Supplemental Digital Content is Available in the Text.
Cross-sectional observational study in a cohort of 232 diabetic polyneuropathy patients confirmed higher severity of neuropathy and predominant loss-of-function sensory profile in painful cases.
Keywords: Painful diabetic neuropathy, Sensory profile, Quantitative sensory testing, Risk factors
Abstract
Different sensory profiles in diabetic distal symmetrical sensory-motor polyneuropathy (DSPN) may be associated with pain and the responsiveness to analgesia. We aimed to characterize sensory phenotypes of patients with painful and painless diabetic neuropathy and to assess demographic, clinical, metabolic, and electrophysiological parameters related to the presence of neuropathic pain in a large cohort of well-defined DSPN subjects. This observational cross-sectional multi-center cohort study (performed as part of the ncRNAPain EU consortium) of 232 subjects with nonpainful (n = 74) and painful (n = 158) DSPN associated with diabetes mellitus of type 1 and 2 (median age 63 years, range 21-87 years; 92 women) comprised detailed history taking, laboratory tests, neurological examination, quantitative sensory testing, nerve conduction studies, and neuropathy severity scores. All parameters were analyzed with regard to the presence and severity of neuropathic pain. Neuropathic pain was positively correlated with the severity of neuropathy and thermal hyposensitivity (P < 0.001). A minority of patients with painful DSPN (14.6%) had a sensory profile, indicating thermal hypersensitivity that was associated with less severe neuropathy. Neuropathic pain was further linked to female sex and higher cognitive appraisal of pain as assessed by the pain catastrophizing scale (P < 0.001), while parameters related to diabetes showed no influence on neuropathic pain with the exception of laboratory signs of nephropathy. This study confirms the value of comprehensive DSPN phenotyping and underlines the importance of the severity of neuropathy for the presence of pain. Different sensory phenotypes might be useful for stratification of patients with painful DSPN for analgesic treatment and drug trials.
1. Introduction
Neuropathic pain (NeuP) has multiple pathophysiological mechanisms that represent potential targets for tailored therapy. Because NeuP is defined as pain caused by a lesion or disease affecting the somatosensory system,39 there have been attempts to identify sensory phenotypes that may reflect these pathophysiological mechanisms38 and to identify NeuP biomarkers. For example, sensory function is lost or reduced in a group of patients, which might be indicative of deafferentation (DA), whereas other patients have evidence of preserved small-fiber function and associated hypersensitivity, a pattern termed the “irritable nociceptor” (IN).9,13
There exist several types of painful neuropathies in patients with diabetes, but painful diabetic distal symmetrical sensory-motor polyneuropathy (pDSPN) as a variant of a typical symmetrical length-dependent DSPN is the most frequent. The findings that characterize pDSPN compared with painless DSPN (nDSPN) have been addressed in many studies, mainly with the goal of better understanding the mechanisms of NeuP in pDSPN, determining risk markers for pain development, and targeting therapy.3,32 The results, however, are mixed and sometimes even controversial, partly because of sample heterogeneity and small sample sizes.3 A recent cross-sectional observational study in a large cohort of patients with DSPN gave the first robust results, providing the rationale for a further phenotyping of pDSPN.38 These results included that pDSPN severity was positively correlated with pain.
To investigate further the question of why some persons with DSPN report no pain while the same disease leads to severe pain in others, we aimed to characterize sensory phenotypes together with clinical and neurophysiological parameters possibly affecting the development of NeuP in a large and well-defined cohort of patients with DSPN. Our objectives were to identify the pattern of symptoms and signs of large and small-fiber dysfunction that would distinguish pDSPN and nDSPN.
2. Material and methods
2.1 Study design and patients
This observational, cross-sectional, multicenter cohort study was part of the international “ncRNAPain consortium” (http://www.ncrna-pain.eu/). It was approved by the respective local authorities: the Ethical committees of the University Hospital Brno (No.602133), and the Rhineland—Palatinate medical association (9142-F), and registered at the German Clinical Trials Register; https://www.germanctr.de/ (Registration Number DRKS00008964).
Patients with diabetes mellitus type 1 or 2 older than 18 years with diagnosed DSPN, or patients with symptoms and signs suggestive of DSPN were recruited from 2 University Diabetes Centers in Brno (Czech Republic), from the Departments of Neurology and Anesthesiology in Würzburg and Department of Neurology in Mainz (Germany), and referred for a single clinical assessment to one of 3 study centers (Departments of Neurology in Brno, Wurzburg, and Mainz). Patients who were referred to our centers were consecutively recruited between January 2015 and June 2016 and agreed to participate in the study. All participants signed written informed consent before inclusion.
All patients first underwent evaluation to confirm the diagnosis of DSPN and their eligibility for the study. During this assessment, patients reported their medical and drug history in detail. They also reported on their age, ethnicity, education, employment status, medical history, date of diabetes diagnosis, its type and treatment. Alcohol consumption was quantified using standard drinks with a cutoff of 3/2 standard drinks/day in men/women.28 Basic clinical parameters were measured for each participant (weight, height, body mass index, and blood pressure). All patients also underwent basic blood tests to exclude other causes of polyneuropathy (vitamin B12 and folate levels, thyroid hormones, serum protein electrophoresis, blood count, serum creatinine, bilirubin and transaminases), glycosylated haemoglobin (HbA1C), and serum lipid spectrum. A structured neurological examination was conducted to assess clinical signs and symptoms of DSPN. Nerve conduction studies (NCS) and skin biopsy with evaluation of intraepidermal nerve fiber density (IENFD) (performed in patients who did not comply with NCS criteria for DSPN–9 cases) were conducted to confirm the diagnosis of DSPN.
Exclusion criteria were:
(1) NeuP due to other cause than DSPN
(2) Central nervous system lesions
(3) History or presence of laboratory abnormalities indicating a disease, condition, or treatment that might be a potential cause of polyneuropathy other than diabetes.
Included were patients with a combination of symptoms (decreased sensation, positive sensory symptoms, like burning or aching pain, mainly in the toes, feet, or legs) or signs (decreased distal sensation, decreased or absent ankle reflexes) of distal symmetrical sensory-motor diabetic neuropathy and abnormalities in either NCS or IENFD, who complied with the criteria for definite DSPN.37
Subjects meeting study inclusion and exclusion criteria then underwent quantitative sensory testing (QST), pain assessment, and evaluation of neuropathy severity and neuropsychological scores and questionnaires. Recruitment of study participants and the criteria used to subdivide the study participants into the different subgroups are shown in the flow diagram (Fig. 1).
Figure 1.
