Abstract
We conducted a phase II, noncomparative, multicenter study to assess the efficacy and safety of allogeneic bone marrow‐derived mesenchymal stromal cells (BM‐MSCs) expanded in vitro for patients with aplastic anemia (AA) refractory to immunosuppressive therapy. Seventy‐four patients from seven centers received allogeneic BM‐MSCs at a dose of 1–2 × 106 cells/kg per week for 4 weeks. Responses were assessed at 0.5, 1, 2, 3, 6, 9, and 12 months after the first cells infusion. Patients with response at 1 month continued to receive four infusions. All patients were evaluable. The overall response rate was 28.4% (95% confidence interval, 19%–40%), with 6.8% complete response and 21.6% partial response. The median times to response of leukocytic, erythrocytic, and megakaryocytic linages were 19 (range, 11–29), 17 (range, 12–25), and 31 (range, 26–84) days, respectively. After median follow‐up of 17 months, overall survival was 87.8%. Seven patients developed transitory and mild headache and fever, but no other adverse events were observed. Antithymocyte globulin used in previous treatment and no activated infection throughout treatment were predictors for response. Allogeneic BM‐MSCs infusion is a feasible and effective treatment option for refractory AA. The trial was registered at www.clinicaltrials.gov as NCT00195624. Stem Cells Translational Medicine 2017;6:1569–1575
Keywords: Bone marrow, Mesenchymal stromal cells, Aplastic anemia, Cellular therapy
Significance Statement.
This study further assessed bone marrow‐derived mesenchymal stromal cells (BM‐MSCs) transfusion for treatment of aplastic anemia (AA) that was refractory to immunosuppressive therapy though larger samples. The results suggest that BM‐MSCs transfusion as a supplementary measure can be used to treat refractory AA. It showed the time to hematopoietic recovery of BM‐MSCs transfusion for refractory AA. It also provided further data that antithymocyte globulin used in previous treatment and no activated infection were the most important predictors for response.
Introduction
Aplastic anemia (AA) is a bone marrow failure syndrome characterized by marrow hypoplasia and exhaustion of hematopoietic precursors, resulting in pancytopenia. Immune‐mediated pathophysiology of destruction of hematopoietic precursors was confirmed by laboratory studies and immunosuppressive therapy (IST) 1. IST is accepted as the first‐line treatment option. However, 30%–40% of patients with severe aplastic anemia (SAA) remain pancytopenic following IST. About 20% of patients with non‐severe aplastic anemia (NSAA) are dependent on transfusion and eventually transform to SAA 2. Most studies have shown that the response rate of SAA to rabbit antithymocyte globulin (ATG) was inferior to that of horse ATG 3. Patients with SAA that is refractory to IST or who have a relapse after IST may undergo allogeneic hematopoietic stem cell transplantation (HSCT). However, about one third of patients do not find a suitable donor for HSCT. Patients aged >50 years are not eligible for transplant. Following HSCT, complications such as graft‐versus‐host disease (GVHD) and graft failure may occur. The 5‐year overall survival (OS) of patients with AA that is refractory to IST is <60% 4.
Bone marrow‐derived mesenchymal stromal cells (BM‐MSCs), as important stromal components of bone marrow, can support hematopoiesis and are multipotent stem cells with the capacity to differentiate into osteoblasts, chondrocytes, adipocytes, and neural cells 5. BM‐MSCs express low levels of major histocompatibility (MHC)‐I but lack expression of MHC‐II surface molecules; therefore, allogeneic BM‐MSCs infusion may have an effect on patients through immune evasion. MSCs also express serine protease inhibitors to evade the host immune response 6, 7. BM‐MSCs exert immunomodulatory effects on activated lymphoid cells, including T cells, B cells, natural killer cells, and dendritic cells 8. Le Blanc et al. 9 first reported infusion of haploidentical BM‐MSCs in a 9‐year‐old boy with grade 4 acute GVHD of the gastrointestinal tract and liver. Now BM‐MSCs have been successfully used for several refractory immune diseases. BM‐MSC also support hematopoiesis. Nestin+ BM‐MSCs are spatially associated with hemopoietic stem cells (HSCs) and adrenergic nerve fibers and highly express HSC maintenance genes 10.
