The nonpsychoactive cannabis-derivative cannabidiol (CBD) reduced nociceptor activity and pain in end-stage osteoarthritis. Prophylactic treatment with CBD prevented joint neuropathy and chronic neuropathic pain.
Keywords: Cannabinoids, Osteoarthritis, Pain, Neuropathy, Inflammation
Abstract
Osteoarthritis (OA) is a multifactorial joint disease, which includes joint degeneration, intermittent inflammation, and peripheral neuropathy. Cannabidiol (CBD) is a noneuphoria producing constituent of cannabis that has the potential to relieve pain. The aim of this study was to determine whether CBD is anti-nociceptive in OA, and whether inhibition of inflammation by CBD could prevent the development of OA pain and joint neuropathy. Osteoarthritis was induced in male Wistar rats (150-175 g) by intra-articular injection of sodium monoiodoacetate (MIA; 3 mg). On day 14 (end-stage OA), joint afferent mechanosensitivity was assessed using in vivo electrophysiology, whereas pain behaviour was measured by von Frey hair algesiometry and dynamic incapacitance. To investigate acute joint inflammation, blood flow and leukocyte trafficking were measured on day 1 after MIA. Joint nerve myelination was calculated by G-ratio analysis. The therapeutic and prophylactic effects of peripheral CBD (100-300 μg) were assessed. In end-stage OA, CBD dose-dependently decreased joint afferent firing rate, and increased withdrawal threshold and weight bearing (P < 0.0001; n = 8). Acute, transient joint inflammation was reduced by local CBD treatment (P < 0.0001; n = 6). Prophylactic administration of CBD prevented the development of MIA-induced joint pain at later time points (P < 0.0001; n = 8), and was also found to be neuroprotective (P < 0.05; n = 6-8). The data presented here indicate that local administration of CBD blocked OA pain. Prophylactic CBD treatment prevented the later development of pain and nerve damage in these OA joints. These findings suggest that CBD may be a safe, useful therapeutic for treating OA joint neuropathic pain.
1. Introduction
The most prominent form of synovial joint disease, osteoarthritis (OA), is characterised by joint degeneration, pain, and in some patients, articular neuropathy.21 Chronic pain associated with OA is a major concern for which there are few viable treatments. The first-line therapy used to treat OA pain is nonsteroidal anti-inflammatory drugs; however, with long-term use their efficacy declines and they can lead to major adverse gastrointestinal and cardiovascular events. Historically, OA has been classified as noninflammatory arthritis; however, there is now overwhelming evidence that synovitis can occur in response to pro-inflammatory mediators being released into the joint.10,11,13,29,32 It is believed that this low-level inflammation contributes to degenerative changes that affect the entire joint leading to the development of peripheral sensitisation and nociceptive pain.18,22,37 In addition to structural defects, there is growing evidence to suggest that approximately 30% of patients with OA have neuropathic pain.1,34 Thus, a therapeutic which can block inflammation, neuropathy, and pain is sorely needed.
The endocannabinoid system (ECS) plays an important physiological role in the regulation of tissue inflammation and pain.23,38 A functional ECS has been demonstrated in the joints of animals36 and humans,31 which acts tonically to maintain joint homeostasis. Immunohistological and pharmacological evidence confirm that cannabinoid 1 (CB1) and cannabinoid 2 (CB2) receptors are expressed on the neurones and microvasculature that supply rat knee joints.23,24,36 In addition, CB2 receptors are colocalized with pronociceptive transient receptor potential vanilloid-1 (TRPV1) channels where, through common intra-cellular pathways, they act together to modulate joint pain.23,24,36 This suggests that drugs which target the ECS have the potential to regulate painful arthritis and inflammatory joint disease.
Cannabidiol is the main noneuphoria producing component of the cannabis plant.26 Pharmacologically, CBD has a complex signalling mechanism whereby it can both activate and silence classical cannabinoid receptors as well as modulate noncanonical cannabinoid receptor pathways. In in vitro studies, CBD has been shown to be an inverse agonist at CB2 receptors,40 and a full antagonist at CB1 receptors40 and G protein-coupled receptor-55 (GPR55).33 In vitro, CBD was found to be an agonist at TRPV13 and transient receptor potential ankyrin 1 (TRPA1),9 which play a central role in the development of OA.27 In musculoskeletal disease models, systemic administration of CBD suppressed the progression of collagen-induced arthritis by reducing inflammatory cytokine production.20 Although these preliminary findings indicate a possible role for CBD in relieving joint inflammation, the local effect of articularly applied CBD on OA and joint pain has not been investigated.
The initial aim of this study was to assess the effect of locally administered CBD on joint pain in animals with end-stage OA. Since acute inflammation can contribute to the long-term development of OA joint pain,32 the ability of CBD to reduce acute OA synovitis and prevent the subsequent progression of persistent OA pain was also investigated. Finally, the effect of prophylactic CBD treatment on OA joint neuropathy was assessed.
2. Methods
2.1. Animals
Male Wistar rats (150-175 g; Charles River Laboratories, Senneville, QC, Canada) were housed in ventilated racks at 22°C ± 2°C on a 12:12 hours light:dark cycle (light-on from 7:00 to 19:00). After arrival at the animal care facility, all rats were permitted at least 1 week to acclimate to their environment. Animals were housed in pairs, cages were lined with woodchip bedding, and animals were provided with environmental enrichment. Standard laboratory chow and water were provided ad libitum. All experimental protocols were approved by the Dalhousie University Committee on the Use of Laboratory Animals, which acts in accordance with Animal Research: Reporting of In Vivo Experiments (ARRIVE) and the standards put forth by the Canadian Council for Animal Care.
2.2. Sodium monoiodoacetate model of osteoarthritis
Animals were deeply anaesthetised (2%-4% isoflurane; 100% oxygen at 1 L/min) until cessation of all sensory reflexes. The right knee joint was shaved, swabbed with 100% ethanol and 50 μL of sodium monoiodoacetate (MIA) (3 mg in saline) was injected into the joint space (intra-articular; i.artic.). The knee was then manually extended and flexed for 30 seconds to disperse the solution throughout the joint.
