Summary
Megakaryocytes (Mgks) are terminally differentiated blood cells specified to produce platelets, whereas hematopoietic stem cells (HSCs) are the most undifferentiated blood cells that retain multipotency to produce all kinds of blood cells. As such, these two cell types reside at the bottom and the top of the hematopoietic hierarchy, respectively. In spite of this distance, they share several important cell surface molecules as well as transcription factors.
In the conventional step‐wise differentiation model, HSCs gradually lose their self‐renewal capacity and differentiate into multipotent progenitors (MPPs), which is the first branch point of myeloid and lymphoid lineage. In this model, common myeloid progenitors can differentiate into bipotent Mgk/erythroid progenitors (MEPs), and MEPs eventually differentiate into unipotent mature Mgks. However, it has been recently reported that a subpopulation within the HSC and MPP compartments demonstrates an Mgk‐biased differentiation potential. These reports imply that revisions to the HSC‐to‐Mgk differentiation pathway should be discussed. In this review, we summarize recent findings about Mgk differentiation from HSCs and discuss future directions in this research field. Stem Cells Translational Medicine 2017;6:1661–1665
Significance Statement.
Transplant experimental models and single‐cell analysis data reveal that the hematopoietic stem cell (HSC) compartment contains a subpopulation with megakaryocyte (Mgk) lineage‐biased potential. Mgk‐biased progenitor cells also can be detected in the multipotent progenitor fraction, suggesting that Mgks could emerge through a non‐step‐wise differentiation pathway from HSCs, especially in stress‐induced hematopoiesis.
Introduction
The hematopoietic system constantly generates a precise number of blood cells with diverse functions. These functions are maintained by hematopoietic stem cells (HSCs), characterized by self‐renewal capacity and multipotent differentiation potential. This rare subpopulation of blood cells provides a huge number of peripheral blood cells throughout an individual's lifetime 1.
Platelets are small, anucleated fragments of blood cells that are generated from polypoid megakaryocytes (Mgks) and play a critical role in homeostasis 2. In the classical “step‐wise” hierarchical hematopoiesis model, HSCs produce committed progenitors with decreasing self‐renewal capacity and restricted lineage differentiation potential. The bifurcation of myeloid/lymphoid lineages first occurs within multipotent progenitors (MPPs) during differentiation. The myeloid lineage progenitors further lose differentiation potential into granulocyte/macrophage and erythroid linages and eventually produce unipotent Mgk progenitors (MKPs) 3. This differentiation model simplifies the complexity of the hematopoietic system and has been widely accepted over the past decades. However, recent studies with novel prospective isolation techniques and single‐cell functional/molecular analyses of HSCs and Mgks have revealed that these two cell types share a remarkable number of surface molecules, transcription factors, and cytokine signaling pathways. This suggests that Mgk might be directly differentiated from HSC, possibly bypassing the Mgk/erythroid progenitor (MEP) stage 4. In this paper, we summarize the recent findings within the topic of megakaryopoiesis and propose a revised road map for Mgk lineage differentiation.
Shared Surface Receptors, Signaling, and Gene Expression Profiles in Hematopoietic Stem Cells and Committed Mgk Lineage Cells
HSCs and Mgks share many common features, as previously described (Fig. 1) 4. As an example, the thrombopoietin (TPO) signaling is one of the most essential cytokine signaling pathways in both HSCs and Mgks. TPO was originally identified as a critical growth factor for platelet production 5. However, studies using Mpl ‐/‐ mice demonstrated that Mpl, which is a TPO receptor, also plays an essential role for maintenance of HSCs 6. HSCs derived from Mpl ‐/‐ mice showed a significantly reduced long‐term repopulating capacity, suggesting that TPO/Mpl signaling is functionally essential in HSC activity 6. Another study demonstrated an indispensable role of TPO/Mpl signaling in HSC quiescence 7. In humans, congenital defects or a loss‐of‐function mutation of Mpl causes severe thrombocytopenia, called congenital amegakaryocytic thrombocytopenia (CAMT). Patients with CAMT have a high risk of bone marrow failure, suggesting that TPO/Mpl signaling is also important for maintenance of human HSCs 4. These data imply that TPO/Mpl signaling might play a critical bridging role between HSCs and Mgks. In addition, other shared signaling pathways between HSCs and Mgks, including CXCR4/CXCL12 [8, 9], Notch 10, 11, and Stem cell factor/c‐Kit signaling 12, have also been reported 4.