Several studies have shown that AA BM‐MSCs have poor potential for proliferation and differentiation, changes in gene expression, and reduced ability to support hematopoiesis in vitro 11, 12. In 2003, Fouillard et al. 13 reported a 68‐year‐old woman patient with SAA refractory to ATG and cyclosporine who was ineligible for allogeneic HSCT and received two infusions of allogeneic MSCs (allo‐MSCs). The bone marrow stroma of the patient improved, but improved hematopoiesis was not observed. In 2010, Jaganathan et al. 14 reported a patient with SAA who received allogeneic HSCT three times and had graft failure each time, although, eventually, the patient had complete hematopoietic recovery after the fourth HSCT with donor‐derived MSCs co‐injection. In our previous study, we evaluated the feasibility, safety, and immunological effects of the intravenous administration of MSCs from a related donor in 18 patients with refractory AA. Our data showed that 33.3% of patients with AA who were refractory to IST achieved complete response (CR) or partial response (PR) to BM‐MSCs treatment 15. On the basis of our previous data, we further conducted a phase II, noncomparative, open‐label, multicenter study to assess the efficacy and safety of allogeneic BM‐MSCs expanded in vitro in patients with AA that was refractory to IST.
Materials and Methods
Study Design
This was a phase II, noncomparative, open‐label, multicenter study (ClinicalTrials.gov Identifier: NCT00195624). The primary objective was to evaluate the overall response rate (ORR), including CR and PR, after BM‐MSCs infusion. Secondary objectives were as follows: time to response (TTR), safety, OS, and the relationship between clinical outcome and baseline prognostic markers. The trial was conducted at seven centers in China and was approved by the ethics committee of each institution. All patients provided written informed consent. Allogeneic BM‐MSCs were supplied by the Center of Cell‐Biological Therapy & Research of Guangzhou General Hospital of Guangzhou Military Command. BM‐MSCs were identified by Guangzhou Saliai Stem Cell Science and Technology Co. Ltd.
Patient Group
In the trial, we included patients with AA refractory to IST in need of treatment according to current criteria 16. The main inclusion criteria were as follows: (a) diagnosed with acquired AA as defined by standard criteria; (b) age ≥16 years; (c) had an incomplete response to ATG and cyclosporine for at least 6 months or only cyclosporine for at least 12 months and did not have a human leukocyte antigen (HLA)‐matched donor available for bone marrow transplantation; (d) peripheral blood counts met at least one of the following criteria at the time of enrollment: hemoglobin <70 g/L, neutrophilic granulocyte <1 × 109/L, or platelet count <30 × 109/L; and (e) adequate hepatic and renal function.
The main exclusion criteria were as follows: (a) participant in any other clinical trial within 4 weeks before study entry; (b) a history of allergic reactions to biological composition; (c) unable to give a truly informed consent; (d) complicated with paroxysmal nocturnal hemoglobinuria; (e) complicated with malignancy during the last 5 years; (f) one or more organs dysfunction; (g) pregnant or breastfeeding women; and (h) HIV‐positive patients.
MSCs Collection, Expansion, and Identification
BM‐MSCs were obtained from 74 healthy donors (48 males and 26 females, ages 16–67 years), including 40 related donors, 27 haploidentical donors, and 7 unrelated donors, after written informed consent. Approximately 30 ml of bone marrow was obtained from each donor. MSCs expansion in vitro was followed according to Schallmoser et al.'s report 17. BM‐MSCs were harvested after 11–25 days. Passage 3‐ Passage 5 (P3‐P5) expanded cells were allowed for infusion. The cells were resuspended in normal saline at a concentration of 3–5 × 106/ml. Quality control of BM‐MSCs include counts, viability, morphology, endotoxin, aseptic culture, immunophenotype. The immunophenotypes were identified according to the criteria of International Society for Cellular Therapy, which defines human MSCs as plastic adherent cells expressing CD105, CD90, and CD73 but that lack the expression of CD45, CD34, CD14 or CD11b, CD79a or CD19, and human leukocyte antigen‐D related surface antigen 18.
Study Treatment
Baseline assessments included a physical examination, blood sampling for hematology, biochemistry, and immunology, bone marrow examination, and cytogenetic analysis. Allogeneic BM‐MSCs were expanded in vitro at a dose of 1–2 × 106/kg per week, for a total of 4 weeks. Patients with a response at 1 month continued to receive four doses. Continuation of cyclosporine was permitted, but other immunosuppressive agents were not permitted. Supportive care included antibiotics and blood transfusions but not hematopoietic growth factors.
Efficacy Evaluation
Blood counts, chemistries, and immunology were monitored weekly in the first 3 months after the first MSCs infusion and then every 3 months. Bone marrow examination and cytogenetic analysis were done at study entry and repeated at 3, 6, and 12 months after the first MSCs infusion.
The primary endpoint was hematologic response at 1 year according to previously reported criteria. CR was defined as the return of all blood counts to normal. PR was defined as improvement so that the patient no longer belonged to the severe status group and no longer required transfusions 19. Secondary endpoints were the TTR, the OS, and clonal evolution at 2 years after the first MSCs infusion.