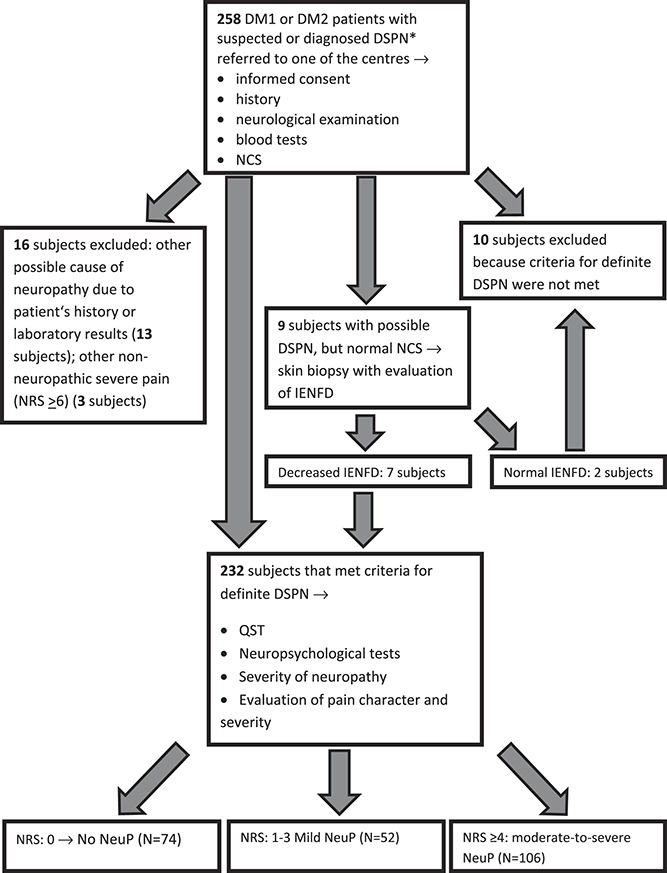
Flow diagram of study participant recruitment and the criteria to subdivide the study participants into the different subgroups. *Diagnosis was made outside one of the centres participating in the study. DM1, diabetes mellitus type 1; DM2, diabetes mellitus type 2; DSPN, diabetic polyneuropathy; IENFD, intraepidermal nerve fiber density; NCS, Nerve conduction studies; NeuP, Neuropathic pain; NRS, numerical rating scale; QST, quantitative sensory testing.
2.2. Structured neurological examination
A comprehensive neurological examination included assessment of light touch (using a brush), pain (pinprick), temperature (TipTherm) and vibration sensation (tuning fork), deep-tendon reflexes, muscle bulk, and motor power using a motor Medical Research Council (mMRC) scale25 modified by professors Sommer and Üçeyler (August 2013, unpublished). The mMRC scoring was extended to the following muscle pairs: elbow extensors, finger extensors, fingers spreading muscles, foot plantar flexors, big toe extensors, and big toe plantar flexors to better account for distal pareses. The mMRC sum score thus ranges from 0 (“total paralysis”) to 120 (“normal strength”). Autonomic signs and symptoms in the limbs (color changes, sweating, skin temperature, trophic changes) were also assessed.
2.3. Nerve conduction studies
The extent of electrophysiological examination was set to comply with the American Association of Electrodiagnostic Medicine (AAEM) definition of distal neuropathy for research purposes.11 Motor NCS of the peroneal, tibial, and ulnar nerves, including 10 consecutive F-waves, antidromic sensory nerve conduction of the sural and radial nerve, and concentric needle electromyography from at least 2 distal lower-extremity muscles and 1 distal upper-extremity muscle, were performed in a standard manner.2 The results were processed according to published reference values accepted by our EMG laboratories.20
2.4. Skin biopsy for intraepidermal nerve fiber density assessment
All skin biopsies were processed in the Brno laboratory. Skin punch biopsy samples were taken from the distal calf, approximately 10 cm above the right lateral malleolus. Frozen sections of 50 μm thickness were cut and immunostained with rabbit polyclonal antibodies to human PGP-9.5 (1:200; Ultraclone, Wellow, United Kingdom) as primary antibody and goat anti-rabbit IgG labelled with fluorescein probe as secondary antibody (1:100; Chemicon, Temecula, CA). The details of specimen removal and staining techniques have been published43 and follow standard recommendations.22 The average IENFD per millimeter of epidermal length was calculated according to current guidelines.22
2.5. Quantitative sensory testing
Skin and muscle sensitivity was tested using the standardized test battery for QST published by the German Research Network on Neuropathic Pain (Deutscher Forschungsverbund Neuropathischer Schmerz, DFNS).27 The standardized quantitative sensory testing assessment published by the DFNS contained 13 different thermal and mechanical tests: cold detection thresholds (CDT); warm detection thresholds (WDT); paradoxical heat sensations (PHS) during the procedure of alternating warm and cold stimuli (thermal sensory limen [TSL]); cold pain thresholds (CPT) and heat pain thresholds (HPT); mechanical detection thresholds for touch (MDT) and vibration (VDT); mechanical pain thresholds (MPT) for pinprick and pressure pain thresholds (PPT); a stimulus–response–function for pinprick sensitivity (mechanical pain sensitivity [MPS]); and dynamic mechanical allodynia (DMA) as well as pain summation to repetitive pinprick stimuli (wind-up ratio). All tests were performed on the dorsum of the foot, except for PPT (sole) and VDT (medial malleolus) on the right.
The Czech version of DFNS instructions have recently been validated, and the applicability of published reference values23 has been confirmed for the Czech population.36 For all parameters, negative (loss of function) as well as positive (gain of function) phenomena were assessed. Definition of QST abnormalities were based on DFNS recommendations24: if the individual z-values were outside of the 95% confidence interval of the reference group (ie, z-scores >1.96 or <−1.96), the values were designated as absolute abnormalities.
2.6. Pain assessment
Assessment of any pain was performed during neurological examination and included its descriptors, distribution, pain duration, intensity, time course, and analgesic treatment. Based on clinical evaluation and results of additional tests (QST, NCS, IENFD), pain in every patient was classified as NeuP and nonneuropathic using the International Association for the Study of Pain (IASP)/Neuropathic Pain Special Interest Group (NeuPSIG) definition.39
Severity of NeuP was quantified using an 11-step numerical rating scale (NRS). We assessed current pain intensity, mean, and minimum and maximum pain intensity during the preceding week. Ongoing analgesic therapy was not stopped before the assessment.
Patients with DSPN were further classified as pDSPN and nDSPN. Participants classified as pDSPN had to have chronic (ie, ≥3 months) peripheral NeuP at the time of the clinical assessment and to meet the criteria of probable or confirmed NeuP according to the updated IASP grading system.14
The severity of NeuP was rated according to the mean NRS during the last week before clinical examination. Study participants were thereafter allocated into 3 groups based on the mean pain intensity last week score: 0: no NeuP (nDSPN); 1 to 3: mild NeuP (pDSPN-m); and 4 to 10: moderate to severe NeuP (pDSPN-s).
