Abstract
The enhanced dosimetric performance of knowledge‐based volumetric modulated arc therapy (VMAT) planning might be jointly contributed by the patient‐specific optimization objectives, as estimated by the RapidPlan model, and by the potentially improved Photon Optimizer (PO) algorithm than the previous Progressive Resolution Optimizer (PRO) engine. As PO is mandatory for RapidPlan estimation but optional for conventional manual planning, appreciating the two optimizers may provide practical guidelines for the algorithm selection because knowledge‐based planning may not replace the current method completely in a short run. Using a previously validated dose–volume histogram (DVH) estimation model which can produce clinically acceptable plans automatically for rectal cancer patients without interactive manual adjustment, this study reoptimized 30 historically approved plans (referred as clinical plans that were created manually with PRO) with RapidPlan solution (PO plans). Then the PRO algorithm was utilized to optimize the plans again using the same dose–volume constraints as PO plans, where the line objectives were converted as a series of point objectives automatically (PRO plans). On the basis of comparable target dose coverage, the combined applications of new objectives and PO algorithm have significantly reduced the organs‐at‐risk (OAR) exposure by 23.49–32.72% than the clinical plans. These discrepancies have been largely preserved after substituting PRO for PO, indicating the dosimetric improvements were mostly attributable to the refined objectives. Therefore, Eclipse users of earlier versions may instantly benefit from adopting the model‐generated objectives from other RapidPlan‐equipped centers, even with PRO algorithm. However, the additional contribution made by the PO relative to PRO accounted for 1.54–3.74%, suggesting PO should be selected with priority whenever available, with or without RapidPlan solution as a purchasable package. Significantly increased monitor units were associated with the model‐generated objectives but independent from the optimizers, indicating higher modulation in these plans. As a summary, PO prevails over PRO algorithm for VMAT planning with or without knowledge‐based technique.
Keywords: knowledge‐based planning, Photon Optimizer, Progressive Resolution Optimizer, RapidPlan, VMAT
1. Introduction
Using the anatomical structures, field geometry, dose metrics, and dose prescription of previous plans as historical experiences to predict the dose–volume objectives for the upcoming patients,1, 2 knowledge‐based treatment planning has been deemed as a promising solution to reduce the subjective inter‐planner varieties,3, 4, 5, 6, 7, 8, 9, 10 enhance the clinical efficiency and quality of prospective plans.2, 11, 12, 13, 14, 15 As a commercial knowledge‐based optimizer, RapidPlan in Eclipse treatment planning system (V13.5 or later, Varian Medical Systems, Palo Alto, CA, USA) has been validated by many publications suggesting superior dosimetric outcomes than the conventional methods.16, 17, 18, 19, 20, 21, 22, 23 However, in addition to the usage of possibly advanced personalized optimization objectives as generated by the dose–volume histogram (DVH) estimation models, the new Photon Optimizer (PO) was introduced to combine and substitute for the two old algorithms, that is, Progressive Resolution Optimizer (PRO) for volumetric modulated arc therapy (VMAT) and Dose Volume Optimizer (DVO) for static field intensity‐modulated radiotherapy (IMRT). By far, no study has been conducted to confirm if the observed dosimetric progress was also partially (if any) attributable to the potentially better PO algorithm.
According to the manufacturer, DVO optimizes the field shape and intensity using a simple gradient optimization to approach the desired dose–volume objectives. The fluences are back‐projected from the derivates of the costs at each cloud point representing the patient volume. PRO optimizes MLC leaf positions and MU/deg based on control points segmentation of the gantry angle. As the optimization progresses, the accuracy of the angle resolution and dose calculation segments increase. Relative to prior optimizers, PO has involved critical changes: the old point cloud model for PRO and DVO has been replaced by a new volume representation, where “structures, DVH calculation and dose sampling are defined spatially by using one single matrix over the image”. However, DVO and PRO have a more powerful dose–volume objective form than the PO in terms of limiting the local minima in the optimization space. More technical details were described by Vanetti et al.24 and Cozzi et al.25 It is noticed that PO is mandatory for RapidPlan to accommodate its geometry‐based expected dose (GED) algorithm, yet is optional for the conventional manual planning where all the optimization features can be used in the same way without a model.
