Abstract
It is important to minimize lung dose during intensity‐modulated radiation therapy (IMRT) of nonsmall cell lung cancer (NSCLC). In this study, an approach was proposed to reduce lung dose by relaxing the constraint of target dose homogeneity during treatment planning of IMRT. Ten NSCLC patients with lung tumor on the right side were selected. The total dose for planning target volume (PTV) was 60 Gy (2 Gy/fraction). For each patient, two IMRT plans with six beams were created in Pinnacle treatment planning system. The dose homogeneity of target was controlled by constraints on the maximum and uniform doses of target volume. One IMRT plan was made with homogeneous target dose (the resulting target dose was within 95%–107% of the prescribed dose), while another IMRT plan was made with inhomogeneous target dose (the resulting target dose was more than 95% of the prescribed dose). During plan optimization, the dose of cord and heart in two types of IMRT plans were kept nearly the same. The doses of lungs, PTV and organs at risk (OARs) between two types of IMRT plans were compared and analyzed quantitatively. For all patients, the lung dose was decreased in the IMRT plans with inhomogeneous target dose. On average, the mean dose, V5, V20, and V30 of lung were reduced by 1.4 Gy, 4.8%, 3.7%, and 1.7%, respectively, and the dose to normal tissue was also reduced. These reductions in DVH values were all statistically significant (P < 0.05). There were no significant differences between the two IMRT plans on V25, V30, V40, V50 and mean dose for heart. The maximum doses of cords in two type IMRT plans were nearly the same. IMRT plans with inhomogeneous target dose could protect lungs better and may be considered as a choice for treating NSCLC.
Keywords: IMRT, non small cell lung cancer, plan optimization, target dose homogeneity, treatment planning
1. INTRODUCTION
Lung cancer is the most frequently diagnosed cancer and the leading cause of cancer death among males. About 85% of lung cancers are NSCLC worldwide.1 Radiotherapy plays an important role in the treatment of locally advanced, unresectable NSCLC.2 Treatment outcome of standard radiotherapy for NSCLC patients has not improved much during the past decades, and the 5‐year relative survival rate is still no more than 20%.3 Local failure often occurs in the primary tumor. Doses higher than the standard 60–66 Gy are required to obtain a better local tumor control.4, 5 But this is limited by the protection of organs at risk. Some patients suffered from severe side effects after radiation therapy.6, 7 Radiation pneumonitis (RP) is the main dose‐limiting complication in radiation therapy for NSCLC, and occurs in 5%–50% of patients.8, 9 In the case of conventionally fractionated radiotherapy, the traditional strategy for minimizing patients' risk is to follow empirically established dose–volume constraints, such as V20 < 30%–35% and mean lung dose (MLD) <20–23 Gy. However, the relationship between RP risk and dosimetical statistics such as MLD varies among institutions. And it also changes when different treatment techniques (i.e., CRT, IMRT, and VMAT) are applied.10
IMRT is a common technology for the treatment of lung cancer.11 The target volume could get higher dose and better conformity index than 3D‐CRT. However, the improvement of uniformity of target dose could increase the volume of the low dose area in nearby OARs. As a result the volume of low dose area in the lung could be significantly increased.12, 13 Shirvani et al found that the proportion of patients under IMRT treatment increased year by year and V20 of lung decreased significantly in IMRT group based on 3986 patients. Compared with 3D–CRT group the adverse reaction of lung occurred at similar rates using IMRT and showed that the lower V20 did not reduce the incidence of RP.14 The reason may be that V5 of lung would be increased as IMRT applied.15 To minimize the risk of RP some new techniques were introduced to reduce the lung dose, including respiratory gated PET/CT, Cyber Knife, VMAT, etc.16, 17, 18, 19
Conventionally, the standard practice is that tumors should be irradiated to an intended uniform, or homogeneous, dose.20 While this optimizes the tumor control probability in the case of homogenous tumors, this is generally not the optimal dose distribution in tumors with spatial variation in radiation sensitivity.21 In addition, dose escalation strategies that involve delivery of uniform doses are typically limited by normal tissue dose tolerance. There have been studies indicating that deliberately using nonuniform radiation doses allows for dose escalation of tumor subvolumes without necessarily increasing the dose which is delivered to adjacent critical structures.22, 23
It still remains unclear whether the dose to OARs could be reduced when the dose nonuniformity in the target area is increased. The goal of this study is to investigate whether it is beneficial to decrease the lung toxicity for NSCLC by increasing target dose inhomogeneity in IMRT plans.
2. METHODS
2.A. Patient data
10 NSCLC patients with lung tumor on the right side treated at our institution between July 2014 and October 2015 were selected in this study which had been approved by ethics committee. The patient characteristics are listed in Table 1. The patient‐related privacy information (e.g., name, identification number, telephone number) has been removed. All the patients were immobilized in the supine position using a thermoplastic mask. Treatment plans were made based on computed tomography (Philips, Brilliance Big Bore) with slice thickness of 3 mm.
Table 1.