Figure 1.
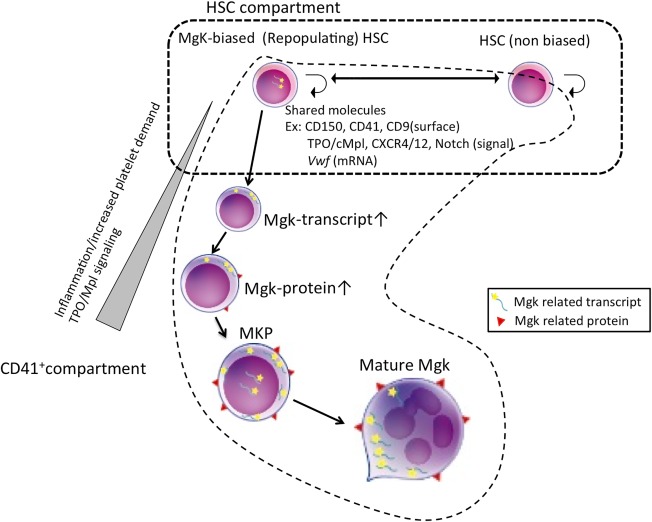
Shared surface receptors and signaling in HSCs, Mgk‐biased HSCs, and committed Mgks. In the immunophenotypic HSC compartment (mainly the CD41+ subpopulation), Mgk‐biased HSCs exist. HSCs, Mgk‐biased HSCs, and MKPs share several surface molecules. Transcripts of Mgk‐related genes, such as Vwf or CD42b, can be detected in Mgk‐biased HSCs, but protein synthesis is inhibited in the steady state. External signaling such as inflammatory cytokine or TPO/Mpl signaling can trigger protein synthesis, induce Mgk lineage differentiation, and generate adequate numbers of platelets during emergency thrombocytosis. Abbreviations: HSC, hematopoietic stem cell; Mgk, megakaryocyte; MKP, Mgk progenitor; TPO, thrombopoietin.
Furthermore, various surface molecules are co‐expressed on HSCs/MPPs and Mgks. Indeed, the majority of surface markers so far used for isolation of MKPs, such as CD150 [13], CD41 [14], and CD9 15, are also expressed in the entire population of, or a fraction of, HSCs. CD150, known as SLAM1, is widely used for purification of mouse HSCs 13 and is also expressed on Mgk lineage cells, including MKPs and platelets 16. CD41 (integrin αIIb) non‐covalently associates with CD61 (integrin β3) to form the integrin αIIbβ3 complex on Mgks and platelets 4, which plays a critical role in thrombus formation 17. Additionally, CD41 is expressed on the surface of a subpopulation of long‐term HSCs (LT‐HSCs) and MPPs in mice. CD41 expression in Lineage‐Sca1+/c‐Kit+ mouse hematopoietic stem/progenitor fraction (LSK) increases with aging and is related to the myeloid differentiation potential of HSCs 14, 18. From the similar expression pattern of these molecules arises the possibility that Mgks may directly differentiate from HSC.