Safety Evaluation
Adverse events were monitored and graded according to the NCI Common Terminology Criteria for Adverse Events version 3.0.
Statistical Analyses
Summary statistics were used to describe the demographic and baseline clinical characteristics of the patients’ treatment responses. The primary endpoint was the ORR. On the basis of a historical ORR rate of 33% with MSCs in refractory AA 15, the desirable ORR rate was 35%. The study had 80% power, with a 5% type I error rate. Log‐rank tests were conducted to identify hematopoietic and immunological changes in different time points. Kaplan‐Meier product‐limit method was used to estimate OS curves, and Fish test was used to compare OS between responders and non‐responders. Multivariate logistic regression model was used to evaluate the effects of risk factors on the probabilities of response. All tests were two‐sided, accepting p < .05 as indicating a statistically significant difference. Data analysis was performed using the SPSS Version 19.0 (IBM, Armonk, NY, https://www.ibm.com/us-en/) and STATA Version 11.0 (STATA, College Station, TX, http://www.stata.com/) statistical software.
Results
Patient Characteristics
Between October 2010 and September 2014, 74 patients were registered from 7 centers. Patients’ characteristics are listed in Table 1. All 74 patients were analyzed for efficacy and safety. Fifty‐three patients (71.6%) completed one course and 21 patients (28.4%) completed two courses. Eleven patients got infection at entry of the study and 14 patients got infection during the study. Patients were on stable doses of cyclosporine, keeping the target trough blood level of cyclosporine to 200–300 ng/ml throughout the study.
Table 1.
Patient demographic and baseline clinical characteristics
| Characteristics | Patients, n (%) |
|---|---|
| Age, years | |
| <20 | 6 (8.11%) |
| 20–40 | 35 (47.30%) |
| 40–60 | 26 (35.13%) |
| >60 | 7 (9.46%) |
| Sex | |
| Male | 40 (54.05%) |
| Female | 34 (45.95%) |
| Type of AA | |
| NSAA | 50 (67.57%) |
| SAA | 24 (32.43%) |
| Duration from diagnosis, months | |
| Median (range) | 26.5 (6–249) |
| Pre‐treatment blood cell count | |
| Median WBC, ×109/L (range) | 2.68 (0.53–7.45) |
| Median HGB, g/L (range) | 67 (43–120) |
| Median PLT, ×109/L (range) | 14 (2–167) |
| Previous therapy of aplastic | |
| CsA | 12 (16.22%) |
| CsA, Andriol | 48 (64.86%) |
| CsA, ATG | 14 (18.92%) |
| Donors of MSCs | |
| Related | 40 (54.05%) |
| Haploidentical | 27 (36.49%) |
| Unrelated | 7 (9.46%) |
Abbreviations: AA, aplastic anemia; ATG, antithymocyte globulin; CsA, cyclosporine; HGB, hemoglobin; MSCs, mesenchymal stromal cells; NSAA, non‐severe aplastic anemia; PLT, platelet; SAA, severe aplastic anemia; WBC, white blood cell.
MSCs Identification
MSCs were plastic‐adherent and were evaluated for morphology in phase contrast microscopy, observing their typical fibroblast morphology (Fig. S1 in the Supporting Information Appendix). MSCs consistently (>92%) expressed the surface markers CD73, CD90, and CD105 and were negative for CD34, CD45, and CD19 (Fig. S2 in the Supporting Information Appendix). BM‐MSCs expanded in vitro have normal capabilities of adipocyte and osteogenic differentiation (Fig. S3 in the Supporting Information Appendix). No significant differences were observed in MSCs expanded with respect to the sex or type of the donor.
Hematologic and Immunologic Responses
At 1 year, the ORR was 28.4% (n = 21; 95% confidence interval [CI], 19%–40%). The CR rate was 6.8% (n = 6; 95% CI, 3%–16%), and the PR rate was 21.6% (n = 15; 95% CI, 13%–33%; Table 2). Figure 1 shows the follow‐up of the blood cell counts and the percentage of CD4+CD25+FOXP3+ regulatory T (Treg) cells of all patients at baseline and at 0.5, 1, 3, 6, 9, and 12 months after MSC transplantation. Compared with the baseline, the improvement of leukocytic linage and megakaryocytic linage was significant (p < .05).
Table 2.
Response to treatment at the 12th month
| Time, months | CR, n | PR, n | ORR (%) |
|---|---|---|---|
| 0.5 | 0 | 4 | 5.41% |
| 1 | 0 | 9 | 12.16% |
| 2 | 1 | 3 | 5.41% |
| 3 | 4 | 0 | 5.41% |
| 6 | 0 | 0 | 0.00% |
| 12 | 0 | 0 | 0.00% |
| Total | 5 | 16 | 28.40% |
Abbreviations: CR, complete remission; PR, partial remission; ORR, overall response rate.