In addition, pain and its impact on everyday life activities was quantified and characterized using the Graded Chronic Pain Scale (GCPS).44 The Neuropathic Pain Symptom Inventory (NPSI),6 a self-administered questionnaire, was applied to evaluate NeuP symptoms. We used the Czech and German validated versions of the NPSI.30,35
2.7. Neuropathy severity and neuropsychological scores and questionnaires
The modified Toronto Clinical Neuropathy Score (mTCNS) was applied as a tool to quantify DSPN. It consists of 6 symptoms scores and 5 sensory test scores, summarized as sum score of symptoms (0-18 points), sum score of sensory tests (0-15 points) and total sum score (0-33 points), and correlates with diabetic neuropathy severity.7 Similarly, the INCAT Overall Disability Sum Score (ODSS) was used to quantify disability in DSPN.25
The Beck Depression Inventory II (BDI II) and the State-Trait Anxiety Inventory Y (STAI Y) were used to measure symptoms of depression and anxiety.34 The Pain Catastrophizing Scale (PCS) was used to assess negative cognitions and appraisals of pain.26
2.8. Standardization of performed evaluations
During the preparation phase of the study, investigators from all 3 centers had 2 meetings focused on the standardized use of all tests and scores, on the use of validated versions of the questionnaires in Czech and German, and on the interpretation and possible pitfalls of all individual items of scores and tests. Evaluation of most of the tests and scores was done by 1 researcher in the Mainz (C.R.) and Würzburg (N.Ü.), and by 3 researchers in the Brno center (J.R., I.S., and I.K.), where the greatest part of the study group was recruited. The data were submitted to a common online database, which forces complete data entry and includes plausibility checks, and data were discussed in regular meetings.
2.9. Statistical evaluation
Standard measures of summary statistics were applied to describe primary data: Continuous parameters were summarized as median (5th-95th percentile range) or mean ± SD. Categorical parameters are expressed as absolute and relative frequencies.
P value represents the comparison of patients with different levels of pain (Kruskal–Wallis test for continuous variables and Fisher exact test for categorical variables). Normal distribution of continuous variables was inspected by graphical tools and confirmed by the Shapiro–Wilks test. The diagnostic power of potential predictors and final model scores were assessed on the basis of receiver operating characteristics (ROC) curves. The ROC analysis was performed using an ROC web calculator10 for curve fitting, SPSS 24.0.0.0.1 (IBM Corporation, 2016) for the area under the curve (AUC) computation and testing and MedCalc 11.1.0.0 (MedCalc Software 1993-2009) for computation of positive and negative predictive value. Significance of ROC analysis was based on the calculated AUC with corresponding 95% confidence interval. The computation was based on binormal assumption. The analyses used logistic regression models to quantify the association between potential predictors and the end point. Both a univariate-adjusted and a multivariate-adjusted approach were followed. The models used maximum likelihood estimation directly comparing the likelihood L0 for the null model when all slope parameters are zero, with the likelihood L1 of the fitted model. Significance of regression coefficients was tested by the help of Wald statistics, which is based on the asymptotic normality property of maximum likelihood estimates (tested on the basis of Chi-square distribution). Spearman correlation analysis was performed to explore associations between findings, clinical scores or pain intensities. The propensity score matching method was used to match subgroups.
3. Results
3.1. Study participants
Of 258 assessed patients, 232 study participants with DSPN were included (Fig. 1). Ten subjects did not meet the criteria for DSPN and 16 subjects met exclusion criteria. According to the presence and intensity of NeuP, study participants were divided into 3 groups: nDSPN (n = 74), (pDSPN-m) (n = 52), and pDSPN-s (n = 106); 66 of the latter had severe pain (NRS ≥ 6). In 21 pDSPN-m patients who responded to analgesic therapy, NRS before administration of analgesic therapy was reported to be ≥4.
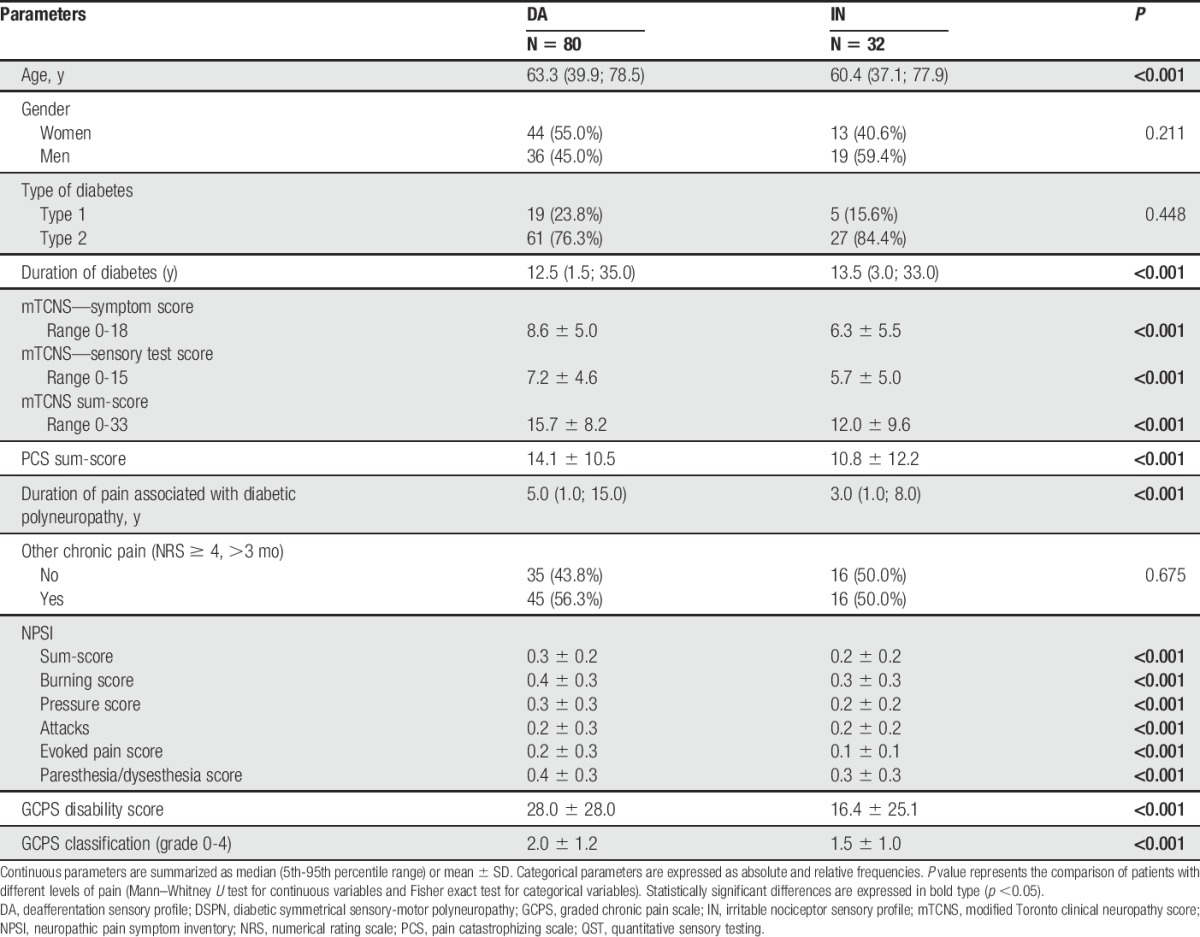
3.2. Demographics and diabetes characteristics
Most participants had type 2 diabetes (172 cases; 74.1%). The median age was 63 (range 21-87) years, and all participants were white, with 92 women (39.7%). Table 1 summarizes demographic and clinical data in relation to subtyping of NeuP. Women were overrepresented in pDSPN-s compared with nDSPN (P = 0.017).
