Abstract
Background
The South-East Asia Region Kala-azar Elimination Programme (KAEP) is expected to enter the consolidation phase in 2017, which focuses on case detection, vector control, and identifying potential sources of infection. Post-kala-azar dermal leishmaniasis (PKDL) is thought to play a role in the recurrence of visceral leishmaniasis (VL)/kala-azar outbreaks, and control of PKDL is among the priorities of the KAEP.
Methodology and principal finding
We reviewed the literature with regard to PKDL in Asia and interpreted the findings in relation to current intervention methods in the KAEP in order to make recommendations. There is a considerable knowledge gap regarding the pathophysiology of VL and PKDL, especially the underlying immune responses. Risk factors (of which previous VL treatments may be most important) are poorly understood and need to be better defined. The role of PKDL patients in transmission is largely unknown, and there is insufficient information about the importance of duration, distribution and severity of the rash, time of onset, and self-healing. Current intervention methods focus on active case detection and treatment of all PKDL cases with miltefosine while there is increasing drug resistance. The prevention of PKDL by improved VL treatment currently receives insufficient attention.
Conclusion and significance
PKDL is a heterogeneous and dynamic condition, and patients differ with regard to time of onset after VL, chronicity, and distribution and appearance of the rash, as well as immune responses (including tendency to self-heal), all of which may vary over time. It is essential to fully describe the pathophysiology in order to make informed decisions on the most cost-effective approach. Emphasis should be on early detection of those who contribute to transmission and those who are in need of treatment, for whom short-course, effective, and safe drug regimens should be available. The prevention of PKDL should be emphasised by innovative and improved treatment for VL, which may include immunomodulation.
Introduction
Kala-azar/visceral leishmaniasis (VL) is endemic in the Indian subcontinent (ISC), affecting the Gangetic plains of India, Bangladesh, and Nepal.
ln 2005, the governments of Bangladesh, India, and Nepal agreed on a regional initiative, the Regional Kala-azar Elimination Programme (KAEP), to eliminate VL as a public health problem from the region by 2015, which has now been extended to 2017 [1]. The factors favourable for the elimination of kala-azar in this region included high endemicity, limited to 1 geographical region in the 3 countries (45 districts in Bangladesh, 52 in India, and 12 in Nepal); the absence of an animal reservoir; Phlebotomus argentipes being the only vector and susceptibility to insecticide; the availability of an accurate rapid diagnostic test (RDT) detecting antibodies against the 39-aminoacid-recombinant kinesin antigen (anti rK39); the use of an oral drug, miltefosine; and strong political commitment from the 3 countries [2]. Subsequently, other efficacious treatments, including combination therapies and single dose liposomal amphotericin B (AmBisome), which replaced miltefosine, became available. The key strategies for elimination were early diagnosis and complete case management; integrated vector management and vector surveillance; effective disease surveillance through passive and active case detection; social mobilisation and building partnerships; and implementation and operational research [3].
This multidisciplinary approach strategy aimed at reaching the elimination target of less than 1 kala-azar case in 10,000 population by 2015. Current figures are promising, with a substantial decline in cases by 59% and a reduction of mortality by 85%. Nepal has eliminated the disease at the district level and sustained the situation for the past 2 years, Bangladesh has achieved the elimination target in more than 90% of endemic districts (upazilas), and India has achieved the target in more than 70% of endemic districts (blocks) [3].
From the attack phase, the KAEP is moving to the consolidation phase, aiming to identify (new) low-endemic foci by active case detection, consolidating vector control measures, and treating potential sources of infection, of which post-kala-azar dermal leishmaniasis (PKDL) is the most important [4,5].
PKDL is a dermal condition that in Asia occurs in a significant percentage (10%–20%) of patients after VL treatment [6]. PKDL consists of painless macular or papulonodular (PN) lesions—or a mix of both—that harbour parasites that may be exposed to the bite of sand flies, thus possibly playing a major role in the transmission cycle [7,8].
PKDL is therefore recognised as a constraint in the elimination effort, and the development of strategies for case finding, diagnosis, and treatment is among the objectives in the KAEP [4].
These strategies are currently not based on solid scientific evidence, with costly case finding, risk of misdiagnosis, and risk of inadequate and unnecessary treatment with potentially toxic drugs as a result.
In this paper, we review the available information on PKDL relevant for the KAEP, identify essential gaps in knowledge, and make recommendations.
Search strategy and selection criteria
We searched the electronic database PubMed for articles published from January 1900 to May 2017 by use of the terms ‘PKDL,’ ‘post-kala-azar dermal leishmaniasis,’ ‘kala-azar,’ ‘visceral leishmaniasis,’ and ‘VL’ paired with the terms ‘India,' ‘Bangladesh,’ ‘Nepal,’ or ‘Indian subcontinent’ in the English language. Relevant articles from the authors’ personal files were identified. From the articles, certain relevant references were searched and included.
Epidemiology
PKDL rate and prevalence of PKDL
The most recent studies (published between 2000 and 2017) are summarised in Table 1.
Table 1. Summary of epidemiological studies published from 2000 to 2017 on prevalence, incidence, and interval between onset of PKDL and VL treatment.a.
| Country | Region | Year | Type of study | Case finding | Prevalence | Incidence | PKDL rate | Interval after VL | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| India | Bihar | 2012 | Community based | active | 4.4/10,000 population (confirmed cases) 7.8/10,000 population (probable cases) |
median: 30 months range: 9–129 months |
[9] | ||
| West Bengal | 2012 | Community based | active | 25/2,435 population point prevalence 1% |
mean: 3.13 years range: 6 months to 14 years <1 year: 3 of 25 |
[10] | |||
| Patna | 2007–2012 | Hospital based | passive | 25 PKDL/8,311 VL cases treated (0.3%) | median: 1.2 years (IQR 0.8–2.2) <12 months: 36% |
[11] | |||
| Patna | not recorded | Hospital-based case series (n = 60) | passive | 3 months to 10 years | [12] | ||||
| New Delhi | 1995–2014 | Hospital-based case series (n = 282) | passive | <12 months: 13% 1–5 years: 56% >5 years: 31% |
[13] | ||||
| Bangladesh | Fulbaria | 2007–2008 | Community based | active | 1/10,000 PY (2002–2004) 21/10,000 PY (2007) |
9.9% | median: 21 months (95% CI 16.4–25.6) | [14] | |
| Fulbaria | 2010–2013 | Community based | active | 12-month FU 0.6% 24-month FU 8.1% 36-month FU 10.2% |
range: 0–36 months |
b |
|||
| Fulbaria | 2007–2010 | Community based | active | 12-month FU 3% 24-month FU 10% 60-month FU 17% |
median: 19 months range: 0–120 months |
[15] | |||
| Trishal | 2010 | Community based | active | 6.2/10,000 population | median: 36 months (IQR 24–48) |
[16] | |||
| Fulbaria | 2007 | Community based | active | 3.2–7.3/1,000 population in 4 paras | 13.9 (entire community); 16% in 4 of 9 paras | 6–24 months: 40% 0–6 months: 20% |
[17] | ||
| Nepal | Endemic districts | 2000–2009 | Retrospective cohort | active | mean 2.4% <2 years: 1.4% <8 years: 3.6% |
23 months (IQR 15–41) | [18] |
||
| Dharan | 1998–2000 | Hospital-based case series (n = 22) | passive | mean: 26.9 ± 11.9 months range: 6–60 months |
[19] |
aNote differences in methodology used: field-based versus hospital-based studies; active versus passive case finding; measurement of prevalence versus incidence; and confirmed versus probable PKDL cases.
bPersonal communication, Koert Ritmeijer, Médecins sans Frontières, to Eduard Zijlstra.