Summary of patient characteristics
| Patient No. | Age | TNM | PTV Volume (cm3) |
|---|---|---|---|
| 1 | 35 | T2N3M0 | 392.9 |
| 2 | 73 | T3N2M0 | 403.1 |
| 3 | 47 | T2N1M0 | 210.8 |
| 4 | 60 | T2N2M0 | 227.5 |
| 5 | 77 | T3N1M0 | 273.4 |
| 6 | 55 | T2N3M0 | 381.0 |
| 7 | 40 | T2N1M0 | 212.9 |
| 8 | 69 | T2N1M0 | 225.3 |
| 9 | 57 | T2N2M1 | 251.6 |
| 10 | 56 | T3N2M0 | 395.8 |
The gross tumor volume (GTV) was delineated by experienced radiation oncologists based on the planning CT (free‐breathing scan) with fluorodeoxyglucose positron emission tomography (FDG‐PET) images as auxiliary references, and consisted of the primary tumor and involved lymph nodes. The clinical target volume (CTV) was created as an expansion of the GTV by 10 mm in mediastinum and 5 mm in lung tissue excluding bony structures and major vessels in accordance with recommendations provided by the Danish Oncology Lung cancer Group (DOLG). The planning target volume was created as a patient specific expansion of the CTV with a margin of 5–10 mm.24, 25 The organs at risk included the heart, the spinal cord, the lungs, and the normal tissues. The prescribed dose was 60 Gy in 2.0 Gy daily fractions.
2.B. Plan optimization
The IMRT plans were made on Pinnacle 9.10 workstation and treated on Elekta Synergy accelerator using 6 MV photon beams. The adaptive convolution algorithm provided by Pinnacle was chosen as the dose calculation engine and the calculation grid resolution was set as 2 × 2 × 2 mm3. The dose of treatment plans was calculated on free‐breathing CT. Two IMRT plans using a static step‐and‐shoot delivery approach were created for each patient. One was with standard homogeneous dose distribution (IMRThomo) for target, and the other was with an inhomogeneous dose distribution (IMRTInho) for target. The two IMRT plans used the same couch and collimator angles, and consisted of six beams with the gantry angles 185°, 215°, 245°, 345°, 15°, and 155° respectively.
For IMRThomo plans, PTV dose was restricted within 95%–107% of the prescribed dose (60 Gy) based on the recommendation of International Commission on Radiation Units. 26 The maximum dose of spinal Cord PRV (planning organ at risk volume) was 45 Gy. V20 Gy and mean dose of the lungs were set to be as low as possible. The homogeneity of the PTV was enforced by increasing the weight of the PTV uniform dose to 100. For IMRTinho plans, the dose constraints were: PTV dose ≥95% of the prescribed dose (60 Gy), maximum dose of 45 Gy to the spinal Cord PRV, V20 Gy and mean dose for the lungs were set to be as low as possible. There were no uniform dose constrains for PTV. For each patient, a “Boost” optimization region was constructed by expanding the GTV with the same expansion margin. There were no limitations on the maximum dose to this region. Details of objectives set for the initial optimization were illustrated in Table 2.
Table 2.
Objective settings for the initial optimization
| Item | ROI name | Group | Objective type | Target(Gy) | Weight |
|---|---|---|---|---|---|
| 1 | PTV | IMRThomo | Maximum dose | 63 | 50 |
| PTV‐Boost | IMRTinho | Maximum dose | 63 | 50 | |
| 2 | PTV | IMRThomo | Uniform dose | 60.5 | 100 |
| IMRTinho | / | / | / | ||
| 3 | PTV | Both | Minimum dose | 59.5 | 90 |
| 4 | PTV | Both | Minimum DVH | 60/95%coverage | 100 |
| 5 | PTV‐3 mm | Both | Minimum dose | 60 | 30 |
| 6 | Lung | Both | Maximum DVH | 5/44% coverage | 30 |
| 7 | Lung | Both | Maximum DVH | 20/18% coverage | 60 |
| 8 | Lung | Both | Maximum DVH | 30/14% coverage | 30 |
| 9 | Lung | Both | Maximum EUD | 11 | 1 |
| 10 | Cord | Both | Maximum dose | 35 | 40 |
| 11 | Cord PRV | Both | Maximum dose | 38 | 60 |
| 12 | Cord PRV | Both | Maximum EUD | 7 | 0.5 |
| 13 | Heart | Both | Maximum DVH | 30/18% coverage | 30 |
| 14 | Heart | Both | Maximum DVH | 40/13% coverage | 30 |
| 15 | Heart | Both | Maximum EUD | 15 | 0.3 |
| 16 | Ring1 | Both | Maximum dose | 59 | 20 |
| 17 | Ring2 | Both | Maximum dose | 56 | 20 |
| 18 | Normal Tissue | Both | Maximum dose | 50 | 20 |
| 19 | Normal Tissue | Both | Maximum EUD | 10 | 0.2 |
Minimum DVH 60/95% coverage: the minimum normalized volume that is radiated by a dose greater than 60 Gy is 95%; Maximum DVH 5/44% coverage: the maximum normalized volume that is radiated by a dose greater than 5 Gy is 44%.
