Abstract
The umbilical cord has become an increasingly used source of mesenchymal stromal cells for preclinical and, more recently, clinical studies. Despite the increased activity, several aspects of this cell population have been under‐appreciated. Key issues are that consensus on the anatomical structures within the cord is lacking, and potentially different populations are identified as arising from a single source. To help address these points, we propose a histologically based nomenclature for cord structures and provide an analysis of their developmental origins and composition. Methods of cell isolation from Wharton's jelly are discussed and the immunophenotypic and clonal characteristics of the cells are evaluated. The perivascular origin of the cells is also addressed. Finally, clinical trials with umbilical cord cells are briefly reviewed. Interpreting the outcomes of the many clinical studies that have been undertaken with mesenchymal stromal cells from different tissue sources has been challenging, for many reasons. It is, therefore, particularly important that as umbilical cord cells are increasingly deployed therapeutically, we strive to better understand the derivation and functional characteristics of the cells from this important tissue source. Stem Cells Translational Medicine 2017;6:1620–1630
Keywords: Wharton's Jelly, Mesenchymal stromal cell, Embryology, Therapy
Significance Statement.
The connective tissue of the human umbilical cord, Wharton's jelly, is garnering increasing attention as a source of mesenchymal stromal cells, and is now being employed in clinical trials. In addition, in the public sector, parents wishing to store (bank) umbilical cord blood are increasingly being offered cord tissue, or the mesenchymal cells therein, as an additional banking service. However, there is little consensus on either the means by which cells are extracted from the tissue or the anatomical descriptors of the tissue itself. We propose, herein, a cord nomenclature‐based robustly on anatomical/histological structure and developmental origins, within the context of providing a foundation for not only the much‐needed methodological transparency in reporting of both basic and clinical studies, but also providing guidelines for the family banking sector.
Introduction
The human umbilical cord is an increasingly popular source of cells being developed for cell therapy. The reasons, often reiterated, are the noninvasive harvest from tissue normally discarded at birth, the relatively high cell yields, and a phenotype that parallels that of mesenchymal stromal cells from other tissue sources. These cells are now being employed in human clinical trials, while also providing a cell source for an increasing number of preclinical and basic studies. Several recent reviews have highlighted the therapeutic efficacy of umbilical cord‐derived mesenchymal stromal cells and their potential advantages over other sources 1, 2, 3, 4, 5. However, although the umbilical cord is structurally and compositionally a much simpler tissue than bone marrow, fat, or placenta, there is little consensus on either the structure of the connective tissue of the human cord or the means by which the cells contained therein are extracted. As the popularity of this abundant cell source increases there is a need to re‐appraise our understanding of the structure of this important organ and provide a foundation for establishing means by which methods of cell extraction, and phenotype, can be compared between those groups conducting not only preclinical, but also clinical, studies (see Fig. 1).
Figure 1.
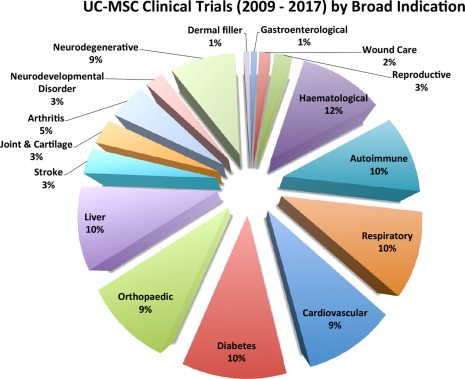
Registered clinical trials (2009–2016) employing human umbilical cord MSCs numbered a total of 109 as of January 2016, based on Clinicaltrials.gov data, although only 34 are currently open. The pie‐chart shows the broad distribution of target indications (excluding those from cord blood). Although “Haematological” indications are the largest group at 12%, the majority of trials rely on the immune modulatory and anti‐inflammatory properties of the cells, rather than a capacity for connective tissue lineage differentiation. These percentages differ from MSC trials employing cells from all tissue sources, where “Neuro‐degenerative” and “Liver” targets represent 60% of the total number of clinical trials. Abbreviation: MSC, mesenchymal stromal cells.
The Structure of the Human Umbilical Cord
In placental mammals, the umbilical cord is a structure that connects the placenta to the developing fetus, thereby providing a source of fetal nourishment. At term, in humans, it is 40–60 cm long, with a girth of 1–2 cm. The structure appears simple with an outer covering of a single layer of amniotic epithelium that encloses a mucoid connective tissue through which three vessels, a vein and two arteries, carry oxygenated and deoxygenated blood between the placenta and fetus, respectively. Unlike other vessels of similar diameter in the human, the umbilical vessels comprise only a tunica intima and media, but no tunica adventitia (see Fig. 2). The adventitial roles, considered to be vascular support and some contractile function, are considered to be fulfilled by the mucoid connective tissue, “Wharton's jelly”—first described by Thomas Wharton in 1656—that also prevents kinking of the vessels during movement of the fetus in the womb; although Wharton himself thought that the Jelly served as a surrogate lymph transport system 6. The jelly contains no other blood or lymph vessels and is not innervated. These characteristics, no adventia and no vessels other than the two arteries and vein, are not typical of other species commonly used in research, as discussed below. Thus, when the same methods to extract cells from umbilical cords are employed across species 7 the variation in structure provides a source of variability in the harvested cell population.
Figure 2.
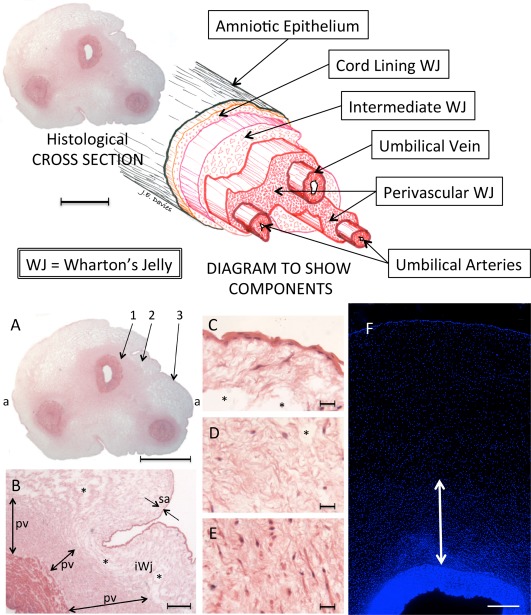
Above: The structure of the human umbilical cord with a three‐dimensional exploded diagram. The diagram was made by directly tracing the outlines of the various features in the histological section, then shifting them along the tilted longitudinal axis. Scale Bar = 5 mm. Below: The human umbilical cord. (A–E): A paraffin embedded section stained with haematoxylin and eosin. (A): Complete cross‐section of the cord showing an outer amniotic epithelium and three vessels contained within Wharton's jelly. The latter is more highly stained in the perivascular zones due to an increase in both cells and matrix. The paucity of staining in those areas beyond the perivascular zones is, in part, due to the presence of clefts [see (B–D)]. Maximum width (a‐a) = 1.8 cm. (B): Enlargement spanning regions 1 and 2 in (A) where the transition from tunica media of the umbilical vein to perivascular Wharton's jelly is clearly seen, as is the transition from perivascular zone to the remainder of Wharton's jelly and the amniotic epithelium. The matrix of the subamniotic Wharton's jelly is marginally denser than that of the intermediate Wharton's jelly, which separates it from the perivascular zone. pv, perivascular zone; sa, subamnion; i, intermediate Wharton's jelly; *, clefts. (C): From area 3 in (A): The amniotic epithelium and the subamniotic Wharton's jelly (sometimes called the “cord lining”). The cells have a stellate morphology. Takechi et al. 27 were the first to report that cells in this subamniotic zone were longer and had more cytoplasmic processes than those in the perivascular zones. *, clefts. (D): From area 2 in (A), the intermediate Wharton's jelly that contains sparse cells, which are small and more rounded in shape, and matrix that surrounds many clefts. *, clefts. (E): From area 1 in (A), the perivascular zone with denser matrix and numerous cells. The latter of varying morphologies, some elongated, some rounded, and others stellate. (F): DAPI (4′,6‐diamidino‐2‐phenylindole) staining of a portion of cord including the vascular tunica media (dense blue structure below), Wharton's Jelly, and the amniotic epithelium (thin dense blue line above). DAPI staining is densest in the perivascular zone and decreases toward the amniotic epithelium. White arrow, perivascular zone. Although the cell density in the amniotic epithelium is clear, there is no obvious increase in cell density in the subamniotic zone compared to that of the iWJ. [Image courtesy of Shiva Hamidian Jahromi]. Scale Bars: A = 5 mm; B = 500 µm; C–E = 100 µm; F = 200 µm. Abbreviation: WJ, Wharton's jelly.
