Abstract
Fecal incontinence (FI) is the involuntary passage of fecal material. Current treatments have limited successful outcomes. The objective of this study was to develop a large animal model of passive FI and to demonstrate sustained restoration of fecal continence using anorectal manometry in this model after implantation of engineered autologous internal anal sphincter (IAS) biosphincters. Twenty female rabbits were used in this study. The animals were divided into three groups: (a) Non‐treated group: Rabbits underwent IAS injury by hemi‐sphincterectomy without treatment. (b) Treated group: Rabbits underwent IAS injury by hemi‐sphincterectomy followed by implantation of autologous biosphincters. (c) Sham group: Rabbits underwent IAS injury by hemi‐sphincterectomy followed by re‐accessing the surgical site followed by immediate closure without implantation of biosphincters. Anorectal manometry was used to measure resting anal pressure and recto‐anal inhibitory reflex (RAIR) at baseline, 1 month post‐sphincterectomy, up to 3 months after implantation and post‐sham. Following sphincterectomy, all rabbits had decreased basal tone and loss of RAIR, indicative of FI. Anal hygiene was also lost in the rabbits. Decreases in basal tone and RAIR were sustained more than 3 months in the non‐treated group. Autologous biosphincters were successfully implanted into eight donor rabbits in the treated group. Basal tone and RAIR were restored at 3 months following biosphincter implantation and were significantly higher compared with rabbits in the non‐treated and sham groups. Histologically, smooth muscle reconstruction and continuity was restored in the treated group compared with the non‐treated group. Results in this study provided promising outcomes for treatment of FI. Results demonstrated the feasibility of developing and validating a large animal model of passive FI. This study also showed the efficacy of the engineered biosphincters to restore fecal continence as demonstrated by manometry. Stem Cells Translational Medicine 2017;6:1795–1802
Keywords: Fecal incontinence, Biosphincter, Large animal model, Bioengineering, Basal tone, Rectoanal inhibitory reflex (RAIR)
Significance Statement.
This study demonstrated the ability to restore fecal continence in large animal models using engineered autologous sphincters. This study has a high impact on patients suffering from fecal incontinence by providing an approach to use the patient's own cells to engineer a new sphincter that will help restore continence.
Introduction
Fecal incontinence (FI) is a devastating clinical condition in which patients lose the ability to control the passage of fecal material, in terms of consistency or frequency of incidence. This is caused by the loss of anal sphincter function. The patient's quality of life becomes highly impaired 1. Patients with FI face many problems including embarrassment, limited social activities, and loss of jobs. Due to psychological consequences, patients become reluctant to report their condition. This results in inaccurate estimation of the prevalence of FI. In a previous report, it was estimated that 8.3% of U.S. adults are affected with FI, with no difference between men and women 2.
Fecal continence is the result of a coordinated interplay between the anorectum, the internal anal sphincter (IAS), the external anal sphincter (EAS), and the enteric nervous system (ENS) 3. The anal basal tone is dominantly (70%–85%) generated by the IAS, which maintains anal closure. The EAS supports the tone of the IAS by squeeze pressure. This being said, it is important to distinguish between (a) passive FI or soiling that is due to damage to the IAS and (b) urge‐related incontinence due to damage to the EAS. Patients with FI can present with damage to the IAS or EAS or both.
Loss of IAS integrity and function due to aging, trauma, anorectal surgery, or medical comorbidity leads to passive FI 4, 5. Current surgical approaches to treat FI include injection of inert bulking agents, sacral nerve stimulation, and colostomy 6, 7. Treatment results are inconsistent and long ‐term efficacy remains poor. Other treatments for FI include implantation of artificial anal sphincters 8, 9, 10. Those studies are limited by the explantation of the device due to infection or malfunction. Overall, treatment strategies for passive FI remain limited. To remedy passive FI due to weakened IAS, restoration of the IAS musculature along with its intrinsic innervation is an essential step for restoration of function. In this study, we focus on these cellular components using a tissue engineering and regenerative medicine approach to treat FI in a large animal model of FI.
Current animal models of human FI have combined IAS injury with EAS and pudendal nerve disruption 11, 12. These injuries seek to mimic EAS and pudendal nerve injury that can occur during childbirth secondary to nerve crush injuries from prolonged labor, controlled episiotomy or traumatic sphincter disruption. These animal models of human FI lack a component of IAS dysfunction.
In this study, we provide a regenerative medicine approach to treat passive FI caused by injury to the IAS. We previously bioengineered intrinsically innervated IASs using IAS smooth muscle cells and enteric neural progenitor cells. Following implantation in rodents, the engineered sphincters became vascularized and maintained their phenotype and functionality 13, 14. The hypothesis of this study was that implantation of engineered autologous biosphincters will reinstate IAS function and restore fecal continence in rabbits with passive FI. The first goal of this study was to create and validate a large animal model of passive FI. The second goal was to restore anorectal function in the model of passive FI using implantation of autologous engineered innervated IAS constructs. The study was divided into three randomized groups of animals. The first group was a non‐treated group with rabbits that had passive FI without any treatment and served as control. The second group consisted of rabbits with passive FI followed by treatment with autologous biosphincters implantation. The third group consisted of rabbits with passive FI followed by sham surgery.
