome. Furthermore, compared to the results of the autologous ASC study, the pro‐inflammatory serum cytokines TNFα and IFNγ did not show any consistent change after ASC administration. It is plausible that an innate immune reaction to the ASCs and/or alloantibody production may have reduced ASC survival time and impeded their immunomodulatory effect which in turn also reduced the success rate and prolonged the clinical response 40. This is in agreement with previous studies that demonstrated that allogenic ASCs do not completely evade immune surveillance 40, 41, 54.
We discovered two potential biomarkers of successful response to autologous ASC therapy in cats with FCGS in our last study: a decreased pretreatment percentage of CD8lo cells, which increased with cure, and increased serum IL6 after ASC infusion. Receptor expression on CD8 cells may dictate whether a CD8 cell has a suppressive or cytotoxic phenotype with decreased CD8 receptor expression (limiting the ability of cells to be classically activated to a cytotoxic phenotype) associated with increased suppressive functions 55. We hypothesized that the induction of CD8lo cells and the concurrent increase in IL6 may be responsible for the generation of T regulatory cells and “reprogramming” of the immune response resulting in long‐term cure in these cats. As such, one objective of the current study was to determine if these changes would be robust and repeatable after allogenic ASC infusion. Although two of the four cats (50%) that responded to allogenic therapy did demonstrate increased CD8lo cells, the change was neither predictable nor robust. There was also no accompanying increase in serum IL6, even in the face of disease cure. These findings suggest that there are multiple mechanisms of action for ASC therapy in cats in the context of inflammatory/immune‐mediated diseases. This is not that surprising given the heterogeneity of the disease and the cells. Further studies, both in vitro and in vivo, are needed to dissect out the interaction of ASCs with dendritic cells/macrophages, T cell subsets (e.g., Th17 cells), and B cells to begin to elucidate the mechanism(s) of action for these very potent cells.
The challenge of understanding how stem cells function is coupled with the need to determine where these cells traffic after administration and how long they persist. The potential to utilize naturally occurring diseases in companion animals for cell tracking studies (with relevant, physiological inflammatory recruitment stimuli) is a strength of these animal models. We found that the IV administration of ASCs to cats with FCGS resulted in the immediate adherence of some cells to the vein close to the site of administration with most cells retained in the lungs and a small proportion of ASCs that trafficked to the inflamed oral cavity. Cell trapping in the lungs was expected and was similar to what has been reported in tracking studies performed in dogs and rodents 52, 56, 57. It is likely that the trapping occurs at the pulmonary capillary level, due to the relative large size of the MSC compared with the capillary lumen diameter. Interestingly, the amount of radioactivity in the oral cavity of clinical patients was higher than in a healthy control cat. This was not surprising given the degree of oral inflammation and rapid cell adherence to the vasculature after administration. Results confirm that at least a subset of cells traffic early on to the site of inflammation. The presence of radioactivity in the urinary tract is probably due to dissociation of the radioactive label from the ASC. We have noted this suboptimal stability of the label in previous studies and it is a clear limitation of scintigraphic tracking using Tc‐HMPAO 57. Overall the head/whole body ratios tended to decrease overtime with lowest values noted 6 hours after injection. The values seem to increase at 24 hours but this should be interpreted with caution due to the lower quality of the signal obtained at 24 hours secondary to the short half‐life (6 hours) of Technetium. Also, the presence of dissociated label increases over time and is likely to affect the later measurements. Previous studies have alluded to similar findings and that ASCs that are trapped in the microcapillary networks have a short life span and exert their action via a paracarine mechanism 56, 58, 59, 60. Finally, given the delayed clinical response as compared to the administration of autologous ASCs, it possible that pulmonary trapping may occur to a greater extent for allogeneic cells as compared to autologous cells, thus decreasing relative transit to the oral mucosa. Altered cell tracking is one consideration for the delayed potency of allogeneic cells.