Nanaev et al. 8 identified three regions within the term human umbilical cord, based on the distribution of extracellular matrix proteins and cytoskeletal features of the stromal cells: the subamniotic zone, Wharton's jelly, and the combined media and adventitia of the blood vessels. However, since adventitia per se is not present, Wharton's jelly comprises all the tissue from the outer margins of the tunica media to the inner surface of the amniotic epithelium. A major focus for Nanaev et al. 8 was the description of clefts within the Wharton's jelly that were considered to be regions devoid of collagen but containing only ground substance, and thought to play an important role in the mechanical properties of the tissue. The presence of clefts provides a useful landmark when examining Wharton's jelly since they are absent from the perivascular and subamniotic zones, but easily visible at low magnification in routine, for example haematoxylin and eosin stained, cross‐sections of cords. Nanaev et al. 8 also observed that the cells of the three zones represented various stages of differentiation of fibroblasts toward the myofibroblast lineage, and also discussed the different embryological origins of the central and subamniotic connective tissues of the cord; although it is worth noting that their work pre‐dated the first publications mentioning stem cells in Wharton's jelly by 7 years 9.
The regional classification of cord anatomy was expanded in the highly cited review of Can and Karahuseyinoglu 10. They identified six zones within the human umbilical cord: (a) the surface (amniotic) epithelium (b) subamniotic stroma (c) clefts (d) intervascular stroma (e) perivascular stroma and (f) the vessels. Three issues are noteworthy here. First, they stated that only the intervascular tissue was classically described as Wharton's jelly, although there is no evidence for this in either the original description by Thomas Wharton in his treatise Adenographia 6, or in subsequent literature. Second, they conclude, by an interpretation of the findings of Nanaev et al. 8 that the subamniotic zone contained immature cells retaining the ability to proliferate whereas the perivascular zone contained highly differentiated cells. In fact, Nanaev et al. 8 noted that the cells in these two zones were from differing embryological origin, and that the subamniotic cells were delayed in their differentiation compared to those in the perivascular zone, which were more numerous (see also Fig. 2). Finally, although the amniotic epithelium was identified as a distinct zone, the endothelial linings of the vessels were not mentioned although umbilical cord endothelial cells had been isolated since 1963 11.
Defining the structural—or anatomical—descriptors of Wharton's jelly is important because their employment, by different authors, is inconsistent. This is nontrivial since some authors claim to extract cells from one region of the cord, to the exclusion of others and report the cell phenotype obtained. Given the above, a hypothetical example could be that three different authors could claim to extract cells from Wharton's jelly when one extracts cells from only the intervascular jelly, the second from the whole of Wharton's jelly, as described herein, and the third from Wharton's jelly excluding the perivascular tissue. Examples of each of these can be found in the extant literature. However, when varying descriptions of the regions/zones of the cord are combined with a lack of sufficient methodological information regarding the isolation techniques employed to harvest the cells, the comparison of cell populations employed by different authors becomes fraught with difficulty. This is not only an academic issue that needs a solution so that questions regarding the putative phenotypes of the cells from various regions/zones of Wharton's jelly could be realistically addressed but, as the umbilical cord becomes a more popular source of cells for clinical trials, some of which are driven by industry, the growing patent estates directed at extraction of cells from the human umbilical cord will also be obliged to face the same challenges of terminology and methodologic transparency.
An example of these variances can be seen in the multiple definitions of the perivascular tissue of the cord. As stated, some authors do not consider this part of Wharton's jelly, but the description of the perivascular zone varies widely as summarized in Table 1, where the order is in increasing perivascular dimension rather than publication date. First, it can be seen that the dimensions of this zone have not changed according to the year of publication, it is also clear that some authors have not mentioned it at all. However, there would seem to be a trend where the smaller dimensions are allotted by authors who have not provided histological evidence of their dimensions, but rather drawn a cartoon figure. Similarly, partial histology, defined here as provision of a histological image of a single, or partial, vessel have returned intermediate values while those examples where full cross‐sectional histology of the cord has been provided, return the highest dimensional values for the perivascular zone. These differences are stark, and range from a perivascular layer only two cells thick 13 to histological evidence of up to 2,000 microns 18 (See Fig. 2). In their paper, Karahuseyinoglu et al. 17 who provide partial histology, designate the arterial perivascular zone as 600 microns. While this does apply to arterial perivascular tissue, that around the vein is thicker, up to 2,000 microns, as seen in Figure 2 and it is noteworthy that it is the umbilical vein that carries oxygenated blood. Thus, although these zones can be easily identified anatomically, other authors have described, or graphically illustrated, them quite differently. For example, Troyer and Weiss 14, Conconi et al. 19, and Coskun and Can 15 all illustrated the perivascular tissue as a “layer” of relatively uniform width that (assuming an average total cord girth of 1.5 cm) was 50–150 microns in width. However, these authors provided no rationale for their choice of these dimensions assigned to the perivascular region.
Table 1.
A comparison of the dimensions cited in the literature for the perivascular tissue of the human umbilical cord arranged in increasing dimensions
| Reference | Width of perivascular zone | Evidence provided | Measurement method |
|---|---|---|---|
| Watson et al. 12 | Not mentioned | Cartoon | Cord cartoon shows only one artery and one vein. |
| Kita et al. 13 | 2 cells thick | Cartoon | Cord cross‐section histology was provided but with only the cord lining membrane labeled. Cartoon shows the perivascular cells as only 2 cells thick. |
| Troyer and Weiss 14 | <250–370 μma | Cartoon | Measurements made from authors' cartoon |
| Coskun and Can 15 | 200–500 μma | Cartoon | Measurements made from authors' cartoon |
| Schugar et al. 16 | 430 ± 120 μm | Partial histology and cartoon | Although authors report direct measurements from histology sections, only partial histology was illustrated. Cartoon figures show averages for specific measurements made. |
| Karahuseyinoglu et al. 17 | Approx. 600 μm | Partial Histology | Authors drew a ring around one umbilical artery that, given their scale bar, would give a perivascular zone of 600 μm width. |
| Subramanian et al. 18 | 350–1,550 μm | Histology | Authors illustrate direct measurement from whole cord section. |
| This text | 750–2,000 μma | Histology | “Thin perivascular areas” were described around each vessel. Measurements made from their provided histology. |
Dimensions have been measured from authors’ cartoon based on the average width of a cord, at term, being 1.5 cm. “Partial histology” refers to a single cord vessel, or part of a cord vessel being illustrated.
In a detailed treatment of the umbilical cord, the average perivascular width was reported by Schugar et al. 16 as 430 ± 120 microns, and supported with partial, arterial, histology. In fact, the perivascular regions are not only more variable in width (as measured from the outer margin of the tunica media of the vessels) but may appear to be joined so that the perivascular region forms a continuum around two or all three of the vessels. However, these joining areas are of a slightly lesser cell density and designated the intervascular Wharton's jelly by Can and Karahuseyinoglu 10. The intervascular areas can be seen to vary from 500 to 830 microns between the perivascular Wharton's jelly in Figure 2. However, it should be noted that these dimensions are highly variable both within the same cord and between different cords, as indeed the girth of individual cords is generally considered to vary from 1 to 2 cm at term 20.