Materials and Methods
Animal Care
All animal surgeries were performed following the guidelines established by the Institutional Animal Care and Use Committee (IACUC) at Wake Forest School of Medicine. Experimental protocols were reviewed and approved by this committee. Female New Zealand white rabbits (weight ranging from 2.5 kg to 3 kg) were selected to develop an animal model of human FI.
Experimental Design
Twenty New Zealand female rabbits were used in this study and were randomly divided into three groups: (a) Non‐treated group (control): 6 rabbits underwent a survival surgery for internal anal hemi‐sphincterectomy to cause passive FI without any further treatments. (b) Treated group: 10 rabbits underwent a survival surgery for internal anal hemi‐sphincterectomy to cause passive FI followed by a second survival surgery (6–8 weeks later) for implantation of biosphincters (treatment). Two of the 10 rabbits in the treated group had complications non‐related to the implant (anesthesia and ulcer complications) and were excluded from the study; therefore all manometric readings following implantation were conducted on the remaining 8 rabbits. (3) Sham group: Four rabbits underwent a survival surgery for internal anal hemi‐sphincterectomy to cause passive FI followed by a second survival surgery (6–8 weeks later) where the surgical site was re‐accessed without implantation of biosphincters. Rabbits were socially housed when they arrived to the facility. Following the internal anal hemi‐sphincterectomy surgery, rabbits were single housed and remained single housed for the remaining of the study for safety and faster recovery (to avoid any fighting between rabbits).
Surgeries
All surgical procedures involving the rabbit were performed under strict aseptic conditions following the species‐specific surgical guidelines set forth by IACUC. Intestinal biopsy and internal anal sphincterectomy was performed on all 20 rabbits.
Small Intestinal Biopsy
All 20 rabbits underwent laparotomy for small intestinal biopsy for harvest of neural progenitor cells. A midline laparotomy was made through the abdominal wall fascia. Two sections separated by 10 cm were identified in the small intestine. An antimesenteric biopsy was taken sharply and passed off the field for culture. The antimesenteric defects were closed with a running 5–0 PDS suture. The closure was leak tested by applying manual pressure at the proximal and distal ends of the closure. Suture line disruptions were reinforced with 5–0 PDS suture in an interrupted fashion. The peritoneal cavity was then irrigated and closed with a running suture on the fascia and interrupted skin closure.
Internal Anal Hemi‐Sphincterectomy
All 20 rabbits (from the three groups) underwent internal anal hemi‐sphincterectomy to develop the model of passive FI. The perianal area was prepped and draped in the standard surgical fashion. A ventral hemicircumferential curvilinear incision was made through the anocutaneous tissue using an 11 blade scalpel. The dissection was carried more proximally to identify the tissue plane between the anal canal submucosa and IAS (Fig. 1). This plane was identified throughout the length of the hemi‐circumferential incision. The IAS was then dissected from the submucosal plane and the overlying EAS for 1 cm. The IAS fibers were then sharply amputated. IAS tissue was then immediately placed in iced‐cold Hank's balanced salt solution (HBSS). Once the IAS had been circumferentially amputated, the incision was closed with interrupted 4–0 Prolene suture. Sutures were removed 10–14 days after surgery. Antibiotics (enrofloxcin) were given at 10 mg/kg intramuscular for 5 days post operatively. Analgesics were given orally for 3 days post operatively.
Figure 1.
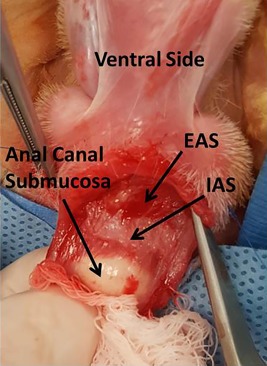
Internal Anal Hemi‐sphincterectomy. Finger is in the anal canal providing traction on the anal canal submucosa. Abbreviations: EAS, external anal sphincter; IAS, internal anal sphincter.
Implantation of Engineered Autologous Biosphincters
Six to eight weeks following the first surgery (intestinal biopsy and sphincterectomy), 10 rabbits (treated group) were scheduled for a second survival surgery (biosphincter implantation). A circumferential incision was made around the anocutaneous junction of the anal canal. The intersphincteric plane was identified and dissected for approximately 1 cm proximally. The most proximal portion of the dissection was marked with a 5–0 prolene suture. Four engineered autologous biosphincters were placed in the intersphincteric space. The engineered biosphincters were stacked circumferentially next to each other around the injury site as full muscle rings. The distal margin of the implanted biosphincters was also marked with a 5–0 prolene suture. The circumferential incision was closed with 5–0 prolene suture to reapproximate the anocutaneous junction.