Figure 1.
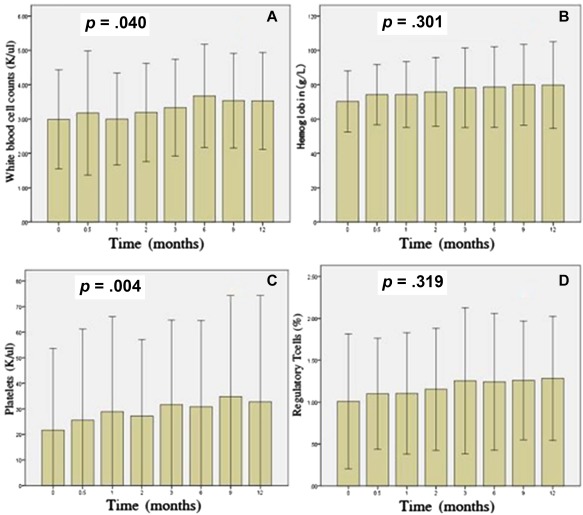
Peripheral blood cells and regulatory T (Treg) cells were quantified at baseline, and 0.5, 1, 3, 6, 9 and 12 months post‐mesenchymal stromal cell treatment. (A–D) show the changes of white blood cell counts, hemoglobin counts, platelet counts, and Treg percentages, respectively.
Bone Marrow Cellular Response
Bone marrow aspiration and bone marrow biopsy specimens were assessed for cellularity. In all patients with hematologic response, ten patients had normalization of cellularity followed for more than 1 year (Fig. 2).
Figure 2.
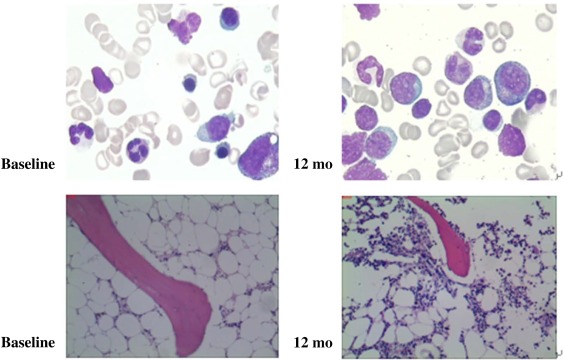
Bone marrow cellularity at baseline and at 12 months in a patient with trilineage responses to bone marrow‐derived mesenchymal stromal cells.
TTR
The median TTR of leukocytic linage was 19 days (range, 11–29). The median TTR of erythrocytic linage was 17 days (range, 12–25). The median TTR of megakaryocytic linage was 31 days (range, 26–84).
OS and Clone Evolution
The median follow‐up among survivors was 17 months (range, 3–24); the 2‐year OS rate was 87.8% (95% CI, 73.0%–95.7%; Fig. 3). Responders were all alive at the follow‐up endpoint. Nine patients died, all of whom were SAA patients. Three patients had progression to myelodysplasia (one with refractory anemia with excess blasts (RAEB)‐I, two with RAEB‐II). The median progression time was 11 months (range, 8–12). Two patients developed clonal cytogenetic abnormalities at 8 and 12 months, respectively. Others had normal karyotype through the study. Chromosome 7 abnormalities developed in one patient; another patient developed complex karyotype.
Figure 3.
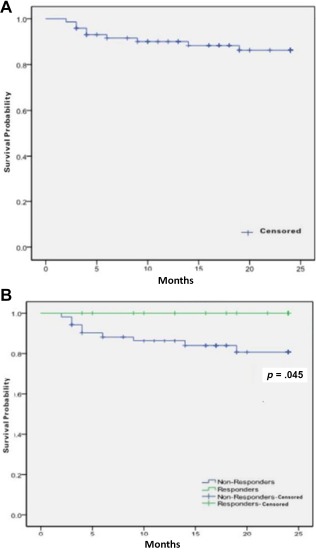
(A): Overall survival for all patients and (B): overall survival between responders and non‐responders.
Safety
Seven patients got fever (five with grade 1 and two with grade 2), two of them complicated with grade 1 headache. All seven patients underwent hemoculture examination, and the results were negative. No other adverse events were observed in the study. At the follow‐up endpoint, nine patients died. One patient with RAEB‐II died of disease progression, two patients died of intracranial hemorrhages, and six patients died of serious infection.