Abbreviations: FU, follow-up; IQR, interquartile range; PKDL, post-kala-azar dermal leishmaniasis; PY, person-year; VL, visceral leishmaniasis.
In India, the exact PKDL rate (i.e., the frequency with which PKDL follows after treatment of VL) is not known exactly because cohort studies with active follow-up of VL cases are not available. Passive case finding yielded a PKDL rate of 0.3% [11].
In Bangladesh, 2 prospective studies showed that PKDL rates increased with longer duration of follow-up: in 1 study, the rate was 10.2% by 3 years and 17% by 5 years, respectively (personal communication, Koert Ritmeijer, Médecins sans Frontières [MSF] Holland, to Eduard Zijlstra). Another study showed increased prevalence from 1 case to 21 cases per 10,000 person-years in 2002–2004 and 2007, respectively [15].
Interval after VL
In contrast to earlier studies in which the interval between treatment of VL and onset of PKDL was thought to be at least 1–3 years or longer, recent information shows that PKDL may occur much earlier, with up to 36% of cases presenting within 1 year after VL [13,17,20,21]. This is more in agreement with the experience in Sudan (which has the highest global PKDL rate, up to 60%), where the interval after VL is 0–13 months [22].
This interval is influenced by previous treatment for VL; in India, in those previously treated with sodium stibogluconate (SSG), the interval was longer as compared with those treated with AmBisome (2.9 years and 1.2 years, respectively) [11]. In another study, the median interval after VL treatment with SSG was 36 months, while this was 48 months and 21 months for those treated for VL with amphotericin deoxycholate or miltefosine, respectively [13].
Age is also important because younger patients (<15 years) were reported to have significantly shorter intervals than the older age group: median intervals were 26 months and 50 months, respectively [16].
Concomitant VL and PKDL, often referred to as para–kala-azar dermal leishmaniasis, has only sporadically been reported in the ISC, while this seems more common in Sudan (up to 18%) [15,23,24].
In 10%–23% of cases, there is no history of previous VL; this is similar to what has been reported from Africa [10,13,14,20,25,26].
Gender balance
While in hospital-based studies in nonendemic areas a male preponderance (80%) is reported among PKDL patients with a male-to-female ratio of 3–4:1, in endemic areas, the ratio seems much more balanced (ratio 1.2–1.9) and not different from that found in VL [10–12,14,27–29].
Active case finding
Because PKDL patients are not ill and the disease may run a chronic course with limited discomfort, many do not report to health facilities or report late. Only up to 25% of patients seek medical treatment for cosmetic reasons. This is concerning because of the possible risk in transmission [18,30,31]. Various strategies have been used for active case detection [10,16,29,32,33].
Using a cluster approach in following up treated VL patients, active case detection was shown to be superior to passive case detection in Bangladesh (Fulbaria and Trishal districts); for PKDL, the yield increased 30-fold, while for VL, this was 1.4-fold, emphasising the lack of urgency felt by PKDL patients to seek medical care. However, in both strategies, patients had already been symptomatic for 345–360 days prior to diagnosis (clinically and by a positive rK39 RDT) [29].
PKDL and transmission
Studies have indicated that P. argentipes is the sole vector of Leishmania donovani in the ISC [34]. It also feeds on animals, mainly cattle, that are not a reservoir for parasites but whose presence influences biting behaviour and breeding sites and thus, indirectly, transmission (Fig 1) [35]. Other animals, including goats, have been suggested as reservoirs, but there is no conclusive evidence as to their role in transmission [36]. After parasites had been demonstrated in sand flies feeding on 2 patients with nodular lesions in a study from 1933, a 30% infection rate of sand flies was reported after feeding on a patient with depigmented (macular) PKDL lesions (the predominant type in the ISC), in which parasites are normally scanty in a slit-skin smear or biopsy [37]. In a later experiment, 53% of sand flies that fed on 4 PKDL patients with nodular or nodulo-ulcerative lesions developed infection; this was thought to be an explanation for a recent VL outbreak in southern West Bengal in the 1980s [7]. These experiments also showed that after feeding on a PKDL patient, the parasite developed in the sand fly in the same way as after feeding on a VL patient and that the strain is capable of causing visceralised disease in mice and hamsters [38]. Recently, 3 PKDL patients from Bangladesh were found infective to sand flies, including a patient with macular lesions. [39]
Fig 1. Endemic area for VL in India (Muzaffarpur).

(A) People live in close contact with animals that may attract sand flies. (B) Typical houses with walls made of mud. VL, visceral leishmaniasis.
Despite this, in a longitudinal study over 2 years in Bihar, a highly endemic area, no cases of VL were observed in households with PKDL. Because the immune status of participants was not assessed but likely to be high, it may be argued that different results could have been obtained in an interepidemic period [26].
Epidemiological modelling
Various models have attempted to describe and quantify the influence of PKDL in transmission of VL in the ISC. Dye (1992) based his model on data collected between 1875 and 1950 in Assam, where, from 1920, cases were treated with tartar emetic and the PKDL rate was 25%. The model predicted that if 0.5% of PKDL cases remained infectious, an epidemic may become endemic [40].
Stauch et al. (2011) based their VL model in the ISC on data from the KALANET trial and assumed a PKDL rate of 3% and a prevalence of 0.5 cases/10,000 [41]. Active case finding and effective short-course treatment of PKDL were recommended. Recently, similar recommendations were made, and the need to determine and quantify the infectivity of PKDL was emphasised because this may influence the impact of insecticide residual spraying (IRS) [42,43].
Pathogenesis
It is likely that PKDL results from the persistence of parasites from VL in the skin, leading to a block in the development of an adequate immune response. Using the (imperfect) helper T cell 2 (Th2)/helper T cell 1 (Th1) dichotomy, the immune response in African PKDL has characteristics of a Th2 response in the skin, while systemically, the Th1 response predominates, the latter being the result of successful VL therapy [44,45]. Alternatively, a new infection by leishmania parasites in an individual who has already acquired a degree of immunity has been suggested [46].