Although autologous and allogeneic cell sources appear to be safe when administered systemically, direct comparison between the two sources are rare 38. However, in terms of clinical outcome, the response of allogeneic therapy for nonresponsive FCGS appears to be delayed as compared to autologous ASC treatment. No direct comparison to other studies is possible; however, other studies also demonstrated improved outcome when using an autologous cell source as compared to allogeneic 61, 62. This delayed response has practical implications when performing a clinical trial in a debilitating and painful disease, such as FCGS, as cat‐owner satisfaction and compliance depend on response to therapy. The delayed response is likely to contribute to a withdrawal from a long term clinical trial.
Conclusion
The use of allogeneic ASCs provides an ideal clinical and commercially applicable solution to stem cell therapy as it can be sold “off‐the shelf,” is readily available, and minimizes the delay in therapy associated with culture time and characterization 38, 39, 58, 63. However, these pilot data suggest that the use of allogeneic cells for this oral inflammatory disease may be associated with inconsistencies in immunomodulation and delayed clinical response as compared to the administration of autologous ASCs. In addition, alterations in serum cytokines and CD8lo cells were not noted suggesting that the potency and mechanism(s) of action for autologous and allogenic ASCs differ in this model of oral inflammation.
Author Contributions
B.A. and D.L.B.: conception and design, financial support, provision of study material or patients, manuscript writing, data analysis and interpretation; K.C.C. and A.S.: manuscript writing, data analysis and interpretation; M.S.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; F.J.M.V.: provision of study material or patients, collection and/or assembly of data, final approval of manuscript; N.J.W.: collection and/or assembly of data, data analysis and interpretation; M.R.L.: collection and/or assembly of data, provision of study material or patients, final approval of manuscript; N.F., W.J.M., N.V.: data analysis and interpretation, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest
Supporting information
Supporting Information
Acknowledgments
This study was supported by the NIH 1R21DE024711‐01, the WINN Feline Foundation for a Miller Trust grant, and by the George and Phyllis Miller Feline Health Trust of the San Francisco Foundation and administered by the Center of Companion Animal Health, UCD. The Stomatitis Disease Activity Index used in this study is a modified version of the SDAI originally developed by Dr. Jamie Anderson. Finally, we thank John Doval and Chrisoula Toupadakis for assistance with the figures and to Jennifer Harrison, S. Jason Peters and Rich F. Larson for the ASC tracking section.
The work was performed at the Department of Surgical and Radiological Sciences and the Department of Pathology, Microbiology and Immunology, School of Veterinary Medicine, University of California, Davis.
References
- 1. Arzi B, Mills‐Ko E, Verstraete FJM et al. Therapeutic efficacy of fresh, autologous mesenchymal stem cells for severe refractory gingivostomatitis in cats. Stem Cells Transl Med 2016;5:75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baron F, Storb R. Mesenchymal stromal cells: A new tool against graft‐versus‐host disease? Biol Blood Marrow Transplant 2012;18:822–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Plessers J, Dekimpe E, Van WM et al. Clinical‐grade human multipotent adult progenitor cells block CD8+ cytotoxic T lymphocytes. Stem Cells Transl Med 2016;5:1607–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thanunchai M, Hongeng S, Thitithanyanont A. Mesenchymal stromal cells and viral infection. Stem Cells Int 2015;2015:860950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. di SD, Guida A, Salerno C et al. Oral lichen planus: A narrative review. Front Biosci (Elite. ED.) 2014;6:370–376. [DOI] [PubMed] [Google Scholar]