2.3. Electrophysiological recording of joint afferents
After OA development (14-19 days after MIA), animals were deeply anaesthetised using urethane (25% solution; 2 g/kg i.p.). Core body temperature was measured by a rectally inserted thermometer and maintained at 37°C ± 1°C by a thermostatically controlled heating blanket (CWE Inc, Ardmore, PA). After loss of the pedal withdrawal reflex, the trachea was cannulated to allow for artificial ventilation with a Harvard rodent respiratory pump (Harvard Apparatus, Holliston, MA) with 100% O2 (stroke volume: 2.5 mL; breath frequency: 52 breaths/min). The left carotid artery was cannulated to allow for continuous measurement of the mean arterial blood pressure. The cannula was attached to an in-line pressure transducer (Kent Scientific Corp, Torrington, CT) attached to a differentially amplified blood pressure monitor (World Precision Instruments, Sarasota, FL). The jugular vein was cannulated for administration of the muscle relaxant gallamine triethiodide (50 mg/kg), which eliminated neural interference from hind limb musculature, and the distal saphenous artery was cannulated for close intra-arterial (i.a.) administration of CBD or vehicle to the knee joint (100 μL injection volume). A specialised clamp was fixed to the mid-shaft of the isolated right femur and attached to a stereotaxic frame to prevent movement of the proximal aspect of the rat hind limb. The right hind paw was then placed in a shoe-like holder that was connected to a force transducer and torque meter (Data Track 244-1-R; Intertechnology, ON, Canada) to standardise the amount of rotational force being applied to the knee joint. A longitudinal skin incision was made along the medial aspect of the hind limb and the reflected skin was sutured to a metal “O” ring to create a pool which was filled with warm mineral oil to prevent tissue desiccation. The medial articular branch of the saphenous nerve was isolated and transected in the inguinal region to prevent spinal reflexes. The epineurium was removed and the nerve teased to isolate fine neurofilaments which were then placed on a platinum recording electrode to measure single-unit activity. To identify a joint afferent fibre and its receptive field, the knee joint was gently probed with a blunt glass rod. The mechanical threshold of each recorded joint afferent was determined by gradually increasing the torque applied to the joint until the fiber elicited an action potential. The conduction velocity of the fibres were determined by electrically stimulating the receptive field with a pair of silver bipolar stimulating electrodes (0.6 Hz, 2 ms pulse width, 1-15 V). The mechanosensitivity of the joint fibre was assessed by applying noxious outward rotations to the knee and counting the number of action potentials elicited during the rotation. Noxious rotation refers to torque occurring outside the normal range but not severe enough to cause soft tissue injury.
2.3.1. Experimental timeline
On day 14 post-MIA induction, 3 sets of noxious rotations, each lasting 5 seconds, were applied 5 minutes apart as a baseline measurement of afferent activity. After close i.a. infusion of CBD (100, 200, or 300 μg in 100 μL) or vehicle (100 μL), joint mechanosensitivity was assessed for an additional 15 minutes. To minimise the use of animals, multiple doses of CBD or vehicle were assessed in each fibre. A washout period of at least 50 minutes was observed between the administration of varying doses of CBD or vehicle to allow afferent firing to return to baseline levels. The percentage change in afferent activity before and after administration of CBD or vehicle was calculated offline using Spike2 software (Cambridge Electronic Design, Cambridge, United Kingdom). All recorded fibres fired in response to close i.a. administration of potassium chloride (KCl; 1 mM, 0.1 mL) at the conclusion of the experiment, confirming that administered drugs had reached the mechanosensory nerve endings and that the recorded fibre was still viable.
2.4. Behavioural pain measurements
2.4.1. Von Frey hair mechanosensitivity
Von Frey hair mechanosensitivity was used as a measure of secondary allodynia. Alert, unanaesthetised animals were placed in a Plexiglas chamber with a metal mesh flooring which allowed access to the plantar surface of each hind paw. After allowing the animal to acclimate until exploratory behaviour ceased (approximately 10 minutes), ipsilateral hind paw mechanosensitivity was assessed using a modification of the Dixon up–down method.5 A von Frey hair was applied perpendicular to the plantar surface of the ipsilateral hind paw (avoiding the toe pads) until the hair flexed; the filament was then held in place for 3 seconds. If there was a positive response (ie, withdrawal, shaking, or licking of the hind paw), the next lower strength hair was applied; if there was a lack of response, the next higher strength hair was applied up to a cut-off of 15 g bending force. The 50% withdrawal threshold was determined using the following formula: 10(Xf + kδ)/10,000; where Xf = value (in log units) of the final von Frey hair used, k = tabular value for the pattern of the last 6 positive and/or negative responses, and δ = mean difference (in log units) between stimuli.
2.4.2. Hind limb incapacitance
To perform dynamic weight bearing (DWB) measurements, animals were placed in a Perspex chamber (model BIO-DWB-AUTO-R; Bioseb, Boulogne, France) with a pressure-sensitive floor and allowed to move freely. Hind limb weight bearing was tracked and recorded over a 3-minute period. Weight borne on the ipsilateral hind paw was calculated as a percentage of the total weight borne on the hind limbs.
2.4.3. Experimental timeline
Animals underwent baseline von Frey hair mechanosensitivity and DWB testing. Separate cohorts were treated on day 14 post-MIA with an i.artic. injection of either vehicle (50 μL) or CBD (100-300 μg/50 μL). In other experimental cohorts, day 14 OA rats were treated with the highest dose of CBD (300 μg/50 μL) and either the CB1 receptor antagonist, AM281 (75 μg/50 μL), the CB2 receptor antagonist, AM630 (75 μg/50 μL), or the TRPV1 receptor antagonist, SB-366791 (30 μg/50 μL) administered locally (subcutaneously; s.c.) over the joint 10 minutes before i.artic. CBD administration. Behavioural pain measurements for these experiments were conducted at 30, 60, 120, 180, and 240 minutes after drug administration. To investigate the prophylactic effects of CBD on OA pain and peripheral neuropathy, a separate cohort of rats was treated with CBD (300 μg/50 μL) or vehicle (50 μL) s.c. over the knee joint 30 minutes before i.artic. injection of MIA (3 mg/50 μL) and once daily on each of the subsequent 3 days; behavioural pain measurements were conducted on days 0, 1, 2, 3, 7, 10, and 14.
2.5. Inflammation measures
Animals were deeply anaesthetised by an intraperitoneal injection of urethane (25% solution; 2g/kg i.p.). A longitudinal incision was made along the ventral skin of the neck to expose the trachea which was cannulated with PE-200 tubing to permit unrestricted breathing. The right carotid artery was also cannulated with PE-30 tubing filled with heparinised saline (1 U/mL) to allow for continuous monitoring of the mean arterial pressure (MAP).
2.5.1. Intravital microscopy
Both hind limbs were immobilised and the capsule of the ipsilateral knee was exposed by surgically removing a small ellipse of the overlying skin and superficial fascia. Physiological buffer (37°C ± 1°C) was immediately and continuously perfused over the exposed joint.
Intravital microscopy was used to assess leukocyte-endothelial interactions within the microcirculation of the knee joint, as described previously.2 The synovial microcirculation was visualised under incident fluorescent light using a Leica DM2500 microscope with a HCX APO L 20X objective and an HC Plan 10X eyepiece giving a final magnification of ×200. In vivo leukocyte staining was achieved by intravenous administration of 0.05% rhodamine 6G (in saline). Straight, unbranched postcapillary venules (15-50 μm in diameter) were chosen for visualisation and 3 fluorescent videos (per time point) were captured for 1 minute each by a Leica DFC 3000 camera (Leica Microsystems Canada Inc, Richmond Hill, ON, Canada). Two measures of leukocyte-endothelial interactions were used to assess articular inflammation: (1) the number of rolling leukocytes to pass an arbitrary line perpendicular to the venule in 1 minute were counted and (2) the number of adherent leukocytes within a 100-μm portion of the venule. Rolling leukocytes were defined as positively stained blood cells travelling slower than the surrounding blood flow, and adherent leukocytes were defined as positively stained cells that remained stationary for a minimum of 30 seconds.