The identification of the perivascular zones is important because, as Schugar et al. 16 reported this zone that measures an average of 430 microns radially around the tunica media, contains almost 45% of all cells in Wharton's jelly, and thus the method by which cells are extracted from Wharton's jelly will be profoundly influenced by the inclusion, or exclusion, of the perivascular cells. It can reasonably be assumed that the perivascular tissue, as seen in Figure 2, will therefore contain considerably more than 45% of all cells in Wharton's jelly.
Clearly, when evaluating a cross‐section of the human umbilical cord, the perivascular zone previously described as the adventitia of the umbilical vessels (Nanaev et al.) 8, and subsequently illustrated by Subramanian et al. 18, or described by Schugar et al. 16 as the densest cell population in Wharton's jelly, can provide a histological/anatomical descriptor as equally obvious as that of the thin amniotic lining. Thus, we provide a nomenclature for the whole cord, based on such anatomical and histological foundations in Table 2, and illustrated in the diagram of Figure 2.
Table 2.
A proposed nomenclature for the structure of the human umbilical cord based on distinguishable histological features
| Wharton's Jelly | Vessels | ||||
|---|---|---|---|---|---|
| Amnion Epithelium | Subamnion | Intermediate WJ | Perivascular WJ (intervascular WJ) | Tunica media | Tunica intima |
| 1–3 cells thick | 100–150 microns thick. Essentially devoid of clefts. Possibly retains derivation from somatopleuric amniotic mesenchyme. | Sparse matrix and cells. Easily distinguished by the presence of numerous clefts containing only ground substance | 200–2,000 microns thick. Essentially devoid of clefts. Appears more dense than Intermediate WJ. Most likely derived from extraembryonic mesoblast | Two layers of smooth muscle orthogonally arranged | Single layer of endothelial cells |
| Cord “lining” | HUCPVCs | HUVECs | |||
| Can be isolated by careful separation from the intermediate WJ, perivascular WJ, and vessels | Theoretically, could be isolated after careful removal of cord lining, perivascular WJ, and vessels | Can be isolated either manually or enzymatically (intervascular WJ cannot be separated from the perivascular WJ) | HUVECs = Human umbilical vein endothelial cells | ||
| HUCPVCs = Human umbilical cord perivascular cells | |||||
The Cord can be divided, as others have suggested, into three major components (a) the amnion (b) Wharton's Jelly and (c) the vessels. Wharton's jelly itself can be divided into three distinct zones (a) the subamnion, (b) the intermediate Wharton's Jelly, and (c) the Perivascular Wharton's Jelly. The amnion and subamniotic Wharton's Jelly has been designated the “cord lining.” Another region between the perivascular Wharton's Jelly surrounding each vessel can be designated the “Intervascular Wharton's Jelly.” However, from a pragmatic perspective it is only the cord lining and the perivascular Wharton's Jelly that can be distinctly dissected from the remaining regions or zones. Finally, the vessels each comprise a smooth muscle wall and an endothelial lining. Abbreviation: WJ, Wharton's jelly.
A Note on the Development of the Umbilical Cord
Much of the information in this section has been extracted from various sections of Gray's Anatomy 21 and provides an update on the account of Nanaev et al. 8 since our understanding of the development of the cord has evolved in recent years.
Although the structure of the umbilical cord would appear relatively simple, its embryological development results in mesenchymal contributions from several sources. In human development, after implantation of the blastocyst at the end of the first week following fertilization, the early fetus is initially connected to the maternal endometrium through the invading trophoblast. This connection rapidly develops into the connecting stalk, which is, therefore, formed from the extraembryonic mesoblast, that covers the amnion, secondary yolk sac, and the wall of the mural trophoblast. Soon, during early hind‐gut development, the connecting stalk mesenchyme is invaded by the allantoic duct, which is a site of vasculogenesis and gives rise to the umbilical vessels that connect with the placental circulation. Thus, as the umbilical cord develops it comprises an outer covering of amniotic epithelium and a coalescence of somatopleuric amniotic mesenchyme with both splanchnopleuric vitellointestinal and allantoic mesenchymes. The latter, with which the umbilical vessels are most intimately associated, the perivascular tissue, could reasonably be assumed to originate from the extraembryonic mesoblast. However, the cord grows rapidly in both length and girth to term and the increase in the volume of Wharton's jelly is commensurate with this growth. Since Schugar et al. 16 have convincingly demonstrated that the majority of mesenchymal cells in the term cord are in the perivascular regions (see above), it would seem reasonable to assume that this region is also the site of the majority of the precursor cell proliferation that drives the increase in Wharton's jelly volume (see also the discussion of Nanaev et al. 8 above). It is also interesting to note that amniotic fluid contains PDGF‐AB 22. Since it is known that some perivascular cells are recruited via (PDGF)‐B/PDGF receptor‐ß signaling, and that umbilical cord perivascular cells have been shown to be PDGF‐Rß+, such signaling may contribute to perivascular cell migration outward from the vasculature. Indeed, comparing the distribution of CD146+ cells in first trimester and term cords, it is clear that Wharton's jelly becomes populated with such cells during gestation (see Fig. 3). CD146, together with NG2 and PDGF‐Rß have been shown to identify a subset of perivascular cells that also express mesenchymal stromal cell markers, in vitro, which would support the hypothesis that the perivascular tissue contains a progenitor cell population that can give rise to the differentiated myofibroblasts that represent the functional phenotype of Wharton's jelly cells.
Figure 3.
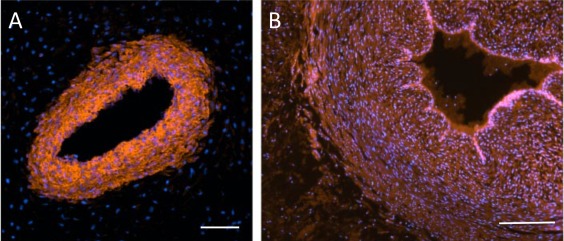
CD146 labeling of a first trimester cord (A) and a term cord (B) counterstained with DAPI. In the first trimester, the smooth muscle vessel wall labels positively, but the surrounding tissue is negative for CD146. On the contrary, at term the vascular endothelium is now highly positive, the vessel wall is still positive, but so also are cells in the perivascular tissue. Scale Bars A, B = 100 µm.
A Note on Comparative Anatomy
Although the purpose of this review is to focus on the human umbilical cord, it is worth noting that some authors have described employing the same cell extraction procedures for human and other mammal species. This is particularly relevant to those using enzymatic cell isolation procedures of more than one of the tissue types in the cord structure, amnion, Wharton's jelly, and vessels, because cord structure, and thus composition, can be quite different between species. Figure 4 provides examples of these differences for human, equine, canine, and porcine cord. In these, the human cord is unique as the equine cord has four major vessels and multiple collateral vessels, a similar composition is seen in the canine cord, while the porcine cord—although superficially similar to the human—displays several small vessels within the Wharton's jelly. The major vessels of the equine cord also, unlike those found in the human cord, possess a well‐developed tunica adventitia.
Figure 4.