The additional contours were as follows: (a) PTV‐3 mm, shrinkage from the PTV by 3 mm; (b) Ring1 and Ring2, the 5‐mm‐wide rings at 5 mm and 10 mm distance, respectively, from the PTV; (c) Normal Tissue, the whole CT volumes excluding the PTV expanded by 20 mm in all directions; (d) Boost, GTV expanded by 2 mm in all directions; (e) PTV‐Boost, the volume of PTV excluding Boost.
2.C. Plan evaluation
IMRThomo and IMRTInho plans were considered acceptable if at least 95% of PTV volume receiving 100% of the prescribed dose. All plans were reviewed and evaluated by one experienced radiation oncologist according to the standard clinical protocol. To quantify the target coverage and dose distribution, various dosimetric metrics were applied as follows: (a) D2% defined as the maximum dose for the PTV and indicated the maximum dose, and D98% indicated the minimum dose; (b) conformity index (CI) and homogeneity index (HI); (c) total number of monitor units; (d) mean dose. For a fair comparison, we normalized IMRTinho and IMRThomo plans to the same level of D98% of PTV. Organs at risk were evaluated in terms of the Dmean and percent of volumes receiving different doses. The percent of volumes V5, V10, V15, V20, V30, V40, V50, and Dmean for Lungs and V25, V30, V40, V50, and Dmean for heart were recorded. The maximum dose and Dmean for cord, cord PRV and normal tissue were also recorded. A margin of 5 mm was added to cord to form the cord PRV.
Normal tissue complication probabilities (NTCP) model was used to evaluate the treatment plans. The model was based on the Lyman–Kutcher–Burman(LKB) model in this study. LKB model is defined with the following equations: 27
| (1) |
| (2) |
| (3) |
where D eff is the dose that, if given uniformly to the entire volume, will lead to the same NTCP as the actual nonuniform dose distribution, TD 50 is the uniform dose given to the entire organ volume that results in 50% complication risk, m is the slope of the curve represented by the integral of the normal distribution, n is a parameter which describes the magnitude of the volume effect, v i is the relative volume related to dose voxel D i. In this calculation, TD 50 = 24.5 Gy, m = 0.18, and n = 0.87 for lung which is given by Pinnacle planning system. All the results were analyzed using the paired t‐test. A P‐value of less than 0.05 was considered statistically significant. SPSS software (IBM SPSS Statistics, version 22) was used for the analyses.
3. RESULTS
For all patients, both IMRThomo and IMRTinho plans were accepted for clinical treatment by the radiation oncologist. Figures 1 and 2 were the comparisons of dose distributions and dose–volume histograms between two plans for one patient. They showed that the uniformities of PTV dose of two plans were different. It was also clear that the mean dose of lung in IMRTinho plan was lower than that in IMRThomo plan, while the doses of the other OARs in IMRTinho plans were nearly the same as those in IMRThomo plans.
Figure 1.
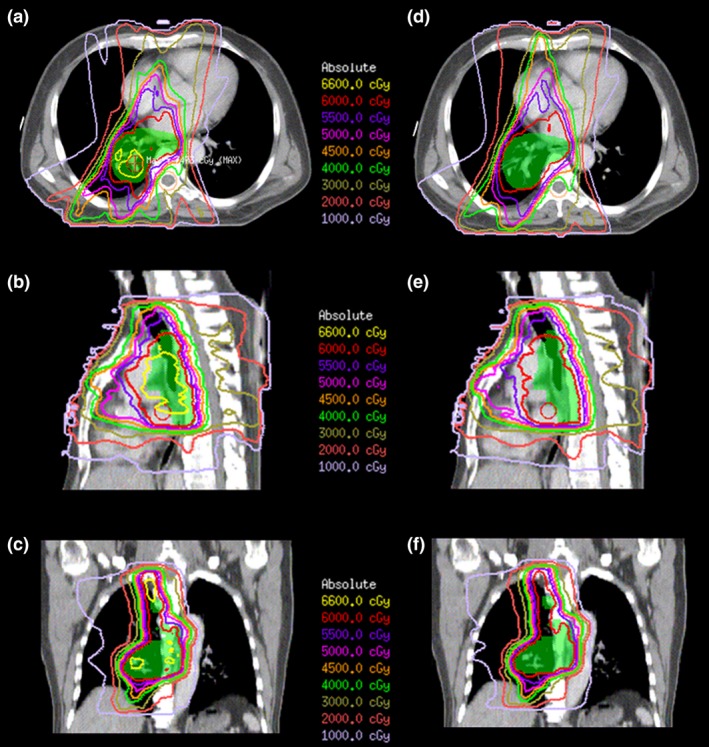
Isodose distribution of IMRT inho (a, b, c) and IMRT homo (d, e, f) plans for one patient with six coplanar beams.
Figure 2.