Sham Surgery
Six to eight weeks following the first surgery (intestinal biopsy and sphincterectomy), 4 rabbits (sham group) were scheduled for a second survival surgery (sham surgery). A circumferential incision was made around the anocutaneous junction of the anal canal. The intersphincteric plane was identified and dissected for approximately 1 cm proximally. The dissection plane was then closed with 5–0 prolene suture without implanting any biosphincters. Sutures were removed 10–14 days after surgery.
Isolation of Enteric Neural Progenitor Cells
The intestinal biopsies were washed extensively with HBSS containing 2X antibiotics/antimycotic. The tissue was minced, washed, and subjected to digestion twice in a mixture containing type II Collagenase and Dispase II. The cells were recovered by centrifugation and washed before being passed through a 70 µm nylon cell strainer. Cells were washed and re‐suspended in neuronal growth medium before being filtered through a 40 µm nylon cell strainer. Cells were plated in neural growth media.
Isolation of IAS Smooth Muscle Cells
IAS tissue excised from the rabbit was rapidly cleaned in ice‐cold carbonated HBSS containing 2× antibiotic/antimycotic (200U/ml Penicillin G, 200 µg/ml Streptomycin and 0.5 µg/ml Amphotericin B) (Invitrogen, Carlsbad CA, www.thermofisher.com) and 50 µg/ml gentamycin. The tissue was finely minced and digested twice using Collagenase type II (Worthington Biochemical, Lakewood, NY, www.worthington-biochem.com) for 1 hour each digest. The cells were collected, washed, and re‐suspended in growth medium before plating onto tissue culture flasks.
Bioengineering Autologous Biosphincters
Four to five weeks following cell culture, smooth muscle cells, and enteric neural progenitor cells obtained from the same rabbit were collected to engineer sphincteric tissues for autologous implantation. The process of engineering the sphincters was described previously 13. Briefly, a total of 200,000 enteric neural progenitor cells/biosphincter were dissociated using accutase and re‐suspended in a collagen/laminin gel mixture containing a final concentration of 10%FBS, 1× DMEM, 10 µg/ml laminin and 0.4 mg/ml type I collagen. One milliliter of the gel mixture was poured on 35 mm Sylgard‐coated petri dishes that have an 8 mm diameter cylindrical post in the center (to create the lumen of the sphincter). The mixture was allowed to gel at 37°C for 15 minutes. In the meantime, a total of 500,000 smooth muscle cells/biosphincter was re‐suspended in 1 ml collagen gel mixture similar to what was described above without the laminin and overlaid on top of the neural gel layer for an additional 20 minutes. Both layers together formed one biosphincter. Constructs were supplemented with neural differentiation medium (Neurobasal‐A medium supplemented with B27 and 1% FBS). Six innervated IAS constructs were bioengineered from each rabbit biopsy. Each rabbit received implantation of four biosphincters (a total of 2 million smooth muscle cells and 800,000 neural progenitor cells).
Quality Control of the Engineered Biosphincters Before Implantation
At day 10–12 post engineering, quality control of the biosphincters was evaluated. Real time force generation was performed to evaluate the physiological functionality of the biosphincters. The protocol for physiological testing has been previously described 15. Briefly, the biosphincters were hooked in an organ bath connected to an isometric force transducer. Biosphincters were maintained at 37°C due to continuous water perfusion through the organ bath. Data acquisition was performed using Powerlab data system and analyzed using GraphPad Prism 5.0. Biosphincters were allowed to generate basal tone before any treatment. Electrical field stimulation (EFS) (5 Hz, 0.5 ms) was performed to stimulate the neurons in the biosphincters. Neurotoxin tetrodotoxin (TTX) was used as an inhibitor of the neural component.
Anorectal Manometry
Anorectal manometry was performed regularly at baseline and at 3 months in the non‐treated group, treated group, and sham group on lightly sedated rabbits. During manometric studies, a catheter with four air charged pressure transducers arranged at the same level circumferentially and 90° apart was used. Following sedation, the rabbits were laid down on their abdomen and the catheter was inserted into the rectum 6 cm deep. The catheter was then withdrawn in 1 cm increments and the area of maximum resting pressure (basal tone) was identified. Baseline pressure was recorded. A balloon attached to the distal aspect of the catheter was used to evaluate the percentage decrease in basal pressure in response to rectal balloon inflation to a volume of 20 mL (RAIR). Data acquisition and analysis was performed using BioVIEW software (Sandhill Scientific, Littleton, CO, http://www.diversatekhealthcare.com/).
Histological Analysis
The surgical anal site was re‐accessed following euthanasia in both the treated and non‐treated groups and the sphincter was resected. The sphincter was fixed in formaldehyde, processed, and paraffin embedded. Cross sections of 6 µm thickness were obtained. Hematoxylin and eosin (H&E) stain was performed for morphological analysis. Immunohistochemistry analysis of the surgical site was also performed. Slides were stained against primary antibodies for smooth muscle actin (smooth muscle) and βIII tubulin (neural marker). Appropriate fluorophore‐conjugated secondary antibodies were then used.