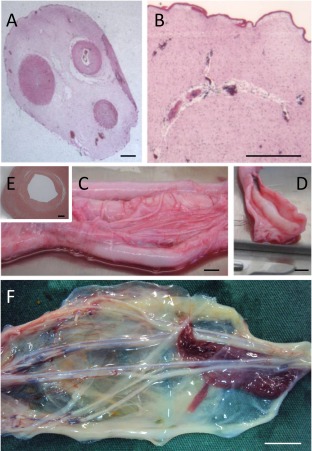
Comparative anatomy of the umbilical cord. (A, B): Porcine, (C‐E): Equine, (F): Canine. (A): Unlike the human cord, the perivascular zones are not clearly demarcated in the porcine cord. (B): In the porcine cord, Wharton's jelly contains many small vascular structures. (C): The equine cord has four major vessels and many co‐lateral and branching vessels. (D): Each of the main vessels has a well‐developed tunica adventitia as seen in this dissected specimen. (E): The tunica adventitia is distinct from the tunica media in this H&E stained cross‐section. (F): Like the equine cord, the canine cord has multiple vessels with co‐lateral branching. The vessels are contained within the amniotic epithelium but, unlike the human, porcine and equine cords, does not from a firm roughly circular but rather a diffuse and flattened structure. [Image courtesy of Dr. Emily Correna Carlo Reis]. Scale Bars: A, B and E = 1 mm; C, D and F = 1 cm.
The Composition of Wharton's Jelly
Wharton's jelly is classified as a connective tissue. Some consider Wharton's jelly a simple connective tissue in contrast to the liquid or skeletal connective tissues 23, but it is most commonly classified as a mucoid 21, or mucous 24, 25, connective tissue. The common property of connective tissues is that they comprise cells surrounded by the extracellular matrix that they have elaborated. Of course, Wharton himself knew nothing of cells within his “Jelly,” as cells were described for the first time 9 years later by Hooke 26 in 1665.
Usually, other cells are also present in the matrix of connective tissues including some form of phagocytic cell type and also those providing vascular and nervous elements. In this respect, the Wharton's jelly of the human cord is unique among connective tissues as it contains only mesenchymal cells that comprise the functional myofibroblasts of the tissue, and their precursors. There are no other cell types described in Wharton's jelly, and no vascular or nervous elements, except the three major vessels of the cord itself.
It was McElreavey et al. 27 who first extracted fibroblast‐like cells from the human umbilical cord, and Takechi et al. 28 reported cells, resembling fibroblasts containing myo‐filaments, and stained positive for both vimentin and desmin. The cell distribution within Wharton's jelly was described as densest in the perivascular area and loose under the amniotic epithelium. Furthermore, the peripheral cells had longer and more numerous cytoplasmic processes than those in the perivascular regions. These morphological differences may point to differences in embryological derivation (see above), although we are not aware of any studies that have explored such issues. These authors, together with others later, observed that the perivascular region appeared to serve the function of an adventia, which is not present the vessels of the human umbilical cord, although can be found in other species—see the comments on the equine cord above. It was over a decade later that several laboratories showed that these cells had the properties of mesenchymal stromal cells (MSCs) 29, 30, 31, 32; (see below for discussion of MSCs).
A connective tissue matrix will comprise a fiber component, most commonly collagen, and a ground substance. Collagen I is the predominant protein 33 in Wharton's jelly. Gogiel et al. 34 showed that Wharton's jelly contains mainly small chondroitin/dermatan sulphate proteoglycans with decorin predominating over biglycan. Meyer reported that 95% of Wharton's jelly is extracellular matrix comprising collagen (3.6), glycoprotein (0.3) hylauronin (0.31) sulfated glycosaminoglycan (0.14) and diffusible plasma proteins (1.2)—all % wet weight 35.
What is the Nutrient Supply for the UC?
The only blood supply in the human umbilical cord is through the umbilical vein and two arteries. The umbilical cord forms at day 26 at which time the amniotic fluid, which is generated from maternal plasma, comprises mostly electrolytes and water. This changes in volume and composition throughout gestation and by about 12 weeks the liquid contains proteins, carbohydrates and lipids to which urea is added when fetal kidney function starts around week 16. Of the many roles of the amniotic fluid, a nutrient contribution to cells of, and beyond, the amniotic membrane is clearly important 36. Thus, the amniotic fluid provides an additional source of nutrients to the cells of the umbilical cord especially those in the subamniotic region, which may explain the small increase in cell density in the outer 100–150 microns of Wharton's jelly.
Wharton's Jelly as a Source of MSCs
Conventional wisdom dictates that the human umbilical cord is a rich source of MSC. Indeed, it is the advent of MSC biology that has driven the increasing interest in umbilical cord tissue as witnessed by the increasing number of publications in the last two decades. Given the embryological derivation of the cord (see above) and the multiple mesenchymal sources that contribute to its formation, it is not surprising that some progenitor populations can be found in every region of Wharton's jelly 18 although what is not yet clear is whether their phenotypes are equivalent. Given the opinion that the vasculature of the cord is the predominant nutrient supply and that perivascular cells are known to migrate away from their vascular niche (see below), it may be that the majority of MSC in Wharton's jelly originate in the perivascular region.
We adhere herein, in concordance with the ISCT position paper of 2006 37, to the term mesenchymal stromal cell (MSC) rather mesenchymal stem cell since it is rarely that authors have demonstrated the stem‐like qualities, of self‐renewal and multilineage differentiation potential of offspring, of the cells they have isolated from the human umbilical cord. One exception to this was the work reported by Sarugaser et al. 38 that did show, at the single cell clonal level, that true self‐renewing cells existed in the perivascular tissue of the cord that gave rise to multilineage offspring—work that was only possible due to the high CFU‐F frequency in the cell populations extracted from the perivascular tissue (see Fig. 5 and “Cell Phenotype” above right). No such studies have been reported with cells from other regions of Wharton's jelly. Furthermore, even the stem cell population itself is heterogeneous as different MSCs had different renewable multilineage differentiation capacity. Thus, employment of the term “mesenchymal stem cell” in the singular is misleading as a family of mesenchymal stem cells were shown to exist.
Figure 5.
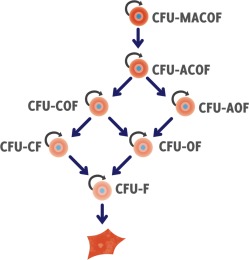
Due to the high CFU‐F frequency, Sarugaser et al. undertook differentiation assays on both parent and daughter single cell derived clonal populations. Various self‐renewing mesenchymal stromal cells types were identified resulting in the hierarchy show here. The semicircular arrows represent populations that are self‐renewing. The default lineage is the fibroblast. Thus, some stem cells self‐renew and parents and daughters both display five‐lineage differentiation while others are capable of only 4, 3, or 2 lineage differentiation. A self‐renewing fibroblast was also identified. Abbreviations: MACOF, muscle, adipo, chondro, osteo, and fibro; WJ, Wharton's jelly.
The yield of MSC from Wharton's jelly has been shown to depend on the method of cell extraction employed. Using a CFU‐F assay as a surrogate MSC measure, this can be illustrated by a comparison of Sarugaser et al. 29 and Lu et al. 32. While the former report a CFU‐F of 1:300 at harvest from an isolated UC perivascular population, Lu et al. extracted their cells by mincing umbilical cords into 1–2 mm 32 fragments, sequentially incubating with collagenase and trypsin and passing the resulting product through filters to obtain cell suspensions which would have contained all cell types in the umbilical cord. They reported a CFU‐F of 1:1,609 which, although an order of magnitude lower than Sarugaser et al., 29 was still positively compared to values of 1:10,000 to <1:100,000 reported for bone marrow MSCs 32, 39. Furthermore, Lu et al. 32 showed that the population doubling (PD) time of 24 hours was stable through to P10; compared to a bone marrow PD of 40 hours. Given the high CFU‐F frequency, it is of interest to consider why the perivascular zone of the umbilical cord has such a high density of stem cells in situ. This could, in part, be explained by the rapid growth of Wharton's jelly that is commensurate with the increase in length and girth of the cord during gestation. This could be borne out by the finding of mitotic figures in early trimester cords but, to our knowledge, such a study has not been undertaken.