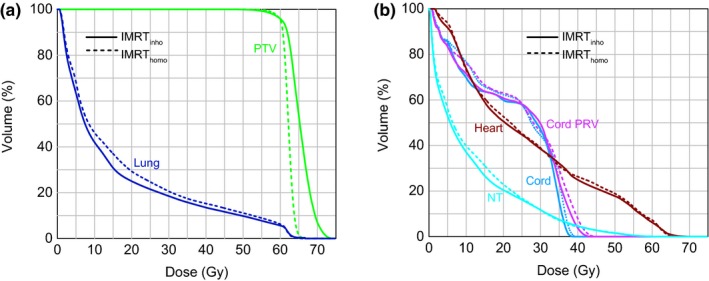
Dose–volume histograms (DVH) for (a) PTV and Lung (b) Heart, Cord, Cord PRV, and NT obtained with IMRT inho and IMRT homo plans.
The dosimetric statistics of PTV in the two IMRT plans were listed in Table 3. IMRThomo exhibited better HI than IMRTinho (P < 0.001). D2%, Mu and mean dose showed significant difference with P < 0.001, P = 0.001, and P < 0.001 respectively. There were no significant differences for D98%(P = 0.876), CI (P = 0.176), number of segments (P = 0.364), and delivery time (P = 0.094) when comparing IMRThomo plans with IMRTinho plans.
Table 3.
Dosimetric parameters comparison of homogeneous and inhomogeneous plans for PTV (mean ± standard deviation)
| PTV | IMRThomo | IMRTinho | P‐value |
|---|---|---|---|
| D2% (Gy) | 64.7 ± 1.6 | 70.1 ± 2.3 | <0.001 |
| D98% (Gy) | 58.3 ± 0.7 | 58.3 ± 0.7 | 0.876 |
| CI | 0.66 ± 0.04 | 0.70 ± 0.06 | 0.176 |
| HI | 0.11 ± 0.04 | 0.19 ± 0.04 | <0.001 |
| MU | 419.7 ± 76.1 | 498.0 ± 61.3 | 0.001 |
| Mean (Gy) | 62.3 ± 0.7 | 66.0 ± 1.9 | <0.001 |
| Segments | 37.2 ± 8.4 | 38.7 ± 6.9 | 0.364 |
| Delivery time(s) | 323 ± 26 | 352 ± 32 | 0.094 |
CI, conformity index; where TVRI is the target volume covered by the 95% prescription dose, TV is the target volume and VRI is the volume of the 95% prescription dose. HI, homogeneity index; HI = (D 2–D 98)/D prescription; MU, monitor units.
The dosimetric statistics of OARs in the two IMRT plans were listed in Table 4. The mean volume of lungs in this study was 3013.3 cm3 (ranged from 2571.4 to 4892.3 cm3). Lung dose in IMRTinho plans was significant reduced. V20 of lungs decreased from 27.9 ± 3.8% (IMRThomo) to 24.2 ± 3.1% (IMRTinho) with P < 0.001, and the MLD decreased from 15.7 ± 2.1 Gy (IMRThomo) to 14.3 ± 2.0 Gy (IMRTinho) (P < 0.001). On average, V5 of lungs decreased from 57.1 ± 8.3% (IMRThomo) to 52.3 ± 8.0% (IMRTinho). V10 of lungs decreased from 42.6 ± 7.1% (IMRThomo) to 38.2 ± 6.8% (IMRTinho), and V30 of lungs decreased from 19.6 ± 2.4% (IMRTinho) to 17.9 ± 2.4% (IMRTinho). Other dose–volume statistics such as V15, V40, and V50 in IMRTinho plans also showed significant decreases while comparing with those in IMRThomo plans. Figure 3 showed averaged difference of dose–volume histograms (IMRThomo – IMRTinho) for lungs with standard deviation. The peak of averaged difference was 5.5% corresponding to the dose of 4 Gy in the low dose area.
Table 4.
Dosimetric parameters comparison between homogeneous and inhomogeneous plans for organs at risk (mean ± standard deviation)
| Organ at risk | IMRThomo | IMRTinho | P‐value |
|---|---|---|---|
| Lung | |||
| V5 (%) | 57.1 ± 8.3 | 52.3 ± 8.0 | 0.005 |
| V10 (%) | 42.6 ± 7.1 | 38.2 ± 6.8 | 0.002 |
| V15 (%) | 34.4 ± 5.7 | 29.9 ± 4.5 | <0.001 |
| V20 (%) | 27.9 ± 3.8 | 24.2 ± 3.1 | <0.001 |
| V30(%) | 19.6 ± 2.4 | 17.9 ± 2.4 | <0.001 |
| V40 (%) | 14.2 ± 2.4 | 13.1 ± 2.7 | 0.001 |
| V50 (%) | 9.5 ± 2.7 | 8.7 ± 2.9 | 0.001 |
| Mean (Gy) | 15.7 ± 2.1 | 14.3 ± 2.0 | <0.001 |
| NTCP | 4.2 ± 1.5% | 2.5 ± 1.2% | <0.001 |
| Heart | |||
| V25(%) | 25.1 ± 10.6 | 24.5 ± 12.3 | 0.543 |
| V30(%) | 21.6 ± 9.3 | 21.3 ± 10.8 | 0.687 |
| V40(%) | 14.4 ± 6.4 | 14.9 ± 8.4 | 0.662 |
| V50(%) | 8.7 ± 4.2 | 9.1 ± 5.7 | 0.733 |
| Mean (Gy) | 16.1 ± 5.8 | 16.0 ± 7.0 | 0.869 |
| Cord | |||
| Max (Gy) | 37.7 ± 5.0 | 37.6 ± 5.8 | 0.860 |
| Cord PRV | |||
| Max (Gy) | 42.1 ± 4.1 | 42.9 ± 4.7 | 0.273 |
| Normal tissue | |||
| Mean (Gy) | 12.0 ± 1.2 | 11.2 ± 1.3 | 0.002 |
Figure 3.