2.5.2. Laser speckle contrast analysis
In the same animals, knee joint blood flow was measured by laser speckle contrast analysis (LASCA) using a PeriCam PSI System (Perimed Inc, Ardmore, PA). At each time point, 1-minute recordings of the exposed knee joint were taken at a working distance of 10 cm with a frame capture rate of 25 images per second. Using dedicated software (PIMSoft, Version 1.5.4.8078), images were averaged to generate 1 perfusion image per second. At the end of the experiment, rats were euthanised and a dead scan of the knee was taken. This “biological zero” value was subtracted from all measurements to account for any Brownian motion in the tissue. Images were analysed offline where mean blood perfusion (perfusion units) in a defined region of interest approximating the knee joint was calculated.
2.5.3. Experimental timeline
Inflammation measures were conducted on day 1 post-MIA induction, which corresponds to the peak of inflammation in this OA model. After baseline intravital microscopy and LASCA recordings (1 minute) a 50-μL bolus of CBD (300 μg) or vehicle (separate cohort) was applied topically over the exposed knee joint. Subsequent recordings were taken at 5, 15, 30, 60, 120, and 180 minutes after drug administration. In separate cohorts, day 1 MIA rats were treated with the highest dose of CBD (300 μg/50 μL) and either the CB1 receptor antagonist, AM281 (75 μg/50 μL), the CB2 receptor antagonist, AM630 (75 μg/50 μL), or the TRPV1 receptor antagonist, SB-366791 (30 μg/50 μL) administered topically over the joint 10 minutes before CBD administration.
2.6. G-ratio analysis of the saphenous nerve
A segment of the saphenous nerve was isolated proximal to the ipsilateral knee joint and placed in 2.5% glutaraldehyde (diluted with 0.1 M sodium cacodylate buffer), and stored at 4°C for at least 1 week. The nerve samples were then removed from the fixative and rinsed 3 times with 0.1 M sodium cacodylate buffer. The samples were fixed in 1% osmium tetroxide for 2 hours, rinsed with distilled water, and then placed in 0.25% uranyl acetate (4°C) overnight. The samples were then dehydrated in a graduated series of acetone (50%, 70%, 95%, and finally 100%). The samples were then dried in 100% acetone for 10 minutes. Epon–araldite resin was used to mount the samples. The samples were placed in a 3:1 ratio of dried 100% acetone to resin for 3 hours, followed by a 1:3 ratio of dried 100% acetone to resin overnight. Next the samples were placed in 100% Epon–araldite resin for 3 hours and cured in an oven at 60°C for 48 hours. Finally, using an LKB Huxley ultramicrotome with a diamond knife, the samples were sectioned into 100 nm thick slices. Cross-sectional slices of nerves were placed onto a copper wire grid consisting of 300 individual squares per inch (each square measuring 83 × 58 μm) and then stained with 2% aqueous uranyl acetate for 10 minutes and finally lead citrate for 4 minutes.
The copper wire grids containing the saphenous nerve sections were inserted into a JEOL JEM 1230 transmission electron microscope (JEOL Corp Ltd, Tokyo, Japan). The microscope was set at a voltage of 80.0 kV, and images were captured at ×2500 using a Hamamatsu ORCA-HR digital camera (Hamamatsu Photonics, Hamamatsu City, Japan). One nerve cross-section image was visually partitioned into 9 quadrants and 3 images were captured (from quadrants 1, 5, and 9). All fibres were assessed using the G-ratio plugin in ImageJ processing software. The G-ratio was calculated using the equation 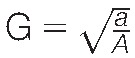 where, a is the internal axonal area and A is the total axonal area of the fibre. The higher the G-ratio the higher the degree of demyelination.
where, a is the internal axonal area and A is the total axonal area of the fibre. The higher the G-ratio the higher the degree of demyelination.
2.7. Drugs and reagents
Cannabidiol (2-[(1R,6R)-3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-5-pentyl-1,3-benzenediol) was obtained from Tocris Bioscience (Bio-Techne, Abingdon, United Kingdom). AM281 (CB1 receptor antagonist; 1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-4-morpholinyl-1H-pyrazole-3-carboxamide) and AM630 (CB2 receptor antagonist; 6-iodo-2-methyl-1-(2-morpholin-4-ylethyl)indol-3-yl]-(4-methoxyphenyl)methanone) were obtained from Cayman Chemicals (Ann Arbor, MI). SB-366791 (N-(3-methoxyphenyl)-4-chlorocinnamide), rhodamine 6G, cremophor, dimethyl sulphoxide (DMSO), urethane, and MIA were obtained from Sigma-Aldrich (St. Louis, MO). Solutions of CBD, AM281, AM630, and SB-366791 were prepared in vehicle (1:1:18; DMSO:cremophor:saline) on the day of use. Rhodamine 6G (0.05%) and MIA were dissolved in saline. Physiological buffer (135 mM NaCl, 20 mM NaHCO3, 5 mM KCl, 1 mM MgSO4*7H2O, pH = 7.4) was prepared in the laboratory.
2.8. Statistical analysis
All data were expressed as mean ± SEM. Data were tested for Gaussian distribution by the Kolmogorov–Smirnov test. All data were normally distributed and were therefore analysed using parametric statistics (2-way analysis of variance (ANOVA), 1-way ANOVA, unpaired 2-tailed Student t test, and paired 2-tailed Student t test). A P value less than 0.05 was considered statistically significant.
3. Results
3.1. Effect of acute administration of cannabidiol on joint afferent mechanosensitivity
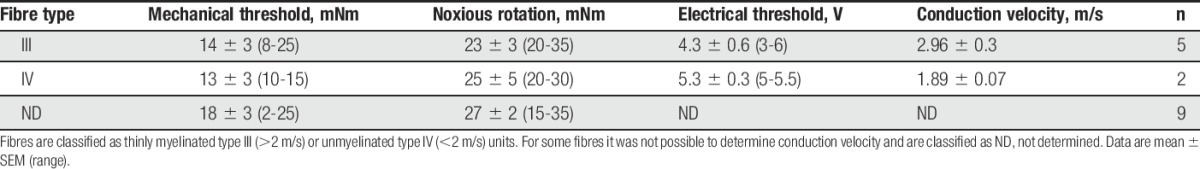
A total of 17 afferent fibres were recorded in this study. Fibres were characterised based on mechanical and electrical threshold, and conduction velocity (summarised in Table 1).
Table 1.
Characterisation of the recorded fibres in the electrophysiology experiments.
On days 14 to 19 post-MIA induction, close i.a. administration of CBD rapidly reduced noxious movement-evoked firing of knee afferent fibres (Fig. 1A) in a dose-dependent manner (P < 0.0001; n = 8, Fig. 1B). The desensitising effect of 300 μg CBD during noxious joint rotation was significant at 3 minutes after drug application and reached a maximum anti-nociceptive effect at 7 minutes (29.3% ± 7.4% change compared with baseline). Although all doses of CBD significantly decreased the mean afferent firing over the course of the 15 minutes assessed, the 300 μg dose was the most effective, decreasing firing by 22.8% ± 1.2% overall (P < 0.0001, n = 8, Fig. 1C).
Figure 1.