Statistical Analysis
Analysis of basal tone and RAIR was analyzed using BioVIEW software (Sandhill Scientific, Littleton, CO). One way ANOVA followed by Tukey's test was used to compare the basal tone and RAIR values recorded regularly at baseline and at 3 months in the non‐treated group, treated group, and sham group. All values are reported as means ± SEM with the corresponding p‐values.
Results
Evaluation of Rabbit Hygiene
All 20 rabbits survived the first survival surgery (intestinal biopsy and sphincterectomy). Following sphincterectomy, changes in fecal control were evident in all 20 rabbits based on fecal staining of the perineum and scatter of fecal material throughout the cage (Fig. 2A, 2B). Manual perineal cleansing was provided to maintain the rabbit's health. Fecal soiling of the cage floor which was once confined to one corner was now spread throughout the cage and indicated FI. Rabbits in the treated group were able to regain their anal hygiene and maintain a clean cage (Fig. 2C, 2D). Their fecal output was confined back to one corner. Of the 10 rabbits in the treated group, 2 rabbits were excluded from the study due to complications. One rabbit died due to an anesthesia complication during the preparation for implantation. The second rabbit was euthanized after developing dehydration and poor feeding 3 weeks post sphincter implantation. Autopsy confirmed gastric and duodenal ulceration. All other rabbits maintained normal weight gain and activity following implantation. There were no signs of wound infection.
Figure 2.
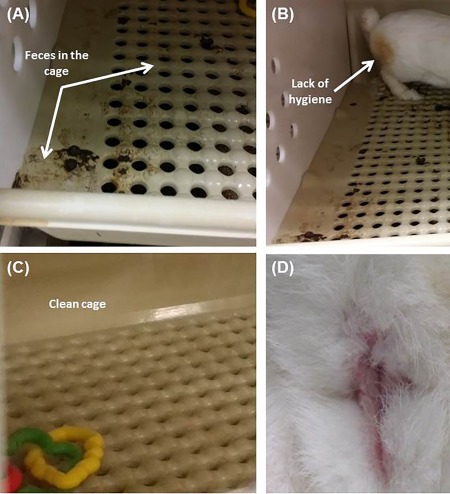
Rabbit hygiene: Rabbits in the non‐treated group had scattered fecal material throughout the cage (A) and fecal staining of the perineum (B). Rabbits in the treated group maintained a clean cage (C) and maintained their hygiene (D).
Quality Control of the Bioengineering Autologous Intrinsically Innervated IAS
One of the engineered constructs was used for quality control and four constructs were used for implantation. All constructs were completely formed at 10 days post engineering with a defined luminal opening of 7 mm diameter. Figure 3A shows a bioengineered biosphincter construct. The constructs were tested for physiological functionality. All constructs were able to generate basal tone, a characteristic of the IAS (Fig. 3B). The average basal tone was 541 ± 13 µN. Innervation of the constructs was demonstrated by relaxation of the smooth muscle following EFS of the neurons. Relaxation was completely abolished in the presence of TTX (data not shown).
Figure 3.
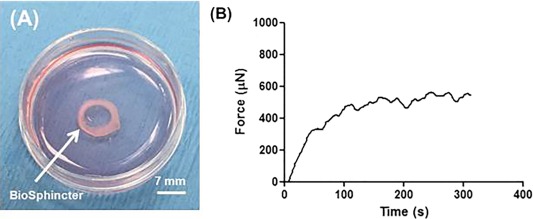
Quality control of the biosphincters. (A): Three dimensional engineered circular biosphincter: The engineered biosphincter has an internal lumen diameter of 7 mm. (B): The engineered IAS constructs were physiologically tested using an organ bath. When the biosphincters were hooked to the organ bath, they generated a spontaneous basal tone over time in the bath. Average basal tone generated by the biosphincters was 541 ± 13 µN. Abbreviation: IAS, internal anal sphincter.
Anorectal Manometry
Anorectal manometry was followed in all groups where basal tone and RAIR were recorded (Table 1).
Table 1.
Summary of the anorectal manometry readings. A total of 20 rabbits underwent sphincterectomy procedure to create the FI model. Of the 20 rabbits, 6 were assigned to the non‐treated group and were followed for up to 3 months without implanting the biosphincters. Ten rabbits were assigned to the treated group. Two of the 10 rabbits in the treated group had complications non‐related to the implant (anesthesia and ulcer complications) and were excluded from the study; therefore all manometric readings following implantation were conducted on the remaining 8 rabbits for up to 3 months. Four rabbits were assigned to the sham group and followed up to 3 months.