Providing knowledge‐based planning is not likely to replace the conventional methods completely in a short run, appreciating the behavior of the new PO against the old optimizers may provide useful guidelines for algorithm selection when RapidPlan is not invoked as a separate executable option. After all, as a purchasable engine, RapidPlan is not available to every Eclipse user, and the model configuration can be a sorely time‐consuming process. In order to assess the different algorithms based on the same (but patient specific) optimization objectives that can produce clinically acceptable plans without interactive manual adjustment, all DVH constraints were derived from our RapidPlan model estimation that has been validated for rectal cancer patients.16, 17 However, this model was configured with features that are not supported by DVO, such as mean dose objective and automatic normal tissue objective; hence, this study only focuses on VMAT planning using PO and PRO algorithms, respectively.
2. Methods
This study was conducted based on Varian Eclipse Treatment Planning System V. 13.5.
2.A. DVH estimation model
As a brief summary of the previous work,16, 17 a DVH estimation model was configured using 81 historical VMAT plans for preoperative rectal cancer patients with simultaneous‐integrated‐boosting (SIB). The library size was determined based on Boutilier's study and the rule‐of‐thumb approach.26 The gross target volume (GTV) was delineated to cover the primary tumor, mesorectal space, and the involved lymph nodes.27 The clinical target volume (CTV) included GTV, presacral region, mesorectal/lateral lymph nodes, internal iliac lymph node chain, and pelvic wall area. Should the anterior organ involvement was suspected, CTV also includes the external iliac lymph nodes, and includes the inguinal lymph nodes when the lower third of the vagina was invaded or major tumor extension into the internal and external anal sphincter was observed.28 Isotropic margins of 5 mm were applied to create planning target volumes (PTVboost from GTV, and PTV from CTV, respectively). Target dose of 50.6 Gy and 41.8 Gy were prescribed to 95% of PTVboost and PTV in 22 fractions, respectively. All plans were created with 1–2 full arc, 5° collimator rotation, and 10 MV photon beams modulated by Varian Millennium 120 multi‐leaf collimator mounted on a Varian Trilogy accelerator. As reported before, the model validation on over 100 historical plans displayed significantly improved dosimetric results than the average clinical level, after applying the RapidPlan‐generated objectives and the PO algorithm.
2.B. VMAT planning using PO and PRO
Thirty clinical VMAT rectal plans that have been manually optimized with PRO were retrospectively selected and reoptimized using the RapidPlan engine (referred as PO plans). Representing the average planning quality, these 30 consecutively treated plans were not used for model development but followed consistent contouring28 and planning protocols as for the training set. Although model estimations are only available with PO, the objectives and priorities calculated from the estimation are applicable to PRO, where the line objectives are represented as a series of points (referred as PRO plans). This approach ensured the clinically acceptable PO, and PRO plans were derived from identical constraints and objectives without interactive human adjustment during the optimization process. All other parameters were maintained as earlier during the optimization as well; hence, the observable discrepancies were mostly ascribed to the disparities of PO and PRO algorithms. Renormalization was performed to assess the organs‐at‐risk (OAR) sparing based on comparable target dose coverage (i.e., 95% PTVboost and PTV were covered by corresponding prescribed dose, respectively).
2.C. Dosimetric evaluation and statistical method
The clinical, PO and PRO plans were evaluated mutually by means of the target homogeneity index (HIPTVboost and HIPTV); the target conformity index (CIPTVboost and CIPTV); dose to 50% of the volume for the femoral head and urinary bladder (D50%_FH and D50_UB), the mean dose (Dmean_FH and Dmean_UB); the hot spot volume receiving over 107% of the prescribed dose to PTVboost (V107%, i.e., V54.14Gy), and the total monitor units (MU). Based on SPSS 21 software (IBM Analytics, Armonk, NY, USA), paired samples t‐test was used to analyze the normally distributed data (Shapiro–Wilk method); otherwise Wilcoxon signed‐rank test was conducted. Significant level was put at P < 0.05 (2‐tailed).