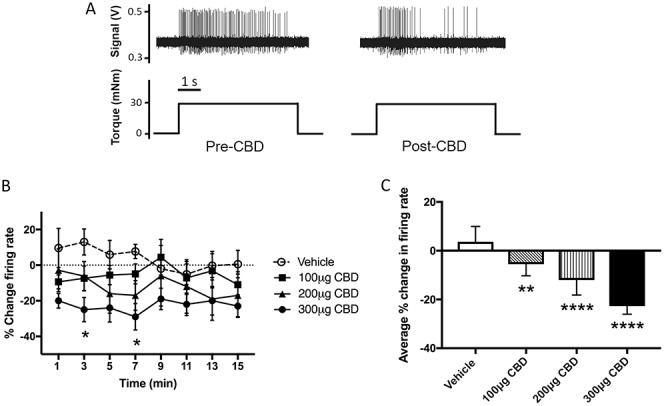
Dose-dependent effect of CBD on joint afferent firing in established OA. Example of a single-unit recording whereby CBD attenuated firing evoked by noxious rotation (A). Cannabidiol (100, 200, or 300 μg i.a.) decreased afferent firing relative to baseline (B). (*P < 0.05 2-way ANOVA with Bonferroni post hoc test; n = 8). The dose-dependent effect of CBD treatment on afferent firing rate was averaged over the 15 minutes after administration (C). (****P < 0.0001, **P < 0.01 1-way ANOVA with Bonferroni post hoc test; n = 8). Data are mean values ± SEM. ANOVA, analysis of variance; CBD, cannabidiol; i.a., intra-arterial; OA, osteoarthritis.
3.2. Effect of acute administration of cannabidiol on sodium monoiodoacetate–induced pain
Intra-articular injection of MIA produced secondary allodynia and weight-bearing deficits in the ipsilateral hind paw and hind limb, respectively, 14 days after injection (P < 0.0001; n = 24; Fig. 2A and P < 0.0001; n = 24; Fig. 2B).
Figure 2.
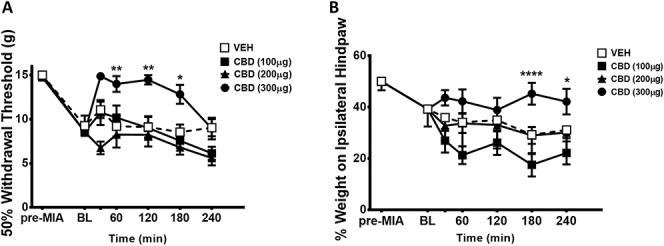
Dose-dependent effect of CBD on pain-related measures in established OA. Intra-articular injection of MIA produced secondary allodynia and weight-bearing deficits in the ipsilateral hind paw and hind limb, respectively, 14 days after MIA injection (****P < 0.0001, 1-way ANOVA with Dunnett post hoc test; n = 24). Cannabidiol (100, 200, or 300 μg i.artic. at BL]) improved hind paw withdrawal threshold (A) and hind limb weight bearing dose-dependently, over 4 hours. (****P < 0.0001, **P < 0.01, *P < 0.05 2-way ANOVA with Bonferroni post hoc test; n = 8). Data are mean values ± SEM. ANOVA, analysis of variance; BL, baseline, CBD, cannabidiol; MIA, sodium monoiodoacetate, OA, osteoarthritis; VEH, vehicle.
When compared with vehicle control, low dose CBD (100, 200 μg) had no effect on withdrawal threshold or hind limb weight bearing (P > 0.05; n = 8; Figs. 2A and B). The 300 μg dose of CBD, however, significantly increased hind paw withdrawal threshold and hind limb weight bearing over the time course tested (P < 0.0001; n = 8; Figs. 2A and B). All subsequent experiments used the 300 μg dose of CBD.
To determine whether CBD was acting locally, 300 μg of the drug was injected into the contralateral knee and the withdrawal threshold was assessed in the ipsilateral joint 1 hour later and hind limb weight bearing was assessed in the ipsilateral joint 3 hour later. It was found that the high dose of CBD administered to the contralateral knee had no effect on ipsilateral hind paw withdrawal thresholds indicating that CBD was not acting centrally in this pain test (P < 0.01; n = 8-10; Fig. 3A). However, in the hind limb weight-bearing test, contralateral CBD was not statistically different from the ipsilateral CBD group (P > 0.05; n = 8-22; Fig. 3B).
Figure 3.
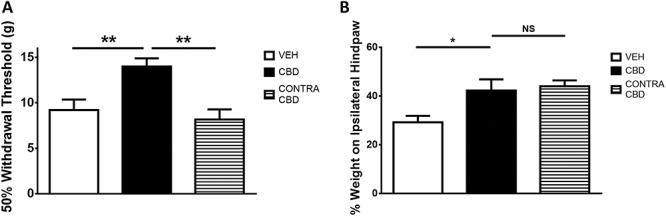
Effect of contralaterally administered CBD on ipsilateral pain behaviour. The improvement in hind paw withdrawal threshold seen with ipsilateral CBD was not observed when CBD (300 μg i.artic.) was administered to the contralateral knee (A). Contralateral CBD did not significantly decrease hind paw weight bearing (B) when compared with ipsilateral CBD. (*P < 0.05, **P < 0.01 1-way ANOVA with Fisher post hoc test; n = 8-9). Data are mean values ± SEM. ANOVA, analysis of variance; CBD, cannabidiol; VEH, vehicle.
The cannabinoid receptor antagonists AM281 and AM630 had no effect on CBD-induced analgesia (P > 0.05; n = 6-8; Figs. 4A and B). Conversely, the TRPV1 antagonist, SB-366791, significantly inhibited the analgesic effect of CBD (P < 0.05; n = 6-8; Fig. 4A) with respect to the hind paw withdrawal threshold, but did not have a significant effect on hind limb weight bearing at 3 hours after injection (P > 0.05; n = 6-22; Fig. 4B).
Figure 4.
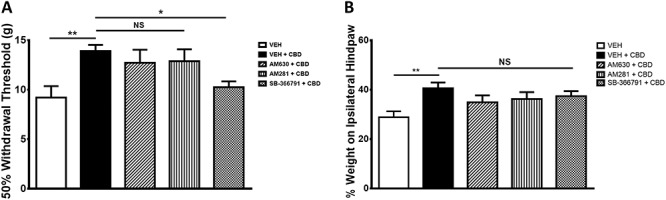
Contribution of cannabinoid and noncannabinoid receptors to the analgesic effects of CBD. Both hind paw withdrawal threshold (A) and hind limb weight bearing (B) were unaltered compared with control after local administration of the CB1 receptor antagonist AM281 (75 μg) or CB2 receptor antagonist AM630 (75 μg). Hind paw withdrawal threshold (A) was reduced compared with control after local administration of the TRPV1 antagonist SB-366791 (30 μg), but hind limb weight bearing (B) was unaffected. (*P < 0.05, **P < 0.01 1-way ANOVA with Fisher post hoc test; n = 6-8). Data are mean values ± SEM. ANOVA, analysis of variance; CBD, cannabidiol; VEH, vehicle.
3.3. Effect of acute administration of cannabidiol on sodium monoiodoacetate–induced inflammation
One day after i.artic. injection of MIA, rolling leukocytes (P < 0.0001; n = 6-12; Fig. 5A), adherent leukocytes (P < 0.0001; n = 6-12; Fig. 5B), and knee joint perfusion were all significantly increased compared with naïve animals (P > 0.05; n = 6-12; Fig. 5C).
Figure 5.
