Abstract
The ex vivo generation of human red blood cells on a large scale from hematopoietic stem and progenitor cells has been considered as a potential method to overcome blood supply shortages. Here, we report that functional human erythrocytes can be efficiently produced from cord blood (CB) CD34+ cells using a bottle turning device culture system. Safety and efficiency studies were performed in murine and nonhuman primate (NHP) models. With the selected optimized culture conditions, one human CB CD34+ cell could be induced ex vivo to produce up to 200 million erythrocytes with a purity of 90.1% ± 6.2% and 50% ± 5.7% (mean ± SD) for CD235a+ cells and enucleated cells, respectively. The yield of erythrocytes from one CB unit (5 million CD34+ cells) could be, in theory, equivalent to 500 blood transfusion units in clinical application. Moreover, induced human erythrocytes had normal hemoglobin content and could continue to undergo terminal maturation in the murine xenotransplantation model. In NHP model, xenotransplantation of induced human erythrocytes enhanced hematological recovery and ameliorated the hypoxia situation in the primates with hemorrhagic anemia. These findings suggested that the ex vivo‐generated erythrocytes could be an alternative blood source for traditional transfusion products in the clinic. Stem Cells Translational Medicine 2017;6:1698–1709
Keywords: Erythrocytes, Hematopoietic stem cells, Expansion and differentiation, Human cord blood, Transplantation, Nonhuman primates
Significance Statement.
We report that functional human erythrocytes can be efficiently produced from cord blood CD34+ cells using a bottle turning device culture system. With the optimized culture conditions, the amount of erythrocytes derived from one cord blood unit (5 million CD34+ cells) could, in theory, be equivalent to 500 blood transfusion units in clinical application. The induced erythrocytes had normal features and functions, and could be continuous to undergo terminal maturation in vivo in the immunodeficient mouse. Moreover, xenotransfusion studies in nonhuman primates with hemorrhagic anemia confirmed the safety and efficiency of the induced erythrocytes. These findings suggested that the ex vivo‐generated erythrocytes could be an alternative blood source for traditional transfusion products in the clinic.
Introduction
Blood transfusion is widely used for various clinical therapies. However, clinical sources of blood are limited, and the supply of blood for transfusion is dependent on blood donations by volunteers. Data from the World Health Organization reveal that an estimated of 108 million blood donations are collected annually worldwide 1. Nevertheless, globally declining fertility rates indicate a gradual reduction in the donor‐eligible population, and blood supply shortages are predicted globally by 2050 2. Furthermore, the risk of infection through transfusion remains an issue. In response to these concerns, the ex vivo production of red blood cells (RBCs) on a large scale from hematopoietic stem and progenitor cells (HSPCs) has been considered as a potential method to overcome the shortage of blood supply.
The ex vivo generation of RBCs can be achieved using optimized media, combinations of cytokines, and stromal cells 3, 4 to simulate the HSPCs niche in vivo. During the last two decades, researchers have successfully expanded and induced erythrocytes from HSPCs in the laboratory, and the functionality of cultured erythrocytes have been confirmed in the immunodeficient mice model 5, 6, 7. In 2011, a study has provided the first proof‐of‐principle in humans for the use of ex vivo‐expanded RBCs for transfusion 8. These findings demonstrated the feasibility of transfusion with cultured RBCs. However, generating large‐scale and clinically applicable RBCs still remains an obstacle because of the large number of cells that are required for transfusion. To address this concern, researchers have focused on maximizing the numerical expansion of RBCs from HSPCs. Neildez‐Nguyen et al. 6 described a process that yielded a 200,000‐fold expansion of erythroid precursor cells derived from cord blood (CB) CD34+ cells (corresponding to approximately 0.5 transfusion units/donation). Although not yet mature, these cells were able to undergo terminal differentiation in a nonobese diabetic/severe combined immunodeficient (NOD/SCID) murine model. Subsequently, Giarratana et al. 7 developed a protocol for the large‐scale ex vivo production of mature human RBCs from HSPCs by coculturing them with mouse MS‐5 cells. The approach can achieve an up to two million‐fold numerical expansion of enucleated erythrocytes. Fujimi et al. 9 also reported a “large‐scale” process for ex vivo generation of RBCs by coculture with human macrophages. The process was capable of generating up to 10 units of RBCs from two umbilical CB collections. However, all of these large‐scale processes were developed in 10‐ml tissue culture flasks; there is a difference between the actual yield and theoretical yield. Furthermore, the dependency on stromal cells hampers the application of this method because of the high complexity and expense 10. To facilitate the translation from laboratory flask‐based RBC culture methods to a bioreactor system, Timmins et al. 11 adapted a feeder‐free protocol for use in an agitated bioreactor system. After 21 days of culture, this approach was able to achieve an amplification of 1.7 × 106‐fold by calculation.
Despite this progress, existing bioreactor technologies are currently insufficient to meet the demands of routine RBC production, and novel techniques need to be developed for efficient RBC production. In addition, majority in vivo studies in the past were performed in murine models, which are not enough for confirming the safety and efficacy of the induced RBCs. Nonhuman primates (NHP) are considered to be more valuable mammalian models 12, 13.
In the present study, we optimized culture conditions and developed a bottle turning device culture system for ex vivo generation of human erythrocytes on a large scale from CB CD34+ cells. We also evaluated the safety and functionality of induced erythrocytes in murine and NHP models.
Materials and Methods
CD34+ Cell Collection
Umbilical CB samples (O‐type) from anonymous specimens were provided by the Suzhou Municipal Hospital Affiliated Nanjing Medical University (Suzhou, China). The study was approved by the Hospital's Ethics Committee and Research Ethics Advisory Committee. CD34+ cells were isolated by super magnetic microbead selection using Mini‐MACS columns (Miltenyi Biotec, Bergisch Gladbach, Germany, http://www.miltenyibiotec.com/). The purity of isolated CD34+ cells ranged from 90% to 99%, as determined by flow cytometry using anti‐human CD34 mAb conjugated with phycoerythrin (PE) (BD Biosciences, NJ, http://www.bdbiosciences.com).
Cell Culture
Isolated CD34+ cells were initially seeded at a density of 1 × 105 cells/ml in 25‐T flasks (Corning) at 37°C with 5% CO2. After 5 days, cell cultivation was transferred into the bottle turning device (HERAcell 240i; Thermo Fisher Scientific Waltham, MA, http://www.thermofisher.com/) (Supporting Information Fig. S1). The bottles were placed horizontally in an incubator at 37°C with 5% CO2 in air, and the bottles were rotated at 0.75 U per minute. Cells were cultured in modified medium (MM) based on Iscove's Modified Dulbecco's medium (IMDM) (Life Technologies, Shanghai, China https://www.lifetechnology.com/) added with nutrition supplements 14, 15, which consisted of putrescine (100 μM; Sigma‐Aldrich, shanghai, China, http://www.sigmaaldrich.com/), selenium (5 ng/ml; Sigma‐Aldrich,), insulin (25 µg/ml; Sigma‐Aldrich), transferrin (200 µg/ml; Sigma‐Aldrich), folic acid (10 µg/ml; Sigma‐Aldrich). The ex vivo expansion and differentiation protocol was comprised of four steps: step 1 (day 0–5), step 2 (day 6–12), step 3 (day 13–18), and step 4 (day 19–21). For each step, different combinations and concentrations of various growth factors (Table 1) including stem cell factor (SCF), thrombopoietin (TPO), fms‐related tyrosine kinase 3 ligand (FL), interleukin (IL)‐3, granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), erythropoietin (EPO), and fetal bovine serum (FBS) (15% vol/vol; Hyclone, Marlborough, MA, https://promo.gelifesciences.com/gl/hyclone/) were added to MM to determine the optimal conditions for ex vivo generation of human erythroid cells. All cytokines were purchased from Biopharmagen Corp. (Suzhou, China, http://www.biopharmagen.com/) unless otherwise specified.
Table 1.