Response Predictors
We confirmed that ATG used in previous treatment and no infection throughout treatment were predictors for response (responders vs. non‐responders, 64.3% vs. 35.7%, 36.7% vs. 63.3%). We evaluated the relationship between ORR and several baseline prognostic markers (Table 1). The ORR was 35.0% and 20.6% for male and female patients, respectively. Among 24 SAA patients, the ORR was 41.7%. The ORR among the 50 NSAA patients was 22.0%. Eight of 14 patients treated with ATG previously and 13 of 60 patients without ATG previously achieved a remission, with an ORR of 57.1% and 21.7%, respectively. Patients with related, haploidentical, and unrelated donors had an ORR of 25.0%, 28.6%, and 33.3%, respectively. In univariate analysis, the ORR was significantly associated with ATG (p = .008) and no infection (p = .026). Multivariate logistic regression analysis for ORR confirmed statistical significance of no infection only (OR = 8.899, 95% CI = 1.649–48.020, p = .011; Fig. 4).
Figure 4.
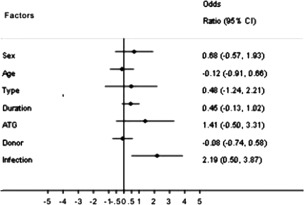
Odds ratio for overall response rate by subgroup. Abbreviations: ATG, antithymocyte globulin; CI, confidence interval.
Discussion
There are no standard therapies for AA refractory to IST and ineligible for HSCT. A second course of ATG plus cyclosporin, alemtuzumab, eltrombopag, and supportive care is usually recommended. For refractory AA, the response rate for rabbit ATG was 33% and 37% for alemtuzumab (humanized anti‐CD52 monoclonal antibody). The 3‐year survival was 60% for rabbit ATG and 83% for alemtuzumab 20, 21. Olnes et al. reported that 44% of refractory AA patients had a hematologic response in at least one lineage at 12 weeks for eltrombopag, an oral thrombopoietin mimetic 22. More than half of patients still have no or minor response to these above therapies. Moreover, expensive medical costs, toxicity, relapse, and clone evolution are also formidable problems.
BM‐MSCs are bone marrow stroma cells capable of multipotential differentiation that constitute the hematopoietic stem cell niche 23, so MSCs play an important role of supporting hematopoiesis. Moreover, MSCs possess powerful immunosuppressive properties. BM‐MSCs from AA patients show the same phenotype characteristics but decreased viability and proliferation and increased adipocytic differentiation 24, 25. BM‐MSCs from AA patients increase the production of hematopoietic inhibitors such as tumor necrosis factor‐α (TNF‐α) and interferon‐γ (IFN‐γ) and suppress Treg cells expansion through reduced transforming growth factor‐β 26. Millar et al. reported that mice given 9–10 Gy of total body irradiation died of bone marrow failure 10–14 days after exposure. Intravenous administration of MSCs improved survival in irradiated mice 27, 28. Normal murine MSCs impair activated dendritic cell through Toll‐like receptor 4, resulting in the decrease of secretion of hematopoietic inhibitors such as TNF‐α and IFN‐γ 29. Several studies have reported that MSCs can induce the generation of Treg cells 30, 31. Several studies about MSCs combined with allogeneic HSCT for severe AA reported that MSCs contributed to hematopoiesis recovery and reduction of GVHD 32. Based on the above research, MSCs infusion maybe a promising method for AA.
In our previous study, BM‐MSCs infusion was an effective treatment for AA, with a 30% response rate 15. In Fouillard et al.'s 13 report, bone marrow stroma of the patient improved but not hematopoiesis. This might be attributable to the sample size. Moreover, the status of that patient is different from our reported patients. The abnormal inflammatory microenvironment of the patient with severe infection might have impacted the effect of MSCs. Different microenvironments can lead to divergent immunogenicity of MSCs 33. Human MSCs exhibit broad‐spectrum antimicrobial effector function mediated by indoleamine 2,3‐dioxygenase 34. In inflammatory microenvironments, MSCs were consumed by excess inflammatory cytokines, and improvement of hematopoiesis was impaired. This was confirmed in our study. Most patients with infection had no response to MSCs. Multivariate logistic regression analysis showed infection was the most important predictor for response.