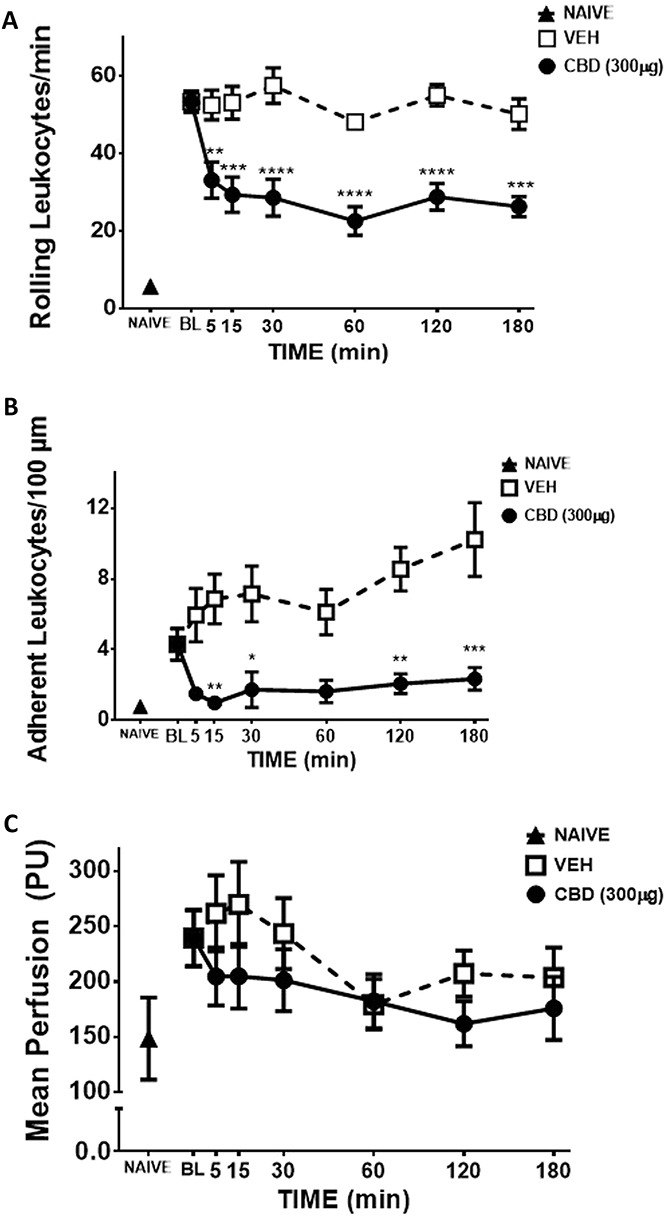
Anti-inflammatory action of CBD on day 1 MIA-induced inflammation. When compared with naïve controls, intra-articular MIA significantly increased rolling (A) and adherent (B) leukocytes, and caused synovial hyperaemia (C) (****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05, P > 0.05, unpaired t test; n = 6-12). Over a 3-hour time course, CBD (300 μg) significantly decreased leukocyte rolling (A) leukocyte adherence (B) and knee joint blood flow (C) when compared to vehicle. (****P < 0.0001, **P < 0.01, *P < 0.05 2-way ANOVA with Bonferroni post hoc test; n = 6). Data are mean values ± SEM. ANOVA, analysis of variance; CBD, cannabidiol; MIA, sodium monoiodoacetate; PU, perfusion unit; VEH, vehicle.
After baseline recordings were completed on day 1 post-MIA induction, topical administration of CBD (300 μg) significantly decreased rolling and adherent leukocytes when compared with vehicle over the 3-hour time course (P < 0.0001; n = 6; Figs. 5A and B). Cannabidiol had a moderate inhibitory effect on synovial hyperaemia (P < 0.05; n = 6; Fig. 5C). The MAP was unaffected by CBD treatment over the 3-hour time course (VEH: 70.7 ± 2.33 mm Hg; CBD: 68.8 ± 2.38 mm Hg), confirming a lack of any systemic effect on blood pressure.
The anti-rolling effect of CBD at 30 minutes was blocked by AM630 and SB-366791 (P < 0.0001, n = 6; Fig. 6A), but not AM281. The anti-adherence effect of CBD on day 1 MIA joints was blocked only by SB-366791(P < 0.001 n = 6; Fig. 6B).
Figure 6.
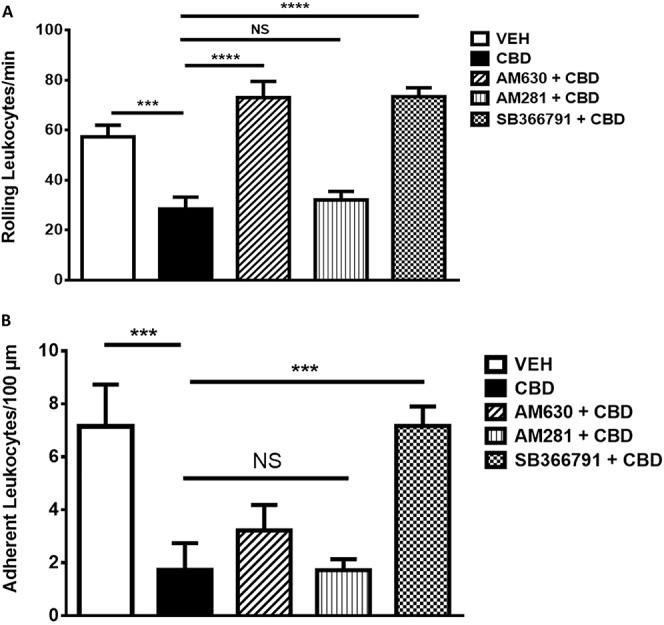
Contribution of cannabinoid and noncannabinoid receptors to the anti-inflammatory effects of CBD. The anti-rolling effect of CBD at 30 minutes was blocked (A) by CB2 receptor antagonist AM630 (75 μg) and TRPV1 antagonist SB-366791 (30 μg), but not CB1 receptor antagonist AM281 (75 μg). The anti-adherence effect of CBD in day 1 MIA joints was blocked by SB-366791 (B). (****P < 0.0001, ***P < 0.001 1-way ANOVA with Fisher LSD post hoc test; n = 60). Data are mean values ± SEM. ANOVA, analysis of variance; CBD, cannabidiol; MIA, sodium monoiodoacetate; VEH, vehicle.
3.4. Prophylactic effect of cannabidiol on sodium monoiodoacetate–induced osteoarthritis pain
Prophylactic treatment of MIA-injected knee joints with CBD (on days 0–3 of MIA) significantly attenuated the development of MIA-induced tactile allodynia during both the acute and late phase of OA development (P < 0.0001; n = 8; Fig. 7A). Conversely, early treatment with CBD had no effect on hind limb weight bearing, when compared with vehicle-treated animals (P > 0.05; n = 8; Fig. 7A).
Figure 7.
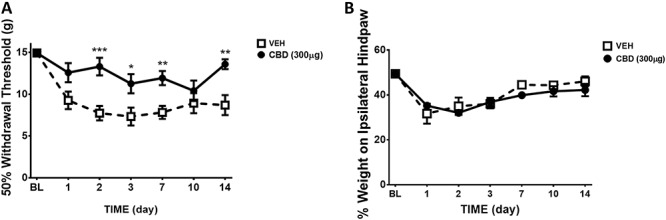
Effect of prophylactic CBD administration on the development of pain over 14 days post-MIA injection. Treating MIA knee joints with CBD (300 μg; s.c.; days 0–3) significantly improved von Frey hair withdrawal threshold over the 14-day development of OA when compared with vehicle (A). Pretreatment of MIA knee joints with CBD had no significant effect on hind limb weight bearing (B) (****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05 2-way ANOVA with Bonferroni post hoc test; n = 8). Data are mean values ± SEM. ANOVA, analysis of variance; CBD, cannabidiol; MIA, sodium monoiodoacetate; OA, osteoarthritis; s.c., subcutaneous; VEH, vehicle.