| Pre‐sphincterectomy | Post‐sphincterectomy | 1 month | 3 months | ||
|---|---|---|---|---|---|
| Post Sphincterectomy | |||||
| Non‐treated group(Mean ± SEM) |
Basal Tone
(mmHg) |
40 ± 3 (n=6) | 23 ±1 (n=6) | 24 ± 3 (n=6) | |
| RAIR (%) | 60 ± 4 (n=6) | 28 ± 3 (n=6) | 33 ± 8 (n=6) | ||
| Post Implant | Post Implant | ||||
| Treated group(Mean ± SEM) |
Basal Tone
(mmHg) |
42 ± 3 (n=10) | 22 ± 1 (n=10) | 45 ± 2 (n=8) | 37 ± 3 (n=8) |
| RAIR (%) | 57 ± 3 (n=10) | 27 ± 5 (n=10) | 51 ± 7 (n=8) | 59 ± 4 (n=8) | |
| Post Sham | Post Sham | ||||
| Sham group(Mean ± SEM) |
Basal Tone
(mmHg) |
30 ± 2 (n=4) | 19 ± 5 (n=4) | 18 ±1 (n=4) | 15 ± 1 (n=4) |
| RAIR (%) | 60 ± 3 (n=4) | 34 ±6 (n=4) | 33 ± 6 (n=4) | 33 ± 4 (n=4) |
Basal Tone
Hemi‐circumferential internal anal sphincterectomy resulted in significant decrease in basal tone in all groups at 1 month post‐sphincterectomy (Fig. 4). In the non‐treated group, basal tone remained low over the study period. Baseline basal tone decreased from 40 ± 3 mmHg (n = 6) to 23 ± 1 mmHg at 1 month (n = 6) and 24 ± 3 mmHg at 3 months (n = 6) post sphincterectomy (p < .0001). Basal tones at 1 and 3 months post‐sphincterectomy were not significantly different from each other (p > .05). In the treated group prior to implantation, anorectal readings were performed on the 10 rabbits following sphincterectomy to confirm their incontinence. Two of the 10 rabbits were excluded from this group due to complications non‐related to the implantation of biosphincters (mentioned above). Anorectal readings following implantation of biosphincters were conducted on the remaining 8 rabbits in the treated group (n = 8). Basal tone significantly increased following implantation of the bioengineered biosphincters (blue bars at 1 and 3 months post‐implantation, p < .05) compared with basal tone following sphincterectomy. Anorectal manometry post‐implantation indicated that basal tone at 3 months (37 ± 3 mmHg, n = 8) was restored back to normal compared with to baseline (p > .05). At 3 months post implant, the basal tone in the treated group was significantly higher than that in the non‐treated group (37 ± 3 mmHg and 24 ± 3 mmHg respectively; p = .0134). In the sham group, basal tone was significantly reduced from 30 ± 2 mmHg (baseline) to 15 ± 1 mmHg at 3‐month time point (n = 4, p = .014). Basal tone at 1 and 3 months post sham was not significantly different from basal tone at 1 and 3 months post‐sphincterectomy (p > .05); however, it was significantly lower than basal tone post‐implantation at 1 and 3 months (p < .05).
Figure 4.

Basal tone changes in rabbits: Rabbits in the non‐treated group (red bars, n = 6) showed a significant sustained decrease (p < .05) in basal tone at 1 and 3 months following sphincterectomy compared with pre‐sphincterectomy. Rabbits in the treated group (blue bars) showed restoration of basal tone at 1 and 3 months following implantation of biosphincters. Basal tone at 3 months post‐sphincterectomy was significantly lower than basal tone in rabbits at 3 months post implant (p < .05). Rabbits in sham group (green bars) showed no significant difference (p > .05) in basal tone compared with the non‐treated group (red bars) but was significantly lower than basal tone in the treated group (blue bars) at 1‐ and 3‐month time point following sham surgery (p < .05).
Rectoanal Inhibitory Reflex (RAIR)
Sphincterectomy also induced significant decrease in RAIR in all rabbits more than a period of 3 months (Fig. 5). RAIR significantly decreased from 60 ± 4% (baseline, n = 6) to 28 ± 3% at 1 month (n = 6) and 33 ± 8% at 3 months (n = 6) post‐sphincterectomy (p < .0001). RAIR at 1 and 3 months post‐sphincterectomy were not significantly different from each other (p > .05). Similar to basal tone, in the treated group RAIR readings were performed on all 10 rabbits following sphincterectomy. Due to the exclusion of the 2 rabbits, RAIR recordings were performed on the remaining 8 rabbits (n = 8). Following implantation of the engineered biosphincters, RAIR was restored to baseline (blue bars at 1 and 3 months, n = 8, p > .05) and was found to be significantly higher than RAIR post‐sphincterectomy at 1 and 3 months in the non‐treated group (p < .001). At 3 months post implant, RAIR in the treated group was significantly higher than that in the non‐treated group (59 ± 4%, n = 8 and 33 ± 8%, n = 6 respectively; p = .013). In the sham group, RAIR was also significantly reduced from 60 ± 3% (baseline) to 33 ± 4% at 3‐month time point (n = 4, p = .0053). RAIR at 1 and 3 months post sham was not significantly different from RAIR at 1 and 3 months post‐sphincterectomy (p > .05); however, it was significantly lower than RAIR post‐implantation at 1 and 3 months (p < .05).