However, two important points should be emphasized.
First, although the CFU‐F frequency at harvest (1:300) is high compared to other tissue sources of MSC, it still means that the majority of cells at harvest, 299 out of 300 are not stem cells. Many of these will be the myofibroblasts that Nanaev et al. 8 identified in five stages of differentiation based on their sequential and additive expression of vimentin, desmin, α‐smooth muscle actin, ‐γsm actin, and sm myosin.
Second, from the therapeutic standpoint, we do not yet know whether the true stem cells within an extracted population are of any particular significance. Certainly, for applications requiring differentiation down a specific connective tissue lineage, the stemness of the population would be important. However, since the majority of targeted clinical applications of MSC rely on their paracrine signaling potential, the differentiation potential of the cells may be either irrelevant to their clinical utility or, worse, could provide clinical complications should unwanted differentiation occur in vivo.
Methods of Cell Isolation from Wharton's Jelly
Umbilical cord tissue is a source for a variety of different cell types: endothelial cells, smooth muscle cells, stromal cells, and epithelial cells and although we focus here on those from Wharton's jelly, some authors have isolated all cell types independently 40, and many have chosen not to isolate the Wharton's jelly from either the vessels or amniotic epithelium before harvesting cells. In the latter cases, therefore, the harvested cell population may demonstrate various degrees of heterogeneity dependent upon the method employed. Conversely, the only cell isolation information provided by some authors is that “MSCs were isolated using the classical adhesion method,” 41 which clearly renders the reader incapable of knowing what cells have been employed. Furthermore, as various authors have used terms describing various regions, or zones, of Wharton's jelly differently (see above) it is rarely possible to glean from the published methods, which cells are specifically being cultured in experiments, or more importantly, employed in clinical trials (see below).
Cell Phenotype
As mentioned, MSCs have been extracted from many tissues and the characterization of the cells, usually takes place after seeding the harvested population in a culture vessel. Indeed, the first of the minimal criteria set out by the ISCT 37 is that the cells are culture adherent. Taken literally, this would mean that MSC can only be described ex vivo; yet conventional wisdom holds that such cells—both mesenchymal cells and mesenchymal stem cells, do exist in vivo. Thus, much has been reported on extracted cell populations, but relatively little is known of their phenotype in vivo. For this reason, some authors have focused on characterizing the cells in situ, since they have been shown to rapidly change their phenotype on entering a cell culture environment, and thus a retrospective analysis does not provide insight into their in vivo phenotype.
Farias et al. 42 specifically distinguished between in situ umbilical cord stromal stem cells (isUCSSC) and cultured UCSSC. They also identified three zones: amnion, Wharton's jelly and vessel walls within the cord, and showed arterial perivascular jelly of about 650 microns depth before and after digestion. Indeed, Farias et al. opined that all cultured umbilical cord stromal stem cells “were 100% positive for CD10…….indicating that they all derived from the Wharton ’ s jelly remaining around the blood vessels.” 42 However, upon tissue digestion, the cells are negative (or lowly expressing) for many of the defined stem cell markers, but after one passage these levels are considerably increased. This is exemplified by Margossian et al. 43, who characterized cells from the Wharton's jelly immediately following isolation by enzymatic digestion and compared to cells grown for one passage on tissue culture plastic. Importantly, expression of CD73, CD90, and CD105, positive markers for MSCs as outlined by the ISCT's minimal criteria for defining MSCs 37, are expressed in only a small fraction of cells in freshly digested tissue, but are greatly increased following a single passage on tissue culture plastic 43. Indeed, it is the very simplicity of the tissue structure of the cord that permits such in situ and in vitro comparisons, which are considerably more challenging with marrow, adipose tissue, or placenta.
Based on the criteria outlined by the Dominici et al. 37, it is necessary for MSCs to be characterized in vitro due to the requirements of plastic adhesiveness, and trilineage differentiation. However, it is interesting to consider that even the generally accepted cell surface marker expression profile requires cell culture to modify the original cell phenotype. Thus, based on these criteria, cells that are isolated are in fact not a population of MSCs, but instead become MSCs following in vitro expansion. So, either culture is selecting for a specific subset of the isolated cells, and thereby enriching them, or else the cells are changing their phenotype. Importantly, this disconnect between MSCs and their in vivo counterparts has been suggested for all MSC sources 44, 45. While this has not hampered the utility of these cells as therapeutics, a greater understanding of MSCs in their natural niche could prove beneficial for advancing the current generation of therapeutics under investigation and provide critical insight into tissue homeostasis and regeneration following injury.
Another consideration relates to the state of oxygenation of the tissue in vivo. The pO2 of oxygenated blood within the umbilical vein is approximately 20%–30% of that within adult arteries 46, and a lack of microvessels within Wharton's jelly, lead to the hypothesis that the stromal cells within the cord reside in a relatively hypoxic environment, a sentiment that has also been postulated by others 47. It is well established that bone marrow (BM)‐derived MSCs grow faster under hypoxic conditions in vitro and have a greater CFU‐F frequency over normoxic conditions 48, 49. However, “hypoxia” in culture often mimics normoxic oxygen tension in vivo 50. In fact, in vivo low O2 does not affect BM CFU‐F, but does increase peripheral blood CFU‐F 51. Indeed, the rich source of MSCs in the cord may, in part, be because MSCs survive better in low oxygen environments than fibroblasts.
Are Wharton's Jelly MSCs Perivascular in Origin?
An influential study by Crisan et al. showed that MSCs could be obtained by isolating populations of perivascular cells from a wide range of adult and fetal tissues 52, creating a strong link between MSCs and a specific location in situ. The term “perivascular cells” commonly includes classically defined pericytes surrounding the microvasculature, as well as other nonpericyte cells within the perivascular niche of the macrovasculature which have also been shown to display properties of MSCs 53, 54, 55. However, recent evidence would suggest a clear functional distinction between pericytes and MSC progenitors in the perivascular niche 56. This may help to explain the case in the umbilical cord where MSCs have been derived from all regions from the perivascular zones to the subamnion. However, as the amniotic fluid provides a source of nutrients to the cord tissue this, together with the disparate embryological origins of the tissue as described above, could explain the nonperivascular distributions of MSCs in cord tissue.
The perivascular zones of the human umbilical cord contain the most abundant source of MSCs (Schugar et al. 16), but Mennan et al. 57 and Subramanian et al. 18 have both shown that cord lining cells exhibit trilineage differentiation potential. The amnion consists solely of epithelial cells, and thus not MSCs 58, but others have also noted stem cell characteristics in this population 59, although caution should be expressed since Miki et al. 59 referenced an extraction technique first described by Akle et al. 60 where they stated that “The amniotic membrane thus obtained consisted of an epithelial monolayer on a basement membrane with an underlying collagen matrix containing a few fibroblasts.”(another example of how harvesting procedures can affect the interpretation of experimental outcomes). Nevertheless, the sub‐amnion is mesenchymal and does contain MSCs 13, 18, 57, 61. Thus, there is evidence, at least in the umbilical cord, that MSCs can be isolated from nonperivascular regions. However, whether these nonperivascular MSCs represent a distinct lineage from those in the perivascular region is unknown.