MSCs have long been reported to be associated with hypoimmunogenicity 35. This property is thought to enable MSC transplantation across MHC barriers. However, recent studies show allo‐MSCs generate antibodies and immune rejection, suggesting that MSCs may not actually be immune privileged. MSCs do not persist following infusion 36. Mice that received syngeneic erythropoietin‐MSCs (EPO‐MSCs) had a persistent increase in hemoglobin, whereas those that received allogeneic EPO‐MSCs had a transient increase in hemoglobin followed by a return to baseline 37. A randomized comparison trial of allogeneic versus autologous BM‐MSCs injected in endocardium in patients with ischemic cardiomyopathy showed that autologous but not allo‐MSCs were associated with an improvement in the 6‐minute walk test and the Minnesota Living With Heart Failure Questionnaire (MLHFQ) score 38. Infusion of allo‐MSCs can induce immune memory and stimulate innate immune responses 39. Furthermore, a complement is engaged on human MSCs’ surface, and MSCs are injured by the complement after their contact with serum 40. Nevertheless, so many successful reports of allo‐MSCs exist. Allo‐MSCs successfully cure diseases, including GVHD, arthritis, lupus, Crohn's disease, myocardial infarction, stroke, acute lung injury, cirrhosis, multiple sclerosis, and amyotrophic lateral sclerosis 41. Whether rejection of donor MSCs influences the efficacy of allo‐MSCs therapies is not known. According to the existing studies on MSCs, immunologic properties, and living animal experiments, we consider allo‐MSC's impact effect of cell therapy. It's a pity that we didn't carry out further genetic analysis to explore the effect with the MSC's donor derivation.
Compared with the TTR of 3–6 months of immunosuppressive agents, such as ATG and cyclosporine, MSCs have much shorter TTR within 1 month. Analogous phenomenon emerged in MSCs for other disease. The mechanism of fast action is not known. We only know that MSCs cannot persist following infusion. The duration of allo‐MSCs was within 20 days 42. For AA, short TTR may contribute to lower risk of serious infection and hemorrhage.
In several factors, we found the efficacy of MSCs was correlated with ATG therapy. Marsh et al. reported the mechanism of AA refractory to ATG, mainly including that the pathogenic mechanism is not immune mediated, and extreme HSC exhaustion and inadequate immune suppression persist 43. We consider in patients refractory to ATG that the first two factors may more contribute to AA, and MSCs could provide supplement for IST.
Most studies show that MSCs infusion is safe. There is no obvious severe adverse event connected with MSCs. In our study, there were only seven patients with transient fever and headache; no other adverse events connected with MSCs were observed. Now, a majority of tracing studies on MSCs reveal that MSCs persist within 20 days in mice in vivo 42. It seems to accommodate with the TTR. Because MSCs could not be implanted forever, long‐term adverse events appear unlikely.
Wei et al. reported that cytomegalovirus (CMV) infects MSCs in culture in vitro 44. The increased chance of virus infection caused by MSCs infusion may be concerning. In our study, there were no patients infected with CMV or Epstein‐Barr virus after MSCs infusion. We consider that MSCs infusion without large doses of immunosuppressive agents may not increase virus infection.
Clonal evolution occurs in 10%–15% of patients with AA; this is more frequent in patients with severe, refractory AA 45. In our cohort, three patients had progression to myelodysplasia (one with RAEB‐I, two with RAEB‐II). Abnormal karyotype was detected in two of them. Other patients with or without response did not undergo clonal evolution by means of cytogenetic analysis. BM‐MSCs didn't increase the clonal evolution opportunity.
Conclusion
Taken as a whole, the response rate of MSCs to AA refractory to IST was comparable to other methods. MSCs have the significant advantage of safety and costs. Our study strongly indicates that it is a promising therapy of MSCs infusion to AA, but the donor alternative, improvement of MSCs properties through improved culture in vitro, and doses of MSCs need to be further studied. Improved MSCs may optimize maximal therapeutic potential.
Author Contributions
Y.P.: conception and design, manuscript writing, collection and/or assembly of data, data analysis and interpretation, final approval of manuscript, clinical care of patients; Y.X.: conception and design, manuscript writing; H.W.X.: conception and design, manuscript writing, collection and/or assembly of data, data analysis and interpretation, final approval of manuscript, clinical care of patients; H.T.: conception and design; D.J.L. and X.D.: conception and design; Q.F.L.: conception and design, collection and/or assembly of data, data analysis and interpretation, final approval of manuscript, clinical care of patients; Z.H.: bone‐marrow‐derived mesenchymal stromal cells culture; L.L.: bone‐marrow‐derived mesenchymal stromal cells quality control; H.J.C., X.H.G., X.Y.W.: bone‐marrow‐derived mesenchymal stromal cells identification; Z.H.L., Y.G., H.B.L., Z.J.J., J.R.L., J.Y.W., D.N.N., X.Z.Z., D.R.X.: collection and/or assembly of data, data analysis and interpretation, final approval of manuscript, clinical care of patients.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Acknowledgments
We thank the clinicians, nurses, and data managers of the seven institutions that entered their patients into the study and provided the necessary data to make this study possible. In addition, we also thank those who spent their time to quality‐control tasks. This study was funded by Key Project of Natural Science Foundation of Guangdong Province (2014A030311006).