3. Results
Without interactive human interference, the patient‐specific optimization objectives generated from the model estimation functioned well with both PO and PRO algorithms, which produced clinically acceptable plans automatically as visually inspected on three‐dimensional dose color wash and DVH distribution.
Numerical dosimetric comparison of plans using various techniques are listed in Tables 1 (target coverage) and 2 (OAR sparing). Relative to the median values, the mean dose deviated by 0.12 Gy and 0.17 Gy to PTVboost, and deviated by 0.34 Gy and 0.09 Gy to PTV, respectively, among different planning techniques. The inter‐group disparities of HIPTVboost, HIPTV, and CIPTV were all within 0.01–0.02. Clinical plans achieved significantly lower CIPTVboost (differed by 0.05–0.06), yet the values for PO and PRO groups were largely comparable (varied by 0.01 only, P > 0.05). Relative to the clinical plans, the usage of RapidPlan‐generated optimization objectives (PO and PRO cases) has also induced: (a) Higher MUs (by 7.92% and 8.17% for PO and PRO); (b) Significant reduction of D50%_FH, Dmean_FH, D50%_UB, and Dmean_UB; (c) Significant reduction or elimination of V107%.
Table 1.
Dosimetric comparison (target coverage) of 30 patients that were planned by: PRO using the manual objectives as in the clinical plans (clinical); PO using the RapidPlan‐generated objectives (PO); and PRO using the RapidPlan‐generated objectives (PRO). Dose unit (Gy)
| Mean | SD | 95% Confidence interval | P | |||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| DPTVboost | Clinical | 52.22 | 0.19 | 52.15 | 52.29 | <0.01a |
| PO | 51.93 | 0.23 | 51.85 | 52.01 | 0.03a | |
| PRO | 52.05 | 0.30 | 51.94 | 52.16 | ||
| DPTV | Clinical | 45.51 | 0.79 | 45.22 | 45.81 | <0.01 |
| PO | 45.85 | 0.75 | 45.57 | 46.13 | 0.19 | |
| PRO | 45.94 | 0.72 | 45.67 | 46.21 | ||
| HIPTVboost | Clinical | 0.06 | 0.01 | 0.06 | 0.06 | <0.01 |
| PO | 0.05 | 0.01 | 0.05 | 0.05 | 0.53 | |
| PRO | 0.05 | 0.01 | 0.04 | 0.05 | ||
| HIPTV | Clinical | 0.26 | 0.01 | 0.26 | 0.26 | <0.01 |
| PO | 0.25 | 0.01 | 0.25 | 0.26 | <0.01 | |
| PRO | 0.26 | 0.01 | 0.26 | 0.26 | ||
| CIPTVboost | Clinical | 1.01 | 0.03 | 1.00 | 1.02 | <0.01a |
| PO | 1.06 | 0.05 | 1.05 | 1.08 | 0.60a | |
| PRO | 1.07 | 0.05 | 1.05 | 1.09 | ||
| CIPTV | Clinical | 1.04 | 0.03 | 1.03 | 1.05 | 0.34 |
| PO | 1.05 | 0.03 | 1.04 | 1.06 | <0.01a | |
| PRO | 1.03 | 0.03 | 1.02 | 1.04 | ||
| MUs | Clinical | 404 | 30 | 392 | 415 | <0.01 |
| PO | 436 | 33 | 423 | 448 | 0.81 | |
| PRO | 437 | 29 | 426 | 448 | ||
Wilcoxon signed‐rank test (abnormal distribution); otherwise paired samples t‐test (normal distribution).
DPTVboost, mean dose to PTVboost; DPTV, mean dose to PTV; HI, homogeneity index; CI, conformity index; SD, standard deviation; and MUs, monitor units.