Figure 5.
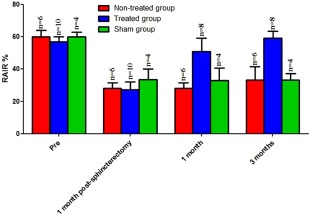
RAIR changes in rabbits: Rabbits in the non‐treated group (red bars) showed a significant sustained decrease (p < .05) in RAIR at 1 and 3 months following sphincterectomy compared with pre‐sphincterectomy. Rabbits in the treated group (blue bars) showed restoration of RAIR at 1 and 3 months following implantation of biosphincters. RAIR at 3 months post‐sphincterectomy was significantly lower than RAIR in rabbits at 3 months post implant (p < .05). Rabbits in sham group (green bars) showed no significant difference (p > .05) in RAIR compared with the non‐treated group (red bars) but was significantly lower than RAIR in the treated group (blue bars) at 1‐ and 3‐month time point following sham surgery (p < .05). Abbreviation: RAIR, Rectoanal inhibitory reflex.
Histological Analysis
In the non‐treated FI model group, H&E analysis of the native anal sphincter demonstrated discontinuity of the IAS smooth muscle at the ventral position of the IAS (where sphincterectomy was performed) (Fig. 6A). Alignment and continuity of the smooth muscle was preserved at the other half of the remaining IAS. Smooth muscle actin stain confirmed the discontinuity in the circular alignment of the IAS (Fig. 6B). βIII tubulin stain showed the presence of neurons in the surgical site (Fig. 6C). In the treated group, the implanted biosphincters were located following euthanasia by tracking the blue prolene sutures used during the implantation surgery to mark the biosphincters. H&E analysis of the implant showed regeneration of continuous circular smooth muscle at the site where sphincterectomy was originally performed (marked by the blue suture) (Fig. 6D). Restoration of smooth muscle alignment and structure were further confirmed using smooth muscle actin stain (Fig. 6E). Innervation was also confirmed by positive stain for βIII tubulin (Fig. 6F). The four biosphincters fused and formed a continuous tissue at the site of implantation. Vascularization of the implant was observed.
Figure 6.
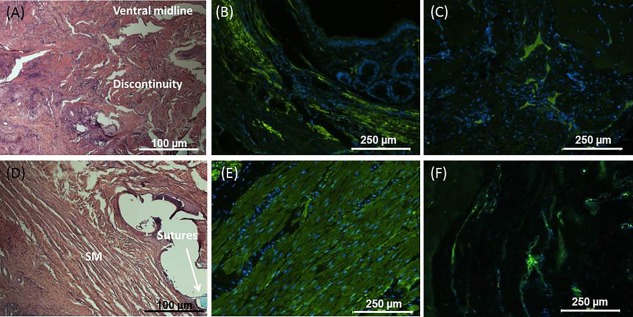
(A): H&E representative figures of the IAS following sphincterectomy (non‐treated group) showed discontinuity at the ventral midline (where semi‐sphincterectomy was performed). (B): Smooth muscle discontinuity was further confirmed using smooth muscle actin. (C): The presence of neurons at the surgical site was confirmed using βIII tubulin. Restoration of IAS circular smooth muscle alignment and structure following implantation in the treated group was confirmed by (D): H&E (blue prolene sutures) and (E): smooth muscle actin stains. (F): Positive stain for βIII tubulin also indicated the presence of neurons at the implant site. Abbreviations: H&E, hematoxylin and eosin; IAS, internal anal sphincter.
Discussion
In this study, we provide a regenerative medicine approach to treat passive FI in rabbits by implanting engineered autologous biosphincters. The first step was to create and validate a large animal model of passive FI. Previous animal models of sphincter injury conducted in rats resulted in immediate decrease in resting and peak pressures 16, 17. However, anal pressures recovered to baseline levels within 14 days. This was attributed to the fact that sphincterotomy wasn't extensive enough to simulate the loss of sphincter muscle seen in humans. Dog and rabbit models of FI have been created with quadrant sphincterectomy by resecting both the EAS and IAS. Studies including these models showed sustained decrease in resting and peak pressures more than 3–6 months 12, 18. Overall, current existing animal models of FI include the IAS and EAS. In our model, IAS only was resected while the EAS was left intact. This caused a sustained decrease in both anal pressure and RAIR; two manometric findings in passive FI patients. Our results confirmed the successful development of a reproducible and reliable animal model of passive human FI. Significant decrease in basal tone and RAIR compared with baseline indicated that the hemi‐sphincterectomy was successful in inducing passive FI in the rabbits and lasted the duration of the study (3 months). The data also showed that possible fibrosis and scarring within the excision bed of the sphincter following sham did not result in any change in basal tone.
The second step of this study was to treat FI induced in these animals. Our lab was the first to successfully engineer and implant IAS constructs in rodents 11, 12. In our rodent studies, we did not create a sphincter injury. Additionally, the engineered sphincters were implanted in a close proximity to the intact native sphincter.