In a closely associated context, a subpopulation of HSCs was reported to express mRNA of von Willebrand factor (vWF) 19, which is mainly found on the surface of injured endothelial cells, mature Mgks, and platelets. On the other hand, CD42b, which is the vWF receptor, was believed to be exclusively expressed on the surface of Mgks and platelets 16. Unlike CD41, immature progenitor cells such as MPPs and LT‐HSCs do not express CD42b in steady‐state conditions 16. Despite such a classical view, we demonstrated CD42b surface expression in a CD34‐/CD41+/CD150+ LSK population, a subset of HSCs, at the hematopoietic recovery phase after 5‐fluorouracil treatment, prompting further investigation 16. Indeed, single‐cell gene expression analysis revealed that a subpopulation of CD41+LSK expresses nuclear CD42b mRNA even in the steady state 16, although it was not clear whether nuclear CD42b mRNA‐positive CD41+ LSKs were functional LT‐HSCs. Furthermore, recent improvement of single‐cell RNA sequencing techniques enables comprehensive gene expression analyses at single‐cell resolution 20. Whole transcriptome analysis using single purified HSCs demonstrated that a subpopulation of HSC compartment expresses Mgk‐specific genes 21.
Another group demonstrated the regulatory mechanism of the expression of Mgk‐related genes in HSCs 22. They showed that the phenotypic HSC compartment contains stem‐like Mgk lineage‐committed progenitors (SL‐MKPs), which share similar characteristics with HSCs but retain platelet‐restricted differentiation capacity. SL‐MKPs reside in the CD41+ subpopulation of phenotypic HSC and express Mgk‐specific mRNAs while translation of these mRNAs into protein is suppressed in SL‐MKPs in the steady state. Inflammation signals, such as activation of the interferon signaling pathway, trigger Mgk differentiation and platelet production through Mgk‐specific mRNA translation in SL‐MKPs. The authors of this paper conclude that cell‐cycle regulators such as p27 and p57 under strict control of FoxO3a suppress translation of such Mgk‐specific mRNAs in the steady state 22. Although further investigation is required, transcriptional/translational regulation of Mgk‐specific genes in the HSC compartment may be a key to understand underlying mechanisms in the commitment to Mgk lineage from HSCs.
Mgk Lineage Commitment Within the HSC Compartment
Heavily shared molecular markers between HSCs and Mgks have raised the possibility that Mgk lineage specification could occur at a more immature stage than that postulated in the classical step‐wise differentiation model 4. In the adult mouse hematopoietic system, HSCs are significantly enriched in either CD34‐/lowLSK 1 or CD150+/CD48‐ LSK cells 13. In the classical differentiation model, the most immature myeloid progenitors, defined as common myeloid progenitors (CMPs), are derived from MPP and differentiate into bipotent granulocyte/macrophage progenitors and MEPs, the latter of which eventually differentiate into MKPs while losing their capacity to differentiate into the erythroid lineage (Fig. 2A) 3. However, it was recently reported that a phenotypic CMP population is highly heterogeneous when analyzed by single‐cell RNA sequencing, while Mgk‐specific genes, particularly transcription factors, are detected in a subpopulation of CMPs, but not in MEPs 23. Indeed, we demonstrated that a CD42b‐positive subpopulation of CMPs represents unipotent MKPs 16. CMPs could be a mixture of heterogeneous progenitors such as unipotent myeloid and Mgk progenitors, which may support the concept that Mgk lineage commitment occurs at stages close to HSCs.
Figure 2.