Culture formula optimization for human erythroid cell expansion
| Group | IMDM | Nutrition supplements | FBS (15% Vol/Vol) | SCF (ng/ml) | EPO (IU/ml) | IL‐3 (ng/ml) | FL (ng/ml) | GM‐CSF (ng/ml) | TPO (ng/ml) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Step 1 | IMDM | + | – | – | – | – | – | – | – | – |
| MM | + | + | – | – | – | – | – | – | – | |
| MM+F | + | + | + | – | – | – | – | – | – | |
| MM+SFT a | + | + | – | 100 | – | – | 100 | – | 50 | |
| MM+F+SFT | + | + | + | 100 | – | – | 100 | – | 50 | |
|
Step 2 Step 2 |
SE3 | + | + | – | 100 | 6 | 20 | – | – | – |
| SE3+F | + | + | + | 100 | 6 | 20 | – | – | – | |
| SE3+F+FL(50) | + | + | + | 100 | 6 | 20 | 50 | – | – | |
| SE3+F+FL(100) | + | + | + | 100 | 6 | 20 | 100 | – | – | |
| SE3+F+FL(150) | + | + | + | 100 | 6 | 20 | 150 | – | – | |
| SE3+F+FL+GM(5) | + | + | + | 100 | 6 | 20 | 100 | 5 | – | |
| SE3+F+FL+GM(10) | + | + | + | 100 | 6 | 20 | 100 | 10 | – | |
| SE3+F+FL+GM(15) a | + | + | + | 100 | 6 | 20 | 100 | 15 | – | |
| SE3+F+FL+GM(20) | + | + | + | 100 | 6 | 20 | 100 | 20 | – | |
| Step 3 | SE | + | + | – | 100 | 6 | – | – | – | – |
| SE+F | + | + | + | 100 | 6 | – | – | – | – | |
| SE+F+IL‐3(5) | + | + | + | 100 | 6 | 5 | – | – | – | |
| SE+F+IL‐3(10) | + | + | + | 100 | 6 | 10 | – | – | – | |
| SE+F+IL‐3(15) | + | + | + | 100 | 6 | 15 | – | – | – | |
| SE+F+IL‐3+FL(25) | + | + | + | 100 | 6 | 10 | 25 | – | – | |
| SE+F+IL‐3+FL(50) a | + | + | + | 100 | 6 | 10 | 50 | – | – | |
| SE+F+IL‐3+FL(100) | + | + | + | 100 | 6 | 10 | 100 | – | – | |
| Step 4 | E | + | + | – | – | 6 | – | – | – | – |
| E+F | + | + | + | – | 6 | – | – | – | – | |
| SE a | + | + | – | 100 | 6 | – | – | – | – | |
| SE+F | + | + | + | 100 | 6 | – | – | – | – | |
Abbreviations: EPO, erythropoietin; FBS, fetal bovine serum; FL, fms‐related tyrosine kinase 3 ligand; GM‐CSF, granulocyte‐macrophage colony‐stimulating factor; IMDM, Iscove's Modified Dulbecco's medium; MM, modified medium; SCF, stem cell factor; TPO, thrombopoietin.
The various combinations and concentrations of growth factors in different groups during the four‐step culture are shown.
The final optimized medium formula in each step.
At regular intervals (1 or 2 days), cultures were demi‐depleted and fed with fresh medium to give a final cell density of 3 × 105 in a minimum volume of 200 ml in each 2 liter‐bottle. In the later stages of culture, total volume increased to 600 ml by dilution feeding in each bottle. For optimization process, cultures were maintained in one bottle. For large‐scale cultures, a total of 106 CB CD34+ as initial cells were expanded to produce 2.9 × 1011 total cells by the use of about 120‐liters medium for subsequent studies.
Cells were stained with 0.4% trypan blue and viable cells were manually counted at various time points using the Count Star cell counting system (Shanghai,China, http://www.countstar.cn/). Cells were stained with Wright‐Giemsa reagents (NJJCBIO, Nanjing, China, http://www.njjcbio.com/) for morphological analysis. Images of the stained cells were taken under a microscope with a digital camera (Olympus, Tokyo, Japan, http://www.olympus‐global.com/). Cells cultured for 21 days were harvested and stored at 4°C for 4 weeks in a Sag‐M preservative‐based solution as previously described 8.
Flow Cytometry
Flow cytometry data were obtained using the FACSVerse system (BD Biosciences) and analyzed using FlowJo software. At least 10,000 events were acquired for each sample. The samples were analyzed for expression of cell surface markers using Fluorescein isothiocyanate (FITC)‐labeled antibodies against CD235a (glycophorin A); phycoerythrin (PE)‐labeled antibodies against CD34 and CD45; allophycocyanin (APC)‐labeled antibodies against CD71. FITC‐, PE‐, or APC‐conjugated isotype‐matched antibodies served as controls. Primary human anti‐RhD antibodies (Bio‐Rad, Shanghai, China) and secondary Alexa Fluor 647‐conjugated rabbit anti‐human antibodies (Abcam, Shanghai, China, http://www.abcam.cn/) were used to detect RhD. All antibodies were purchased from Biopharmagen Corp. (Suzhou, China) unless otherwise specified. Cells were stained with the nucleic acid stain LDS‐751 (Life Technologies) to identify enucleated cells (LDS‐ cells) as described previously 6, 8.
Colony‐Forming Unit Assay
Colony‐forming unit (CFU) assays of unexpanded human CB CD34+ cells and cells cultured for 5 days, 9 days, and 12 days were performed as described previously 16. Cells were incubated at 37°C in 5% CO2 with >95% humidity for approximately 14 days. Burst‐forming unit‐erythroid (BFU‐E), CFU‐erythroid (CFU‐E), and CFU‐granulocyte‐monocyte (CFU‐GM) were counted using a bright‐field microscope. A colony with >100 cells was counted as a positive colony.
Quantitative Reverse Transcription‐Polymerase Chain Reaction
Cultured cells were harvested at various time points. Total RNA was extracted from cells using RNAiso Plus reagent (Takara, Dalian, China, http://www.takara.com.cn). Reverse transcription was performed using the RevertAid First Strand cDNA Synthesis System (Thermo Fisher Scientific) in a 20 µl reaction volume. After reverse transcription, 180 µl of water was added to the reaction mixture, and 1 µl of aliquot was used for each reaction. Quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) was performed using Power SYBR Green PCR Master Mix (Life Technologies,) and StepOne Plus Real‐Time PCR system (Applied Biosystems, Life Technologies). β‐actin was used as an internal control. Primers (Supporting Information Table 2) were designed and generated by Sangon Biotech (Shanghai, China, http://sangon.bioon.com.cn/).
Hemoglobin Content Detection
The hemoglobin content of cultured cells and RBCs from a healthy volunteer was quantified photometrically at 540 nm using Drabkin's reagent (Sigma‐Aldrich).
Functional Analysis of Hemoglobin
Oxygen equilibrium curves were determined using a Hemox‐Analyzer (TSC Scientific Corp, NY, USA, http://www.tcssci.com/). The gas phase gradients were obtained using nitrogen and room air, and the curves were run in both directions. Human peripheral blood cells and umbilical cord blood cells were used as the controls. CD34+ cells derived from CB that were cultured for 21 days were collected, washed, and subjected to analysis.
Animals
Male NOD/SCID mice (specific pathogen‐free; 6 weeks old, weight 16.0–18.0 g), obtained from the Experimental Animal Center of Soochow University (Suzhou, China), were used for transplantation. The experimental protocols used for mice were approved by the Institutional Animal Care and Use Committees of Soochow University (IACUC permit number: SYXK (Su) 2013‐0064).
Cynomolgus primates (5–6 years old, weight 3.5–6 kg) were obtained from the Medical Primate Research Center of the Institute of Medical Biology, Chinese Academy of Medical Sciences and were housed and bred according to the Guidelines of the Experimental Animals Ethics Committee at the Institute of Medical Biology of the Chinese Academy of Medical Sciences (Permit Number YISHENGLUNZI [2014] 07), which complied with the humane regulations of replacement, refinement, and reduction (the 3 Rs) 17.