Recently, an association was found, which needs further study, between chronic environmental arsenic exposure and the development of PKDL [47].
The main factors in the pathogenesis of PKDL, including host- and parasite-related factors, are summarised in S1 Table.
Clinical features and diagnosis
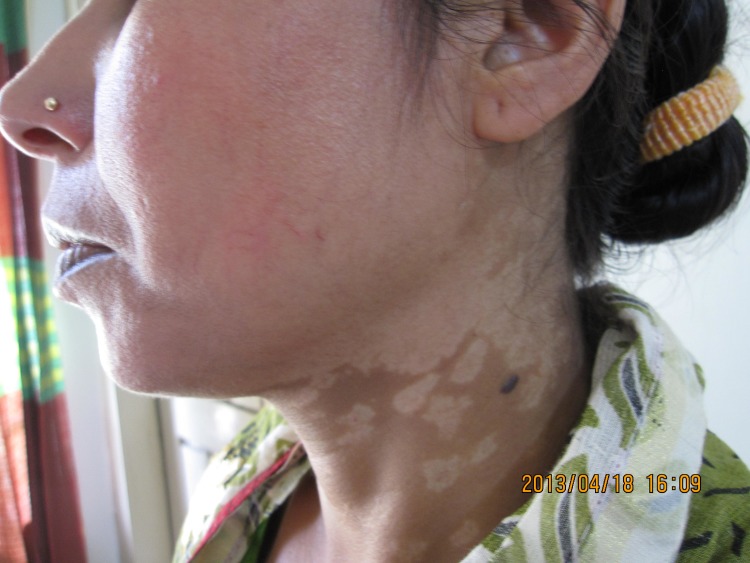
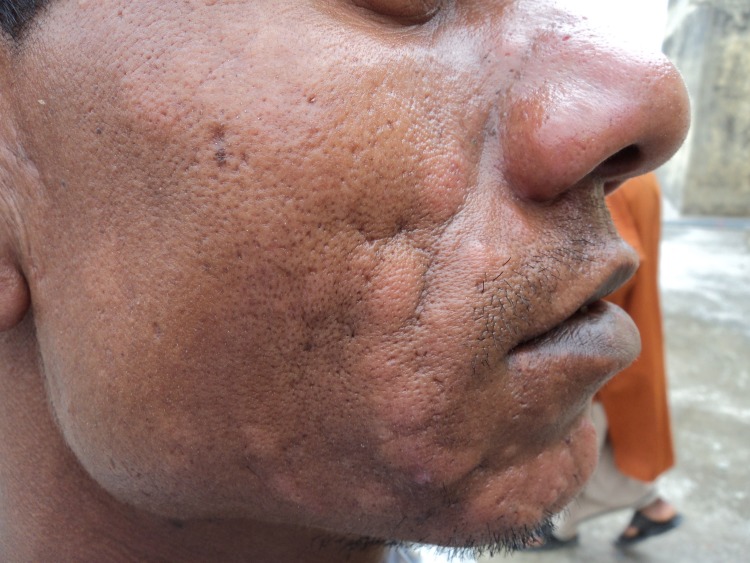
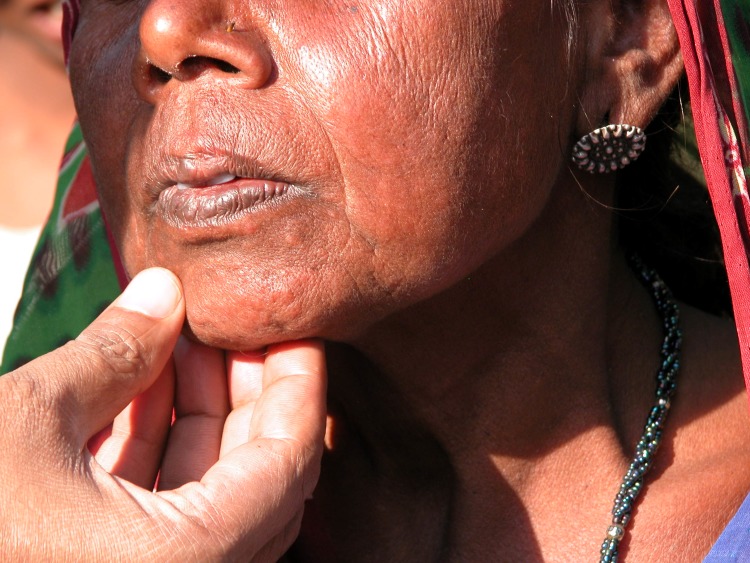
An atlas on the clinical presentation and differential diagnosis of PKDL has been published [48]. In field studies, macular lesions, which can spread to involve most parts of the body, are most common. In hospital-based studies, often cases with a long duration of PKDL are reported with extensive papular, nodular, or mixed presentations; special forms include erythrodermic, warty, lupoid, and tumour-like forms (Figs 2–4).
Fig 2. PKDL from Bangladesh: confluent macular rash involving most of the face (courtesy of Dr. Dinesh Mondal).
PKDL; post-kala-azar dermal leishmaniasis.
Fig 4. PKDL from Bangladesh: PN rash with infiltration of the cheek and chin.
PKDL, post-kala-azar dermal leishmaniasis; PN, papulonodular.
Fig 3. PKDL from India: discrete papules and infiltration of the chin, resulting in a plaque.
PKDL, post-kala-azar dermal leishmaniasis.
The main differential diagnosis is with leprosy and vitiligo, but many other skin conditions can be mistaken for PKDL and vice versa, thus complicating case finding and management [48].
A clinical algorithm for diagnosis under field conditions has been proposed, but confirmation is often necessary [49].
Parasitological diagnosis can be done by slit-skin smear or by biopsy. In slit-skin smears, macular lesions will often fail to show parasites, while PN lesions will be positive in 20%–40% [13,50]. The yield is higher in a biopsy (imprint smears): 91% of PN lesions and 40% of macular lesions will show parasites [12]. Culture and immunohistochemical (IHC) staining improve routine haematoxylin–eosin staining [27].
Serological tests in the blood, such as the direct agglutination test (DAT) and rK39 RDT, may be used for screening; it is not clear whether antibodies may persist from previous VL or indicate the diagnosis of PKDL [10,51]. rK39 RDT can also be done on material from a slit-skin smear with 100% sensitivity and specificity [52].
Recently, serum adenosine deaminase (ADA) levels were found to be raised in PKDL and decreased during treatment [53].