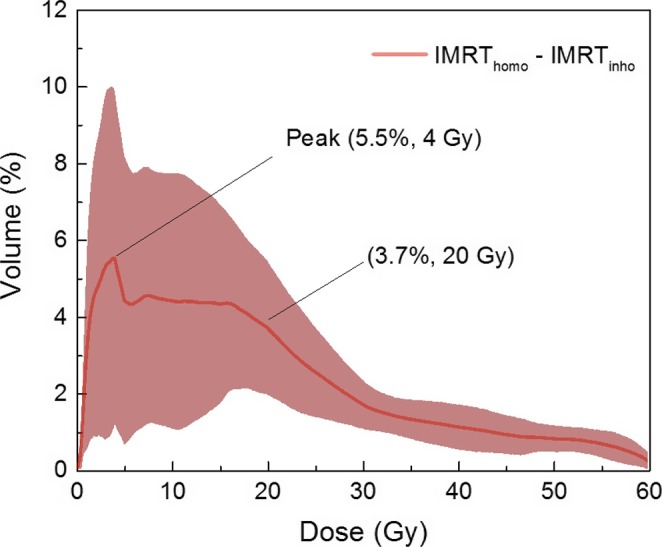
Averaged difference of dose–volume histograms (IMRT homo–IMRT inho) for lung with standard deviation. The standard deviation has been drawn as lines with fill area.
For heart, the differences of V25, V30, V40, and V50 values between IMRThomo and IMRTinho plans were within 0.6%, and all the P values of statistical parameters were more than 0.05. Therefore, there were no significant differences between the IMRThomo and IMRTinho plans on V25, V30, V40, V50 and mean dose. As to cord and cord PRV, the maximum dose were 37.7 ± 5.0 Gy and 42.1 ± 4.1 Gy in IMRThomo plans, while those were 37.6 ± 5.8 Gy and 42.9 ± 4.7 Gy for IMRTinho plans. And the P values were 0.860 and 0.273, indicating the maximum dose of cord and cord PRV in the both plans had no significant difference. For normal tissue, IMRTinho plans showed lower doses than IMRThomo plans. The mean doses in the two plans were 12.0 ± 1.2 Gy and 11.2 ± 1.3 Gy with P = 0.002.
4. DISCUSSION
Nielsen et al have used inhomogeneous dose distributions as a way to increase tumor control probability.28 The goal of this study was to investigate if increasing target dose inhomogeneity could be beneficial to decrease the lung toxicity for NSCLC using IMRT plans. For this purpose, IMRTinho plans were developed with no uniform dose constraints for PTV and no maximum dose constrains for GTV. As shown in Table 3, CIs of the two IMRT plans were nearly the same and HIs were significantly different. For IMRThomo plans mean dose to the PTV was 62.3 ± 0.7 Gy, whereas for IMRTinho plans that was 66.0 ± 1.9 Gy. In certain cases, IMRTinho plans could increase the dose to tumor. Although the increased dose was only about 3.7 Gy, it might be beneficial to improve local control of tumor.
The correlation between severe RP and MLD was reported by several authors.29, 30, 31 In a planning study by Murshed, it was suggested that if MLD was decreased by 2 Gy the risk of RP could be reduced by 10%.32 It was reported that introducing V5 constraint could significantly decreased the lethal pneumonitis. Claude et al showed that only MLD, V20 and V30 were predictive of the severe RP.33 They suggested that a large panel of thresholds from low to high dose could provide advantages.
The result shown in Fig. 3 and Table 4 suggested that lower lung dose values and NTCP could be obtained from low to high dose region in the IMRTinho plans. Relaxing target dose homogeneity could lead to a significant reduction (3.7% absolute difference) in the lung volume receiving doses <20 Gy which was in the low dose region. IMRTinho plans showed an average decrease of V5 with 4.8% absolute difference compared with IMRThomo plans. Therefore, decreased volume of lung was irradiated at low dose area. In the high dose region, IMRTinho plans could give a dose reduction to lung by about 10%. So the risk of radiation pneumonitis could potentially be decreased. The mean dose, V30 and V40 were often used as indicators for heart toxicity. The equal heart toxicity was expected for both homogeneous and inhomogeneous plans in this study because their mean dose, V30 and V40 were similar. The maximum dose of cord and cord PRV in the two plans were kept similar. While lung dose reduced, normal tissues dose was also decreased in IMRTinho plans. This study demonstrated that reducing dose to lungs was possible without increasing the dose to the other OARs.