- 6. Omar AA, Hietanen J, Kero M et al. Oral lichen planus and chronic junctional stomatitis: Differences in lymphocyte subpopulations. Acta Odontol Scand 2009;67:366–369. [DOI] [PubMed] [Google Scholar]
- 7. Lavanya N, Jayanthi P, Rao UK et al. Oral lichen planus: An update on pathogenesis and treatment. J Oral Maxillofac Pathol 2011;15:127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kol A, Arzi B, Athanasiou KA et al. Companion animals: Translational scientist's new best friends. Sci Transl Med 2015;7:308ps21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoffman AM, Dow SW. Concise review: Stem cell trials using companion animal disease models. Stem Cells 2016;34:1709–1729. [DOI] [PubMed] [Google Scholar]
- 10. Girard N, Servet E, Biourge V et al. Periodontal health status in a colony of 109 cats. J Vet Dent 2009;26:147–155. [DOI] [PubMed] [Google Scholar]
- 11. Jennings MW, Lewis JR, Soltero‐Rivera MM et al. Effect of tooth extraction on stomatitis in cats: 95 cases (2000–2013). J Am Vet Med Assoc 2015;246:654–660. [DOI] [PubMed] [Google Scholar]
- 12. Arzi B, Murphy B, Cox DP et al. Presence and quantification of mast cells in the gingiva of cats with tooth resorption, periodontitis and chronic stomatitis. Arch Oral Biol 2010;55:148–154. [DOI] [PubMed] [Google Scholar]
- 13. Harley R, Gruffydd‐Jones TJ, Day MJ. Immunohistochemical characterization of oral mucosal lesions in cats with chronic gingivostomatitis. J Comp Pathol 2011;144:239–250. [DOI] [PubMed] [Google Scholar]
- 14. Healey KA, Dawson S, Burrow R et al. Prevalence of feline chronic gingivo‐stomatitis in first opinion veterinary practice. J Feline Med Surg 2007;9:373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Winer JN, Arzi B, Verstraete FJ. Therapeutic management of feline chronic gingivostomatitis: A systematic review of the literature. Front Vet Sci 2016;3:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pedersen NC. Inflammatory oral cavity diseases of the cat. Vet Clin North Am Small Anim Prac 1992;22:1323–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dowers KL, Hawley JR, Brewer MM et al. Association of Bartonella species, feline calicivirus, and feline herpesvirus 1 infection with gingivostomatitis in cats. J Feline Med Surg 2010;12:314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hennet PR, Camy GA, McGahie DM et al. Comparative efficacy of a recombinant feline interferon omega in refractory cases of calicivirus‐positive cats with caudal stomatitis: A randomised, multi‐centre, controlled, double‐blind study in 39 cats. J Feline Med Surg 2011;13:577–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lommer MJ, Verstraete FJ. Concurrent oral shedding of feline calicivirus and feline herpesvirus 1 in cats with chronic gingivostomatitis. Oral Microbiol Immunol 2003;18:131–134. [DOI] [PubMed] [Google Scholar]
- 20. Borjesson DL, Peroni JF. The regenerative medicine laboratory: Facilitating stem cell therapy for equine disease. Clin Lab Med 2011;31:109–123. [DOI] [PubMed] [Google Scholar]
- 21. Dominici M, Le BK, Mueller I et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 22. Ben‐Ami E, Berrih‐Aknin S, Miller A. Mesenchymal stem cells as an immunomodulatory therapeutic strategy for autoimmune diseases. Autoimmun Rev 2011;10:410–415. [DOI] [PubMed] [Google Scholar]
- 23. Corcione A, Benvenuto F, Ferretti E et al. Human mesenchymal stem cells modulate B‐cell functions. Blood 2006;107:367–372. [DOI] [PubMed] [Google Scholar]
- 24. Peroni JF, Borjesson DL. Anti‐inflammatory and immunomodulatory activities of stem cells. Vet Clin North Am Equine Pract 2011;27:351–362. [DOI] [PubMed] [Google Scholar]
- 25. Singer NG, Caplan AI. Mesenchymal stem cells: Mechanisms of inflammation. Annu Rev Pathol 2011;6:457–478. [DOI] [PubMed] [Google Scholar]