PCR, nested PCR, and RLFP have all been demonstrated to be sensitive and can be done in material obtained by a slit-skin smear [10,16,54,55]. Quantitative PCR (qPCR) is sensitive (96%–100%) and allows monitoring of parasite reduction as a result of treatment [13,56,57]. New readouts such as loop-mediated isothermal amplification (LAMP) may be more appropriate for use in the field; recently, an adaptation using a closed-loop assay potentially reduced contamination and also showed potential as a test of cure in PKDL [52,58]. In contrast to degraded DNA, the presence of parasite DNA that can be amplified indicates live parasites.
Treatment
Recommendations for treatment of PKDL have been published in WHO guidelines [59].
Currently, in the ISC, PKDL cases are treated according to national guidelines with miltefosine for 12 weeks; this regimen (50 mg twice per day) has a cure rate of 78% (intention-to-treat [ITT] analysis) and 93% (per protocol [PP] analysis). Shorter administration for 8 weeks leads to lower cure rates, while a higher daily dose (50 mg 3 times daily) or longer duration (16 weeks) showed higher cure rates of 96%–100%, although at the expense of increased gastrointestinal side effects [60–63].
As in VL, increasing resistance to miltefosine has also been reported in PKDL [10,64]. Parasites in pretreatment PKDL patients show more tolerance to miltefosine than VL samples [65]. Tolerance increases further after treatment of PKDL [66]. A higher parasite load pretreatment is a risk factor for relapse [66,67]. Relapses have also been described after paromomycin treatment [9].
In the Médecins sans Frontières (MSF) programme at Fulbaria, over 1,400 patients with clinically diagnosed PKDL were treated on an outpatient basis with 6 infusions of 5 mg/kg AmBisome administered over 3 weeks (total dose 30 mg/kg). Ninety-six percent of patients had macular lesions. Complete recovery of nodular and papular lesions and a complete or significant repigmentation of macular lesions were observed in 86.5% of patients at 12-month follow-up. Concerns were raised regarding potential complications with rhabdomyolysis [68]. A subsequent prospective cohort study on 110 patients (of whom 97% had macular lesions), using the same regimen to better categorise the safety profile of the regimen, resulted in 6 patients developing severe hypokalaemia during treatment; however, none were symptomatic nor developed rhabdomyolysis. Of 88 patients completing 12-month follow-up, 59 (80%) showed substantial or complete cure. In contrast, the same AmBisome regimen had similar efficacy outcomes (n = 161) but did not lead to severe hypokalaemia in India (personal communication, Koert Ritmeijer, MSF, Amsterdam, the Netherlands, to Eduard Zijlstra). Repeated courses (20 infusions for 3 courses) of conventional amphotericin B have been shown to produce good cure rates (>90%), but nephrotoxicity remains a problem [69].
Immunochemotherapy has been explored in Sudan; SSG for 40 days with alum-precipitated autoclaved L. major plus BCG vaccine or placebo showed better cure rates in the vaccine arm at 87% versus 53% cure. It was noted that leishmanin skin test (LST) conversion was a good surrogate marker for cure [70]. A third-generation vaccine developed to induce cluster of differentiation (CD)8+ T cells is expected to undergo study in the treatment of PKDL in Sudan in the near future [71].
Recurrence of VL after PKDL in non-immunocompromised patients is uncommon and was estimated to occur in 1 out of 700 cases [72].
Assessment of cure
While the parasite load in PKDL is lower than in VL, a long duration of antileishmanial therapy is often given because there is no conclusive laboratory or clinical marker other than the disappearance of the lesions. In the case of macular lesions, this may take 12–18 months [10,12]. While in histological specimens after 3 months of treatment (SSG 10 mg/kg/day for 90 days) parasites can no longer be detected by microscopy, the mononuclear cell infiltrate still persists [12]. Both LAMP and qPCR could be useful, and the latter allows the comparison of the parasite (load) before and after treatment [52].
As in VL, serological tests are not useful for assessing cure; in 1 study, the DAT positivity rate decreased from 75% to 66% after treatment [12].
Discussion
Current tools, research gaps, and needs for PKDL control are summarised in Table 2.
Table 2. PKDL—Summary of current tools and needs for the KAEP.
| Intervention | Tool | In place | Research gap | Need |
|---|---|---|---|---|
| Transmission | • xenodiagnosis | • PKDL: who should be treated: 1. acute versus chronic 2. macular versus papulonodular 3. limited versus extensive • comparison of contribution of PKDL, VL, ex-VL, asymptomatics, HIV–VL • quantitative parasite load (qPCR) for infectivity |
• xenodiagnosis studies to quantify the risk of transmission | |
|
Screening and Dx biomarker |
• clinical Dx • rK39 RDT • PCR |
• WHO self-learning course • WHO Manual for Health Workers • clinical Dx and rK39 RDT |
• validated clinical algorithm • sensitive and specific serological test • biomarker for cure - PCR field application - immunological marker |
• teaching of health workers • validation of clinical algorithm • point-of-care test • studies for biomarker |
| Treatment | • drugs: MF, AMB • immunomodulator |
• universal policy for treatment with MF | • efficacy of current MF regimen (incl. resistance) • optimal regimen of AMB • alternative oral drug(s) • development of immunomodulator • drug penetration in the skin |
• surveillance for resistance • safe, short course, oral drugs • candidate immunomodulator • PK studies with skin biopsies |
|
Prevention • by detection of VL and PKDL • by optimal VL treatment • by bed nets • by vector control |
• case finding • mono or combo treatment • LLINs • IRS |
• active case finding for VL • single-dose AMB • LLIN distribution • IRS |
• sustainability • PKDL rate for each VL treatment regimen • understanding of underlying immune responses • possible mode of action of immune modulator • long-term studies on efficacy, acceptability • efficacy of current intervention with DDT • efficacy of other insecticides |
• political commitment • immunochemotherapy studies • alternative insecticides • other control measures |
Abbreviations: AMB, AmBisome; Dx, diagnosis; IRS, indoor residual insecticide spraying; KAEP, Kala-azar Elimination Programme; LLIN, long-lasting insecticide-impregnated net; MF, miltefosine; PK, pharmacokinetics; PKDL, post-kala-azar dermal leishmaniasis; qPCR, quantitative PCR; RDT, rapid diagnostic test; rK39, recombinant K39; VL, visceral leishmaniasis.
Importance of the immune response
The natural history of PKDL suggests that it is not a static but a dynamic condition in which the parasite load, onset and appearance of the clinical presentation, and tendency to self-heal vary with the developing immune response [22,73]. The roles of UV light and concurrent diseases such as malnutrition, tuberculosis, malaria, or reinfection are unclear and may prevent the establishment of a Th1 response or reverse an established Th1 response to a mixed Th1/Th2 response, thus possibly inducing an inflammatory response around ‘dormant’ parasites in the skin [74]. The role of repeated exposure to leishmanial infection is unclear, but it may also influence the immune responses. More information is needed on the leishmanial strains isolated from VL and PKDL patients in various presentations and the role (if any) of endosymbiotic infection.