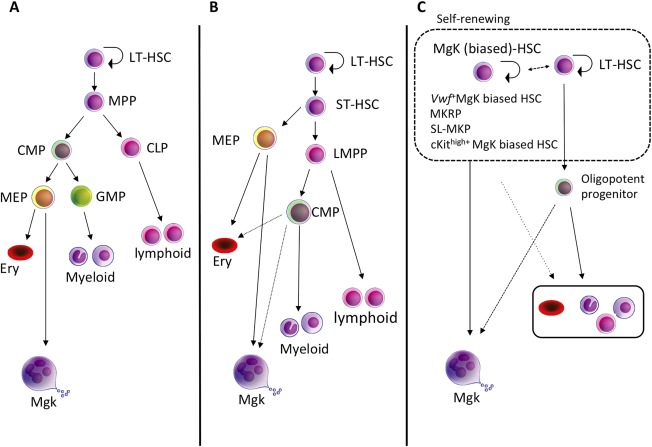
The models of Mgk differentiation from HSCs. (A): The classical “step‐wise” hierarchical hematopoiesis model. The bifurcation of myeloid/lymphoid lineage first occurs in MPPs, and MKPs eventually generate from MEPs as the progeny of CMPs. (B): Alternative model based on the identification of LMPPs. The bifurcation of Mgk lineage first occurs during differentiation from HSCs. LMPPs lose almost all Mgk/erythroid differentiation potential. (C): Proposed model from recent reports. An immunophenotypic‐defined HSC population contains a functionally heterogeneous, Mgk‐biased/restricted subpopulation of HSCs that directly gives rise to MKPs and bypassse the MEP stage. Abbreviations: CLP, common lymphoid progenitor; CMP, common myeloid progenitor; Ery, erythroid; HSC, hematopoietic stem cells; LMPP, lympho‐myeloid primed progenitor; LT‐HSC, long‐term hematopoietic stem cells; MEP, Mgk/erythroid progenitor; MgK, megakaryocytes; MKRP, Mgk‐repopulating progenitors; MPP, multipotent progenitor; SL‐MKP, stem‐like Mgk committed progenitor; ST‐HSC, short term HSC.
There are multiple lines of evidence showing that the megakaryopoietic pathway is bifurcated earlier than assumed in the classical model (Fig. 2B) 10, 19, 24, 25. Flt3+LSKs are characterized as lymphoid‐primed multipotent progenitors (LMPPs), which notably lose Mgk/erythroid differentiation potential, retaining granulocyte/macrophage potential 24. While Mgk/erythroid differentiation potential from LMPPs has been shown before, the actual capacity of LMPPs to produce Mgk is reportedly significantly lower than that of HSCs or MPPs 18, 26, 27. Additionally, it was reported that Notch signaling plays an important role in Mgk lineage specification from HSCs in an in vitro co‐culture system 10. In this report, the authors suggested that Notch signaling promotes the alternative Mgk differentiation pathway from HSCs, which could bypass the MEP stage.
More recently, several studies using novel single‐cell functional analysis methods suggested that mouse LT‐HSC populations are functionally heterogeneous and the commitment into Mgk lineage is already made within a subpopulation of the LT‐HSC compartment (Fig. 2C) 25. Indeed, 10%–15% of the highly purified LT‐HSC population can directly differentiate into Mgk without cell division in single‐cell in vitro culture. The same population also includes highly proliferating cells producing 100–1,000 single‐lineage Mgks 16, 25, 28. Furthermore, the paired daughter assay shows Mgks can be differentiated from HSCs even at the first cell division 25. Although in vitro assays do not always perfectly simulate in vivo hematopoiesis, these data do support the possibility that the subpopulation of HSCs possesses Mgk‐biased/restricted capacity, and Mgks may directly differentiate from them.