It may be that these nonperivascular MSCs are not distinct from the perivascular MSCs, but are instead simply a population of cells migrating away from the vasculature. This process is well characterized in adult tissue where perivascular cells migrate from the vasculature to participate in the fibrotic repair and wound healing 62, 63, 64. Moreover, MSC transport via the circulation occurs normally in adults, albeit at low frequency. Chong et al (2012) 65 harvested 1–2 million peripheral blood monocytes from 2 ml of human peripheral blood that yielded 0.5–1 million culture adherent cells after 2 weeks. In vivo, such migration may be enhanced by tissue injury 66, and also in the developing fetus, as evidenced by the isolation of MSCs from umbilical cord blood, albeit at variable rates of success 67, 68. Thus, there is evidence of MSCs migrating away from their perivascular niche during development. However, if nonperivascular MSCs within the umbilical cord are indeed derived from those in a perivascular niche, it would be expected that their migration would be directed by a chemotactic gradient. One possible mechanism for this, as mentioned above, is through the PDGF/PDGFRβ axis. Signaling through this pathway results in a potent chemotactic and mitogenic response seen in pericyte recruitment, both during development 69, 70, and following tissue injury 71, 72. In cell culture, it has been reported that amniotic epithelial cells produce PDGF‐B 73, 74, and therefore, could potentially serve as a stimulus for perivascular migration in vivo. Whether such a response does truly occur during development, however, has never been investigated. Recruitment of perivascular cells does fit well with the demand for matrix production within the Wharton's jelly to minimize forces on the umbilical cord vessels which might otherwise impair circulation. Similarly, as discussed above, abundant matrix production as seen in fibrotic disease also involves pericyte recruitment.
While we have speculated that some nonperivascular cells within the umbilical cord are derived from a perivascular lineage, currently there is no specific marker or set of markers to identify specific populations of nonperivascular cells within the umbilical cord that give rise to MSCs.
UC MSCs as Therapeutics for Human Disease
As illustrated in Figure 1, umbilical cord clinical trials (excluding those using cord blood) have been targeted at fourteen broad groups of medical indications. The percentage split between indications clearly illustrates a preponderance of trials for autoimmune, cardiovascular, haematological, hepatic, neurodegenerative, and orthopedic targets, each of which commands approximately 10% of the total number.
The Clinicaltrials.gov data for the above trials provides no details of the methods employed to extract umbilical cord cells and thus it is not possible to provide any comparative assessment of the individual therapeutic products. However, five publications have resulted, to date, from these clinical studies from which some information regarding the cell populations can be obtained. Lv et al. 75 [NCT01343511] obtained cells from a corporate source where umbilical cords were first cut into 2–3 cm3 pieces, which were then dissected to obtain 1–4 mm3 pieces of Wharton's jelly used for explant cultures. Jin et al. 76 [NCT01360164] refers to a previous study that describes cutting the cord into 1–2 mm pieces and undertaking explant cultures in growth factor‐enriched medium 77. Wang et al. 78, 79 [NCT01741857] published two papers on their trial using UC cells in systemic lupus erythematosus. In one the reader is referred to the corresponding author for cell harvesting details; although the other paper described the cutting of cord into 1 mm pieces, which were then used in 10% serum‐containing explant cultures. In contrast, Wang et al. 80 [NCT01547091] refer to a previous publication 32) where cords were minced into 1–2 mm3 fragments, sequentially digested in collagenase and trypsin, and expanded in low glucose medium containing 5% fetal bovine serum. Finally, Zhang et al. 81 [NCT01213186] describes first removing the vessels from the cord, dicing the Wharton's jelly for explant culture, but also references an earlier paper which reverts to the method of Lu et al. 32 in which neither vessel, nor amniotic membrane, removal is mentioned. Clearly, those harvesting methods that relied on whole cord pieces in either explant cultures or enzyme digests will start with a heterogeneous population of all cells in the umbilical cord. On the contrary those that isolated only Wharton's jelly started with a more homeogeneous cell population. However, without more precise details it is not possible to comment on the exact population employed in either these clinical studies or the majority of preclinical and basic studies that also lack the requisite information. In addition, if the vessels are removed rapidly from the cord, the perivascular Wharton's jelly is removed with the vessels. On the contrary, precise dissection to separate the perivascular jelly from the tunica media of the vessels can result in a harvest that includes the perivascular, intravascular and subamniotic Wharton's jelly. Nevertheless, all authors referred to their cells as mesenchymal stem cells.
Each of these trials returned positive results, NCT01343511 reported an increased therapeutic effect of UCMSCs over cord blood mononuclear cells alone; NCT01360164 confirmed safety and a possible delay in the progression of spinocerebellar ataxia; NCT01741857 reported a satisfactory response in systemic lupus erythematosus; NCT01213186 improved host immune reconstitution in immune nonresponders and NCT01547091 administration of umbilical cord cells with disease‐modifying antirheumatic drugs provided persistent clinical benefits for patients with rheumatoid arthritis. However, it should also be noted that none of these small studies were blinded, none were randomized and only some were controlled. Furthermore, while all studies used an intravenous route of administration, two also employed intrathecal delivery. The I.V. dose varied from 0.5 × 106 to 4 × 107 cells/Kg body weight. Taken together, these studies do point to the administration of umbilical cord cells being safe and possibly providing some therapeutic benefit, although definitive information will not be available until large scale randomized, double blinded, placebo‐controlled trials are undertaken.
Concluding Remarks
Wharton's jelly‐derived mesenchymal stromal cells have reached the significant milestone of entering many clinical trials for a diverse spectrum of medical indications. However, it will be some time before the initial, and promising, clinical results are translated into robust findings based on randomized, double blinded, placebo‐controlled trials. Clearly, the interpretation of both reported and future work in this burgeoning field of health care will rely on greater transparency in the methods of cell extraction employed. To facilitate this, it will also be essential to arrive at a consensus on the anatomical structure of the cord, and particularly that of the zones of Wharton's Jelly, from which the cells are extracted. To this end, we have proposed herein a nomenclature based on the readily visible anatomical/histological structure of the human umbilical cord. However, our knowledge of the derivation and developmental phenotype of cells in Wharton's jelly remains scant; and opportunities exist for major contributions to our understanding by both embryologists and developmental biologists. Increasing numbers of both scientists and clinicians are interested in this tissue, generally discarded at birth, as a source of cells for therapy. Indeed, Wharton's jelly is among the simplest of human connective tissues, and provides an apparently more homogeneous harvested cell population than other mesenchymal tissue sources. Thus, there is an urgent need to understand more fully both the derivation, and functional phenotype, of this increasingly important source of cells.
Author Contributions
J.E.D.: conception and design, assembly of data, analysis, and interpretation, manuscript writing, final approval of manuscript; J.T.W.: assembly of data, analysis, and interpretation, manuscript writing; A.K.: manuscript writing.
Disclosure of Potential Conflicts of Interest
J.E.D. and A.K. are officers, and shareholders in Tissue Regeneration Therapeutics Inc. (TRT). J.T.W. has worked as a Natural Sciences and Engineering Research Council of Canada (NSERC) Ph.D. Scholar at TRT, but declares no conflicts. We are grateful to TRT for the data employed in Figures 1 and 3.