Prevention of PKDL
The best way to reduce the incidence of PKDL may very well lie in identifying the best treatment for VL that induces a strong and lasting Th1 response so as to prevent PKDL from developing. Currently, it is not known which drug (combination) has the lowest PKDL rate. The 13% PKDL rate after single dose AmBisome (the current VL regimen) is a reason for concern [3]. An immunomodulator that can supplement VL drug treatment should have priority. A candidate immunomodulator (CpG-D35) for cutaneous leishmaniasis that may also be useful in PKDL but is not expected to enter clinical studies in the near future is under development [75].
Management of PKDL
More information is needed on the proportion of PKDL patients that self-cure, skin penetration and immunomodulatory effects of drugs used, and duration of treatment. The clinical cure of the lesions probably lags behind parasitological cure and subsequent immunological cure with the switch from a mixed Th1/Th2 response to a Th1 response (S1 Fig) [16]. A biomarker for cure is needed.
Short, ambulant treatment courses are needed, preferably with (an) oral drug(s). The current treatment with miltefosine is of long duration, and there are reports of increasing failure [13]. For any regimen, safety is of paramount importance because PKDL patients are mostly asymptomatic except for the rash. This is particularly important from an ethical point of view if patients are treated who otherwise may self-heal over time.
Contribution to transmission
For the design of a cost-effective control strategy, the contribution of each category of PKDL patients should be studied: acute versus chronic, macular versus papular versus nodular, and limited versus extensive disease. In each clinical presentation, infectivity of the blood should also be studied. This can only be done by xenodiagnosis studies that quantify the potential for infectivity. For this purpose, insectaria have been established at Muzaffarpur (India) and Mymensingh (Bangladesh).
Clearly, this information is essential for the design and application of mathematical models in which the potential contribution of PKDL to transmission should be balanced and quantified against that of other clinical entities in the spectrum of VL: VL cases, ex-VL cases, HIV–VL coinfection, and asymptomatic individuals. Also, here, understanding the dynamics is important because the contribution of each may vary in the course of the condition as well as over time in the local epidemiological context [76,77]. On the same note, there is also confusion about cured VL and PKDL cases reentering the pool of susceptible individuals, while the current understanding supports that cure provides lasting immunity; however, continuing exposure seems necessary.
Other epidemiological information such as seasonality of sand flies and their behaviour, human migration, and the potential increase of HIV coinfection are among other factors to be explored. Dye and Wolpert recognised the influence of factors such as earthquakes, influenza, and malaria on the statistics of fever (VL or other) deaths, thus emphasising the dynamics over time [78].
In conclusion, the current optimal results obtained so far in the KAEP are fragile and must be consolidated before commitment is lost. History has shown that PKDL is a crucially important factor in the control of VL in the ISC. For the short term, a strategy for active case finding; the validation and implementation of diagnostic tools such as LAMP or qPCR; optimal treatment for VL aiming at the lowest possible PKDL rates; and the identification of short, safe, and effective treatment for PKDL are essential for the VL elimination efforts in the ISC to succeed and be sustained. For the long term, immunological studies and infectivity studies may provide insight in more targeted and more efficient approaches.
Key learning points
The immune responses at various manifestations of PKDL need to be described (acute versus chronic, macular versus PN, limited versus extensive).
The contribution of various manifestations of PKDL in transmission needs to be studied in xenodiagnosis studies and compared to the contribution of asymptomatics, VL patients, HIV-coinfected VL patients, and ex-VL patients, including parasite load by qPCR.
Prevention of PKDL by optimal VL treatment is crucial.
Optimal treatment for PKDL should be based on a safe oral drug with good skin penetration and immunomodulatory properties; it may include an immunomodulator.
A point-of-care biomarker for cure needs to be identified to define the optimal duration of treatment for PKDL.
Top 5 papers
Banjara MR, Kroeger A, Huda MM, Kumar V, Gurung CK, et al. (2015) Feasibility of a combined camp approach for vector control together with active case detection of visceral leishmaniasis, post kala-azar dermal leishmaniasis, tuberculosis, leprosy and malaria in Bangladesh, India and Nepal: an exploratory study. Trans R Soc Trop Med Hyg 109: 408–415.
Hirve S, Boelaert M, Matlashewski G, Mondal D, Arana B, et al. (2016) Transmission Dynamics of Visceral Leishmaniasis in the Indian Subcontinent—A Systematic Literature Review. PLoS Negl Trop Dis 10: e0004896.
WHO. (2011) Regional Strategic Framework for Elimination of kala-azar from the South-east Asia Region (2011–2015). SEA-CD-239.
WHO. Post-kala-azar dermal leishmaniasis: a manual for case management and control. http://apps.who.int/iris/bitstream/10665/78608/1/9789241505215_eng.pdf (accessed March 23, 2016).
Zijlstra EE (2016) The immunology of post-kala-azar dermal leishmaniasis (PKDL). Parasit Vectors 9: 464
Supporting information
(DOCX)
(DOCX)
Funding Statement
The authors received no specific funding for this work.
References
- 1.http://www.searo.who.int/mediacentre/events/governance/rc/rc67_report.pdf.
- 2.Regional strategic framework for elimination of VL from SEA Region (2005–2015). New Delhi: WHO-SEARO. SEA-VBC-85 (Rev.1) p.
- 3.WHO Kala-azar Elimination Programme. Report of a WHO consultation of partners. Geneva, 10–11 February 2015.
- 4.WHO. (2011) Regional Strategic Framework for Elimination of kala-azar from the South-east Asia Region (2011–2015). SEA-CD-239.
- 5.www.searo.who.int/entity/vector_borne_tropical_diseases/documents/SEA-CD-239/en/.