- 26. Arzi B, Kol A, Murphy B et al. Feline foamy virus adversely affects feline mesenchymal stem cell culture and expansion: Implications for animal model development. Stem Cells Dev. 2014;24:814–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beggs KJ, Lyubimov A, Borneman JN et al. Immunologic consequences of multiple, high‐dose administration of allogeneic mesenchymal stem cells to baboons. Cell Transplant 2006;15:711–721. [DOI] [PubMed] [Google Scholar]
- 28. Carrade DD, Borjesson DL. Immunomodulation by mesenchymal stem cells in veterinary species. Comp Med 2013;63:207–217. [PMC free article] [PubMed] [Google Scholar]
- 29. Martinello T, Bronzini I, Maccatrozzo L et al. Canine adipose‐derived‐mesenchymal stem cells do not lose stem features after a long‐term cryopreservation. Res Vet Sci 2011;91:18–24. [DOI] [PubMed] [Google Scholar]
- 30. Quimby JM, Webb TL, Gibbons DS et al. Evaluation of intrarenal mesenchymal stem cell injection for treatment of chronic kidney disease in cats: A pilot study. J Feline Med Surg 2011;13:418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Quimby JM, Webb TL, Habenicht LM et al. Safety and efficacy of intravenous infusion of allogeneic cryopreserved mesenchymal stem cells for treatment of chronic kidney disease in cats: Results of three sequential pilot studies. Stem Cell Res Ther 2013;4:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vieira NM, Brandalise V, Zucconi E et al. Isolation, characterization, and differentiation potential of canine adipose‐derived stem cells. Cell Transplant 2010;19:279–289. [DOI] [PubMed] [Google Scholar]
- 33. Kang MH, Park HM. Evaluation of adverse reactions in dogs following intravenous mesenchymal stem cell transplantation. Acta Vet Scand 2014;56:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kol A, Wood JA, Carrade Holt DD et al. Multiple intravenous injections of allogeneic equine mesenchymal stem cells do not induce a systemic inflammatory response but do alter lymphocyte subsets in healthy horses. Stem Cell Res Ther 2015;6:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee CC, Ye F, Tarantal AF. Comparison of growth and differentiation of fetal and adult rhesus monkey mesenchymal stem cells. Stem Cells Dev 2006;15:209–220. [DOI] [PubMed] [Google Scholar]
- 36. Rosselli DD, Mumaw JL, Dickerson V et al. Efficacy of allogeneic mesenchymal stem cell administration in a model of acute ischemic kidney injury in cats. Res Vet Sci 2016;108:18–24. [DOI] [PubMed] [Google Scholar]
- 37. Lalu MM, McIntyre L, Pugliese C et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): A systematic review and meta‐analysis of clinical trials. PLoS One 2012;7:e47559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat Biotechnol 2014;32:252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Trounson A, McDonald C. Stem cell therapies in clinical trials: Progress and challenges. Cell Stem Cell 2015;17:11–22. [DOI] [PubMed] [Google Scholar]
- 40. Griffin MD, Ryan AE, Alagesan S et al. Anti‐donor immune responses elicited by allogeneic mesenchymal stem cells: What have we learned so far? Immunol Cell Biol 2013;91:40–51. [DOI] [PubMed] [Google Scholar]
- 41. Owens SD, Kol A, Walker NJ et al. Allogeneic mesenchymal stem cell treatment induces specific alloantibodies in horses. Stem Cells Int 2016;2016:5830103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moll G, Rasmusson‐Duprez I, von BL et al. Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem Cells 2012;30:1565–1574. [DOI] [PubMed] [Google Scholar]
- 43. Lommer MJ. Efficacy of cyclosporine for chronic, refractory stomatitis in cats: A randomized, placebo‐controlled, double‐blinded clinical study. J Vet Dent 2013;30:8–17. [DOI] [PubMed] [Google Scholar]
- 44. Trela JM, Spriet M, Padgett KA et al. Scintigraphic comparison of intra‐arterial injection and distal intravenous regional limb perfusion for administration of mesenchymal stem cells to the equine foot. Equine Vet J 2014;46:479–483. [DOI] [PubMed] [Google Scholar]