The esophagus was often located close to or may be part of the target volume. In this case, plan optimization might lead to high dose at the esophagus which must be avoided by adding more constraints to restrict it. The hot spots which were close to cord should be paid special attention and these hot spots should be avoided as possible in the process of plan optimization. The impact of daily setup errors on dose distribution was estimated by modifying the position of the isocenter point on planning CT slices of one patient. Six plans with same beamlets and MUs of IMRTinho were created, and the prescribed dose was 10 Gy in 2.0 Gy daily fractions for each plan. Isocenter was shifted toward the head, foot, left, right, anterior, and posterior, respectively, by 5 mm in each plan. The total “estimated” plan (IMRTinho+5 mm) was composed of the six plans. Dose distribution between IMRTinho+5 mm and IMRTinho plans was compared as shown in Fig. 4. We can see that the position of hot spots remains unchanged and big hot spots become smaller due to edge blurring effect from the Fig. 4. Some small hot spots that appear in IMRTinho plan don't manifest in the dose distribution received by the patient due to day to day setup variations. There is little change in lung and GTV doses between IMRTinho plans and IMRTinho+5 mm plans as shown in Fig. 5.
Figure 4.
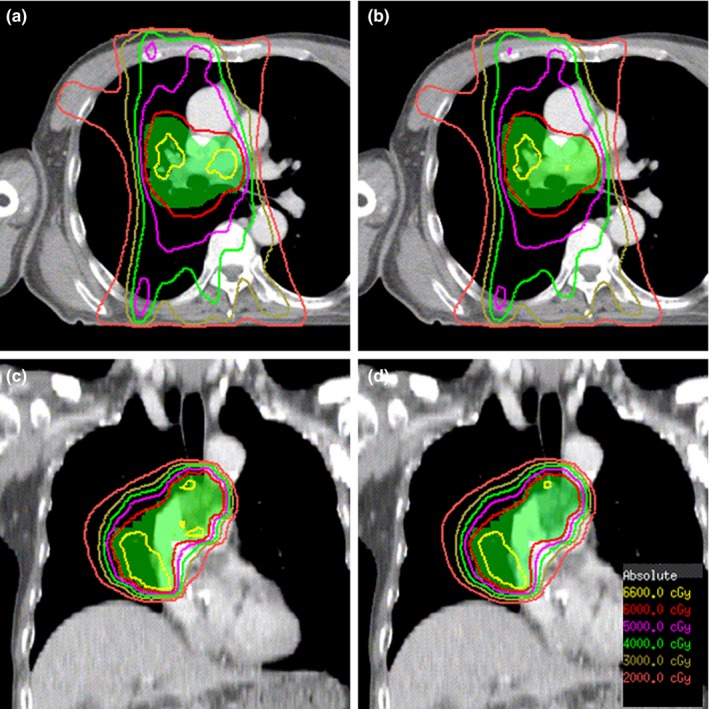
Dose distribution of IMRT inho and IMRT inho+5 mm plans.
Figure 5.
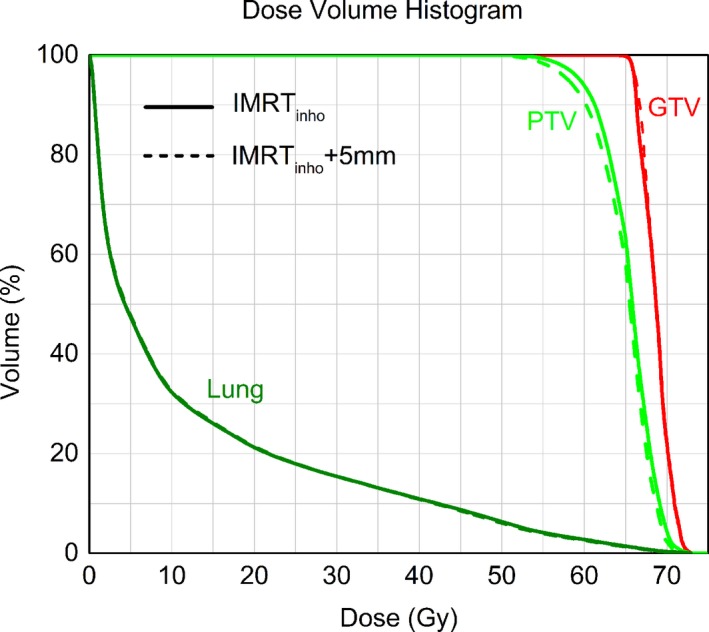
Dose–volume histograms for PTV, GTV, and Lung obtained with IMRT inho and IMRT inho+5 mm plans.
Respiratory motion and tumor shrinkage throughout treatment can further affect the dose to the tumor and the hot spots may move toward nearby critical structures. The use of daily localization with CBCT could detect changes in the target and ensure the safety of OARs.34 As the spatial distribution of biological properties in tumors such as proliferation and hypoxia are known to be nonuniform, the dose distribution that maximizes tumor control probability for a given tumor is also nonuniform. Regions of high radiosensitivity may correspond to regions of high tumor proliferation, whereas regions of high radioresistance may correspond to regions of tumor hypoxia. Based on positron emission tomography images and IMRTinho plans, these regions might be controlled to get inhomogeneous dose distribution to increase tumor control probability. Besides NSCLC, this method should be valid in other cancers such as esophageal cancer, liver cancer, etc., which will be investigated in the future studies.