Table 1.
Summary of demographic and clinical parameters.
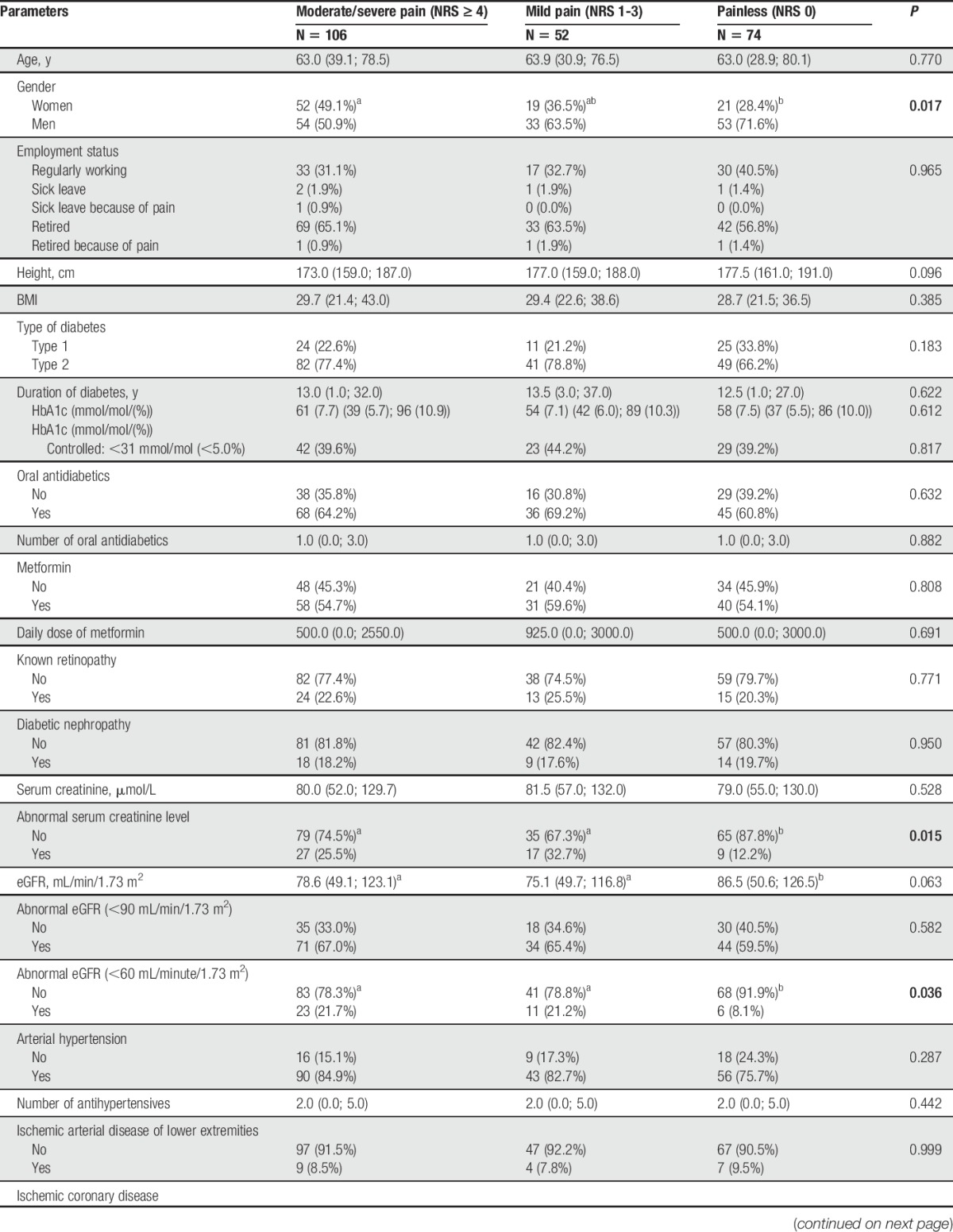
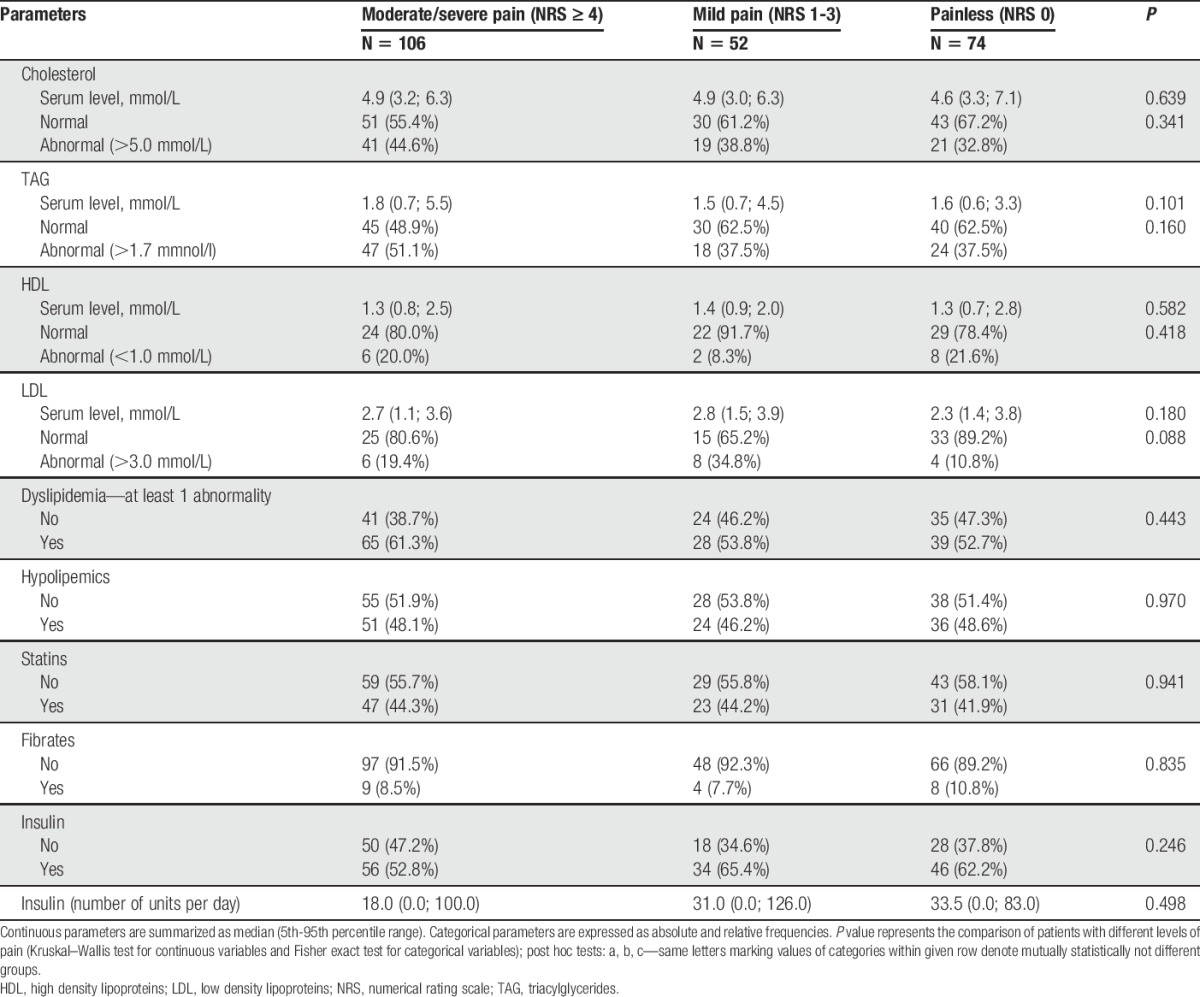
Laboratory data are given in Table 2 (with possible relation to diabetes) and Suppl. Table 1 (additional laboratory data; available online at http://links.lww.com/PAIN/A474). Regarding diabetes mellitus–related data, the only difference between pDSPN and nDSPN was the higher number of abnormally increased serum creatinine (P < 0.015) and abnormally decreased estimated glomerular filtration rate (P = 0.036) as markers of possible diabetic nephropathy in pDSPN.