Studies in the NOD/SCID Murine Model
After sublethal irradiation (radioactive source: 60Co; dose: 2.5 Gy; radioactive intensity: 0.38 Gy/minute), NOD/SCID mice were intraperitoneally injected with human type O RBCs (4–5 × 109 cells per mouse) to saturate the reticuloendothelial system 7. After 24 hours, the mice were injected intraperitoneally with 21‐day cultured cells (4 × 109 cells per mouse, experimental group, n = 4) or normal human RBCs (4 × 109 cells per mouse, positive control, n = 4), which had been previously washed, filtered through a deleukocyting filter, and labeled with CFSE (Life Technologies). Peripheral blood samples were then collected from the retro‐orbital plexus at different time points after injection and colabeled with LDS‐751, anti‐CD71 antibodies, or anti‐RhD antibodies for analysis by flow cytometry. Continuous observation lasted 6 months to evaluate the safety of cultured cells.
Studies in the NHP Model
Slide agglutination tests were performed using NHP serum and human RBCs (O‐type). Primates that showed no agglutination or weak agglutinate reaction to human RBCs (O‐type) were selected to be candidates for the subsequent experiments. Before xenotransfusion, primate's whole blood was collected depending on individual weight for modeling hemorrhagic anemia (as shown in Table 2). To reducing immunosuppression 18, all primates were injected intravenously with cobra venom factor (CVF) (Kmbiogen, Kunming, China, http://www.biogen.net.cn/) (0.05 mg/kg) at 24 hours before xenotransfusion, dexamethasone (0.5 mg/kg) and CVF (0.02 mg/kg) at half an hour before xenotransfusion, and dexamethasone (0.5 mg/kg) at 1 hour after xenotransfusion, respectively. As shown in Table 2, total primates (n = 9) were divided into four groups. As negative control, primates (n = 3) were injected with 706‐hydroxyethyl starch after blood collection; for positive controls (positive control I, n = 2; positive control II, n = 2), primates were transfused with normal human RBCs (O‐type) derived from fresh whole blood; for experimental group (n = 2), primates were transplanted with 21‐day cultured cells. All cells were previously washed, filtered through a deleukocyting filter, and suspended in the 706‐hydroxyethyl starch for xenotransfusion. For positive control I, the amount of transfused RBCs was equal to the collected amount of RBCs; for positive control II and experimental group, the amount of transfused cells was half of the collected amount of RBCs, and cells were labeled with FITC‐microbeads (Lumigenex, Suzhou, China, http://www.lumigenex.com/) as described previously 19. Labeling efficiency of FITC‐microbeads was approximately 100%, as confirmed by flow cytometry.
Table 2.
Summary of relevant information of the primates before/after xenotransplantation
| Characteristics | Positive control Ia | Positive control IIb | Negative controlc | Experimental groupd | |||||
|---|---|---|---|---|---|---|---|---|---|
| Age, yr | 6 | 5 | 5 | 5 | 5 | 6 | 6 | 5 | 5 |
| Sex | male | male | male | male | male | male | male | male | male |
| Weight (kg) | 5.6 | 4.6 | 4.2 | 4.0 | 5.0 | 4.6 | 4.8 | 3.6 | 4.0 |
| Before xenotransplantation | |||||||||
| RBC counts (109/ml) | 5.81 | 5.53 | 6.01 | 5.89 | 5.86 | 5.95 | 5.75 | 5.42 | 5.60 |
| Hemoglobin content (g/l) | 152 | 148 | 150 | 145 | 137 | 153 | 143 | 147 | 152 |
| LDH (U/l) | 390.5 | 353.2 | 276.4 | 306.0 | 432.9 | 281.0 | 347.0 | 397.8 | 294.8 |
| Collected peripheral Blood(ml) | 56 | 46 | 42 | 40 | 50 | 46 | 48 | 36 | 40 |
| Collected RBC number (109) | 325.36 | 254.38 | 252.42 | 235.60 | 293.00 | 273.70 | 276.00 | 195.12 | 224.00 |
|
Xenotransplanted cell number
(After deleukocyting) |
|||||||||
| Total cells (109) | 325.36 | 254.38 | 126.21 | 117.80 | — | — | — | 97.56 | 112.00 |
| Enucleated cells (109) | 325.36 | 254.38 | 126.21 | 117.80 | — | — | — | 54.63 | 62.72 |
| FITC microbeads labeling efficiency, % | — | — | 97% | 98% | — | — | — | 98% | 96% |
| RBC source | Human peripheral blood |
Human peripheral blood |
Human peripheral blood | Human peripheral Blood | — | — | — |
Cultured RBCs |
Cultured RBCs |
Abbreviations: —, not applicable; FITC, fluorescein isothiocyanate; LDH, lactate dehydrogenase; RBC, red blood cell; yr, year.
Positive control I: primates (n = 2) were transfused with RBCs derived from normal human peripheral blood. The amount of transplanted RBCs was equal to the collected.
Positive control II: primates (n = 2) were transfused with RBCs derived from normal human peripheral blood. The amount of transplanted RBCs was half of the collected.
Negative control: primates (n = 3) were treated with 706‐hydroxyethyl starch after blood collection.
Experimental group: primates (n = 2) were transfused with 21‐day cultured cells after blood collection. The amount of transplanted cells was half of the collected.
Routine whole blood tests were performed using SysmexXT‐2000iv (Sysmex corporation, Kobe, Japan, http://www.sysmex.com/) to monitor hematopoietic recovery, including the number of RBCs and hemoglobin content. Lactate dehydrogenase (LDH) of primates was measured by Mindray BS‐200 Automatic Biochemistry Analyzer (Mindray, Shenzhen, China, http://www.mindray.com/cn/). FITC fluorescent cells were detected by flow cytometry.
Statistical Analysis
One‐way analysis of variance followed by Dunnett's multiple comparison test was used for comparison among the various treatment groups. Results were considered statistically significant when the p value was less than .05.
Results
Culture Condition Optimization for Ex Vivo Generation of Human Erythrocytes From CB CD34+ Cells
We optimized a four‐step protocol for the ex vivo expansion and differentiation of human erythrocytes from CB CD34+ cells (Table 1). Various groups with different medium formulas were assessed in each step.
In step 1, isolated CD34+ cells were expanded for 5 days to produce an increased amount of HSPCs. The highest expansion fold was observed in the MM +SFT group, which consisted of IMDM, nutrition supplements, SCF at 100 ng/ml, FL at 100 ng/ml, and TPO at 50 ng/ml. The fold increase in CD34+ cell proliferation was 20 ± 2.4, and the CD34+ percentage was maintained at 80% ± 4.3%. Although the MM+F+SFT group had the highest expansion fold of total cells, the ratio of CD34+ cells was rapidly decreased because of the effect of FBS (Fig. 1A). Therefore, the MM+SFT group was selected for CD34+ cell expansion in step 1.
Figure 1.