In this large animal study, basal tone and RAIR were restored to baseline (prior to sphincterectomy) following implantation of biosphincters. RAIR is a coordinated response of the anal sphincter in response to rectal distension which causes a brief contraction of the EAS followed by prolonged relaxation of the IAS. Therefore, RAIR is a sampling reflex mediated by the IAS 19. Also, presence of RAIR is an indication of an intact myenteric plexus 20. Therefore the neurons of the myenteric plexus are essential for sensing the distention of the rectum causing RAIR to occur. Passive FI caused by IAS dysfunction results in exaggerated IAS relaxation in response to rectal distension 21. Alterations in RAIR are a clinical measure of IAS dysfunction in patients with FI 21, 22. Following rectal distension using an inflated balloon, RAIR was restored to normal levels in rabbits in the treated group. The restoration of this important inhibitory nNOS‐mediated inhibitory reflex indicated the integration of the intrinsic neural component of the engineered biosphincters with the native tissue and restored the integrity of the neuronal plexus necessary for the reflex. The results from the sham group showed no significant improvement of the manometric readings, indicating that the scarring and fibrosis due to the second surgery did not restore basal tone or RAIR. Compared to control and sham groups, manometry readings in the treated groups confirmed that the biosphincters were viable and functional in vivo with maintenance of both the muscle and neural components. Current studies in the lab are investigating tagging both types of cells for future in vivo tracking of the cells following implantation of the biosphincters. Histologically, the restoration of the IAS smooth muscle alignment and structure was confirmed in the treated group, whereas in the non‐treated group, discontinuity was clear. Both groups showed positive stain for βIII tubulin indicating the presence of neurons at the surgical site; however, additional biochemical studies may be needed to quantify and compare the neural population between the two groups.
Previous studies attempted to treat FI using different regenerative medicine approaches. In another study, myogenic stem cells improved external sphincter function after transection of both the internal and external sphincter only when injected along with a biogel scaffold 2 weeks after sphincter damage 23, 24. Other studies conducted in dog models of FI with internal and external sphincter injury have also injected myoblasts incorporated in polycaprolactone beads 25. The resting and contractile pressures were improved. The novelty of our study is in (a) developing and validating a large animal model of passive FI and (b) engineering autologous biosphincters to remedy passive FI in rabbits.
This study provides promising and exciting outcomes to treat passive FI. Our future studies will include longer follow up for the non‐treated, treated and sham groups. Preliminary results showed promising results of maintenance of animal continence over longer period of time following implantation of biosphincters. Additional animals are needed to further confirm our observations. Overall, this study demonstrates that the engineered biosphincters restored dynamic function of the IAS by way of restored basal tone and RAIR and thus restore anorectal motility necessary for fecal continence.
In conclusion, this regenerative medicine approach has a promising potential for wide clinical application in the aging population with passive FI. This study demonstrated an autologous treatment for passive FI that restores IAS function to baseline. Patients presenting with passive FI will have a new therapeutic approach using autologous cells to engineer the biosphincters. Implantation of the functional biosphincters into incontinent patients provides a new methodology to replace or augment the function of the existing deficient IAS. For translational purposes, we have been successful in identifying medical grade collagen and recombinant laminin as well as scalable techniques to reach clinical sizes. Characterization of the scaled up biosphincters with medical grade reagents are currently under investigation. All those studies are necessary to justify a clinical trial. Our 3‐month follow up study is promising with longer term results needed for confirmation. Long‐term follow‐up is currently under investigation in our lab.
Authors Contributions
J.B., E.Z., K.N.B.: Conception and design of the study, generation, collection, assembly, analysis and/or interpretation of data, drafting or revision of the manuscript, approval of the final version of the manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Acknowledgments
This work was supported by Army, Navy, NIH, Air Force, VA and Health Affairs to support the AFIRM II effort, under Award No. W81XWH‐13‐2‐0052; GU 7 and NIH/NIDDK R01DK071614 and R42DK105593. The authors would like to thank Dr. Sita Somara, Dr. Shreya Raghavan and Dr. Stephen Rego for their help in this study.