Table 2.
Summary of laboratory parameters (related to diabetes).
3.3. Pain characteristics and questionnaires
All pDSPN patients reported descriptors typical of neuropathic pain.6,15 In all cases, pain was present symmetrically and distally in the feet, and in some patients, also in the hands.
Study participants in all groups reported additional pain of nonneuropathic origin, mostly low back pain, musculoskeletal pain, or headache (Table 3).
Table 3.
Summary of pain-related parameters, questionnaires, and neuropsychological scales.
As expected from the group definition, patients classified as pDSPN-s had higher NeuP intensities (Table 3) in comparison with those classified as pDSPN-m. Similarly, all subscores and the summary disability score of GCPS and the GCPS classification grade showed higher values in pDSPN-s compared with pDSPN-m and nDSPN.
Study participants with pDSPN-s reported greater severity of symptoms across all parameters of the NPSI compared with those with pDSPN-m (Table 3).
Psychological scales reflecting pain catastrophizing, depression, and anxiety (PCS, BDI II, STAI-S, and STAI-T) were higher (sum scores and some individual items) in pDSPN compared with nDSPN (BDI II, STAI-S, and STAI-T), and showed higher scores (PCS) in pDPS-s compared with pDSPN-m (Table 3).
3.4. Analgesic treatment
There was increased reported analgesic use in study participants with NeuP, and especially of those analgesics specific for treatment of neuropathic pain, that is, gabapentinoids and serotonin-norepinephrine reuptake inhibitors (mostly duloxetine) (Suppl. Table 2, available online at http://links.lww.com/PAIN/A474). These analgesics were prescribed only in a very small number of patients with nDSPN (in 4 patients—5.4%). Of 158 patients with pDSPN, 70 patients were treated with standard analgesics recommended in painful diabetic neuropathy, whereas 88 subjects received either nonrecommended analgesia or no treatment (Suppl. Table 2, available online at http://links.lww.com/PAIN/A474).
3.5. Clinical examination
Qualitative and semiquantitative sensory examination disclosed more frequent abnormalities representing loss of function (hypo/anesthesia) in pDSPN-s in comparison with nDSPN (cold and warm hypoesthesia, P < 0.006 and <0.011; pallhypoesthesia, P ≤ 0.006), and in comparison with the pDSPN-m subgroup (tactile hypoesthesia and mechanical hypoalgesia, P < 0.001) (Suppl. Table 3, available online at http://links.lww.com/PAIN/A474). Motor deficits expressed as the mMRC score of the lower extremities were greater in pDSPN-s compared with both nDSPN and pDSPN-m (P < 0.001). Autonomic signs/symptoms were not different between pain subgroups (Suppl. Table 3, available online at http://links.lww.com/PAIN/A474).
3.6. Quantitative sensory testing
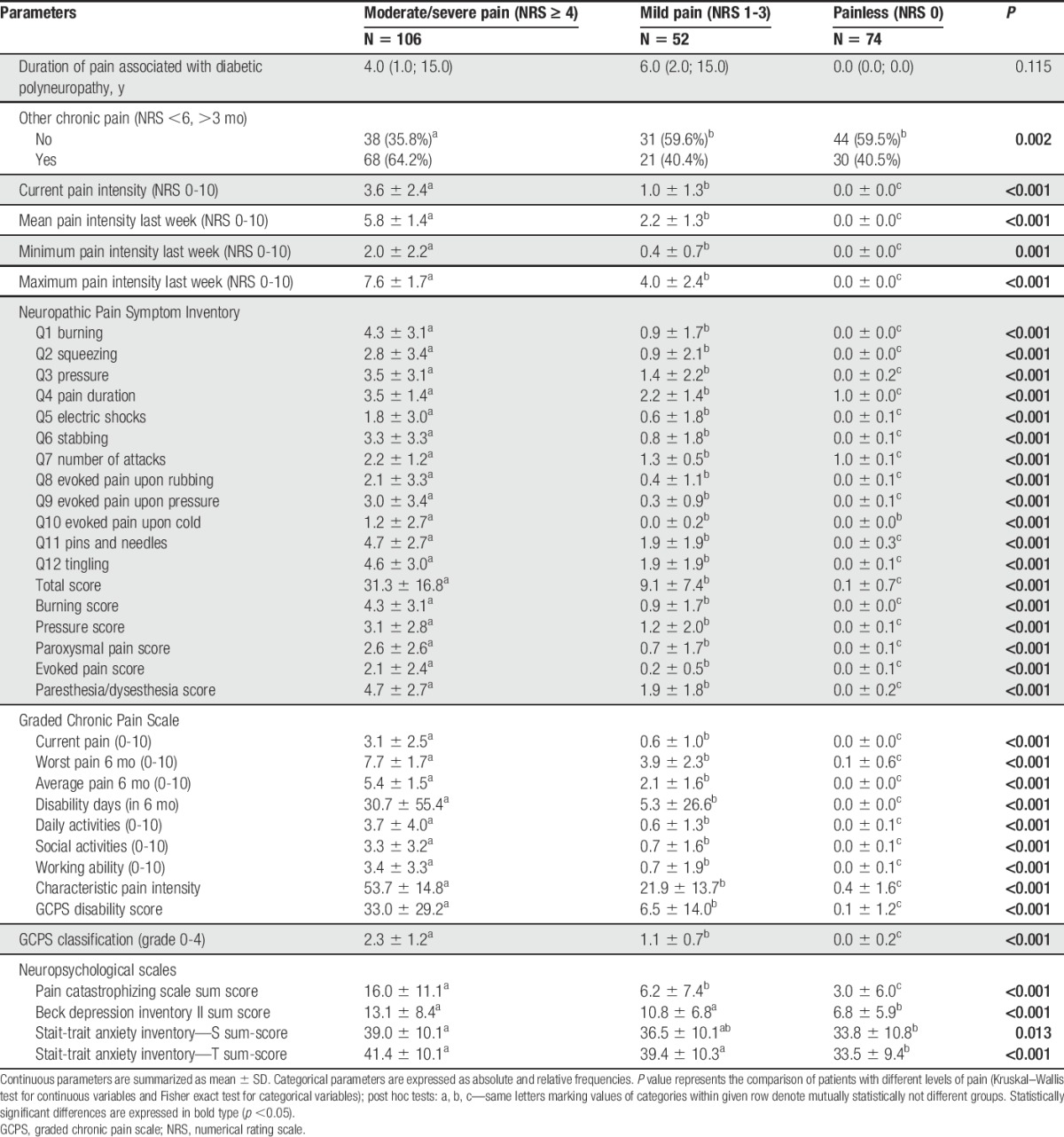
A substantial proportion of individual Z-scores in patients with DSPN fell outside the normative range (below −2 z-score). Group comparisons revealed differences in the loss of function direction in all 3 subgroups with the lowest z-score values (greatest loss) in pDSPN-s and the highest z-score values (the least loss) in nDSPN. We found differences between pDSPN-s and nDSPN for all thermal QST modalities (CDT, WDT, TSL, CPT, and HPT; P < 0.01), but also between pDSPN-s and pDSPN-m (CDT, WDT, TSL, and HPT; P < 0.05), and between pDSPN-m and nDSPN (WDT and TSL; P < 0.01, CDT; P < 0.05) (Suppl. Table 4, available online at http://links.lww.com/PAIN/A474; Fig. 2).