- 45. Hare JM, Fishman JE, Gerstenblith G et al. Comparison of allogeneic vs autologous bone marrow‐derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The Poseidon randomized trial. JAMA 2012;308:2369–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pezzanite LM, Fortier LA, Antczak DF et al. Equine allogeneic bone marrow‐derived mesenchymal stromal cells elicit antibody responses in vivo. Stem Cell Res Ther 2015;6:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Isakova IA, Dufour J, Lanclos C et al. Cell‐dose‐dependent increases in circulating levels of immune effector cells in rhesus macaques following intracranial injection of allogeneic MSCs. Exp Hematol 2010;38:957–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rigol M, Solanes N, Roura S et al. Allogeneic adipose stem cell therapy in acute myocardial infarction. Eur J Clin Invest 2014;44:83–92. [DOI] [PubMed] [Google Scholar]
- 49. Schu S, Nosov M, O'Flynn L et al. Immunogenicity of allogeneic mesenchymal stem cells. J Cell Mol Med 2012;16:2094–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nankivell BJ, Alexander SI. Rejection of the kidney allograft. N Engl J Med 2010;363:1451–1462. [DOI] [PubMed] [Google Scholar]
- 51. Wood KJ, Goto R. Mechanisms of rejection: Current perspectives. Translantation 2012;93:1–10. [DOI] [PubMed] [Google Scholar]
- 52. Cao J, Hou S, Ding H et al. In vivo tracking of systemically administered allogeneic bone marrow mesenchymal stem cells in normal rats through bioluminescence imaging. Stem Cells Int 2016;2016:3970942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Spees JL, Gregory CA, Singh H et al. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol Ther 2004;9:747–756. [DOI] [PubMed] [Google Scholar]
- 54. Isakova IA, Lanclos C, Bruhn J et al. Allo‐reactivity of mesenchymal stem cells in rhesus macaques is dose and haplotype dependent and limits durable cell engraftment in vivo. Plos One 2014;9:e87238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Trautmann A, Ruckert B, Schmid‐Grendelmeier P et al. Human CD8 T cells of the peripheral blood contain a low CD8 expressing cytotoxic/effector subpopulation. Immunology 2003;108:305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Saat TC, van den Engel S, Bijman‐Lachger W et al. Fate and effect of intravenously infused mesenchymal stem cells in a mouse model of hepatic ischemia reperfusion injury and resection. Stem Cells Int 2016;2016:5761487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Spriet M, Hunt GB, Walker NJ et al. Scintigraphic tracking of mesenchymal stem cells after portal, systemic intravenous and splenic administration in healthy beagle dogs. Vet Radiol Ultrasound 2015;56:327–334. [DOI] [PubMed] [Google Scholar]
- 58. Eggenhofer E, Benseler V, Kroemer A et al. Mesenchymal stem cells are short‐lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol 2012;3:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fischer UM, Harting MT, Jimenez F et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first‐pass effect. Stem Cells Dev 2009;18:683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hoogduijn MJ, Roemeling‐van RM, Engela AU et al. Mesenchymal stem cells induce an inflammatory response after intravenous infusion. Stem Cells Dev 2013;22:2825–2835. [DOI] [PubMed] [Google Scholar]
- 61. Alyea E, Weller E, Schlossman R et al. Outcome after autologous and allogeneic stem cell transplantation for patients with multiple myeloma: Impact of graft‐versus‐myeloma effect. Bone Marrow Transplant 2003;32:1145–1151. [DOI] [PubMed] [Google Scholar]
- 62. Yun S, Vincelette ND, Abraham I et al. Outcome comparison of allogeneic versus autologous stem cell transplantation in transformed low‐grade lymphoid malignancies: A systematic review and pooled analysis of comparative studies. Acta Haematol 2016;136:244–255. [DOI] [PubMed] [Google Scholar]
- 63. Ankrum J, Karp JM. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol Med 2010;16:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