- 6.Zijlstra EE, Musa AM, Khalil EA, el-Hassan IM, el-Hassan AM (2003) Post-kala-azar dermal leishmaniasis. Lancet Infect Dis 3: 87–98. [DOI] [PubMed] [Google Scholar]
- 7.Addy M, Nandy A (1992) Ten years of kala-azar in west Bengal, Part I. Did post-kala-azar dermal leishmaniasis initiate the outbreak in 24-Parganas? Bull World Health Organ 70: 341–346. [PMC free article] [PubMed] [Google Scholar]
- 8.Thakur CP, Kumar A, Mitra G, Thakur S, Sinha PK, et al. (2008) Impact of amphotericin-B in the treatment of kala-azar on the incidence of PKDL in Bihar, India. Indian J Med Res 128: 38–44. [PubMed] [Google Scholar]
- 9.Singh RP, Picado A, Alam S, Hasker E, Singh SP, et al. (2012) Post-kala-azar dermal leishmaniasis (PKDL) in visceral leishmaniasis-endemic communities in Bihar, India . Trop Med Int Health. [DOI] [PubMed] [Google Scholar]
- 10.Ganguly S, Saha P, Chatterjee M, Roy S, Ghosh TK, et al. (2015) PKDL—A Silent Parasite Pool for Transmission of Leishmaniasis in Kala-azar Endemic Areas of Malda District, West Bengal, India. PLoS Negl Trop Dis 9: e0004138 doi: 10.1371/journal.pntd.0004138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burza S, Sinha PK, Mahajan R, Sanz MG, Lima MA, et al. (2014) Post Kala-Azar dermal leishmaniasis following treatment with 20 mg/kg liposomal amphotericin B (Ambisome) for primary visceral leishmaniasis in Bihar, India. PLoS Negl Trop Dis 8: e2611 doi: 10.1371/journal.pntd.0002611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verma N, Bimal S, Das VN, Pandey K, Singh D, et al. (2015) Clinicopathological and Immunological Changes in Indian Post Kala-Azar Dermal Leishmaniasis (PKDL) Cases in relation to Treatment: A Retrospective Study. Biomed Res Int 2015: 745062 doi: 10.1155/2015/745062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramesh V, Kaushal H, Mishra AK, Singh R, Salotra P (2015) Clinico-epidemiological analysis of Post kala-azar dermal leishmaniasis (PKDL) cases in India over last two decades: a hospital based retrospective study. BMC Public Health 15: 1092 doi: 10.1186/s12889-015-2424-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahman KM, Islam S, Rahman MW, Kenah E, Ghalib CM, et al. (2010) Increasing incidence of post-kala-azar dermal leishmaniasis in a population-based study in Bangladesh. Clin Infect Dis 50: 73–76. doi: 10.1086/648727 [DOI] [PubMed] [Google Scholar]
- 15.Islam S, Kenah E, Bhuiyan MA, Rahman KM, Goodhew B, et al. (2013) Clinical and immunological aspects of post-kala-azar dermal leishmaniasis in Bangladesh. Am J Trop Med Hyg 89: 345–353. doi: 10.4269/ajtmh.12-0711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mondal D, Nasrin KN, Huda MM, Kabir M, Hossain MS, et al. (2010) Enhanced case detection and improved diagnosis of PKDL in a Kala-azar-endemic area of Bangladesh. PLoS Negl Trop Dis. 2010 October 5;4(10). pii: e832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.International Center for Diarrheal Disease Research B (2007) Post kala-azar dermal leishmaniasis: new observations challenge previous assumptions. Health and Science Bulletin, ICDDR,B; 5:6–12. [Google Scholar]
- 18.Uranw S, Ostyn B, Rijal A, Devkota S, Khanal B, et al. (2011) Post-kala-azar dermal leishmaniasis in Nepal: a retrospective cohort study (2000–2010). PLoS Negl Trop Dis 5: e1433 doi: 10.1371/journal.pntd.0001433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garg VK, Agrawal S, Rani S, Joshi A, Agarwalla A, et al. (2001) Post-kala-azar dermal leishmaniasis in Nepal. Int J Dermatol 40: 179–184. [DOI] [PubMed] [Google Scholar]
- 20.Islam S, Ashraful Alam Bhuiyan M, Bern C (2011) Post-Kala-Azar dermal leishmaniasis in Mymensingh, Bangladesh. Am J Trop Med Hyg 85: 193–194. doi: 10.4269/ajtmh.2011.11-0128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thakur CP (1984) Epidemiological, clinical and therapeutic features of Bihar kala-azar (including post kala-azar dermal leishmaniasis). Trans R Soc Trop Med Hyg 78: 391–398. [DOI] [PubMed] [Google Scholar]
- 22.Musa AM, Khalil EA, Raheem MA, Zijlstra EE, Ibrahim ME, et al. (2002) The natural history of Sudanese post-kala-azar dermal leishmaniasis: clinical, immunological and prognostic features. Ann Trop Med Parasitol 96: 765–772. doi: 10.1179/000349802125002211 [DOI] [PubMed] [Google Scholar]
- 23.Zijlstra EE, Khalil EA, Kager PA, El-Hassan AM (2000) Post-kala-azar dermal leishmaniasis in the Sudan: clinical presentation and differential diagnosis. Br J Dermatol 143: 136–143. [DOI] [PubMed] [Google Scholar]
- 24.Ramesh V (2007) Post-kala-azar dermal leishmaniasis with visceral leishmaniasis, or a rare presentation of visceral leishmaniasis with extensive skin manifestations. Int J Dermatol 46: 1326 doi: 10.1111/j.1365-4632.2007.03481.x [DOI] [PubMed] [Google Scholar]
- 25.Zijlstra EE, el-Hassan AM, Ismael A (1995) Endemic kala-azar in eastern Sudan: post-kala-azar dermal leishmaniasis. Am J Trop Med Hyg 52: 299–305. [DOI] [PubMed] [Google Scholar]
- 26.Das VN, Pandey RN, Siddiqui NA, Chapman LA, Kumar V, et al. (2016) Longitudinal Study of Transmission in Households with Visceral Leishmaniasis, Asymptomatic Infections and PKDL in Highly Endemic Villages in Bihar, India. PLoS Negl Trop Dis 10: e0005196 doi: 10.1371/journal.pntd.0005196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salotra P, Sreenivas G, Beena KR, Mukherjee A, Ramesh V (2003) Parasite detection in patients with post kala-azar dermal leishmaniasis in India: a comparison between molecular and immunological methods. J Clin Pathol 56: 840–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozaki M, Islam S, Rahman KM, Rahman A, Luby SP, et al. (2011) Economic consequences of post-kala-azar dermal leishmaniasis in a rural Bangladeshi community. Am J Trop Med Hyg 85: 528–534. doi: 10.4269/ajtmh.2011.10-0683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Das AK, Harries AD, Hinderaker SG, Zachariah R, Ahmed B, et al. (2014) Active and passive case detection strategies for the control of leishmaniasis in Bangladesh. Public Health Action 4: 15–21. doi: 10.5588/pha.13.0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh RP, Picado A, Alam S, Hasker E, Singh SP, et al. (2012) Post-kala-azar dermal leishmaniasis in visceral leishmaniasis-endemic communities in Bihar, India. Trop Med Int Health 17: 1345–1348. doi: 10.1111/j.1365-3156.2012.03067.x [DOI] [PubMed] [Google Scholar]
- 31.Mondal D, Khan MG (2011) Recent advances in post-kala-azar dermal leishmaniasis. Curr Opin Infect Dis 24: 418–422. doi: 10.1097/QCO.0b013e32834a8ba1 [DOI] [PubMed] [Google Scholar]