Table 2.
Dosimetric comparison (OAR sparing) of 30 patients that were planned by: PRO using the manual objectives as in the clinical plans (clinical); PO using the RapidPlan‐generated objectives (PO); and PRO using the RapidPlan‐generated objectives (PRO). Dose unit (Gy)
| Mean | SD | 95% Confidence interval | P | ||||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| D50%_FH | Clinical | 15.10 | 2.28 | 14.25 | 15.95 | <0.01 | |
| PO | 10.16 | 2.65 | 9.18 | 11.15 | 0.06 | ||
| PRO | 10.43 | 2.95 | 9.33 | 11.53 | |||
| Dmean_FH | Clinical | 16.27 | 2.10 | 15.49 | 17.05 | <0.01 | |
| PO | 12.31 | 1.71 | 11.67 | 12.95 | <0.01 | ||
| PRO | 12.56 | 1.87 | 11.86 | 13.25 | |||
| D50%_UB | Clinical | 28.05 | 2.60 | 27.08 | 29.02 | <0.01a | |
| PO | 19.18 | 1.96 | 18.45 | 19.91 | <0.01 | ||
| PRO | 20.23 | 2.05 | 19.47 | 21.00 | |||
| Dmean_UB | Clinical | 29.21 | 2.10 | 28.42 | 29.99 | <0.01 | |
| PO | 22.35 | 2.02 | 21.59 | 23.10 | <0.01 | ||
| PRO | 23.12 | 2.09 | 22.34 | 23.90 | |||
| V107% | Clinical | 0.04 | 0.11 | 0.00 | 0.08 | 0.01a | |
| PO | Not observed | 0.32a | |||||
| PRO | 0.01 | 0.05 | ‐0.01 | 0.03 | |||
Wilcoxon signed‐rank test (abnormal distribution); otherwise paired samples t‐test (normal distribution).
SD, standard deviation; D50%, dose to the 50% volume of the structure; Dmean, mean dose; FH, femoral head, UB, urinary bladder, and V107%, volume receiving over 107% of the prescribed dose.
Figure 1 displays the mean DVH plots of the 30 patients as grouped by the plan types. The clinical, PI and PRO plans are plotted as solid, dotted, and dashed lines, respectively. Adequate target dose coverage (prescription: 50.6 Gy and 41.8 Gy to 95% of PTVboost and PTV, respectively) was achieved by all three techniques, yet the dose to the OARs was significantly reduced using RapidPlan‐generated objectives (PO and PRO plans) than the manual settings (clinical plans).
Figure 1.
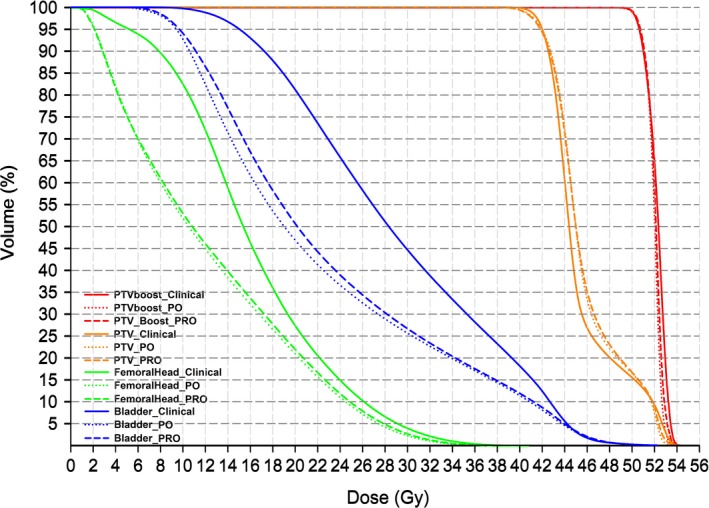
The mean DVHs of 30 patients: the solid, dotted, and dashed lines indicate the clinical, PO and PRO plans, respectively.