3.5. Cannabidiol prophylaxis and sodium monoiodoacetate–induced peripheral nerve damage
Treatment of OA knees with CBD during the acute inflammatory phase of the MIA model (days 0–3 of MIA) inhibited saphenous nerve demyelination on day 14 compared with vehicle-treated knees (P < 0.05; n = 6-8; Fig. 8B).
Figure 8.
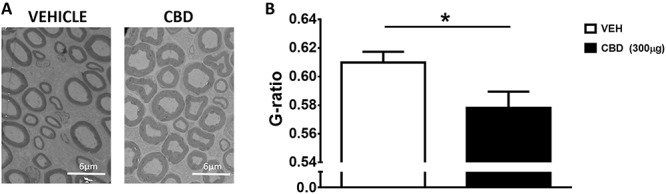
Prophylactic CBD reduces joint nerve demyelination in MIA-induced OA. Representative sections of electron micrographs of axons found in saphenous nerves taken at day 14 from MIA treated with vehicle (A) (days 0–3), or CBD (300 μg; days 0–3). (B) G-ratio calculations showing that MIA-induced axonal demyelination is prevented by CBD treatment. Scale bar is 6 μm. (*P < 0.05 unpaired t test; n = 6 = 8). Data are presented as mean values ± SEM. CBD, cannabidiol; MIA, sodium monoiodoacetate; OA, osteoarthritis; VEH, vehicle.
4. Discussion
Pain and disease progression are poorly managed in many patients with OA because of the multifactorial nature of the disease. Intra-articular injection of MIA produces monoarthritis with several features that resemble human OA, including joint pain, intermittent inflammation, and joint nerve damage. This study aimed to address, for the first time, whether the inflammatory and neuropathic pain associated with MIA could be blocked by local administration of the noneuphoria producing phytocannabinoid CBD.
It has previously been shown that the pain associated with the MIA model of OA is mediated in part by the sensitisation of joint afferent fibres.35,37 Peripheral administration of CBD dose-dependently decreased joint afferent firing on day 14 after MIA injection. These electrophysiology data confirm that CBD has a peripheral site of action in knee joints. Because all recordings were made from Aδ or C fibres during noxious movement of the knee, this suggests that CBD can inhibit the mechanosensitivity of joint nociceptors.
In end-stage OA, intra-articular injection of 300 μg of CBD improved unrestrained hind limb weight bearing and hind paw withdrawal threshold (Fig. 2). These observations, along with our electrophysiology data, assert that CBD acts locally in the joint to reduce joint mechanical pain as revealed by improved weight bearing as well as a reduction in centrally mediated secondary allodynia as determined by hind paw withdrawal threshold. Contralateral injection of CBD had no discernible effect on ipsilateral secondary allodynia confirming that the analgesic effect of intra-articular CBD was localised to the site of administration for this pain test. The anti-nociceptive effect of low dose CBD (100 and 200 μg) observed with electrophysiology was not seen in the behavioural pain assessments. This may be because electrophysiology is a highly sensitive technique that detects subtle response to test agents in the periphery, whereas pain behaviours are more complex and encompass the entire pain pathway. The rationale for using two pain behavioural tests in this study was to interrogate different aspects of the pain pathway. Dynamic incapacitance is a measure of spontaneous pain that is associated with joint degeneration or inflammation arising from peripheral sensitisation.4,28 In contrast, von Frey hairs were used to investigate evoked, reflexive responses (ie, paw withdrawal, shake, and lick) at a site distal to the injured joint.28 This secondary allodynia is a consequence of central sensitisation in late stages of the MIA model,16 and can be indicative of nerve injury. Thus, it seems that local injection of CBD is effective at reducing direct nociceptive and inflammatory pain in the joint as well as ameliorating neuropathic features of OA pain.
Both CB1 and CB2 receptor antagonists failed to block the CBD-mediated improvements in hind paw withdrawal threshold and weight bearing. Although CBD has been shown to act as an inverse agonist at CB2 receptors and a full antagonist at CB1 receptors,40 it has also been shown to act through GPR55, serotonin receptors (eg, 5-HT1A), and various transient receptor potential ion channels. Transient receptor potential vanilloid-1 is known to be involved in MIA-induced peripheral sensitisation,17 therefore, antagonist experiments were performed to test the involvement of this ion channel in CBD-mediated analgesia. Here, the TRPV1 antagonist SB-366791 attenuated the secondary allodynia imparted by CBD in established OA. This mechanism of action has been previously reported in in vitro studies using human embryonic kidney (HEK 293) cells and using cell membranes from mouse and rat brains.3 In vivo, TRPV1 antagonism has also been shown to block the pain-relieving effect of CBD in a model of carrageenan-induced paw oedema7 and in the chronic constriction injury model of neuropathic pain.6 Although these data show that the action of CBD is mediated in part by TRPV1, it remains unclear if CBD is acting directly on TRPV1 or if there is an indirect mechanism occurring in the joint. Cannabidiol has been shown to inhibit fatty acid amide hydrolase (FAAH) and the uptake of anandamide.3 Inhibition of FAAH and anandamide reuptake would elevate anandamide levels in the joint which if high enough could ultimately lead to the activation of TRPV1.3
Intra-articular injection of MIA produced an acute inflammatory response on day 1 after injection. This acute phase of inflammation was evinced by an increase in leukocyte trafficking and a moderate increase in joint blood flow. Local application of CBD significantly reduced these acute, inflammatory changes corroborating what has previously been reported in other inflammatory models.8,12,20 Oral administration of CBD, for example, has been shown to be anti-inflammatory and anti-hyperalgesic in the carrageenan model of plantar oedema.8 Malfait et al., showed that systemic administration of CBD, both intraperitoneally and orally, suppressed disease severity and decreased serum inflammatory cytokine levels in the collagen model of rheumatoid arthritis.20 Moreover, CBD administered by a transdermal gel reduced joint swelling, immune cell infiltration, synovial membrane thickening, and the synthesis of pro-inflammatory biomarkers in the Freund complete adjuvant model of inflammatory arthritis.12 The data presented here demonstrate for the first time that CBD has the capacity to reduce the inflammatory flares associated with OA.
The inhibitory effect of CBD on leukocyte trafficking was blocked by the TRPV1 antagonist SB-366791. Opening of TRPV1 ion channels causes the peripheral release of inflammatory neuropeptides which promote neurogenic inflammation and enhanced leukocyte trafficking in joints.19,41 Thus, the anti-inflammatory effects of CBD observed here could be due to desensitisation of TRPV1 ion channels as has been shown elsewhere.14 The anti-rolling effect of CBD on joint leukocytes was also blocked by AM630 suggesting that CB2 receptors may be involved in opposing leukocyte capture in day 1 MIA joints. Zhao et al. showed that activation of CB2 receptors can inhibit the expression of P-selectin which is the adhesion molecule responsible for leukocyte rolling.43 Whether CBD inhibits joint P-selectin activity by a CB2 receptor mechanism requires further investigation.