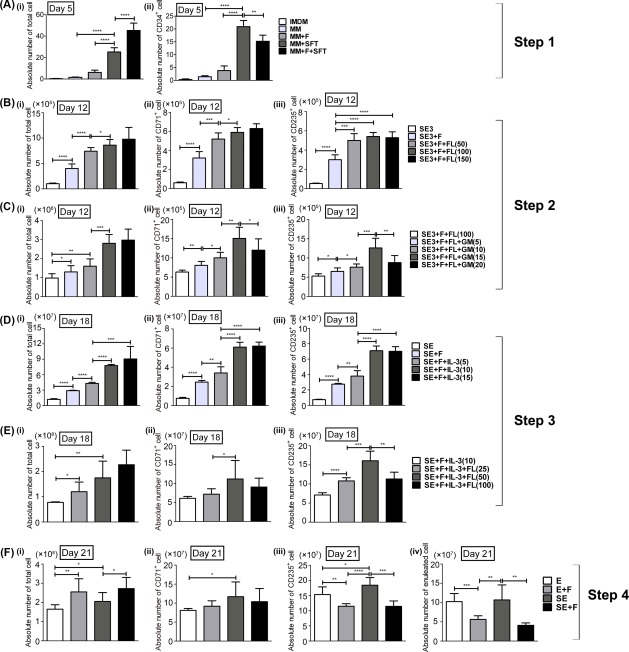
Culture condition optimization for ex vivo generation of human erythrocytes from CB CD34+ cells. Yields of total cells, CD34+ cells, CD71+ cells, CD235a+ cells, and enucleated cells were calculated in case one CD34+ cells were seeded on day 0. (A): For step 1, isolated CD34+ cells were cultured for 5 days in different medium formulas, the absolute numbers of (Ai) total cells and (Aii) CD34+ cells were calculated on day 5. (B, C): Step 2 was initiated with cells derived from the MM+SFT group (IMDM + 100 ng/ml SCF + 100 ng/ml FL + 50 ng/ml TPO) of step 1. (B) Absolute numbers of (Bi) total cells, (Bii) CD71+ cells, and (Biii) CD235a+ cells were calculated on day 12 with FL ranging from 0 to 150 ng/ml in SE3 + F medium (IMDM + nutrition supplements + FBS + 100 ng/ml SCF + 6 IU/ml EPO + 20 ng/ml IL‐3). (C) Absolute numbers of (Ci) total cells, (Cii) CD71+ cells, and (Ciii) CD235a+ cells were calculated on day 12 with GM‐CSF ranging from 0 to 20 ng/ml in SE3+F+FL(100) medium (SE3 + F medium supplemented with 100 ng/ml FL). (D, E): Step 3 was initiated with cells derived from the SE3+F+FL+GM(15) group (SE3+F+FL(100) medium supplemented with 15 ng/ml GM‐CSF) of step 2. (D) Absolute numbers of (Di) total cells, (Dii) CD71+ cells, and (Diii) CD235a+ cells were calculated on day 18 in different medium formulas with IL‐3 ranging from 0 to 15 ng/ml in SE+F (IMDM + nutrition supplements + FBS + 100 ng/ml SCF + 6 IU/ml EPO) medium. (E) Absolute numbers of (Ei) total cells, (Eii) CD71+ cells, and (Eiii) CD235a+ cells were calculated on day 18 with FL concentrations ranging from 0 to 100 ng/ml in SE+F+IL‐3(10) medium (SE+F medium supplemented with 10 ng/ml IL‐3). (F): Step 4 was initiated with cells derived from the SE+F+IL‐3+FL(50) group (SE+F+IL‐3(10) medium supplemented with 50 ng/mL FL) of step 3. (F) Absolute numbers of (Fi) total cells, (Fii) CD71+ cells, (Fiii) CD235a+ cells, and (Fiv) enucleated cells were calculated on day 21 with different medium formulas. Results are presented as mean ± SD of six independent experiments. *, p < .05; **, p < .01; ***, p < .001. One‐way analysis of variance followed by Dunnett's multiple comparison test. Abbreviations: IMDM, Iscove's Modified Dulbecco's medium; MM, modified medium.
The expanded HSPCs were induced to differentiate to the erythroid lineage in step 2. Cytokines such as SCF, EPO and IL‐3 have been used most frequently for erythroid differentiation. On the other hand, FL is known to contribute to the expansion of erythroid progenitors 3 and prevent apoptosis 20, 21. To identify the optimal combinations and concentrations of growth factors for generating human erythroid progenitors ex vivo, FL (50–150 ng/ml) and GM‐CSF (5–20 ng/ml) were added to IMDM supplemented with the nutrition supplements and SCF at 100 ng/ml, EPO at 6 IU/ml, and IL‐3 at 20 ng/ml (Table 1). The addition of FBS and FL in SE3 medium significantly improved cell yield (Fig. 1B). The concentration of FL selected was 100 ng/ml since there was no significant difference in CD71+ and CD235a+ cell numbers with 100 ng/ml FL compared with those with 150 ng/ml FL (Fig. 1B). SE3+F+FL (100) with GM‐CSF at 15 ng/ml demonstrated a significant expansion of CD71+ and CD235a+ cells. When the concentration of GM‐CSF increased to 20 ng/ml, CD71+ and CD235a+ cell numbers decreased, which might be attributed to the myeloid differentiation effect of GM‐CSF 22 (Fig. 1Cii, 1Ciii).
In step 3, further proliferation and differentiation of erythroid cells were induced by a specific cytokine combination. To achieve a high level of erythrocyte production, IL‐3 (5–15 ng/ml) and FL (25–100 ng/ml) were added to the medium supplemented with SCF and EPO, which are commonly used for erythroid maturation, to determine an appropriate combination and concentration for RBC expansion and maturation. IL‐3 at 10 ng/ml and FL at 50 ng/ml significantly enhanced CD235a+ cell expansion when added to the medium supplemented with FBS and nutrition supplements (Fig. 1D, 1E).
In the final step of maturation, FBS was excluded for its multilineage differentiation effects. Although there was no significant difference between the E group and SE group in enucleated cell number, the SE group still had a higher CD235a+ cell number (Fig. 1F). Therefore, the SE group was selected in the last step. Overall, the final optimized culture formula for human erythroid cell expansion and differentiation was IMDM supplemented with nutrition supplements and sequential combinations of cytokines (MM+SFT for step 1, SE3+F+FL+GM(15) for step 2, SE+F+IL‐3+FL(50) for step 3, and SE for step 4) as shown in Table 1.
Large‐Scale Generation of Human Erythrocytes
Ex vivo large‐scale erythropoiesis from CB CD34+ cells was performed in the bottle turning device culture system with the selected cytokine combinations (Supporting Information Fig. S1). 2.9 × 1011 total cells with a 50.0% enucleation rate (LDS– cells) were produced from 106 CB CD34+ cells by the use of about 120 liter medium for subsequent studies. As shown in the growth curve (Fig. 2A), the expansion fold of total cells increased slowly during the initial culture period (day 0 to day 5). Then, from day 5 to day 15, cells entered an exponential growth phase, in which cells maintained a high proliferating rate and vigorous growth. An approximate of 2.9 × 106‐fold and 8.9 × 107‐fold increase in cells was achieved by day 12 and day 15, respectively. Cells had a slow expansion rate from day 18, and the amplification of total cells reached a plateau of approximately 2 × 108‐fold (1.4–2.5 × 108‐fold) by day 21. When cultures were continued, a decrease in cells growth related to cell mortality was observed from day 21 (data not shown).
Figure 2.
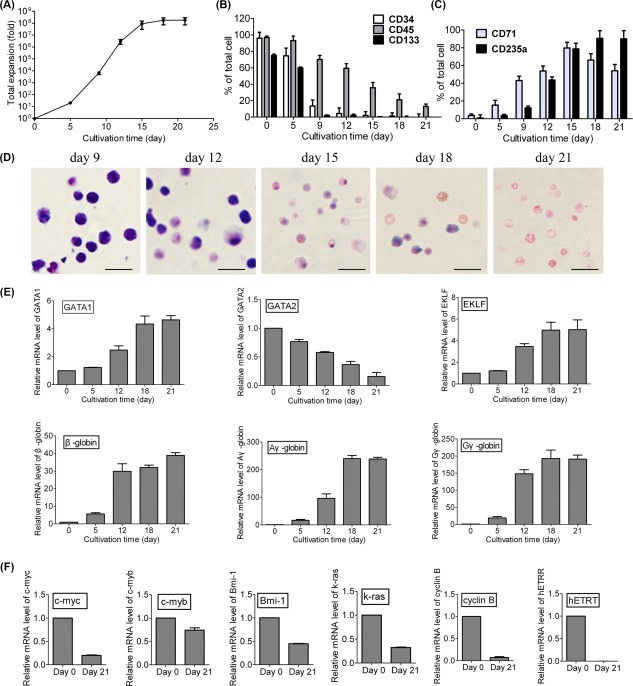
Large‐scale generation of human erythrocytes and characterization analysis. (A): Expansion folds of total cells during the 21‐day culture. Isolated CD34+ cells were cultured under selected culture conditions. Expansion was calculated as a fold increase (after/before expansion) in cell counts on a specific day (day 0, 5, 9, 12, 15, 18, and 21). Flow cytometric analysis of the expression of (B) hematopoietic stem cell markers (CD34, CD133, and CD45) and (C) erythroid markers (CD235a and CD71) in cultured cells. Data are mean ± SD, n = 4. (D): Representative photomicrograph of cells on day 9, 12, 15, 18, and 21 with Wright‐Giemsa staining. Scale bar = 20 μm. (E): Expression profiles of erythroid‐specific genes (GATA1, GATA2, EKLF, β‐globin, γG‐globin, and γA‐globin) on the indicated days. (F): Expression of certain representative proto‐oncogenes (c‐myc, c‐myb, Bmi‐1, k‐ras, cyclin B, and hETRT) in the 21‐day cultured cells. Data are mean ± SD; n = 4. Gene expression was normalized to β‐actin expression.