Taking the clinical plans as baseline, Table 3 breaks down the relative improvement of OAR sparing as contributed by the new objectives and the optimizers, which differs by an order of magnitude.
Table 3.
A breakdown of relative contributions to the improved OAR sparing by the new objectives and optimizers, where total improvement = (Dclinical−DPO)/Dclinical; objective contribution = (Dclinical−DPRO)/Dclinical; and optimizer contribution = (DPRO−DPO)/Dclinical
| D50%_FH | Dmean_FH | D50%_UB | Dmean_UB | |
|---|---|---|---|---|
| Total improvement (%) | 32.72 | 24.34 | 31.62 | 23.49 |
| Objective contribution (%) | 30.93 | 22.80 | 27.88 | 20.85 |
| Optimizer contribution (%) | 1.79 | 1.54 | 3.74 | 2.64 |
D50%, dose to the 50% volume of the structure; Dmean, mean dose; FH, femoral head, UB, urinary bladder; Dclinical, DPO and DPRO, dose parameters corresponding to the clinical, PO and PRO plans.
4. Discussion
With adequate dose coverage to 95% PTVboost and PTV by renormalization, PO and PRO methods have significantly lowered the target mean dose than clinical plans, indicating reduced hot spots in the targets and improved dose homogeneity (Table 1). Although statistically significant, the magnitudes of the numerical difference were relatively small; hence, may be clinically negligible, as echoed by Fig. 1, demonstrating that prescription was selected, such that no difference between the clinical plans and reoptimized plans occurred to bias any results with the OAR dose comparison. The similar CIPTVboost between the PO and PRO plans suggested that the smaller value of the clinical plans should be mostly attributable to different objective settings rather than the optimization algorithms.
Consistent with earlier publications,10, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 macroscopic and significant (P < 0.05) improvements of OAR sparing and hot spot control were observed after substituting the objectives from patient‐specific estimations for the trial‐and‐error manual adjustment. The objective‐induced advantages were largely transferable from PO engine to the conventional PRO algorithm, yet the performance of PRO is slightly inferior than the PO. The more adjacent DVH lines between the PO (dotted) and PRO (dashed) than the clinical (solid) plans as shown in Fig. 1 echoed the aforementioned numerical observations.
Although RapidPlan is a purchasable package, PO algorithm should be available for all Eclipse users of version 13.5 or later. Therefore, it is of patients’ maximal benefit to select PO as the preferential optimizer for VMAT planning, even if knowledge‐based solution is not purchased or the models are not yet ready (custom model configuration is treatment site‐specific and sorely time‐consuming). The slight improvement of OAR sparing by a few percent may not be clinically significant, but it complies with “as low as reasonably achievable” (ALARA) principle at little or no extra cost of effort.
The lesser impact of the optimizer suggested that for the Eclipse users of older versions (before 13.5), transplanting the model‐estimated and patient‐specific objectives from other RapidPlan‐equipped centers can be a transitional option to improve the VMAT plan quality immediately even with PRO algorithm. Surely this should be performed with caution on the basis of consistent contouring/planning protocols, and thorough clinical validations.
The optimizers had little if any impact on the consumed MUs (ΔPRO−PO = 1 MU, P = 0.81). However, MUs increased considerably and significantly (Δ > 32 MUs, P < 0.01) after the model‐estimated objectives were introduced, indicating higher modulation in PO and PRO plans than the clinical plans. More MUs might be associated with smaller MLC apertures and lower agreement between the calculation and delivery of the intended dose distribution.24, 29 Computation‐based analysis has excluded the inferior deliverability of knowledge‐based plans,14 yet physical measurement should still be desirable to gain more confidence. In addition, when more DVH estimation models become available, the comparative assessment could be performed for DVO algorithm and for other lesion sites.
This study is potentially limited by the nondeterministic nature of the optimization process: slightly different results may be obtained even if the same algorithm and constraints were utilized. However, this random and minor uncertainty should be largely canceled out by the averaging during the statistical analysis, and the systematic discrepancies should be mostly attributable to the different optimizers.