A central hypothesis of this study was that early inhibition of OA-related inflammation with CBD would reduce the development of persistent joint pain. Prophylactic treatment of OA joints with CBD on days 1 to 3 after MIA induction prevented secondary allodynia at day 14, but had no effect on hind limb weight bearing. Inflammation associated with MIA diminishes by day 7,4 therefore the pain associated with end-stage OA in this model is largely due to joint degeneration and peripheral neuropathy. Thus, by abolishing early inflammation with prophylactic treatment, CBD attenuates central sensitisation and neuropathic pain development in OA.
Previous studies have shown that MIA-induced OA causes peripheral nerve damage.25,39 Demyelination of the ipsilateral saphenous nerve was confirmed by an increase in G-ratio, purporting MIA-induced peripheral neuropathy compared with saline control animals.25 This study showed that prophylactic treatment with CBD during the early inflammatory phase of MIA prevented this loss of nerve myelin 14 days later, suggesting that blockade of inflammatory flares during OA could protect against joint nerve damage. The G-ratio data would benefit from future studies examining the expression of a biomarker for peripheral nerve damage to further support this finding.
The findings presented here and elsewhere support the concept that MIA recapitulates the neuropathic aspect of OA pain, which is found in approximately 30% of patients.1,34 CBD treatment may be a beneficial therapeutic for the population of patients who experience neuropathic arthritis, and are refractory to currently used first- and second-line analgesics. Several cannabis compounds, including CBD, have been shown to be neuroprotective in other musculoskeletal disorders. In a preclinical model of multiple sclerosis, CBD was shown to improve clinical recovery and rotarod scores in animals, correlating with and indicative of a neuroprotective effect.30 In addition, CBD and ∆9-tetrahydrocannabinol have both been implicated in slowing the progression and promoting the survival of neurones in a preclinical model of amyotrophic lateral sclerosis.15,42 These studies, in addition to the results presented here, highlight the potential utility of CBD as an analgesic and neuroprotective agent in OA.
Cannabidiol is a noneuphoria producing compound and has a more desirable side effect profile compared with other cannabinoid compounds and commonly prescribed analgesics. Animal studies where CBD was administered systemically showed that the animals had no signs of adverse side effects.12,20 For example, exploratory behaviour in rats was not altered by systemic CBD, indicating limited central effects of treatment.12 Our study shows for the first time that CBD is an effective anti-nociceptive and anti-inflammatory agent when administered locally around the joint. Successful relief of OA symptoms by peripherally administered CBD suggests a therapeutic option that has a low chance of adverse effects which is more desirable for patients.
5. Conclusions
This study showed for the first time that local CBD administration inhibited pain and peripheral sensitisation in established OA. Topical treatment with CBD reduced leukocyte trafficking and joint hyperaemia during the early stages of MIA. By attenuating this initial inflammatory response with CBD, end-stage OA pain and peripheral neuropathy were abrogated. Thus, CBD may be a safe therapeutic to treat OA pain locally as well as block the acute inflammatory flares that drive disease progression and joint neuropathy.
Conflict of interest statement
The authors have no conflicts of interest to declare.
This work was supported by an operating grant provided by The Arthritis Society.
Ethics: All experimental protocols were approved by the Dalhousie University Committee on Laboratory Animals, which acts in accordance with the standards put forth by the Canadian Council for Animal Care.
Availability of data and materials: The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Acknowledgements
The technical assistance of Allison Reid is gratefully acknowledged.
Author contributions: H. T. Philpott conducted the pain behaviour experiments, the inflammation measurements (IVM and LASCA), performed the G-ratio measurements, analysed data, and helped draft the manuscript. M. O'Brien conducted all electrophysiology experiments, analysed the data, and helped draft the manuscript. J. J. McDougall conceived the study, participated in its design and coordination, helped analyse data, and helped draft the manuscript. All authors read and approved the final manuscript.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Ahmed S, Magan T, Vargas M, Harrison A, Sofat N. Use of the painDETECT tool in rheumatoid arthritis suggests neuropathic and sensitization components in pain reporting. J Pain Res 2014;7:579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Andruski B, McCafferty DM, Ignacy T, Millen B, McDougall JJ. Leukocyte trafficking and pain behavioral responses to a hydrogen sulfide donor in acute monoarthritis. Am J Physiol Regul Integr Comp Physiol 2008;295:R814–20. [DOI] [PubMed] [Google Scholar]
- [3].Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, Moriello AS, Davis JB, Mechoulam R, Di Marzo V. Molecular targets for cannabidiol and its synthetic analogues: effect of vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol 2001;134:845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bove SE, Calcaterra SL, Brooker RM, Huber CM, Guzman RE, Juneau PL, Schrier DJ, Kilgore KS. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthr Cartil 2003;11:821–30. [DOI] [PubMed] [Google Scholar]
- [5].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53:55–63. [DOI] [PubMed] [Google Scholar]
- [6].Comelli F, Giagoni G, Bettoni I, Colleoni M, Costa B. Antihyperalgesic effect of a cannabis sativa extract in a rat model of neuropathic pain: mechanisms involved. Phytother Res 2008;22:1017–24. [DOI] [PubMed] [Google Scholar]
- [7].Costa B, Giagnoni G, Franke C, Trovato AE, Colleoni M. Vanniloid TRPV1 receptor mediates the antihyperalgesic effect of the nonpsychoactive cannabinoid, cannabidiol, in a rat model of acute inflammation. Br J Pharmacol 2004;143:247–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Costa B, Colleoni M, Conti S, Parolaro D, Franke C, Trovato AE, GIagnoni G. Oral anti-inflammatory activity of cannabidiol, a non-psychoactive constituent of cannabis, in acute carrageenan-induced inflammation in the rat paw. Naunyn Schmiedebergs Arch Pharmacol 2004;369:294–9. [DOI] [PubMed] [Google Scholar]
- [9].De Petrocellis L, Ligresti A, Moriello AS, Allara M, Bisogno T, Petrosino S, Stott CG, Di Marzo V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol 2011;163:1479–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Grönblad M, Konttinen YT, Korkala O, Liesi P, Hukkanen M, Polak JM. Neuropeptides in the synovium of patients with rheumatoid arthritis and osteoarthritis. J Rheumatol 1988;15:1807–10. [PubMed] [Google Scholar]