Erythroid differentiation was morphologically examined by CFU assay and cell staining. CFU assay (Supporting Information Table 1) showed that over 90% of the total colonies were BFU‐E, or CFU‐E on day 9 and day 12, suggesting that these expanded cells were induced toward the erythroid lineage. The proliferation of the generated erythroid progenitors and their subsequent differentiation and maturation were also assessed by flow cytometric analysis. Isolated CD34+ cells (day 0) expressed a high level of HSPCs markers (CD34, CD133, and CD45), and as expected, the expression of erythroid markers (CD71 and CD235a) was low or undetectable. After 21 days of differentiation, the percents of CD34+ and CD133+ decreased significantly and remained weakly positive (1%–2%), and the CD45+ percentage of the cell population also declined to 12.9 ± 2.8% (Fig. 2B). On the other hand, the expression of CD71 showed a rapid increase with a peak on day 15 (79.8% ± 6.3%) and continuous decrease following the differentiation process. The expression of CD235a increased gradually and maintained a high level after that (Fig. 2C). On day 21, the cultured cells strongly expressed CD235a (90.1% ± 6.2%) and weakly expressed CD71 (54.0% ± 7.2%). Cell staining showed that the cell population exhibited a pure erythroid phenotype. Enucleated RBCs could be observed in this population (Fig. 2D).
Analysis of Gene Expression
At various time points of the culture process, the expressions of erythroid‐specific genes (GATA1, GATA2, EKLF, β‐globin, Aγ‐globin, and Gγ‐globin) were detected by qRT‐PCR. As shown in Figure 2E, GATA1 expression gradually increased during erythroid differentiation, whereas GATA2 expression gradually decreased following cell maturation. EKLF, a β‐globin gene transcription factor, exhibited increased expression during differentiation. In addition, the upregulation of Aγ‐globin, Gγ‐globin, and β‐globin was also observed in the cultured erythrocytes. These results indicated that the induced erythrocytes were able to produce fetal‐type hemoglobin (Aγ‐globin, Gγ‐globin) and adult‐type hemoglobin (β‐globin).
The expression levels of typical hematologic disease‐associated proto‐oncogenes (c‐myc, c‐myb, Bmi‐1, K‐ras, cyclin B, and hETRT) were analyzed before and after differentiation. The results showed that the expression levels of these genes in cultured erythrocytes were lower than of those in unexpanded CB CD34+ cells, indicating that the proto‐oncogenes were not activated in the cultured cells after differentiation (Fig. 2F).
Synthesis and Functionality of Hemoglobin
From days 9 to 21, the hemoglobin content of cultured cells increased from 15.3 ± 1.5 pg per cell to 31.5 ± 2.4 pg per cell, which was similar to the content of normal human RBCs (27–33 pg per cell) (Fig. 3A). Furthermore, after centrifugation, the color of the cell pellet changed from light pink to red, which also suggested that hemoglobin synthesis was upregulated following differentiation (Fig. 3B).
Figure 3.
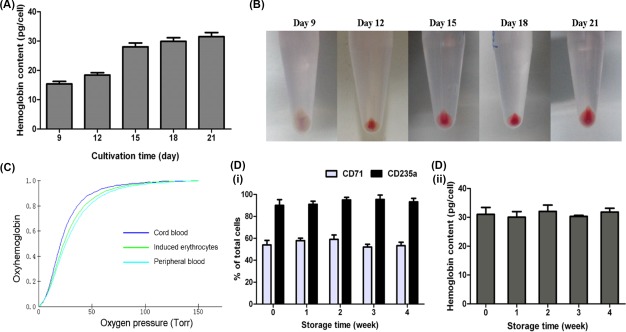
Synthesis and functionality of hemoglobin in the induced erythrocytes. (A): Hemoglobin content and (B) cell pellets of the cultured cells on day 9, 12, 15, 18, and 21. Data are mean ± SD, n = 4. (C): A representative oxygen equilibrium curve of 21‐day induced erythrocytes. Red blood cells derived from cord blood and normal human peripheral blood were used as controls. (D): Long‐term storage of the cultured erythrocytes. Cells were harvested on day 21 and conserved at 4°C in a preservative solution for up to 4 weeks. The (Di) CD71 and CD235a expression and (Dii) hemoglobin content of stored cells were evaluated during storage. Data are mean ± SD, n = 3.
The functionality of hemoglobin from induced cells was analyzed by oxygen equilibrium curves. Although erythrocytes derived from three sources (human CB, human peripheral blood and cultured cells) had different combinations of HbA and HbF, similar hemoglobin dissociation curves were observed (Fig. 3C). This result showed that ex vivo induced RBCs could produce hemoglobin with oxygen binding and dissociation abilities that were equivalent to RBCs produced in vivo.
Long‐Term Storage of Induced Erythrocytes
Cultured erythrocytes were harvested on day 21 and conserved at 4°C in a preservative solution for 4 weeks. The expressions of erythroid markers and hemoglobin content of the erythrocytes were well maintained throughout the storage period (Fig. 3D), indicating that the status of these cells remained unchanged during storage.
In Vivo Maturation of Cultured Cells in the NOD/SCID Murine Model
To follow the in vivo fate of cultured cells, we injected CFSE‐labeled cells collected on day 21 into NOD/SCID mice. Before injection into NOD/SCID mice, 68.6% and 53.4% of the cells were CD71+ and LDS–, respectively. After injection, cultured erythrocytes persisted in the circulation to the same extent as normal human RBCs; CFSE+ cells were detected in the peripheral blood of mice during 3 days post‐injection in both groups of animals (Fig. 4A). The kinetics of CD71+ cells of the CFSE+ population showed a daily decrease (39.0% ± 7.3% of the CFSE+ cells on the first day post‐injection, 17.4% ± 5.5% on the second day, and 3.8% ± 2.3% on the third day) (Fig. 4B, 4C). At the same time, the enucleation of transplanted cells was observed by the conversion of CFSE+LDS+ cells to CFSE+LDS– cells; LDS– cells of the CFSE+ population increased from 62.0% ± 8.2% on the first day post‐injection to 97.1% ± 2.3% on the third day post‐injection (Fig. 4D, 4E). On the third day post‐injection, all CFSE+ cells expressed the human RhD blood group antigen (Fig. 4F). These results indicated that the cultured erythrocytes were able to undergo terminal maturation in vivo. Moreover, 6 months after transplantation, all mice continued to survive with no apparent abnormalities.
Figure 4.