References
- 1. Joerger‐Messerli MS, Marx C, Oppliger B et al. Mesenchymal stem cells from Wharton's jelly and amniotic fluid. Best Pract Res Clin Obstet Gynaecol 2016;31:30–44. [DOI] [PubMed] [Google Scholar]
- 2. Kalaszczynska I, Ferdyn K. Wharton's jelly derived mesenchymal stem cells: Future of regenerative medicine? Recent findings and clinical significance. Biomed Res Int 2015;2015:430847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ding D‐C, Chang Y‐H, Shyu W‐C et al. Human umbilical cord mesenchymal stem cells: A new era for stem cell therapy. Cell Transplant 2015;24:339–47. [DOI] [PubMed] [Google Scholar]
- 4. Nagamura‐Inoue T, He H. Umbilical cord‐derived mesenchymal stem cells: Their advantages and potential clinical utility. World J Stem Cells 2014;6:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. El Omar R, Beroud J, Stoltz J‐F et al. Umbilical cord mesenchymal stem cells: The new gold standard for mesenchymal stem cell‐based therapies? Tissue Eng Part B Rev 2014;20:523–544. [DOI] [PubMed] [Google Scholar]
- 6. Wharton TW. Adenographia. Translated by Freer S. Oxford, UK: Oxford University Press, 1996, 1656:243. [Google Scholar]
- 7. Mitchell KE, Weiss ML, Mitchell BM et al. Matrix cells from Wharton's jelly form neurons and glia. Stem Cells 2003;21:50–60. [DOI] [PubMed] [Google Scholar]
- 8. Nanaev AK, Kohnen G, Milovanov AP, et al. Stromal differentiation and architecture of the human umbilical cord. Placenta 1997;18:53–64. [DOI] [PubMed] [Google Scholar]
- 9. Walker JT, Keating AK, Davies JE. Stem cells: Umbilical cord/Wharton's jelly derived In: Redl H, et al. eds. Tissue Engineering and Regeneration: Cell Engineering and Regeneration. Heidelberg: Springer, 2017. (in press). [Google Scholar]
- 10. Can A, Karahuseyinoglu S. Concise review: Human umbilical cord stroma with regard to the source of fetus‐derived stem cells. Stem Cells 2007;25:2886–2895. [DOI] [PubMed] [Google Scholar]
- 11. Maruyama Y. The human endothelial cell in tissue culture. Zeitschrift fiir Zellforsch 1963;60:69–79. [DOI] [PubMed] [Google Scholar]
- 12. Watson N, Divers R, Kedar R et al. Discarded Wharton jelly of the human umbilical cord: A viable source for mesenchymal stromal cells. Cytotherapy 2015;17:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kita K, Gauglitz GG, Phan TT et al. Isolation and characterization of mesenchymal stem cells from the sub‐amniotic human umbilical cord lining membrane. Stem Cells Dev 2010;19:491–502. [DOI] [PubMed] [Google Scholar]
- 14. Troyer DL, Weiss ML. Concise review: Wharton's jelly‐derived cells are a primitive stromal cell population. Stem Cells 2008;26:591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coskun H, Can A. The assessment of the in vivo to in vitro cellular transition of human umbilical cord multipotent stromal cells. Placenta 2015;36:232–239. [DOI] [PubMed] [Google Scholar]
- 16. Schugar RC, Chirieleison SM, Wescoe KE et al. High harvest yield, high expansion, and phenotype stability of CD146 mesenchymal stromal cells from whole primitive human umbilical cord tissue. J Biomed Biotechnol 2009;2009:789526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karahuseyinoglu S, Cinar O, Kilic E et al. Biology of stem cells in human umbilical cord stroma: In situ and in vitro surveys. Stem Cells 2007;25:319–331. [DOI] [PubMed] [Google Scholar]
- 18. Subramanian A, Fong C‐Y, Biswas A et al. Comparative characterization of cells from the various compartments of the human umbilical cord shows that the Wharton's jelly compartment provides the best source of clinically utilizable mesenchymal stem cells. PLoS One 2015;10:e0127992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Conconi MT, Liddo RD, Tommasini M et al. Phenotype and differentiation potential of stromal populations obtained from various zones of human umbilical cord: An overview. 2011;4:6–20. [Google Scholar]
- 20. Elhassani SB. The umbilical cord: Care, anomalies, and diseases. South Med J 1984;77:730–736. [DOI] [PubMed] [Google Scholar]
- 21. Standring S, Gray H. Gray's Anatomy: The Anatomical Basis of Clinical Practice. 40th ed. (Susan Standring, ed.). Churchill Livingstone: Elsevier, 2008.
- 22. Candilera V, Bouchè C, Schleef J et al. Lung growth factors in the amniotic fluid of normal pregnancies and with congenital diaphragmatic hernia. J Matern Neonatal Med 2016;29:2104–2108. [DOI] [PubMed] [Google Scholar]
- 23. Crow WB. A Synopsis of Biology. 2nd ed. Bristol: John Wright & Sons, 1964. [Google Scholar]
- 24. Foster CL, ed. Hewer's Textbook of Histology for Medical Students. 8th ed London: William Heinemann, 1962. [Google Scholar]
- 25. Junqueira LC, Carneiro J, Contopoulos AN. Basic Histology. 2nd ed Los Altos CA: Lange Medical Publications, 1971. [Google Scholar]
- 26. Hooke RC. Observ. XVIII. Of the schematisme or texture of cork, and of the cells and pores of some other such frothy bodies. In: Micrographia, or, Some physiological descriptions of minute bodies made by magnifying glasses with observations and inquiries thereupon. Printed by Martyn J and Allestry J for The Royal Society, London, England, 1665:268–282.
- 27. McElreavey KD, Irvine AI, Ennis KT et al. Isolation, culture, and characterisation of fibroblast‐like cells derived from the Wharton's Jelly portion of human umbilical cord. Biochem Soc Trans 1991;19:29S. [DOI] [PubMed] [Google Scholar]
- 28. Takechi K, Kuwabara Y, Mizuno M. Ultrastructural and immunohistochemical studies of Wharton's jelly umbilical cord cells. Placenta 1993;14:235–245. [DOI] [PubMed] [Google Scholar]
- 29. Sarugaser R, Lickorish D, Baksh D et al. Human umbilical cord perivascular (HUCPV) cells: A source of mesenchymal progenitors. Stem Cells 2005;23:220–229. [DOI] [PubMed] [Google Scholar]
- 30. Weiss ML, Troyer DL. Stem cells in the umbilical cord. Stem Cell Rev 2006;2:155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Conconi MT, Burra P, Di Liddo R et al. CD105(+) cells from Wharton's jelly show in vitro and in vivo myogenic differentiative potential. Int J Mol Med 2006;18:1089–1096. [PubMed] [Google Scholar]
- 32. Lu L, Zhao Q, Wang X et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis‐supportive function and other potentials. Haematologica 2006;91:1017–1026. [PubMed] [Google Scholar]
- 33. Bańkowski E. Collagen of the umbilical cord and its alteration in EPH‐gestosis (preeclampsia). Proc Indian Acad Sci Chem Sci 1999;111:207–213. [Google Scholar]
- 34. Gogiel T, Bańkowski E, Jaworski S. Proteoglycans of Wharton's jelly. Int J Biochem Cell Biol 2003;35:1461–1469. [DOI] [PubMed] [Google Scholar]
- 35. Meyer FA. Wharton's jelly of the umbilical cord In: Comper WD, ed. Extracellular Matrix. Amsterdam: Harwood Academic Publishers, 1996:443–456. [Google Scholar]
- 36. Underwood MA, Gilbert WM, Sherman MP. Amniotic fluid: Not just fetal urine anymore. J Perinatol 2005;25:341–348. [DOI] [PubMed] [Google Scholar]
- 37. Dominici M, Blanc K Le, Mueller I et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 38. Sarugaser R, Hanoun L, Keating A et al. Human mesenchymal stem cells self‐renew and differentiate according to a deterministic hierarchy. PLoS One 2009;4:e6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 2007;213:341–347. [DOI] [PubMed] [Google Scholar]
- 40. Hayward CJ, Fradette J, Galbraith T et al. Harvesting the potential of the human umbilical cord: Isolation and characterisation of four cell types for tissue engineering applications. Cells Tissues Organs 2012;197:37–54. [DOI] [PubMed] [Google Scholar]
- 41. Qiu Y, Guo J, Mao R et al. TLR3 preconditioning enhances the therapeutic efficacy of umbilical cord mesenchymal stem cells in TNBS‐induced colitis via the TLR3‐Jagged‐1‐Notch‐1 pathway. Mucosal Immunol 2016;1–16. doi: 10.1038/mi.2016.78 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 42. Farias VA, Linares‐Fernández JL, Peñalver JL et al. Human umbilical cord stromal stem cell express CD10 and exert contractile properties. Placenta 2011;32:86–95. [DOI] [PubMed] [Google Scholar]