Data from a single‐cell transplant report demonstrated that purified HSC populations (CD34‐LSK) contain several lineages of biased HSCs, such as myeloid‐biased, lymphoid‐biased, and Mgk/erythroid‐biased HSCs 25. These data implied that fate decision to a specific lineage from multipotent HSCs might be made at the LT‐HSC level and also suggested that conventional, step‐wise, hierarchal, hematopoietic differentiation models might need to be revised. They also demonstrated that the CD150+/CD41+ subpopulation of a mouse LT‐HSC population (CD150+/CD41+/CD34‐ LSK) contains Mgk‐restricted repopulating cells (MKRPs), which showed restricted Mgk/platelet differentiation capacity and self‐renewal capacity in vivo. SL‐MKPs (as discussed previously) also reside as a subpopulation of the CD41+LT‐HSC population (CD41med/CD150+/CD48‐/c‐Kit+/Lin‐) 22. Indeed, our single‐cell culture data revealed that the frequency of pure Mgk colonies from the CD150+/CD41+ LSK population was much higher than that of the CD41‐LSK population 16. We also demonstrated that continuous TPO/Mpl signaling was key for Mgk differentiation from HSCs, bypassing bipotent MEPs 16. Additional clarity comes from studies (using Vwf as a genetic marker) that showed a subpopulation of Vwf‐positive HSCs that possesses Mgk‐biased differentiation capacity and resides at the apex of the HSC hierarchy; these cells expand within the HSC compartment during aging 19, 21. These data also supported the concept that the fate decision of the Mgk lineage is made at the LT‐HSC level. Our group also demonstrated that the CD150+/CD41+LSK population expresses Vwf at the single‐cell level, and the CD41+LSK subpopulation showed exclusively high Mgk differentiation capacity 16.
Shin and colleagues reported that c‐Kithigh+HSC (CD150+/CD34‐LSK) showed impaired self‐renewal activity and megakaryocytic bias 29. This population produces Mgks and platelets more rapidly in vitro and in vivo, and c‐Kitlow HSCs were shown to be more primitive and quiescent than c‐Kithigh+HSCs, implying that the Mgk‐biased subpopulation of HSCs was not at the apex of the hematopoietic hierarchy 29. To clarify, if two distinct subsets of Mgk‐biased HSCs exist at the apex and the sub‐apex of the hematopoietic system, further investigation is required. In actuality, these concepts were mainly based on data from transplant/culture using a purified subpopulation of HSCs, which only reflected the highly stressed hematopoietic condition.
According to the results from a Flt3 lineage tracing a mouse model that genetically marks the progeny of Flt3‐expressing cells, all lineages (including platelets and erythrocytes) can be produced from Flt3+hematopoietic progenitors like the LMPP fraction in vivo 30. Although the possibility of transient flt3 promoter activation at the HSC level might be a concern, the fate decision mechanism that specifies lineage from HSCs in the steady state might be different from stress conditions such as hematopoietic reconstitution after transplant or chemotherapy. Further analyses would be needed for understanding platelet generation from steady‐state HSCs.
Megakaryocytopoiesis in the Human Hematopoietic System
The classical, stepwise hematopoietic differentiation model has been widely accepted to be the same in humans as in mice 31. However, an alternative differentiation model was also suggested in the human hematopoietic system, which would mirror the mouse system 32, 33. Using human cord blood and bone marrow cells, single‐cell analysis similarly revealed that the Mgk lineage fate decision was made within the CD34+/CD38‐HSC‐enriched compartment and that the CD34+/CD38+CMP/MEP populations are both heterogeneous 34, retaining less differentiation capacity into Mgks 32. They also demonstrated that Mgks are generated from intermediate oligopotent progenitors in human fetal hematopoiesis, suggesting that the Mgk lineage differentiation systems in fetal liver and adult bone marrow are distinct from each other 32. Further studies are required to prove the existence of Mgk‐biased HSCs or progenitor cells in the human hematopoietic system, given that in vivo functional analysis at the single‐cell level is much more difficult in humans than in mice 35.
Conclusion
Several recent studies demonstrated that the commitment to the Mgk lineage is decided at a much earlier stage than previously expected. The existence of lineage‐biased HSCs, including Mgk‐biased/restricted HSCs, is also demonstrated. Although the physiological roles of this unique class of cells in hematopoiesis and hematologic diseases are still not fully elucidated (especially in the human hematopoietic system), these findings provide new insights into the hematopoietic regulatory mechanism.
Author Contributions
H.N., K.N., and S.C.: manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Acknowledgments
This work was supported by the Grants‐in‐Aid for Scientific Research (KAKENHI) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (23118503, and 22390191 to S.C.; 22790896 and 24790959 to H.N.), and by the Uehara Memorial Foundation, Yasuda Medical Foundation, and SENSHIN Medical Research Foundation to H.N. We thank Dr. Bryan J. Mathis of the Medical English Communications Center (University of Tsukuba) for critical proofreading of this manuscript.