References
- 1. Young NS, Maciejewski J. The pathophysiology of acquired aplastic anemia. N Engl J Med 1997;336:1365–1372. [DOI] [PubMed] [Google Scholar]
- 2. Kwon JH, Kim I, Lee YG et al. Clinical course of non‐severe aplastic anemia in adults. Int J Hematol 2010;91:770–775. [DOI] [PubMed] [Google Scholar]
- 3. Scheinberg P, Nunez O, Weinstein B et al. Horse versus rabbit antithymocyte globulin in severe acquired apalstic anemia. N Engl J Med 2011;365:430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Valdez JM, Scheinberg P, Nunez O et al. Decreased infection‐related mortality and improved survival in severe aplastic anemia in the past two decades. Clin Infect Dis 2011;52:726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Menabde G, Gogilashvili K, Kakabadze Z et al. Bone marrow‐derived mesenchymal stem cell plasticity and their application perspectives. Georgian Med News 2009:71–76. [PubMed] [Google Scholar]
- 6. De Schauwer C, Piepers S, Van de Walle GR et al. In search for cross‐reactivity to immunophenotype equine mesenchymal stromal cells by multicolor flow cytometry. Cytometry A 2012;81:312–323. [DOI] [PubMed] [Google Scholar]
- 7. El Haddad N, Heathcote D, Moore R et al. Mesenchymal stem cells express serine protease inhibitor to evade the host immune response. Blood 2011;117:1176–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol 2012;12:383–396. [DOI] [PubMed] [Google Scholar]
- 9. Le Blanc K, Rasmusson I, Sundberg B et al. Treatment of severe acute graft‐versus‐host disease with third party haploidentical mesenchymal stem cells. Lancet 2004;363:1439–1441. [DOI] [PubMed] [Google Scholar]
- 10. Méndez‐Ferrer S, Michurina TV, Ferraro F et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010;466:829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chao YH, Peng CT, Harn HJ et al. Poor potential of proliferation and differentiation in bone marrow mesenchymal stem cells derived from children with severe aplastic anemia. Ann Hematol 2010;89:715–723. [DOI] [PubMed] [Google Scholar]
- 12. Li J, Yang S, Lu S et al. Differential gene expression profile associated with the abnormality of bone marrow mesenchymal stem cells in aplastic anemia. PLoS One 2012;7:e47764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fouillard L, Bensidhoum M, Bories D et al. Engraftment of allogeneic mesenchymal stem cells in the bone marrow of a patient with severe idiopathic aplastic anemia improves stroma. Leukemia 2003;17:474–476. [DOI] [PubMed] [Google Scholar]
- 14. Jaganathan BG, Tisato V, Vulliamy T et al. Effects of MSC co‐injection on the reconstitution of aplastic anemia patient following hematopoietic stem cell transplantation. Leukemia 2010;24:1791–1795. [DOI] [PubMed] [Google Scholar]
- 15. Xiao Y, Jiang ZJ, Pang Y et al. Efficacy and safety of mesenchymal stem cell treatment from related donors for patients with refractory aplastic anemia. Cytotherapy 2013;15:760–766. [DOI] [PubMed] [Google Scholar]
- 16. Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood 2012;120:1185–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schallmoser K, Rohde E, Reinisch A et al. Rapid large‐scale expansion of functional mesenchymal stem cells from unmanipulated bone marrow without animal serum. Tissue Eng Part C Methods 2008;14:185–196. [DOI] [PubMed] [Google Scholar]
- 18. Krampera M, Galipeau J, Shi Y et al. Immunological characterization of multipotent mesenchymal stromal cells–The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy 2013;15:1054–1061. [DOI] [PubMed] [Google Scholar]
- 19. Camitta BM, Thomas ED, Nathan DG et al. Severe aplastic anemia: A prospective study of the effect of early marrow transplantation on acute mortality. Blood 1976;48:63–70. [PubMed] [Google Scholar]
- 20. Marsh JC, Bacigalupo A, Schrezenmeier H et al. Prospective study of rabbit antithymocyte globulin and cyclosporine for aplastic anemia from the EBMT Severe Aplastic Anaemia Working Party. Blood 2012;119:5391–5396. [DOI] [PubMed] [Google Scholar]
- 21. Scheinberg P, Nunez O, Weinstein B et al. Activity of alemtuzumab monotherapy in treatment‐naive, relapsed, and refractory severe acquired aplastic anemia. Blood 2012;119:345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Olnes MJ, Scheinberg P, Calvo KR et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med 2012;367:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frenette PS, Pinho S, Lucas D et al. Mesenchymal stem cell: Keystone of the hematopoietic stem cell niche and a stepping‐stone for regenerative medicine. Annu Rev Immunol 2013;31:285–316. [DOI] [PubMed] [Google Scholar]