Figure 2.
Median and interquartile ranges of Z-scores for thermal QST parameters in patients with no neuropathic pain, mild and moderate/severe neuropathic pain. Kruskal–Wallis test, post hoc comparisons: *P < 0.05, **P < 0.01. CDT, cold detection threshold; CPT, cold pain threshold; HPT, heat pain threshold; QST, quantitative sensory testing; TSL, thermal sensory limen; WDT, warm detection threshold.
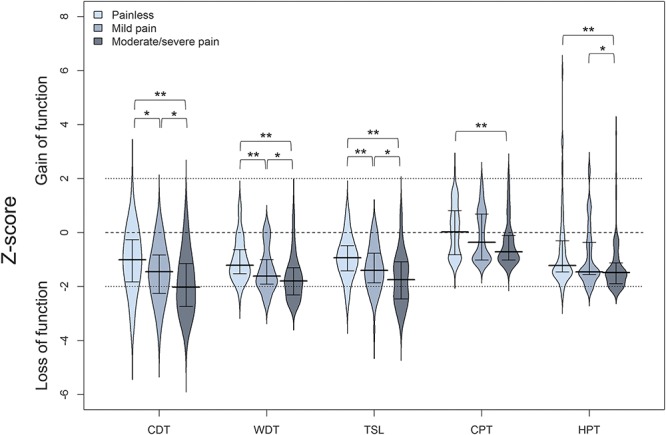
Quantitative sensory testing parameters for sensation of mechanical stimuli showed the same trend with less pronounced differences between subgroups. Group comparisons revealed a greater loss for MDT in pDSPN-s compared with nDSPN (P < 0.01) and pDSPN-m (P < 0.05), for MPS and MPT in pDSPN-s compared with nDSPN (P < 0.01, and P < 0.05, respectively) (Suppl. Table 4, available online at http://links.lww.com/PAIN/A474; Fig. 3).
Figure 3.
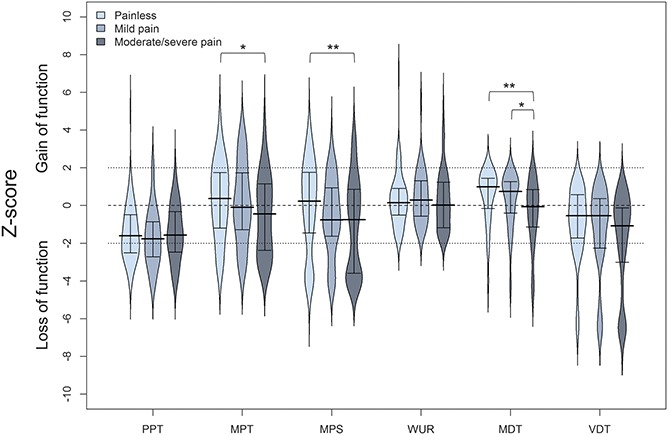
Median and interquartile ranges of Z-scores for mechanical QST parameters in patients with no neuropathic pain, mild and moderate/severe neuropathic pain. Kruskal–Wallis test, post hoc comparisons: *P < 0.05, **P < 0.01. MDT, mechanical detection threshold; MPS, mechanical pain sensitivity; MPT, mechanical pain threshold; PPT, pressure pain threshold; VDT, vibration detection threshold; WUR, wind-up ratio.
We did not observe a higher proportion of “gain” abnormalities in pDSPN with the exception of DMA and PHS (P < 0.05) (Fig. 3). Dynamic mechanical allodynia was found in 12 patients with pDSPN (7.6%), that is, in 8 (7.5%) patients with pDSPN-s and 4 (7.7%) patients with pDSPN-m. The patients with allodynia did not differ regarding demographic and clinical data, other QST data, NCS, and severity of DSPN (data not shown). In the NPSI, 31.9% of patients with pDSPN reported evoked pain (ie, allodynia), but only 7.6% had clinical evidence of brush-evoked allodynia. Paradoxical heat sensations was observed in all groups and was more frequent in pDSPN-s (53.8%) compared with pDSPN-m and nDSPN (P < 0.05) (Suppl. Table 4, available online at http://links.lww.com/PAIN/A474).
In summary, QST data from the feet displayed abnormalities in 93.1% of the subjects in the whole DSPN cohort. The percentage of any QST abnormality was higher in pDSPN compared with nDSPN (P < 0.05, Suppl. Table 4, available online at http://links.lww.com/PAIN/A474). The frequency of abnormal QST values (below 2 SD of healthy controls) is shown in Fig. 4. The overall pattern for both thermal and mechanical parameters expressed both as a mean z-score and proportion of patients with abnormal results was of “loss of function” type.
Figure 4.