References
- 1. Ema H, Morita Y, Yamazaki S et al. Adult mouse hematopoietic stem cells: Purification and single‐cell assays. Nat Protoc 2006;1:2979–2987. [DOI] [PubMed] [Google Scholar]
- 2. Machlus KR, Thon JN, Italiano JE Jr. Interpreting the developmental dance of the megakaryocyte: A review of the cellular and molecular processes mediating platelet formation. Br J Haematol 2014;165:227–236. [DOI] [PubMed] [Google Scholar]
- 3. Akashi K, Traver D, Miyamoto T et al. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 2000;404:193–197. [DOI] [PubMed] [Google Scholar]
- 4. Huang H, Cantor AB. Common features of megakaryocytes and hematopoietic stem cells: What's the connection? J Cell Biochem 2009;107:857–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaushansky K, Lok S, Holly RD et al. Promotion of megakaryocyte progenitor expansion and differentiation by the c‐Mpl ligand thrombopoietin. Nature 1994;369:568–571. [DOI] [PubMed] [Google Scholar]
- 6. Kimura S, Roberts AW, Metcalf D et al. Hematopoietic stem cell deficiencies in mice lacking c‐Mpl, the receptor for thrombopoietin. Proc Natl Acad Sci USA 1998;95:1195–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bersenev A, Wu C, Balcerek J et al. Lnk controls mouse hematopoietic stem cell self‐renewal and quiescence through direct interactions with JAK2. J Clin Invest 2008;118:2832–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Avecilla ST, Hattori K, Heissig B et al. Chemokine‐mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med 2004;10:64–71. [DOI] [PubMed] [Google Scholar]
- 9. Greenbaum A, Hsu YM, Day RB et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem‐cell maintenance. Nature 2013;495:227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mercher T, Cornejo MG, Sears C et al. Notch signaling specifies megakaryocyte development from hematopoietic stem cells. Cell Stem Cell 2008;3:314–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cornejo MG, Mabialah V, Sykes SM et al. Crosstalk between NOTCH and AKT signaling during murine megakaryocyte lineage specification. Blood 2012;118:1264–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaushansky K. Determinants of platelet number and regulation of thrombopoiesis. Hematology Am Soc Hematol Educ Program 2009:147–152. [DOI] [PubMed] [Google Scholar]
- 13. Kiel MJ, Yilmaz OH, Iwashita T et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 2005;121:1109–1121. [DOI] [PubMed] [Google Scholar]
- 14. Gekas C, Graf T. CD41 expression marks myeloid‐biased adult hematopoietic stem cells and increases with age. Blood 2013;121:4463–4472. [DOI] [PubMed] [Google Scholar]
- 15. Karlsson G, Rörby E, Pina C et al. The tetraspanin CD9 affords high‐purity capture of all murine hematopoietic stem cells. Cell Rep 2013;4:642–648. [DOI] [PubMed] [Google Scholar]
- 16. Nishikii H, Kanazawa Y, Umemoto T et al. Unipotent megakaryopoietic pathway bridging hematopoietic stem cells and mature megakaryocytes. Stem Cells 2015;33:2196–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coller BS, Shattil SJ. The GPIIb/IIIa (integrin alphaIIbbeta3) odyssey: A technology‐driven saga of a receptor with twists, turns, and even a bend. Blood 2008;112:3011–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miyawaki K, Arinobu Y, Iwasaki H et al. CD41 marks the initial myelo‐erythroid lineage specification in adult mouse hematopoiesis: Redefinition of murine common myeloid progenitor. Stem Cells 2015;33:976–987. [DOI] [PubMed] [Google Scholar]