- 32.Banjara MR, Kroeger A, Huda MM, Kumar V, Gurung CK, et al. (2015) Feasibility of a combined camp approach for vector control together with active case detection of visceral leishmaniasis, post kala-azar dermal leishmaniasis, tuberculosis, leprosy and malaria in Bangladesh, India and Nepal: an exploratory study. Trans R Soc Trop Med Hyg 109: 408–415. doi: 10.1093/trstmh/trv031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh SP, Hirve S, Huda MM, Banjara MR, Kumar N, et al. (2011) Options for active case detection of visceral leishmaniasis in endemic districts of India, Nepal and Bangladesh, comparing yield, feasibility and costs. PLoS Negl Trop Dis 5: e960 doi: 10.1371/journal.pntd.0000960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukhopadhyay AK, Mishra RN (1991) Development of Leishmania donovani in Phlebotomus argentipes & Ph. papatasi fed on kala-azar patients in Bihar. Indian J Med Res 93: 152–154. [PubMed] [Google Scholar]
- 35.Bern C, Hightower AW, Chowdhury R, Ali M, Amann J, et al. (2005) Risk factors for kala-azar in Bangladesh. Emerg Infect Dis 11: 655–662. doi: 10.3201/eid1105.040718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh N, Mishra J, Singh R, Singh S (2013) Animal reservoirs of visceral leishmaniasis in India. J Parasitol 99: 64–67. doi: 10.1645/GE-3085.1 [DOI] [PubMed] [Google Scholar]
- 37.Napier LE, Smith ROA, Das Gupta CR, Mukherjee S (1933) The infection of Phlebotomus argentipes from dermal leishmanial lesions. Indian J Med Res XXI: 173–177. [Google Scholar]
- 38.Gupta AK, Narayan S, Verma N, Thakur AK, Das P (2012) Viscerotropic potential of parasites isolated from post-kala-azar dermal leishmaniasis cases: an experimental evidence. J Vector Borne Dis 49: 266–267. [PubMed] [Google Scholar]
- 39.Molina R, Ghosh D, Carrillo E, Monnerat S, Bern C, et al. (2017) Infectivity of Post-Kala-azar Dermal Leishmaniasis Patients to Sand Flies: Revisiting a Proof of Concept in the Context of the Kala-azar Elimination Program in the Indian Subcontinent. Clin Infect Dis. 2017 May 18 doi: 10.1093/cid/cix245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dye C (1992) Leishmaniasis epidemiology: the theory catches up. Parasitology 104 Suppl: S7–18. [DOI] [PubMed] [Google Scholar]
- 41.Stauch A, Sarkar RR, Picado A, Ostyn B, Sundar S, et al. (2011) Visceral leishmaniasis in the Indian subcontinent: modelling epidemiology and control. PLoS Negl Trop Dis 5: e1405 doi: 10.1371/journal.pntd.0001405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le Rutte EA, Coffeng LE, Bontje DM, Hasker EC, Ruiz Postigo JA, et al. (2016) Feasibility of eliminating visceral leishmaniasis from the Indian subcontinent: explorations with a set of deterministic age-structured transmission models. Parasit Vectors 9: 24 doi: 10.1186/s13071-016-1292-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rock KS, le Rutte EA, de Vlas SJ, Adams ER, Medley GF, et al. (2015) Uniting mathematics and biology for control of visceral leishmaniasis. Trends Parasitol 31: 251–259. doi: 10.1016/j.pt.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 44.Ismail A, Khalil EA, Musa AM, El Hassan IM, Ibrahim ME, et al. (2006) The pathogenesis of post kala-azar dermal leishmaniasis from the field to the molecule: does ultraviolet light (UVB) radiation play a role? Med Hypotheses 66: 993–999. doi: 10.1016/j.mehy.2005.03.035 [DOI] [PubMed] [Google Scholar]
- 45.Zijlstra EE (2016) The immunology of post-kala-azar dermal leishmaniasis (PKDL). Parasit Vectors 9: 464 doi: 10.1186/s13071-016-1721-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Napier LE, Das Gupta CR (1930) A clinical study of post-kala-azar dermal leishmaniasis. Indian Med Gaz 65: 249–257. [PMC free article] [PubMed] [Google Scholar]
- 47.Das S, Mandal R, Rabidas VN, Verma N, Pandey K, et al. (2016) Chronic Arsenic Exposure and Risk of Post Kala-azar Dermal Leishmaniasis Development in India: A Retrospective Cohort Study. PLoS Negl Trop Dis 10: e0005060 doi: 10.1371/journal.pntd.0005060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.World Health Organization. Zijlstra EE, Alvar J (2012) The Post Kala-azar Dermal Leishmaniasis (PKDL) Atlas. A manual for health workers.
- 49.Adams ER, Versteeg I, Leeflang MM (2013) Systematic Review into Diagnostics for Post-Kala-Azar Dermal Leishmaniasis (PKDL). J Trop Med 2013: 150746 doi: 10.1155/2013/150746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh A, Ramesh V, Ramam M (2015) Histopathological characteristics of post kala-azar dermal leishmaniasis: a series of 88 patients. Indian J Dermatol Venereol Leprol 81: 29–34. doi: 10.4103/0378-6323.148562 [DOI] [PubMed] [Google Scholar]
- 51.Salotra P, Singh R (2006) Challenges in the diagnosis of post kala-azar dermal leishmaniasis. Indian J Med Res 123: 295–310. [PubMed] [Google Scholar]
- 52.Verma N, Singh D, Pandey K, Das VN, Lal CS, et al. (2013) Comparative evaluation of PCR and imprint smear microscopy analyses of skin biopsy specimens in diagnosis of macular, papular, and mixed papulo-nodular lesions of post-kala-azar dermal leishmaniasis. J Clin Microbiol 51: 4217–4219. doi: 10.1128/JCM.01482-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vijayamahantesh, Amit A, Dikhit MR, Pandey RK, Singh K, et al. (2016) Elevated Serum ADA Activity as a Marker for Diagnosis and Prognosis of Visceral Leishmaniasis and Post Kala-Azar Dermal Leishmaniasis in Indian Patients. PLoS ONE 11: e0154117 doi: 10.1371/journal.pone.0154117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salotra P, Sreenivas G, Pogue GP, Lee N, Nakhasi HL, et al. (2001) Development of a species-specific PCR assay for detection of Leishmania donovani in clinical samples from patients with kala-azar and post-kala-azar dermal leishmaniasis. J Clin Microbiol 39: 849–854. doi: 10.1128/JCM.39.3.849-854.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Osman OF, Oskam L, Kroon NC, Schoone GJ, Khalil ET, et al. (1998) Use of PCR for diagnosis of post-kala-azar dermal leishmaniasis. J Clin Microbiol 36: 1621–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumar D, Ramesh V, Verma S, Ramam M, Salotra P (2009) Post-kala-azar dermal leishmaniasis (PKDL) developing after treatment of visceral leishmaniasis with amphotericin B and miltefosine. Ann Trop Med Parasitol 103: 727–730. doi: 10.1179/000349809X12554106963438 [DOI] [PubMed] [Google Scholar]
- 57.Verma S, Kumar R, Katara GK, Singh LC, Negi NS, et al. (2010) Quantification of parasite load in clinical samples of leishmaniasis patients: IL-10 level correlates with parasite load in visceral leishmaniasis. PLoS ONE 5: e10107 doi: 10.1371/journal.pone.0010107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verma S, Singh R, Sharma V, Bumb RA, Negi NS, et al. (2017) Development of a rapid loop-mediated isothermal amplification assay for diagnosis and assessment of cure of Leishmania infection. BMC Infect Dis 17: 223 doi: 10.1186/s12879-017-2318-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.WHO. Post-kala-azar dermal leishmaniasis: a manual for case management and control. http://apps.who.int/iris/bitstream/10665/78608/1/9789241505215_eng.pdf (accessed March 23, 2016).