5. Conclusions
By differentiating the dosimetric improvement contributed by the knowledge‐based objectives and the new optimizer, this work suggests that PO prevails over PRO algorithm for VMAT planning, with or without RapidPlan solution.
Acknowledgments
This work was supported by Beijing Natural Science Foundation (7172048); National Natural Science Foundation of China (11505012); Beijing Municipal Administration of Hospitals' Youth Programme (QML20151004) and Special Fund for Quality Scientific Research in the Public Welfare (201510001‐02). The authors thank Sha Li, Meijiao Wang, Qiaoqiao Hu, Jian Gong, Zhongsu Feng, Zhuolun Liu, Jian Zhang, Tian Li, Xiaoyu Xiang, and Yuliang Huang for their assistance.
Fan Jiang and Hao Wu contributed equally to this work.
References
- 1. Shiraishi S, Moore KL. Knowledge‐based prediction of three‐dimensional dose distributions for external beam radiotherapy. Med Phys. 2016;43:378–387. [DOI] [PubMed] [Google Scholar]
- 2. Zarepisheh M, Long T, Li N, et al. A DVH‐guided IMRT optimization algorithm for automatic treatment planning and adaptive radiotherapy replanning. Med Phys. 2014;41:061711. [DOI] [PubMed] [Google Scholar]
- 3. Chanyavanich V, Das SK, Lee WR, et al. Knowledge‐based IMRT treatment planning for prostate cancer. Med Phys. 2011;38:2515. [DOI] [PubMed] [Google Scholar]
- 4. Zhu X, Ge Y, Li T, et al. A planning quality evaluation tool for prostate adaptive IMRT based on machine learning. Med Phys. 2011;38:719. [DOI] [PubMed] [Google Scholar]
- 5. Wu B, McNutt T, Zahurak M, et al. Fully automated simultaneous integrated boosted‐intensity modulated radiation therapy treatment planning is feasible for head‐and‐neck cancer: a prospective clinical study. Int J Radiat Oncol Biol Phys. 2012;84:e647–e653. [DOI] [PubMed] [Google Scholar]
- 6. Lian J, Yuan L, Ge Y, et al. Modeling the dosimetry of organ‐at‐risk in head and neck IMRT planning: an inter technique and inter institutional study. Med Phys. 2013;40:121704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu B, Pang D, Simari P, et al. Using overlap volume histogram and IMRT plan data to guide and automate VMAT planning: a head‐and neck case study. Med Phys. 2013;40:021714. [DOI] [PubMed] [Google Scholar]
- 8. Yang Y, Ford EC, Wu B, et al. An overlap‐volume‐histogram based method for rectal dose prediction and automated treatment planning in the external beam prostate radiotherapy following hydrogel injection. Med Phys. 2013;40:011709. [DOI] [PubMed] [Google Scholar]
- 9. Good D, Lo J, Lee WR, et al. A knowledge‐based approach to improving and homogenizing intensity modulated radiation therapy planning quality among treatment centers: an example application to prostate cancer planning. Int J Radiat Oncol Biol Phys. 2013;87:176–181. [DOI] [PubMed] [Google Scholar]
- 10. Berry SL, Ma R, Boczkowski A, et al. Evaluating inter‐campus plan consistency using a knowledge based planning model. Radiother Oncol. 2016;120:349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bolan C. Expediting the treatment planning process. Appl Radiat Oncol. 2013;12:19–23. [Google Scholar]
- 12. Moore K, Scott Brame R, Low D, Mutic S. Experience based quality control of clinical intensity modulated radiotherapy planning. Int J Radiat Oncol Biol Phys. 2011;81:545–551. [DOI] [PubMed] [Google Scholar]
- 13. Appenzoller L, Michalski J, Thorstad W, Mutic S, Moore K. Predicting dosevolume histograms for organs‐at‐risk in IMRT planning. Med Phys. 2012;39:7446–7461. [DOI] [PubMed] [Google Scholar]
- 14. Hussein M, South CP, Barry MA, et al. Clinical validation and benchmarking of knowledge‐based IMRT and VMAT treatment planning in pelvic anatomy. Radiothera Oncol. 2016;120:473–479. [DOI] [PubMed] [Google Scholar]