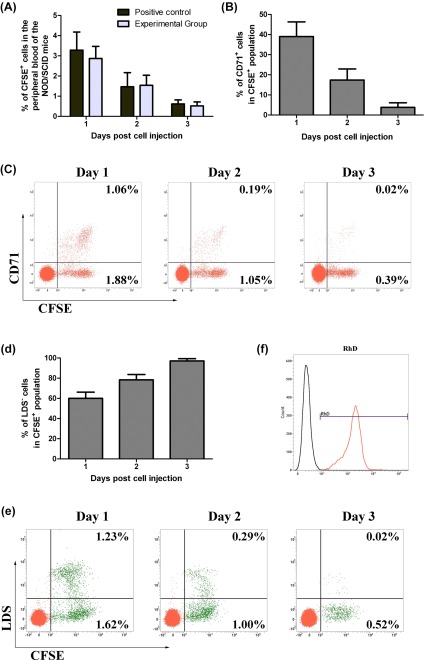
In vivo maturation of the cultured cells in NOD/SCID mice. (A): Percentage of CFSE+ cells in mouse peripheral blood on days 1 to 3 after cell injection. (B): Kinetics of CD71+ cells of the CFSE+ population in mouse peripheral blood after cell injection. (C): Flow cytometric profiles of CD71+ and CFSE+ cells in one representative experiment. Horizontal axis, CFSE detection; vertical axis, CD71 detection. (D): Kinetics of LDS– cells of the CFSE+ cell population after injection. (E): Flow cytometric profiles of LDS+ and CFSE+ cells in one representative experiment. Horizontal axis, CFSE detection; vertical axis, LDS detection. Data are mean ± SD, n = 4. (F): The expression of the RhD antigen in CFSE+ cells in one representative experiment on day 3 post‐injection. CFSE+ cells were co‐labeled with anti‐RhD antibody (red histogram) or its control isotype (black histogram). Abbreviations: CFSE, 5(6)‐Carboxyfluorescein diacetate N‐succinimidyl ester; LDS, laser dye styryl; NOD/SCID, nonobese diabetic/severe combined immunodeficient; RhD, Rhesus antigen D.
Safety Assessment in the NHP Model
To assess the safety and efficacy of induced erythrocytes, a NHP model was used. For modelling hemorrhagic anemia model, the primate's whole blood was collected before xenotransplantation. In month 18 post transplantation, these primates continued to survive with no apparent abnormalities. Parameters of complete blood counts, blood chemistries, appetite, weight gain, and behavior were all normal, which implied that transfusion of cultured cells had no adverse effects on primates.
Xenotransfusion in the NHP Model
After blood collection, RBC counts and hemoglobin content declined remarkably in all primates, which were 82%–85% of the initial values (Fig. 5A, 5B). In the positive control I, after xenotransplantation, RBC counts and hemoglobin content showed a rapid increase and remained at 90% of the initial total amount during 30 hours after transfusion. Then, from hours 30 to 72 post transfusion, the RBC counts and hemoglobin content showed downward trends, which might be due to the immune elimination of human RBCs by primates and the continuous blood collection during the experimental process. In contrast, in the negative control group treated with 706‐hydroxyethyl starch, both of the RBCs count and hemoglobin content showed continuously downward trends during 54 hours after xenotransfusion. Moreover, 144 hours were required for the RBC counts and hemoglobin contents to recover to the normal level for the positive control I. Whereas, 240 hours were required for the negative control group (Fig. 5A, 5B). These results indicated that transfusion of human RBCs could enhance the hematological recovery of the NHP with hemorrhagic anemia.
Figure 5.
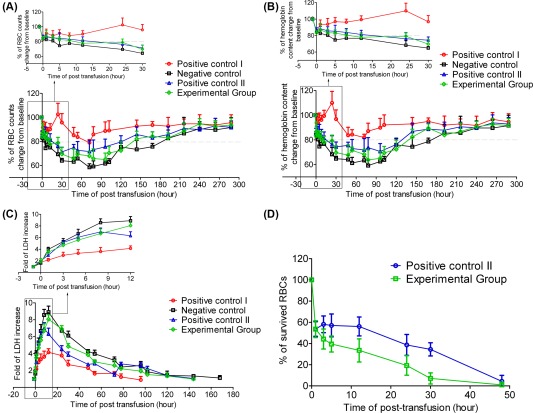
Hematological recovery in nonhuman primates after xenotransfusion. RBC counts (A) and hemoglobin content (B) in primates which received human derived erythrocytes (Positive control I, n = 2; positive control II, n = 2; experimental group, n = 2) or 706‐hydroxyethyl starch (negative control, n = 3). The initial amount of RBC counts and hemoglobin content before xenotransfusion were regarded as 100%. For positive control I and positive control II, primates were transfused with normal human RBCs isolated from peripheral blood (O‐type); for experimental group, primates were transfused with 21‐day cultured erythrocytes. The amount of transfused cells for positive control I was equal to the collected amount, and the amount of transfused cells for positive control II and experimental group was half of the collected amount. Data are mean ± SD. (C): The increased folds of LDH in the peripheral blood of primates at various time points post xenotransfusion. Data are mean ± SD. (D): FITC microbeads‐florescent cells were detected by flow cytometry in the peripheral blood of primates that received normal human RBCs (positive control II), and 21‐day cultured erythrocytes (experimental group). The percentage of FITC‐florescent cells in primates' blood samples which were collected on 5 minutes post xenotransfusion was regarded as 100%. Results are presented as mean ± SD. Abbreviations: LDH, lactate dehydrogenase; RBC, red blood cells.
Primates in positive control II and experimental group showed a similar trend on the change of RBC counts and hemoglobin content (Fig. 5A, 5B). Moreover, the results showed that cultured erythrocytes survived in the circulation of primates to the same extent level as normal human RBCs (Fig. 5D). The recovery of RBC counts and hemoglobin content in these two groups was slower than that in positive control I because of only half amount of transfused cells. At various time points after xenotransfusion, RBCs counts and hemoglobin content in experimental group were slightly lower than that of positive control II, which might be due to the induced erythrocytes containing part of immature cells. However, primates in both of the positive control II and experiment group showed a quicker hematological recovery than those in the negative control group. Complete blood counts showed that the period of RBC counts and hemoglobin content below 80% of the initial amount was from hours 12 to 120 post transfusion and from hours 24 to 120 in the experimental group and positive control II, respectively, while the period was from hours 3 to 168 in the negative control group (Fig. 5A, 5B).
Blood biochemical examination showed that LDH in all primates increased obviously during 10 hours after blood collection, indicating the increase of anaerobic metabolism induced by hypoxemia. The LDH peak was 9‐fold of the initial value in negative control group compared with 4.15‐fold in the positive control I, 6.98‐fold in the positive control II, and 8.06‐fold in the experimental group (Fig. 5C). The 96 hours required for the LDH to come down to normal level in positive control I compared with 144 hours in positive control II and experimental group, and 168 hours in negative control group. These results indicated that xenotransfusion of human derived erythrocytes ameliorated the hypoxia situation the hypoxia situation in the primates with hemorrhagic anemia.
Discussion
In the current study, we developed a four‐step protocol for ex vivo large‐scale generation of erythrocytes from HSPCs. Under our experimental conditions, a combination of 20‐fold amplified CD34+ cells in the first step with subsequent steps of erythroid differentiation generated over 2 × 108 erythrocytes from one input of CB CD34+ cell population. This result indicated that the yield of erythrocytes derived from one cord blood unit (5 million CD34+ cells) could, in theory, be equivalent to 500 blood transfusion units in clinical applications. In comparison with previous studies 3, 11, 23, 24, a higher numerical expansion was achieved in a shorter culture period. In addition to the clinically relevant cell yield, ex vivo‐generated erythrocytes can be closely monitored and controlled in the laboratory to reduce infectious risks, which can be a promising solution in addressing the shortage of safe blood for transfusion.
Given the large number of cells required for clinical use, erythrocyte production in tissue culture flasks would not be feasible or sufficient. Therefore, we scaled up the cell culture process from flasks to a bottle turning device system, which could hold nearly 10 liters of medium with 16 turning bottles in one customized incubator. Dissolved oxygen level is one of the key factors that can affect HSPC differentiation ex vivo 25, 26. In this study, the rotation speed of the bottles was adjusted to maintain an appropriate medium dissolved oxygen level. Moreover, in comparison with a static culture system, the dynamic culture system was able to prevent cells from conglutination or forming clusters. To our knowledge, this is the first report on using a bottle turning device to scale up ex vivo erythropoiesis. In comparison with the small volume culture system (flask‐based culture), the yield data obtained from this culture system would be more reliable for actual large‐scale practice.