- 60.Sundar S, Singh A, Chakravarty J, Rai M (2015) Efficacy and safety of miltefosine in treatment of post-kala-azar dermal leishmaniasis. ScientificWorldJournal 2015: 414378 doi: 10.1155/2015/414378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sundar S, Sinha P, Jha TK, Chakravarty J, Rai M, et al. (2013) Oral miltefosine for Indian post-kala-azar dermal leishmaniasis: a randomised trial. Trop Med Int Health 18: 96–100. doi: 10.1111/tmi.12015 [DOI] [PubMed] [Google Scholar]
- 62.Ramesh V, Katara GK, Verma S, Salotra P (2011) Miltefosine as an effective choice in the treatment of post-kala-azar dermal leishmaniasis. Br J Dermatol 165: 411–414. doi: 10.1111/j.1365-2133.2011.10402.x [DOI] [PubMed] [Google Scholar]
- 63.Ghosh S, Das NK, Mukherjee S, Mukhopadhyay D, Barbhuiya JN, et al. (2015) Inadequacy of 12-Week Miltefosine Treatment for Indian Post-Kala-Azar Dermal Leishmaniasis. Am J Trop Med Hyg 93: 767–769. doi: 10.4269/ajtmh.14-0721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sundar S, Singh A, Rai M, Prajapati VK, Singh AK, et al. (2012) Efficacy of miltefosine in the treatment of visceral leishmaniasis in India after a decade of use. Clin Infect Dis 55: 543–550. doi: 10.1093/cid/cis474 [DOI] [PubMed] [Google Scholar]
- 65.Bhandari V, Kulshrestha A, Deep DK, Stark O, Prajapati VK, et al. (2012) Drug susceptibility in Leishmania isolates following miltefosine treatment in cases of visceral leishmaniasis and post kala-azar dermal leishmaniasis. PLoS Negl Trop Dis 6: e1657 doi: 10.1371/journal.pntd.0001657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramesh V, Singh R, Avishek K, Verma A, Deep DK, et al. (2015) Decline in Clinical Efficacy of Oral Miltefosine in Treatment of Post Kala-azar Dermal Leishmaniasis (PKDL) in India. PLoS Negl Trop Dis 9: e0004093 doi: 10.1371/journal.pntd.0004093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Girgla HS, Marsden RA, Singh GM, Ryan TJ (1977) Post-kala-azar dermal leishmaniasis. Br J Dermatol 97: 307–311. [DOI] [PubMed] [Google Scholar]
- 68.Marking U, den Boer M, Das AK, Ahmed EM, Rollason V, et al. (2014) Hypokalaemia-induced rhabdomyolysis after treatment of post-Kala-azar dermal Leishmaniasis (PKDL) with high-dose AmBisome in Bangladesh-a case report. PLoS Negl Trop Dis 8: e2864 doi: 10.1371/journal.pntd.0002864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rabi Das VN, Siddiqui NA, Pal B, Lal CS, Verma N, et al. (2017) To evaluate efficacy and safety of amphotericin B in two different doses in the treatment of post kala-azar dermal leishmaniasis (PKDL). PLoS ONE 12: e0174497 doi: 10.1371/journal.pone.0174497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Musa AM, Khalil EA, Mahgoub FA, Elgawi SH, Modabber F, et al. (2008) Immunochemotherapy of persistent post-kala-azar dermal leishmaniasis: a novel approach to treatment. Trans R Soc Trop Med Hyg 102: 58–63. doi: 10.1016/j.trstmh.2007.08.006 [DOI] [PubMed] [Google Scholar]
- 71.Osman M, Mistry A, Keding A, Gabe R, Cook E, et al. (2017) A third generation vaccine for human visceral leishmaniasis and post kala azar dermal leishmaniasis: First-in-human trial of ChAd63-KH. PLoS Negl Trop Dis 11: e0005527 doi: 10.1371/journal.pntd.0005527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sen Gupta PC, Mukherjee AM (1968) Recurrence of kala-azar associated with post-kala-azar dermal leishmaniasis. J Indian Med Assoc 50: 1–5 passim. [PubMed] [Google Scholar]
- 73.Haldar JP, Ghose S, Saha KC, Ghose AC (1983) Cell-mediated immune response in Indian kala-azar and post-kala-azar dermal leishmaniasis. Infect Immun 42: 702–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ismail A, El Hassan AM, Kemp K, Gasim S, Kadaru AE, et al. (1999) Immunopathology of post kala-azar dermal leishmaniasis (PKDL): T-cell phenotypes and cytokine profile. J Pathol 189: 615–622. doi: 10.1002/(SICI)1096-9896(199912)189:4<615::AID-PATH466>3.0.CO;2-Z [DOI] [PubMed] [Google Scholar]
- 75.http://www.dndi.org/diseases-projects/portfolio/cpg-d35/.
- 76.Hirve S, Boelaert M, Matlashewski G, Mondal D, Arana B, et al. (2016) Transmission Dynamics of Visceral Leishmaniasis in the Indian Subcontinent—A Systematic Literature Review. PLoS Negl Trop Dis 10: e0004896 doi: 10.1371/journal.pntd.0004896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saha P, Ganguly S, Chatterjee M, Das SB, Kundu PK, et al. (2017) Asymptomatic leishmaniasis in kala-azar endemic areas of Malda district, West Bengal, India. PLoS Negl Trop Dis 11: e0005391 doi: 10.1371/journal.pntd.0005391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dye C, Wolpert DM (1988) Earthquakes, influenza and cycles of Indian kala-azar. Trans R Soc Trop Med Hyg 82: 843–850. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)