- 19. Sanjuan‐Pla A, Macaulay IC, Jensen CT et al. Platelet‐biased stem cells reside at the apex of the haematopoietic stem‐cell hierarchy. Nature 2013;502:232–236. [DOI] [PubMed] [Google Scholar]
- 20. Gawad C, Koh W, Quake SR. Single‐cell genome sequencing: Current state of the science. Nat Rev Genet 2016;17:175–188. [DOI] [PubMed] [Google Scholar]
- 21. Grover A, Sanjuan‐Pla A, Thongjuea S et al. Single‐cell RNA sequencing reveals molecular and functional platelet bias of aged haematopoietic stem cells. Nat Commun 2016;7:11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haas S, Hansson J, Klimmeck D et al. Inflammation‐induced emergency megakaryopoiesis driven by hematopoietic stem cell‐like megakaryocyte progenitors. Cell Stem Cell 2015;17:422–434. [DOI] [PubMed] [Google Scholar]
- 23. Paul F, Arkin Y, Giladi A et al. Transcriptional heterogeneity and lineage commitment in myeloid progenitors. Cell 2015;163:1663–1677. [DOI] [PubMed] [Google Scholar]
- 24. Adolfsson J, Månsson R, Buza‐Vidas N et al. Identification of Flt3+ lympho‐myeloid stem cells lacking erythro‐megakaryocytic potential a revised road map for adult blood lineage commitment. Cell 2005;121:295–306. [DOI] [PubMed] [Google Scholar]
- 25. Yamamoto R, Morita Y, Ooehara J et al. Clonal analysis unveils self‐renewing lineage‐restricted progenitors generated directly from hematopoietic stem cells. Cell 2013;154:1112–1126. [DOI] [PubMed] [Google Scholar]
- 26. Arinobu Y, Mizuno S, Chong Y et al. Reciprocal activation of GATA‐1 and PU.1 marks initial specification of hematopoietic stem cells into myeloerythroid and myelolymphoid lineages. Cell Stem Cell 2007;1:416–427. [DOI] [PubMed] [Google Scholar]
- 27. Forsberg EC, Serwold T, Kogan S et al. New evidence supporting megakaryocyte‐erythrocyte potential of flk2/flt3+ multipotent hematopoietic progenitors. Cell 2006;126:415–426. [DOI] [PubMed] [Google Scholar]
- 28. Roch A, Trachsel V, Lutolf MP. Brief report: Single‐cell analysis reveals cell division‐independent emergence of megakaryocytes from phenotypic hematopoietic stem cells . Stem Cells 2015;33:3152–3157. [DOI] [PubMed] [Google Scholar]
- 29. Shin JY, Hu W, Naramura M et al. High c‐Kit expression identifies hematopoietic stem cells with impaired self‐renewal and megakaryocytic bias. J Exp Med 2014;211:217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boyer SW, Schroeder AV, Smith‐Berdan S et al. All hematopoietic cells develop from hematopoietic stem cells through Flk2/Flt3‐positive progenitor cells. Cell Stem Cell 2011;9:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Manz MG, Miyamoto T, Akashi K et al. Prospective isolation of human clonogenic common myeloid progenitors. Proc Natl Acad Sci USA 2002;99:11872–11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Notta F, Zandi S, Takayama N et al. Distinct routes of lineage development reshape the human blood hierarchy across ontogeny. Science 2016;351:aab2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sanada C, Xavier‐Ferrucio J, Lu YC et al. Adult human megakaryocyte‐erythroid progenitors are in the CD34+CD38mid fraction. Blood 2016;128:923–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Psaila B, Barkas N, Iskander D et al. Single‐cell profiling of human megakaryocyte‐erythroid progenitors identifies distinct megakaryocyte and erythroid differentiation pathways. Genome Biol 2016;17:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goyama S, Wunderlich M, Mulloy JC. Xenograft models for normal and malignant stem cells. Blood 2015;125:2630–2640. [DOI] [PubMed] [Google Scholar]