5. CONCLUSION
It was demonstrated that relaxing the constraints on maximum and uniform dose in the target volume could significantly reduce the dose to lungs from low to high dose region. With this approach, the risk of radiation pneumonitis could potentially be decreased. In addition, it could increase the mean dose to tumor, which might be beneficial to improve local control of tumor by radiotherapy. IMRT plans with inhomogeneous target dose could protect lungs better and may be considered as a choice for NSCLC treatment.
CONFLICT OF INTEREST
All authors approved the final manuscript, and declared that they have no potential conflicts of interest to this work.
ACKNOWLEDGMENTS
This work was supported by Beijing Hope Run Special Fund of Cancer Foundation (NO. LC2014B09). We also thank the funding supports by National Natural Science Foundation of China (NO. 81502649, 11275270) and China National Key Projects of Research and Development (2016YFC0904600).
REFERENCES
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. [DOI] [PubMed] [Google Scholar]
- 2. Yaqub F. ASTRO issues new guidance for radiotherapy in NSCLC. The Lancet Oncol. 2015;16:e268. [DOI] [PubMed] [Google Scholar]
- 3. De Angelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE–5‐a population‐based study. The Lancet Oncol. 2014;15:23–34. [DOI] [PubMed] [Google Scholar]
- 4. Belderbos J, Walraven I, Van D, Verheij M, Ruysscher D. Radiotherapy dose and fractionation for stage III NSCLC. The Lancet Oncol. 2015;16:e156–e157. [DOI] [PubMed] [Google Scholar]
- 5. Hallqvist A, Bergman B, Nyman J. Health related quality of life in locally advanced NSCLC treated with high dose radiotherapy and concurrent chemotherapy or cetuximab–pooled results from two prospective clinical trials. Radiother Oncol. 2012;104:39–44. [DOI] [PubMed] [Google Scholar]
- 6. Barriger R, Forquer J, Henderson M, Johnstone P, Fakiris A. A dosimetric analysis of radiation pneumonitis in non‐small‐cell lung cancer patients treated with stereotactic body radiotherapy. Int J Radiat Oncol. 2009;75:S107–S108. [DOI] [PubMed] [Google Scholar]
- 7. Luhua W, Wei J, Jianrong D, et al. Relationship between dosimetric parameters and radiation pneumonitis in patients with non‐small cell lung cancer who received postoperative radiotherapy. Int J Radiat Oncol. 2008;72:S428. [Google Scholar]
- 8. Farr K, Khalil A, Knap M. Radiation pneumonitis and treatment outcome in radical radiotherapy of stage iii non small cell lung cancer. Eur J Cancer. 2011;47:S605–S606. [Google Scholar]
- 9. Marks LB, Bentzen SM, Deasy JO, et al. Radiation dose‐volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S70–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tucker SL, Jin HK, Wei XO, et al. Impact of toxicity grade and scoring system on the relationship between mean lung dose and risk of radiation pneumonitis in a large cohort of patients with non‐small cell lung cancer. Int J Radiat Oncol. 2010;77:691–698. [DOI] [PubMed] [Google Scholar]
- 11. Scarbrough TJ, Ting JY, McClure JA, et al. Intensity‐modulated radiation therapy (IMRT) for non‐small cell lung cancer (NSCLC): treatment outcomes from a small, elderly cohort. Int J Radiat Oncol. 2006;66:S492–S493. [Google Scholar]
- 12. Fleckenstein J, Eschler A, Kremp K, Kremp S, Rube C. Dose distribution and tumor control probability in out‐of‐field lymph node stations in intensity modulated radiotherapy (IMRT) vs 3D‐conformal radiotherapy (3D‐CRT) of non‐small‐cell lung cancer: an in silico analysis. Radiat Oncol. 2015;10:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schwarz M, Van der Geer J, Van Herk M, Lebesque JV, Mijnheer BJ, Damen EM. Impact of geometrical uncertainties on 3D CRT and IMRT dose distributions for lung cancer treatment. Int J Radiat Oncol Biol Phys. 2006;65:1260–1269. [DOI] [PubMed] [Google Scholar]
- 14. Shirvani SM, Jiang J, Gomez DR, Chang JY, Buchholz TA, Smith BD. Intensity modulated radiotherapy for stage III non‐small cell lung cancer in the United States: predictors of use and association with toxicities. Lung Cancer. 2013;82:252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kay C, Kim JY, Jung JY, Kim KJ. Significance of low dose distribution in developing radiation pneumonitis after hypofractionated radiotherapy using helical tomotherapy for pulmonary metastases. J Thorac Oncol. 2012;7:S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosca F, Kirk M, Soto D, Sall W, McIntyre J. Reducing the low‐dose lung radiation for central lung tumors by restricting the IMRT beams and arc arrangement. Med Dosim. 2012;37:280–286. [DOI] [PubMed] [Google Scholar]