The high cost of ex vivo generating RBCs is a major obstacle for the clinical application. To address this issue, we modified the medium for HSPCs expansion and differentiation to cut the cost. By calculation, the cost to produce a unit (200 ml) of blood with our modified medium is about $6,000 (excluding the cost of cytokines), which is estimated to be 1/30 or even less than that of producing a unit of blood with commercially available medium 27 in the market. On the other hand, we have also attempted to optimize the culture protocol to improve the proliferation/differentiation capacity of HSPCs [Y. Zhang, B. Shen, X. Guan et al., manuscript submitted]. Because of the limited proliferative ability of lineage‐restricted downstream cells, we added SCF, TPO, and Flt‐3 to promote the amplification of CB CD34+ cells during the initial culture period. Considering HSPCs reside in hypoxic niches within bone marrow and cord blood, hypoxia (3% O2) culture condition was tested, aiming to get a better amplification of multipotent and erythroid committed progenitors 28, 29, while our data did not show significant improvement for the ultimate production of RBCs compared to the condition in atmospheric O2 (unpublished data). It might be explained that essentially all experiments, such as cell isolation and the processes for functional analysis, are performed in nonphysiologic ambient air. We will be actively pursuing this approach by introducing an O2‐, and CO2‐controlled glove box to collect and manipulate CB CD34+ cells in native conditions of hypoxia.
It has been suggested that EPO is a key regulator of erythroid differentiation 30. However, EPO alone is insufficient for large‐scale amplification of HSPCs and erythroid cells 31, 32. Therefore, SCF and EPO, which are commonly used for ex vivo erythroid differentiation, and other cytokines (IL‐3, FL, and GM‐SCF) were added at optimized concentrations to stimulate the expansion of erythroid progenitors and maturation into erythrocytes 33, 34, 35, 36. In the last step of erythroid cell maturation, FBS and other cytokines (IL‐3, FL, and GM‐SCF) were excluded because of their multilineage differentiation effects. Moreover, the removal of FBS also improved process security and eliminated the risk of disease transmission. Finally, based on the selected optimized culture conditions, human CB CD34+ cells were efficiently induced to differentiate into erythrocytes after 21 days of culture.
During differentiation, flow cytometric analyses demonstrated that CD235a, CD71, and CD34 expressions in CB‐derived erythrocytes were consistent with previous results regarding erythrocyte induction mechanisms 37, 38. The gene expression profile of cultured cells was also analyzed by qRT‐PCR at various time points. In comparison with initial CD34+ cells, generated erythrocytes had similar or lower expression levels of proto‐oncogenes including c‐myc, c‐myb, Bmi‐1, K‐ras, cyclin B, and hETRT, thus strongly suggesting that the ex vivo‐generated erythrocytes were unlikely tumorigenic. GATA1 is a transcription factor that determines erythroid differentiation and survival as well as hemoglobin gene expression 39, and GATA1 inhibits GATA2 expression. As shown in Figure 2, GATA2 expression was downregulated following EPO stimulation. EKLF binds specifically to the β‐globin promoter 40, and these transcription factors are closely related to hemoglobin gene expression. Therefore, the upregulation of β‐globin in the induced erythrocytes was supported by the increased expression of EKLF. Previous studies have suggested that the original sources of HSPCs appear to possess different propensities for hemoglobin production. CB‐ and mobilized PB‐derived erythrocytes predominantly express γ and β globin chains 7, 41, 42, respectively. In our study, γ/β ratio was 90:10 in cultured erythrocytes by qRT‐PCR analyses, which is consistent with previous results. We also monitored hemoglobin synthesis at the protein level. Hemoglobin synthesis begins at the erythroid precursor stage 43. As expected, hemoglobin was detectable in the day‐9 cell population. Progressive erythroid maturation was accompanied by an increasing level of hemoglobin, and on day 21, the hemoglobin content of induced erythrocytes was similar to that of normal RBCs produced in vivo. For the functional analysis of hemoglobin, induced RBCs showed similar oxygen equilibrium curves to those obtained with healthy adult peripheral blood or umbilical cord blood. Furthermore, the hemoglobin content of cells stored for 4 weeks was similar to that of recent ex vivo‐generated erythrocytes, indicating that the induced cells were stable and functional.
Although the ex vivo‐generated erythrocytes were not fully mature, we were able to confirm their capacity to undergo terminal maturation in vivo using the NOD/SCID murine model, as demonstrated by the absence of membrane CD71 protein and nucleic material. Further studies in NHP confirmed that induced erythrocytes were capable of oxygen‐carrying ability, and the cultured erythrocytes could enhance hematological recovery and ameliorate the hypoxia situation in the primates in a hemorrhagic anemia model. Moreover, xenotransfusion of cultured cells revealed no adverse effect in mice and primates. All mice and primates continued to survive with no apparent abnormalities during 6 months and 18 months after transplantation, respectively, demonstrating the long‐term safety of the induced erythrocytes. These results indicated that xenotransfusion of human erythrocytes were capable of alleviating the hypoxia situation in the primates with hemorrhagic anemia. These findings suggested that the ex vivo‐generated erythrocytes could be considered as a potential substitute for clinical blood transfusion to overcome blood supply shortages, especially for the treatment of chronic anemia or patients with rare blood types, as well as some urgent occasions of blood transfusion.
Conclusion
We have established a large‐scale culture system to produce functional human erythrocytes ex vivo. The results of our study suggest that the ex vivo‐generated RBCs retain the normal features and functionality as the in vivo‐generated RBCs and could be an alternative RBC source for traditional transfusion RBC products in clinical applications.
Author Contributions
Y.J. and X.D.: conception and design, financial support, administrative support, data analysis and interpretation, manuscript writing, final approval of manuscript; Y.Z. and C.W.: conception and design, provision of study material or patients, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; L.W., B.S., and X.G.: collection and/or assembly of data, data analysis and interpretation, final approval of manuscript; J.T. and Z.R.: administrative support, data analysis and interpretation, final approval of manuscript; Y.M. and W.D.: data analysis and interpretation, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
C.W. and Z.R. are employees of Biopharmagen Corp. Y.J. has a leadership position with Biopharmagen Corp. The other authors indicated no potential conflicts of interest.
Supporting information
Supporting Information
Supporting Information Figure 1
Supporting Information Table 1
Supporting Information Table 2
Acknowledgments
We thank our coworkers in the laboratory for their valuable discussions and suggestions. This work was supported by State Scientific Key Projects for New Drug Research and Development (2011ZX09102–010‐04 and 2011ZX09401–027), International Cooperation and Exchange Program (2013DFA30830), China.
This article was published online on 15 June 2017. The logo for the Cord Blood Association incorrectly appeared on the article and has now been removed because the authors are not members of the association. This notice is included in the online version to indicate this version was corrected 05 October 2017.
References
- 1. World Health Organization . Global Database on Blood Safety: Summary Report 2011. Available at http://www.who.int/bloodsafety/global_database/GDBS_Summary_Report_2011.pdf?ua=1. Last accessed December 2016.