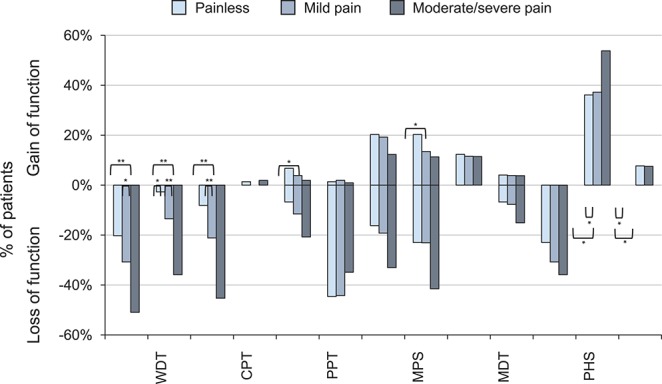
Loss and gain of functions (based on QST values outside the normal range according to DFNS reference data) in patients with no neuropathic pain, mild and moderate/severe neuropathic pain (body region–foot). Fisher exact test, post hoc comparison: *P < 0.05, **P < 0.01. CDT, cold detection threshold; CPT, cold pain threshold; DFNS, Deutscher Forschungsverbund Neuropathischer Schmerz; DMA, dynamic mechanical allodynia; HPT, heat pain threshold; MDT, mechanical detection threshold; MPS, mechanical pain sensitivity; MPT, mechanical pain threshold; PHS, paradoxical heat sensation; PPT, pressure pain threshold; QST, quantitative sensory testing; TSL, thermal sensory limen; VDT, vibration detection threshold; WDT, warm detection threshold; WUR, wind-up ratio.
The concept of the “irritable nociceptor” profile is of a sensory phenotype with preserved small-fiber function (cold, warm, and pinprick sensitivity) together with hyperalgesia, whereas the “deafferentation” profile is dominated by thermal or mechanical sensory loss (irrespective of the presence of gain).3,18 In pDSPN, using this definition, a DA profile prevailed (found in 53.8%), whereas an IN profile was less frequent (in 14.6%); 31.6% of patients did not fall into any of these definitions.
The comparison of subgroups of patients with painful DSPN with different sensory QST profiles showed that those with a DA profile had a higher severity of neuropathy (expressed as mTCNS scores, P < 0.001), longer duration of pain associated with DSPN (P < 0.001), and higher NPSI and GCPS sum scores (P < 0.001) (Table 4).
Table 4.
Comparison of patient characteristics between sensory QST phenotypes in painful DSPN patients.
3.7. Severity of diabetic neuropathy
The modified Toronto Clinical Neuropathy Score scores indicated a higher degree of disability in patients with pDSPN-s compared with pDSPN-m and nDSPN (Table 5). Namely, all individual symptom items and the mTCNS sum score of symptoms and the mTCNS total sum score discriminated all pain subgroups (P < 0.001), whereas the mTCNS sensory tests were able to discriminate pDSPN-s only (with the exception of thermal sensation that showed differences between all 3 subgroups) (P < 0.001) (Table 5 and Fig. 5 and Suppl. Fig. 1, 2; available online at http://links.lww.com/PAIN/A475).
Table 5.
Severity of diabetic neuropathy (mTCNS and ODSS scores).
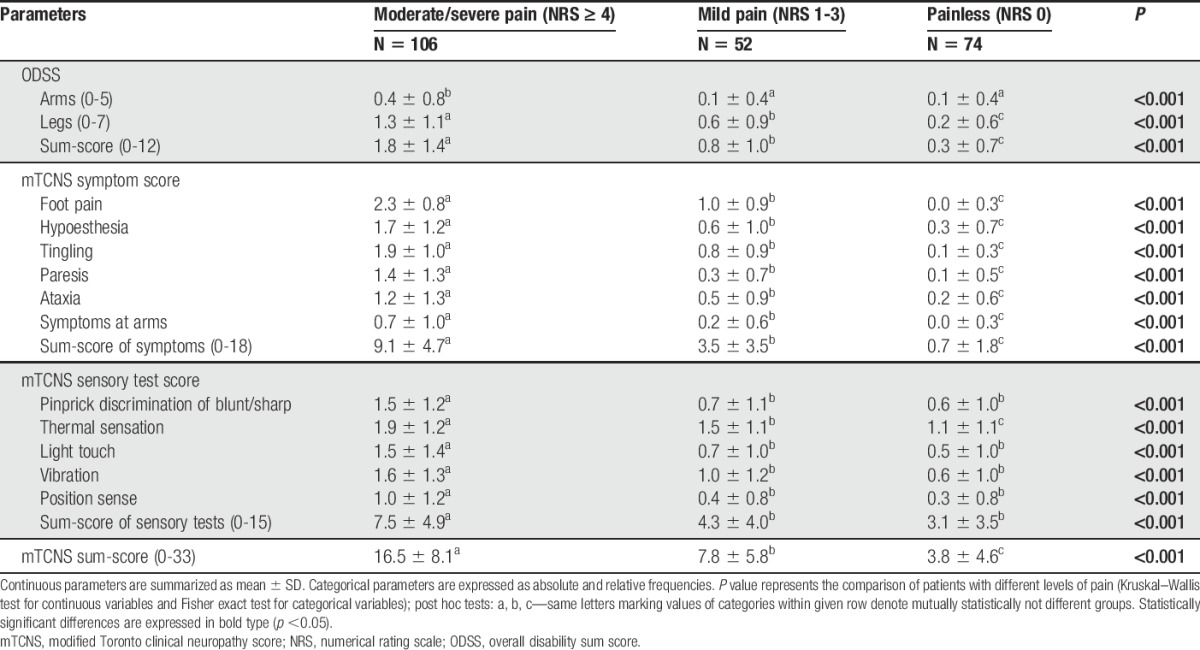
Figure 5.
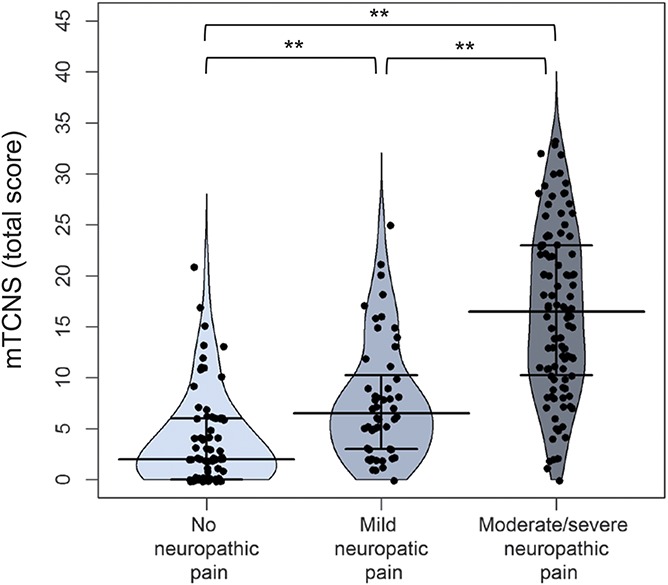
Median and interquartile ranges of modified Toronto Clinical Neuropathy Score (mTCNS) total score in patients with no neuropathic pain, mild and moderate/severe neuropathic pain. Kruskal–Wallis test, post hoc comparison: *P < 0.05, **P < 0.01.
Similarly, the ODSS discriminated all 3 pain subgroups (legs score and sum score) or the pDSPN-s subgroup (arms score, P < 0.001; Table 5 and Suppl. Fig. 3, available online at http://links.lww.com/PAIN/A475), and the ODSS sum score correlated with severity of pain (r = 0.60; P < 0.001).