- 17. Siva S, Devereux T, Hardcastle N, et al. Feasibility of IMRT planning to reduce dose to functional lung using respiratory gated (4D) Gallium‐68 Perfusion PET/CT. Int J Radiat Oncol Biol Phys 2014;90Supplement:S618–S619. [Google Scholar]
- 18. Ding CX, Chang CH, Haslam J, Timmerman R, Solberg T. A dosimetric comparison of stereotactic body radiation therapy techniques for lung cancer: robotic versus conventional linac‐based systems. J Appl Clin Med Phys. 2010;11:212–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chan MKH, Kwong DLW, Law GML, et al. Dosimetric evaluation of four‐dimensional dose distributions of CyberKnife and volumetric‐modulated arc radiotherapy in stereotactic body lung radiotherapy. J Appl Clin Med Phys. 2013;14:136–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Webb S, Evans PM, Swindell W, et al. A proof that uniform dose gives the greatest TCP for fixed integral dose in the planning target volume. Phys Med Biol. 1994;39:2091–2098. [DOI] [PubMed] [Google Scholar]
- 21. Eschmann SM, Paulsen F, Reimold M, et al. Prognostic impact of hypoxia imaging with 18F‐misonidazole PET in non‐small cell lung cancer and head and neck cancer before radiotherapy. J Nucl Med. 2005;46:253–260. [PubMed] [Google Scholar]
- 22. Søvik A, Malinen E, Bruland ØS, et al. Optimization of tumour control probability in hypoxic tumours by radiation dose redistribution: a modelling study. Phys Med Biol. 2007;52:499–513. [DOI] [PubMed] [Google Scholar]
- 23. Chen GP, Ahunbay E, Schultz C, et al. Development of an inverse optimization package to plan nonuniform dose distributions based on spatially inhomogeneous radiosensitivity extracted from biological images. Med Phys. 2007;34:1198–1205. [DOI] [PubMed] [Google Scholar]
- 24. Knap MM, Hoffmann L, Nordsmark M, Vestergaard A. Daily cone‐beam computed tomography used to determine tumour shrinkage and localisation in lung cancer patients. Acta Oncol. 2010;49:1077–1084. [DOI] [PubMed] [Google Scholar]
- 25. Bertelsen A, Hansen O, Brink C. Does VMAT for treatment of NSCLC patients increase the risk of pneumonitis compared to IMRT ? – A planning study. Acta Oncol. 2012;51:752–758. [DOI] [PubMed] [Google Scholar]
- 26. Gregoire V, Mackie TR. ICRU committee on volume and dose specification for prescribing, recording and reporting special techniques in external photon beam therapy: conformal and IMRT. Radiother Oncol. 2005;76:S71. [Google Scholar]
- 27. Semenenko V, Li X. Lyman‐Kutcher‐Burman NTCP model parameters for radiation pneumonitis and xerostomia based on combined analysis of published clinical data. Phys Med Biol. 2008;53:737–755. [DOI] [PubMed] [Google Scholar]
- 28. Nielsen TB, Hansen O, Schytte T, Brink C. Inhomogeneous dose escalation increases expected local control for NSCLC patients with lymph node involvement without increased mean lung dose. Acta Oncol. 2014;53:119–125. [DOI] [PubMed] [Google Scholar]
- 29. Palma DA, Senan S, Tsujino K, et al. Predicting symptomatic radiation pneumonitis after concurrent chemoradiation therapy for non‐small cell lung cancer: results of an international individual patient data meta‐analysis. Int J Radiat Oncol. 2012;84:S549–S549. [Google Scholar]
- 30. Rodriques G, Lock M, D'Souza D, Yu E, Van D. Prediction of radiation pneumonitis by dose‐volume histogram parameters in lung cancer – a systematic review. Radiother Oncol. 2004;73:S26–S26. [DOI] [PubMed] [Google Scholar]
- 31. Piotrowski T, MateckaNowak M, Milecki P. Prediction of radiation pneumonitis: dose‐volume histogram analysis in 62 patients with non‐small cell lung cancer after three‐dimensional conformal radiotherapy. Neoplasma. 2005;52:56–62. [PubMed] [Google Scholar]
- 32. Murshed H, Liu HH, Liao Z, et al. Dose and volume reduction for normal lung using intensity‐modulated radiotherapy for advanced‐stage non‐small‐cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;58:1258–1267. [DOI] [PubMed] [Google Scholar]
- 33. Khalil AA, Hoffmann L, Moeller DS, Farr KP, Knap MM. New dose constraint reduces radiation‐induced fatal pneumonitis in locally advanced non‐small cell lung cancer patients treated with intensity‐modulated radiotherapy. Acta Oncol. 2015;54:1343–1349. [DOI] [PubMed] [Google Scholar]
- 34. Rao M, Wu J, Cao D, et al. Dosimetric impact of breathing motion in lung stereotactic body radiotherapy treatment using image‐modulated radiotherapy and volumetric modulated arc therapy. Int J Radiat Oncol Biol Phys. 2012;83:e251–e256. [DOI] [PubMed] [Google Scholar]