- 2. Ali A, Auvinen MK, Rautonen J. The aging population poses a global challenge for blood services. Transfusion 2010;50:584–588. [DOI] [PubMed] [Google Scholar]
- 3. Baek EJ, Kim HS, Kim S et al. In vitro clinical‐grade generation of red blood cells from human umbilical cord blood CD34+ cells. Transfusion 2008;48:2235–2245. [DOI] [PubMed] [Google Scholar]
- 4. Xi J, Li Y, Wang R et al. In vitro large scale production of human mature red blood cells from hematopoietic stem cells by coculturing with human fetal liver stromal cells. BioMed Res Int 2013;2013:807863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hiroyama T, Miharada K, Sudo K et al. Establishment of mouse embryonic stem cell‐derived erythroid progenitor cell lines able to produce functional red blood cells. PLoS One 2008;3:e1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neildez‐Nguyen TM, Wajcman H, Marden MC et al. Human erythroid cells produced ex vivo at large scale differentiate into red blood cells in vivo. Nat Biotechnol 2002;20:467–472. [DOI] [PubMed] [Google Scholar]
- 7. Giarratana MC, Kobari L, Lapillonne H et al. Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat Biotechnol 2005;23:69–74. [DOI] [PubMed] [Google Scholar]
- 8. Giarratana MC, Rouard H, Dumont A et al. Proof of principle for transfusion of in vitro‐generated red blood cells. Blood 2011;118:5071–5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fujimi A, Matsunaga T, Kobune M et al. Ex vivo large‐scale generation of human red blood cells from cord blood CD34+ cells by co‐culturing with macrophages. Int J Hematol 2008;87:339–350. [DOI] [PubMed] [Google Scholar]
- 10. Shah S, Huang X, Cheng L. Concise review: Stem cell‐based approaches to red blood cell production for transfusion. Stem Cells transl Med 2014;3:346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Timmins NE, Athanasas S, Gunther M et al. Ultra‐high‐yield manufacture of red blood cells from hematopoietic stem cells. Tissue Eng Part C Methods 2011;17:1131–1137. [DOI] [PubMed] [Google Scholar]
- 12. Trobridge GD, Kiem HP. Large animal models of hematopoietic stem cell gene therapy. Gene Ther 2010;17:939–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim S, Kim N, Presson AP et al. Dynamics of HSPC repopulation in nonhuman primates revealed by a decade‐long clonal‐tracking study. Cell Stem Cell 2014;14:473–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buono KD, Goodus MT, Guardia Clausi M et al. Mechanisms of mouse neural precursor expansion after neonatal hypoxia‐ischemia. J Neurosci 2015;35:8855–8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Covey MV, Levison SW. Leukemia inhibitory factor participates in the expansion of neural stem/progenitors after perinatal hypoxia/ischemia. Neuroscience 2007;148:501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Giarratana MC, Kobari L, Neildez Nguyen TM et al. Cell culture bags allow a large extent of ex vivo expansion of LTC‐IC and functional mature cells which can subsequently be frozen: Interest for a large‐scale clinical applications. Bone Marrow Transplant 1998;22:707–715. [DOI] [PubMed] [Google Scholar]
- 17. Aglietta M, Bertolini F, Carlo‐Stella C et al. Ex vivo expansion of hematopoietic cells and their clinical use. Haematologica 1998;83:824–848. [PubMed] [Google Scholar]
- 18. Tan YX, Ji SP, Lu YP et al. [Preliminary study on xenotransfusion from porcine red blood cell into Rhesus monkey]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2006;14:150–155. [PubMed] [Google Scholar]
- 19. Guan X, Qin M, Zhang Y et al. Safety and efficacy of megakaryocytes induced from hematopoietic stem cells in murine and nonhuman primate models. Stem Cell Transl Med 2016,5:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rossi B, Zanolin E, Vincenzi C et al. Effect of addition of FLT‐3 ligand and megakaryocyte growth and development factor on hemopoietic cells in serum‐free conditions. Stem Cells Dev 2004;13:362–371. [DOI] [PubMed] [Google Scholar]
- 21. Murray LJ, Young JC, Osborne LJ et al. Thrombopoietin, flt3, and kit ligands together suppress apoptosis of human mobilized CD34+ cells and recruit primitive CD34+ Thy‐1+ cells into rapid division. Exp Hematol 1999;27:1019–1028. [DOI] [PubMed] [Google Scholar]
- 22. Mossadegh‐Keller N, Sarrazin S, Kandalla PK et al. M‐CSF instructs myeloid lineage fate in single haematopoietic stem cells. Nature 2013;497:239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leberbauer C, Boulme F, Unfried G et al. Different steroids co‐regulate long‐term expansion versus terminal differentiation in primary human erythroid progenitors. Blood 2005;105:85–94. [DOI] [PubMed] [Google Scholar]
- 24. Miharada K, Hiroyama T, Sudo K et al. Efficient enucleation of erythroblasts differentiated in vitro from hematopoietic stem and progenitor cells. Nat Biotechnol 2006;24:1255–1256. [DOI] [PubMed] [Google Scholar]
- 25. Hevehan DL, Papoutsakis ET, Miller WM. Physiologically significant effects of pH and oxygen tension on granulopoiesis. Exp Hematol 2000;28:267–275. [DOI] [PubMed] [Google Scholar]
- 26. Rogers HM, Yu X, Wen J et al. Hypoxia alters progression of the erythroid program. Exp Hematol 2008;36:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. StemSpan™ SFEM Serum‐free medium . STEMCELL Technologies Inc. Available at https://www.stemcell.com/stemspan-sfem.html. Last accessed December 2016.
- 28. Mantel CR, O'Leary HA, Chitteti BR et al. Enhancing hematopoietic stem cell transplantation efficacy by mitigating oxygen shock. Cell 2015;161:1553–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vlaski M, Lafarge X, Chevaleyre J et al. Low oxygen concentration as a general physiologic regulator of erythropoiesis beyond the EPO‐related downstream tuning and a tool for the optimization of red blood cell production ex vivo. Exp Hematol 2009;37:573–84. [DOI] [PubMed] [Google Scholar]
- 30. Zeuner A, Martelli F, Vaglio S et al. Concise review: Stem cell‐derived erythrocytes as upcoming players in blood transfusion. Stem Cells 2012;30:1587–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Munugalavadla V, Kapur R. Role of c‐Kit and erythropoietin receptor in erythropoiesis. Crit Rev Oncol Hematol 2005;54:63–75. [DOI] [PubMed] [Google Scholar]
- 32. Arcasoy MO, Jiang X. Co‐operative signalling mechanisms required for erythroid precursor expansion in response to erythropoietin and stem cell factor. Br J Haematol 2005;130:121–129. [DOI] [PubMed] [Google Scholar]
- 33. Fan J, Ding X, Jiang Y. A novel monoclonal antibody of human stem cell factor inhibits umbilical cord blood stem cell ex vivo expansion. J Hematol Oncol 2012;5:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shen B, Jiang W, Fan J et al. Residues 39–56 of stem cell factor protein sequence are capable of stimulating the expansion of cord blood CD34+ cells. PLoS One 2015;10:e0141485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jiang XS, Chai C, Zhang Y et al. Surface‐immobilization of adhesion peptides on substrate for ex vivo expansion of cryopreserved umbilical cord blood CD34+ cells. Biomaterials 2006;27:2723–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Drouet M, Mathieu J, Grenier N et al. The reduction of in vitro radiation‐induced Fas‐related apoptosis in CD34+ progenitor cells by SCF, FLT‐3 ligand, TPO, and IL‐3 in combination resulted in CD34+ cell proliferation and differentiation. Stem Cells 1999;17:273–285. [DOI] [PubMed] [Google Scholar]
- 37. Baek EJ, Kim HS, Kim JH et al. Stroma‐free mass production of clinical‐grade red blood cells (RBCs) by using poloxamer 188 as an RBC survival enhancer. Transfusion 2009;49:2285–2295. [DOI] [PubMed] [Google Scholar]
- 38. Kim HO, Baek EJ. Red blood cell engineering in stroma and serum/plasma‐free conditions and long term storage. Tissue Eng Part A 2012;18:117–126. [DOI] [PubMed] [Google Scholar]
- 39. Ferreira R, Ohneda K, Yamamoto M et al. GATA1 function, a paradigm for transcription factors in hematopoiesis. Mol Cell Biol 2005;25:1215–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang W, Kadam S, Emerson BM et al. Site‐specific acetylation by p300 or CREB binding protein regulates erythroid Kruppel‐like factor transcriptional activity via its interaction with the SWI‐SNF complex. Mol Cell Biol 2001;21:2413–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Di Baldassarre A, Di Rico M, Di Noia A et al. Protein kinase Calpha is differentially activated during neonatal and adult erythropoiesis and favors expression of a reporter gene under the control of the (A)gamma globin‐promoter in cellular models of hemoglobin switching. J Cell Biochem 2007;101:411–424. [DOI] [PubMed] [Google Scholar]
- 42. Zhang CC, Lodish HF. Insulin‐like growth factor 2 expressed in a novel fetal liver cell population is a growth factor for hematopoietic stem cells. Blood 2004;103:2513–2521. [DOI] [PubMed] [Google Scholar]
- 43. Migliaccio AR, Masselli E, Varricchio L et al. Ex‐vivo expansion of red blood cells: How real for transfusion in humans? Blood Rev 2012;26:81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information Figure 1
Supporting Information Table 1
Supporting Information Table 2