- [11].Guermazi A, Roemer FW, Hayashi D, Crema MD, Niu J, Zhang Y, Marra MD, Katur A, Lynch JA, El-Khoury GY, Baker K, Hughes LB, Nevitt MC, Felson DT. Assessment of synovitis with contrast-enhanced MRI using a whole-joint semiquantitative scoring system in people with, or at high risk of, knee osteoarthritis: the MOST study. Ann Rheum Dis 2011;70:805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hammell D, Zhang L, Ma F, Abshire S, Mcilwrath S, Stinchcomb A, Westlund K. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain 2015;20:936–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hill CL, Hunter DJ, Niu J, Clancy M, Guermazi A, Genant H, Gale D, Grainger A, Conaghan P. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis 2007;66:1599–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Iannotti FA, Hill CL, Leo A, Alhusaini A, Soubrane C, Mazzarella E, Russo E, Whalley BJ, Di Marzo V, Stephens GJ. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS Chem Neurosci 2013;5:1131–41. [DOI] [PubMed] [Google Scholar]
- [15].Iuvone T, Espsito G, De Fillippis D, Scuderi C, Steardo L. Cannabidiol: a promising drug for neurodegenerative disorders? CNS Neurosci Ther 2009;1:65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kelly S, Dobson KL, Harris J. Spinal nociceptive reflexes are sensitized in the monosodium iodoacetate model of osteoarthritis pain in the rat. Osteoarth Cartil 2013;21:1327–35. [DOI] [PubMed] [Google Scholar]
- [17].Kelly S, Chapman RJ, Woodhams S, Sagar DR, Turner J, Burston JJ, Bullock C, Paton K, Huang J, Wong A, McWilliams DF, Okine BN, Barrett DA, Walsh DA, Chapman V. Increased function of pronociceptive TRPV1 at the level of the joint in a rat model of osteoarthritis pain. Ann Rheum Dis 2015;74:252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Krustev E, Rioux D, McDougall JJ. Mechanisms and mediators that drive arthritis pain. Curr Osteoporos Rep 2015;13:216–24. [DOI] [PubMed] [Google Scholar]
- [19].Krustev E, Muley MM, McDougall JJ. Endocannabinoids inhibit neurogenic inflammation in murine joints by a non-canonical cannabinoid receptor mechanism. Neuropeptides 2017;64:131–5. [DOI] [PubMed] [Google Scholar]
- [20].Malfait AM, Gallily R, Sumariwalla PF, Malik AS, Andreakos E, Mechoulam R, Feldmann M. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci 2000;97:9561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].McDougall JJ. Arthritis and pain. Neurogenic origin of joint pain. Arthritis Res Ther 2006;8:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].McDougall JJ, Andruski B, Schuelert N, Hallgrimsson B, Matyas JR. Unravelling the relationship between age, nociception and joint destruction in naturally occurring osteoarthritis of Dunkin Hartley Guinea pigs. PAIN 2009;141:222–32. [DOI] [PubMed] [Google Scholar]
- [23].McDougall JJ. Cannabinoids and pain control in the periphery. In: Cairns B, editors. Peripheral receptor targets for analgesia: novel approaches to pain. Hoboken, NJ: John Wiley & Sons, 2009. p. 325–45. [Google Scholar]
- [24].McDougall JJ. Peripheral analgesia: hitting pain where it hurts. BBA Mol Basis Dis 2011;1812:459–67. [DOI] [PubMed] [Google Scholar]
- [25].McDougall JJ, Albacete S, Schülert N, Mitchell PG, Lin C, Oskins JL, Biu H, Chambers MG. Lysophosphatidic acid provides a missing link between osteoarthritis and joint neuropathic pain. Osteoarth Cartil 2017;25:926–34. [DOI] [PubMed] [Google Scholar]
- [26].Mechoulam R, Gaoni Y. Hashish. IV. The isolation and structure of cannabinolic, cannabidiolic and cannabigerolic acids. Tetrahedron 1965;21:1223–9. [DOI] [PubMed] [Google Scholar]
- [27].Moilanen LJ, Hamalainen M, Nummenmaa E, Ilmarinen P, Vuolteenaho K, Nieminen RM, Lehtimaki L, Moilanen E. Monosodium iodoacetate-induced inflammation and joint pain are reduced in TRPA1 deficient mice–potential role of TRPA1 in osteoarthritis. Osteoarth Cartil 2015;23:2017–26. [DOI] [PubMed] [Google Scholar]
- [28].Muley MM, Krustev E, McDougall JJ. Preclinical assessment of inflammatory pain. CNS Neurosci Ther 2016;22:88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Poole AR. An introduction to the pathophysiology of osteoarthritis. Front Biosci 1999;4:D662–70. [DOI] [PubMed] [Google Scholar]
- [30].Pryce G, Riddall DR, Selwood DL, Giovannoni G, Baker D. Neuroprotection in experimental autoimmune encephalomyelitis and progressive multiple sclerosis by cannabis-based cannabinoids. J Neuroimmune Pharmacol 2015;10:281–92. [DOI] [PubMed] [Google Scholar]
- [31].Richardson D, Pearson RG, Kurian N, Latif ML, Garle MJ, Barrett DA, Kendall DA, Scammell BE, Reeve AJ, Chapman V. Characterisation of the cannabinoid receptor system in synovial tissue and fluid in patients with osteoarthritis and rheumatoid arthritis. Arthritis Res Ther 2008;10:R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, Sokolove J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol 2016;12:580–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol 2007;152:1092–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Schomberg D, Ahmed M, Miranpuri G, Olson J, Resnick D. Neuropathic pain: role of inflammation, immune response, and ion channel activity in central injury mechanisms. Ann Neurosci 2012;19:125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Schuelert N, McDougall JJ. Electrophysiological evidence that the vasoactive intestinal peptide receptor antagonist VIP6-28 reduces nociception in an animal model of osteoarthritis. Osteoarth Cartil 2006;14:1155–62. [DOI] [PubMed] [Google Scholar]
- [36].Schuelert N, McDougall JJ. Cannabinoid-mediated antinociception is enhanced in rat osteoarthritis knees. Arthritis Rheum 2008;58:145–53. [DOI] [PubMed] [Google Scholar]
- [37].Schuelert N, McDougall JJ. Grading of monosodium iodoacetate-induced osteoarthritis reveals a concentration-dependent sensitization of nociceptors in the knee joint of the rat. Neurosci Lett 2009;465:184–8. [DOI] [PubMed] [Google Scholar]
- [38].Schuelert N, Zhang C, Mogg AJ, Broad LM, Hepburn DL, Nisenbaum ES, Johnson MP, McDougall JJ. Paradoxical effects of the cannabinoid CB2 receptor agonist GW405833 on rat osteoarthritic knee joint pain. Osteoarth Cartil 2010;18:1536–43. [DOI] [PubMed] [Google Scholar]
- [39].Thakur M, Rahman W, Hobbs C, Dickenson AH, Bennett DL. Characterisation of a peripheral neuropathic component of the rat monoiodoacetate model of osteoarthritis. PLoS One 2012;7:e33730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol 2007;150:613–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Varga A, Nemeth J, Szabo A, McDougall JJ, Zhang C, Elekes K, Pinter E, Szolcsanyi J, Helyes Z. Effects of the novel TRPV1 receptor antagonist SB366791 in vitro and in vivo in the rat. Neurosci Lett 2005;385:137–42. [DOI] [PubMed] [Google Scholar]
- [42].Weydt P, Hong S, Witting A, Moller T, Stella N, Kliot M. Cannabinol delays symptom onset in SOD1 (G93A) transgenic mice without affecting survival. Amyotroph Lateral Scler Other Motor Neuron Disord 2005;6:182–4. [DOI] [PubMed] [Google Scholar]
- [43].Zhao Y, Yuan Z, Liu Y, Xue J, Tian Y, Liu W, Zhang W, Shen Y, Xu W, Liang X, Chen T. Activation of cannabinoid CB2 receptor ameliorates atherosclerosis associated with suppression of adhesion molecules. J Cardiovasc Pharmacol 2010;55:292–8. [DOI] [PubMed] [Google Scholar]