References
- 1. Bartlett L, Nowak M, Ho Y‐H. Impact of fecal incontinence on quality of life. World J Gastroenterol 2009;15:3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Whitehead WE, Borrud L, Goode PS et al. Fecal incontinence in US adults: Epidemiology and risk factors. Gastroenterology 2009;137:512–517e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rao SS. Pathophysiology of adult fecal incontinence. Gastroenterology 2004;126:S14–S22. [DOI] [PubMed] [Google Scholar]
- 4. Huebner M, Margulies RU, Fenner DE et al. Age effects on internal anal sphincter thickness and diameter in nulliparous females. Dis Colon Rectum 2007;50:1405–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lindsey I, Jones OM, Smilgin‐Humphreys M et al. Patterns of fecal incontinence after anal surgery. Dis Colon Rectum 2004;47:1643–1649. [DOI] [PubMed] [Google Scholar]
- 6. Maslekar S, Smith K, Harji D et al. Injectable collagen for the treatment of fecal incontinence: Long‐term results. Dis Colon Rectum 2013;56:354–359. [DOI] [PubMed] [Google Scholar]
- 7. Hull T, Giese C, Wexner SD et al. Long‐term durability of sacral nerve stimulation therapy for chronic fecal incontinence. Dis Colon Rectum 2013;56:234–245. [DOI] [PubMed] [Google Scholar]
- 8. Darnis B, Faucheron J‐L, Damon H et al. Technical and functional results of the artificial bowel sphincter for treatment of severe fecal incontinence: Is there any benefit for the patient? Dis Colon Rectum 2013;56:505–510. [DOI] [PubMed] [Google Scholar]
- 9. Wang X, DaSilva G, Maron DJ et al. Outcomes of reimplantation of the artificial Bowel Sphincter. Dis Colon Rectum 2016;59:122–126. [DOI] [PubMed] [Google Scholar]
- 10. Hong KD, Dasilva G, Kalaskar SN et al. Long‐term outcomes of artificial bowel sphincter for fecal incontinence: A systematic review and meta‐analysis. J Am Coll Surg 2013;217:718–725. [DOI] [PubMed] [Google Scholar]
- 11. Healy C, O'Herlihy C, O'Brien C et al. Experimental models of neuropathic fecal incontinence: An animal model of childbirth injury to the pudendal nerve and external anal sphincter. Dis Colon Rectum 2008;51:1619–1626. [DOI] [PubMed] [Google Scholar]
- 12. Kang S‐B, Lee HS, Lim JY et al. Injection of porous polycaprolactone beads containing autologous myoblasts in a dog model of fecal incontinence. J Korean Surg Soc 2013;84;216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gilmont RR, Raghavan S, Somara S et al. Bioengineering of physiologically functional intrinsically innervated human internal anal sphincter constructs. Tissue Eng Part A 2014;20:1603–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Raghavan S, Miyasaka EA, Gilmont RR et al. Perianal implantation of bioengineered human internal anal sphincter constructs intrinsically innervated with human neural progenitor cells. Surgery 2014;155:668–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raghavan S, Gilmont RR, Miyasaka EA et al. Successful implantation of bioengineered, intrinsically innervated, human internal anal sphincter. Gastroenterology 2011;141:310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salcedo L, Damaser M, Butler R et al. Long‐term effects on pressure and electromyography in a rat model of anal sphincter injury. Dis Colon Rectum 2010;53:1209–1217. [DOI] [PubMed] [Google Scholar]
- 17. Zutshi M, Salcedo LB, Zaszczurynski PJ et al. Effects of sphincterotomy and pudendal nerve transection on the anal sphincter in a rat model. Dis Colon Rectum 2009;52:1321–1329. [DOI] [PubMed] [Google Scholar]
- 18. Kajbafzadeh A‐M, Elmi A, Talab SS et al. Functional external anal sphincter reconstruction for treatment of anal incontinence using muscle progenitor cell auto grafting. Dis Colon & Rectum 2010;53:1415–1421. [DOI] [PubMed] [Google Scholar]
- 19. Zbar AP, Khaikin M. Should we care about the internal anal sphincter? Dis Colon Rectum 2012;55:105–108. [DOI] [PubMed] [Google Scholar]
- 20. Rao S, Azpiroz F, Diamant N et al. Minimum standards of anorectal manometry. Neurogastroenterol Motil 2002;14:553–559. [DOI] [PubMed] [Google Scholar]
- 21. Kaur G, Gardiner A, Duthie GS. Rectoanal reflex parameters in incontinence and constipation. Dis Colon Rectum 2002;45:928–933. [DOI] [PubMed] [Google Scholar]
- 22. Zbar AP, Aslam M, Gold DM et al. Parameters of the rectoanal inhibitory reflex in patients with idiopathic fecal incontinence and chronic constipation. Dis Colon Rectum 1998;41:200–208. [DOI] [PubMed] [Google Scholar]
- 23. White AB, Keller PW, Acevedo JF et al. Effect of myogenic stem cells on contractile properties of the repaired and unrepaired transected external anal sphincter in an animal model. Obstet Gynecol 2010;115:815–823. [DOI] [PubMed] [Google Scholar]
- 24. Montoya TI, Acevedo JF, Smith B et al. Myogenic stem cell‐laden hydrogel scaffold in wound healing of the disrupted external anal sphincter. Int Urogynecol J 2015;26:893–904. [DOI] [PubMed] [Google Scholar]
- 25. Oh H‐K, Lee HS, Lee JH et al. Functional and histological evidence for the targeted therapy using biocompatible polycaprolactone beads and autologous myoblasts in a dog model of fecal incontinence. Dis Colon Rectum 2015;58:517–525. [DOI] [PubMed] [Google Scholar]