- 15. Chang ATY, Hung AWM, Cheung FWK, et al. Comparison of planning quality and efficiency between conventional and knowledge‐based algorithms in nasopharyngeal cancer patients using intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2016;95:981–990. [DOI] [PubMed] [Google Scholar]
- 16. Wu H, Jiang F, Yue H, Zhang H, Wang K, Zhang Y. Applying a RapidPlan model trained on a technique and orientation to another: a feasibility and dosimetric evaluation. Radiat Oncol. 2016;11:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu H, Jiang F, Yue H, Li S, Zhang Y. A dosimetric evaluation of knowledge‐based VMAT planning with simultaneous integrated boosting for rectal cancer patients. J Appl Clin Med Phys. 2016;17:6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fogliata A, Belosi F, Clivio A, et al. On the pre‐clinical validation of a commercial model‐based optimisation engine: application to volumetric modulated arc therapy for patients with lung or prostate cancer. Radiothera Oncol. 2014;113:385–391. [DOI] [PubMed] [Google Scholar]
- 19. Nwankwo O, Mekdash H, Sihono DSK, et al. Knowledge‐based radiation therapy (KBRT) treatment planning versus planning by experts: validation of a KBRT algorithm for prostate cancer treatment planning. Radiat Oncol. 2015;10:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fogliata A, Nicolini G, Clivio A, et al. A broad scope knowledge based model for optimization of VMAT in esophageal cancer: validation and assessment of plan quality among different treatment centers[J]. Radiat Oncol. 2015;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fogliata A, Nicolini G, Bourgier C, et al. Performance of a knowledge‐based model for optimization of volumetric modulated arc therapy plans for single and bilateral breast irradiation[J]. PLoS One. 2015;10:e0145137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tol JP, Delaney AR, Dahele M, et al. Evaluation of a knowledge‐based planning solution for head and neck cancer. Int J Radiat Oncol Biol Phys. 2015;91:612–620. [DOI] [PubMed] [Google Scholar]
- 23. Fogliata A, Wang PM, Belosi F, et al. Assessment of a model based optimization engine for volumetric modulated arc therapy for patients with advanced hepatocellular cancer. Radiat Oncol. 2014;9:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vanetti E, Nicolini G, Nord J, et al. On the role of the optimization algorithm of RapidArc® volumetric modulated arc therapy on plan quality and efficiency. Med Phys. 2011;38:5844–5856. [DOI] [PubMed] [Google Scholar]
- 25. Cozzi L, Dinshaw KA, Shrivastava SK, et al. A treatment planning study comparing volumetric arc modulation with RapidArc and fixed field IMRT for cervix uteri radiotherapy. Radiat Oncol. 2008;89:180–191. [DOI] [PubMed] [Google Scholar]
- 26. Boutilier JJ, Craig T, Sharpe MB, et al. Sample size requirements for knowledge‐based treatment planning. Med Phys. 2016;43:1212–1221. [DOI] [PubMed] [Google Scholar]
- 27. Roels S, Duthoy W, Haustermans K, et al. Definition and delineation of the clinical target volume for rectal cancer. Int J Radiat Oncol Biol Phys. 2006;65:1129–1142. [DOI] [PubMed] [Google Scholar]
- 28. Li JL, Ji JF, Cai Y, et al. Preoperative concomitant boost intensity‐modulated radiotherapy with oral capecitabine in locally advanced mid‐low rectal cancer: a phase II trial. Radiother Oncol. 2012;102:4–9. [DOI] [PubMed] [Google Scholar]
- 29. Giorgia N, Antonella F, Eugenio V, et al. What is an acceptably smoothed fluence? dosimetric and delivery considerations for dynamic sliding window IMRT. Radiat Oncol. 2007;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]