- 24. El‐Mahgoub ER, Ahmed E, Afifi RA et al. Mesenchymal stem cells from pediatric patients with aplastic anemia: Isolation, characterization, adipogenic, and osteogenic differentiation. Fetal Pediatr Pathol 2014;33:9–15. [DOI] [PubMed] [Google Scholar]
- 25. Tripathy NK, Singh SP, Nityanand S. Enhanced adipogenicity of bone marrow mesenchymal stem cells in aplastic anemia. Stem Cells Int 2014;2014:276862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li J, Lu S, Yang S et al. Impaired immunomodulatory ability of bone marrow mesenchymal stem cells on CD4(+) T cells in aplastic anemia. Results Immunol 2012;2:142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Millar JL, Stephens TC, Wist EA. An explanation for the ability of cytotoxic drug pretreatment to reduce bone marrow related lethality of total body irradiation (TBI). Int J Radiat Oncol Biol Phys 1982;8:581–583. [DOI] [PubMed] [Google Scholar]
- 28. Abdel‐Mageed AS, Senagore AJ, Pietryga DW et al. Intravenous administration of mesenchymal stem cells genetically modified with extracellular superoxide dismutase improves survival in irradiated mice. Blood 2009;113:1201–1203. [DOI] [PubMed] [Google Scholar]
- 29. Chiesa S, Morbelli S, Morando S et al. Mesenchymal stem cells impair in vivo T‐cell priming by dendritic cells. Proc Natl Acad Sci USA 2011;108:17384–17389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ma S, Xie N, Li W et al. Immunobiology of mesenchymal stem cells. Cell Death Differ 2014;21:216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Akiyama K, Chen C, Wang D et al. Mesenchymal‐stem‐cell‐induced immunoregulation involves FAS‐ligand‐/FAS‐mediated T cell apoptosis. Cell Stem Cell 2012;10:544–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu Y, Cao Y, Li X et al. Cotransplantation of haploidentical hematopoietic and umbilical cord mesenchymal stem cells for severe aplastic anemia: Successful engraftment and mild GVHD. Stem Cell Res 2014;12:132–138. [DOI] [PubMed] [Google Scholar]
- 33. Wang Y, Chen X, Cao W et al. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat Immunol 2014;15:1009–1016. [DOI] [PubMed] [Google Scholar]
- 34. Meisel R, Brockers S, Heseler K et al. Human but not murine multipotent mesenchymal stromal cells exhibit broad‐spectrum antimicrobial effector function mediated by indoleamine 2,3‐dioxygenase. Leukemia 2011;25:648–654. [DOI] [PubMed] [Google Scholar]
- 35. Klyushnenkova E, Mosca JD, Zernetkina V et al. T cell responses to allogeneic human mesenchymal stem cells: Immunogenicity, tolerance, and suppression. J Biomed Sci 2005;12:47–57. [DOI] [PubMed] [Google Scholar]
- 36. Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat Biotechnol 2014;32:252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Eliopoulos N, Stagg J, Lejeune L et al. Allogeneic marrow stromal cells are immune rejected by MHC class I‐ and class II‐mismatched recipient mice. Blood 2005;106:4057–4065. [DOI] [PubMed] [Google Scholar]
- 38. Hare JM, Fishman JE, Gerstenblith G et al. Comparison of allogeneic vs autologous bone marrow‐derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The POSEIDON randomized trial. JAMA 2012;308:2369–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zangi L, Margalit R, Reich‐Zeliger S et al. Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells 2009;27:2865–2874. [DOI] [PubMed] [Google Scholar]
- 40. Li Y, Lin F. Mesenchymal stem cells are injured by complement after their contact with serum. Blood 2012;120:3436–3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ankrum J, Karp JM. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol Med 2010;16:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zangi L, Margalit R, Reich‐Zeliger S et al. Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells 2009;27:2865–2874. [DOI] [PubMed] [Google Scholar]
- 43. Marsh JC, Kulasekararaj AG. Management of the refractory aplastic anemia patient: What are the options? Blood 2013;122:3561–3567. [DOI] [PubMed] [Google Scholar]
- 44. Wei G, Lin M, Cai Z et al. Cytomegalovirus infection in mesenchymal stem cells and their activation could be enhanced by nuclear factor‐κB inhibitor pyrrolidinedithiocarbamate in vitro. Transplant Proc 2011;43:1944–1949. [DOI] [PubMed] [Google Scholar]
- 45. Socié G, Rosenfeld S, Frickhofen N et al. Late clonal diseases of treated aplastic anemia. Semin Hematol 2000;37:91–101. [PubMed] [Google Scholar]