- 43. Margossian T, Reppel L, Makdissy N et al. Mesenchymal stem cells derived from Wharton's jelly: Comparative phenotype analysis between tissue and in vitro expansion. Biomed Mater Eng 2012;22:243–254. [DOI] [PubMed] [Google Scholar]
- 44. Murray IR, West CC, Hardy WR et al. Natural history of mesenchymal stem cells, from vessel walls to culture vessels. Cell Mol Life Sci 2014;71:1353–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Crisan M, Corselli M, Chen C‐W et al. Multilineage stem cells in the adult: A perivascular legacy? Organogenesis 2011;7:101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Carreau A, Hafny‐Rahbi BE, Matejuk A et al. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med 2011;15:1239–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lavrentieva A, Majore I, Kasper C et al. Effects of hypoxic culture conditions on umbilical cord‐derived human mesenchymal stem cells. Cell Commun Signal 2010;8:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Santos F Dos, Andrade PZ, Boura JS et al. Ex vivo expansion of human mesenchymal stem cells: A more effective cell proliferation kinetics and metabolism under hypoxia. J Cell Physiol 2010;223:27–35. [DOI] [PubMed] [Google Scholar]
- 49. Boyette LB, Creasey OA, Guzik L et al. Human bone marrow‐derived mesenchymal stem cells display enhanced clonogenicity but impaired differentiation with hypoxic preconditioning. Stem Cells Transl Med 2014;3:241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ivanovic Z. Hypoxia or in situ normoxia: The stem cell paradigm. J Cell Physiol 2009;219:271–275. [DOI] [PubMed] [Google Scholar]
- 51. Rochefort GY, Delorme B, Lopez A et al. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells 2006;24:2202–2208. [DOI] [PubMed] [Google Scholar]
- 52. Crisan M, Yap S, Casteilla L et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008;3:301–313. [DOI] [PubMed] [Google Scholar]
- 53. Tintut Y, Alfonso Z, Saini T et al. Multilineage potential of cells from the artery wall. Circulation 2003;108:2505–2510. [DOI] [PubMed] [Google Scholar]
- 54. Hoshino A, Chiba H, Nagai K et al. Human vascular adventitial fibroblasts contain mesenchymal stem/progenitor cells. Biochem Biophys Res Commun 2008;368:305–310. [DOI] [PubMed] [Google Scholar]
- 55. Corselli M, Chen CW, Sun B et al. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev 2012;21:1299–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Guimarães‐Camboa N, Cattaneo P, Sun Y et al. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell. 2016;20:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mennan C, Wright K, Bhattacharjee A et al. Isolation and characterisation of mesenchymal stem cells from different regions of the human umbilical cord. Biomed Res Int 2013;2013:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jeschke MG, Gauglitz GG, Phan TT et al. Umbilical cord lining membrane and Wharton's jelly‐derived mesenchymal stem cells: The similarities and differences. Open Tissue Eng Regen Med J 2011;4:21–27. [Google Scholar]
- 59. Miki T, Lehmann T, Cai H et al. Stem cell characteristics of amniotic epithelial cells. Stem Cells 2005;23:1549–1559. [DOI] [PubMed] [Google Scholar]
- 60. Akle CA, Adinolfi M, Welsh KI et al. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet 1981;2:1003–1005. [DOI] [PubMed] [Google Scholar]
- 61. Gonzalez R, Griparic L, Umana M et al. An efficient approach to isolation and characterization of pre‐ and postnatal umbilical cord lining stem cells for clinical applications. Cell Transplant 2010;19:1439–1449. [DOI] [PubMed] [Google Scholar]
- 62. Humphreys BD, Lin S‐L, Kobayashi A et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 2010;176:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dulauroy S, Di Carlo SE, Langa F et al. Lineage tracing and genetic ablation of ADAM12+ perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat Med 2012;18:1262–1270. [DOI] [PubMed] [Google Scholar]
- 64. Hung C, Linn G, Chow Y‐HH et al. Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med 2013;188:820–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chong P‐P, Selvaratnam L, Abbas AA et al. Human peripheral blood derived mesenchymal stem cells demonstrate similar characteristics and chondrogenic differentiation potential to bone marrow derived mesenchymal stem cells. J Orthop Res 2012;30:634–642. [DOI] [PubMed] [Google Scholar]
- 66. Jung KH, Chu K, Lee ST et al. Multipotent PDGFR‐beta ‐expressing cells in the circulation of stroke patients. Neurobiol Dis 2011;41:489–497. [DOI] [PubMed] [Google Scholar]
- 67. Zeddou M, Briquet A, Relic B et al. The umbilical cord matrix is a better source of mesenchymal stem cells (MSC) than the umbilical cord blood. Cell Biol Int 2010;34:693–701. [DOI] [PubMed] [Google Scholar]
- 68. Zhang X, Hirai M, Cantero S et al. Isolation and characterization of mesenchymal stem cells from human umbilical cord blood: Reevaluation of critical factors for successful isolation and high ability to proliferate and differentiate to chondrocytes as compared to mesenchymal stem cells from bone marrow and adipose tissue. J Cell Biochem 2011;112:1206–1218. [DOI] [PubMed] [Google Scholar]
- 69. Hellström M, Kalén M, Lindahl P et al. Role of PDGF‐B and PDGFR‐beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 1999;126:3047–3055. [DOI] [PubMed] [Google Scholar]
- 70. Stratman AN, Schwindt AE, Malotte KM et al. Endothelial‐derived PDGF‐BB and HB‐EGF coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood 2010;116:4720–4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lin SL, Chang FC, Schrimpf C et al. Targeting endothelium‐pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am J Pathol 2011;178:911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rajkumar VS, Shiwen X, Bostrom M et al. Platelet‐derived growth factor‐beta receptor activation is essential for fibroblast and pericyte recruitment during cutaneous wound healing. Am J Pathol 2006;169:2254–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Grzywocz Z, Pius‐Sadowska E, Klos P et al. Growth factors and their receptors derived from human amniotic cells in vitro. Folia Histochem Cytobiol 2014;52:163–170. [DOI] [PubMed] [Google Scholar]
- 74. Jin E, Kim T‐H, Han S et al. Amniotic epithelial cells promote wound healing in mice through high epithelialization and engraftment. J Tissue Eng Regen Med 2016;10:613–622. [DOI] [PubMed] [Google Scholar]
- 75. Lv Y, Zhang Y, Liu M et al. Transplantation of human cord blood mononuclear cells and umbilical cord‐derived mesenchymal stem cells in autism. J Transl Med 2013;11:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jin J, Liu Z, Lu Z, et al. Safety and efficacy of umbilical cord mesenchymal stem cell therapy in hereditary spinocerebellar ataxia. Curr Neurovasc Res 2013;10:11–20. [DOI] [PubMed] [Google Scholar]
- 77. Lu Z, Ye D, Qian L et al. Human umbilical cord mesenchymal stem cell therapy on neuromyelitis optica. Curr Neurovasc Res 2012;9:250–255. [DOI] [PubMed] [Google Scholar]
- 78. Wang D, Feng X, Lu L et al. A CD8 T cell/indoleamine 2,3‐dioxygenase axis is required for mesenchymal stem cell suppression of human systemic lupus erythematosus. Arthritis Rheumatol 2014;66:2234–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang D, Li J, Zhang Y et al. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: A multicenter clinical study. Arthritis Res Ther 2014;16:R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang L, Wang L, Cong X et al. Human umbilical cord mesenchymal stem cell therapy for patients with active rheumatoid arthritis: Safety and efficacy. Stem Cells Dev 2013;22:3192–3202. [DOI] [PubMed] [Google Scholar]
- 81. Zhang Z, Fu J, Xu X et al. Safety and immunological responses to human mesenchymal stem cell therapy in difficult‐to‐treat HIV‐1‐infected patients. AIDS 2013;27:1283–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]