3.8. The relation between clinical examination and quantitative sensory testing
Quantitative sensory testing thermal and mechanical parameters correlated well with the clinical scores when considering all study participants, that is, including those with and without NeuP (Suppl. Table 5, available online at http://links.lww.com/PAIN/A474). The mTCNS sum score correlated negatively with QST z-scores (P < 0.01 for CDT, WDT, TSL, CPT, HPT, MPT, MPS, MDT, and VDT; P < 0.05 for PPT and wind-up ratio). Similarly, the ODSS sum score correlated negatively with most QST z-scores (P < 0.001 for CDT, WDT, TSL, CPT, HPT, and MPS; P < 0.05 for MPT). Finally, the mMRC sum score correlated negatively with most thermal QST z-scores (P < 0.01 for CDT, WDT, TSL; P < 0.05 for CPT). Therefore, the higher the clinical scores, that is, more severe the DSPN, the greater was the loss of sensation on QST parameters. The correlation was stronger for thermal QST parameters.
3.9. Nerve conduction studies and skin biopsies
Nerve conduction studies were abnormal in all but 7 DSPN cases, in whom the diagnosis of DSPN was based on abnormal IENFD findings. There were no differences in any of the evaluated NCS parameters between pain subgroups (Suppl. Table 6, available online at http://links.lww.com/PAIN/A474).
IENFD was determined in 9 DSPN cases with normal NCS and was abnormal in 7 cases, in whom the diagnosis of DSPN was based on IENFD decrease; the median IENFD was 2.8 (range 0-10.0) fibers/mm.
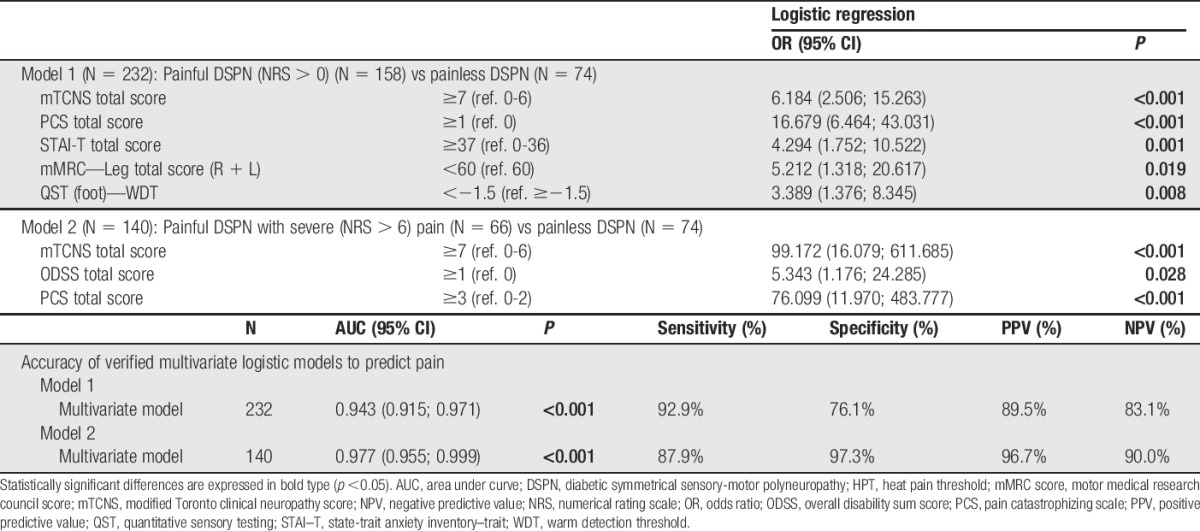
3.10. Logistic models to predict pain
Using multivariate logistic regression analysis and the most robust independent predictors (mTCNS total score ≥7, mMRC leg total score <60, WDT z-score <1.5 and abnormal serum creatinine), it was possible to find a model predicting the presence of NeuP with high sensitivity (>90%) and moderate specificity (76%) (Table 6). Accuracy of the predictive models in terms of sensitivity, specificity, and positive and negative predictive value was even higher in model 2, predicting/discriminating pDSPN with severe pain compared with painless cases and based on DSPN severity predictors (mTCNS total score ≥7, and ODSS total score >1; Table 6).
Table 6.
Multivariate logistic regression predictive models and its accuracy.
As mTCNS scores reflecting neuropathy severity seem to be the strongest predictors of NeuP, we compared characteristics of the pDSPN and nDSPN subgroups matched for the severity of neuropathy. Patients with pDSPN were more frequently women (P < 0.001; Suppl. Table 7, only selected parameters with significant differences included; available online at http://links.lww.com/PAIN/A474).
4. Discussion
This study reports detailed phenotyping using structured neurological examination and QST in a well-defined largest-ever published cohort of patients with DSPN. Key findings are that the presence and severity of NeuP was associated with more advanced diabetic neuropathy leading to higher disability and more frequent and advanced dysfunction of sensory fibers. No correlation was found between NeuP and factors related to diabetes and its control with the exception of laboratory signs of nephropathy. Our study, conducted in a large independent cohort, confirms key findings of a recent large-scale cohort study of DSPN subjects,38 and adds additional information.
There is a continuous effort to uncover distinct sensory profiles specific not only for the presence of NeuP as such, but also for predicting the response to therapy. Quantitative sensory testing findings in previous studies revealed a heterogeneity of sensory profiles in different pain syndromes, and some sensory profiles have been suggested to represent specific pathophysiological mechanisms.4,24 In a large cohort of 343 patients with painful polyneuropathy of different etiologies, 83.4% had a QST abnormality.24 Gain-of-function abnormalities were found in 31.2% and loss-of-function abnormalities in 72.9%.24 In previously described DSPN cohorts, loss-of-function abnormalities of small fiber–mediated and large fiber–mediated sensory modalities disclosed by not only QST, but also by clinical and electrophysiological methods was predominant, whereas gain-of-function abnormalities were only found in a minority,21,42 which corresponds to our findings.
Correlations of thermal QST abnormalities (CDT and WDT thresholds) with the severity of NeuP in patients with diabetic neuropathy have been reported previously in a smaller study.21 Others41 suggested that severe neuropathy is associated with an increased risk of developing pDSPN, although their small cohort was hospital-based and thus selective. In a cohort of diabetic patients with a low prevalence of neuropathy, pDSPN was more likely to occur in those with clinically manifest neuropathy.31 In a smaller community-based study, 51 patients with pDSPN had a higher severity of neuropathy compared with patients with painless DSPN.8
The PiNS study38 was the first that used the DFNS QST protocol in a large cohort of DSPN subjects with and without NeuP and correlated sensory profiles with the severity of NeuP, measures of neuropathy severity, and disability. In this study, the QST pattern of diabetic neuropathy was consistent with loss of function in both large and small sensory fibers. Furthermore, study participants with moderate to severe NeuP had the greatest loss of function in QST and more severe neuropathy assessed with neuropathy disability scores.38 Our results are in concordance with all these findings. The DA QST profile was predominant in our patients with pDSPN, and the frequency and severity of thermal loss correlated not only with the presence and severity of NeuP, but also to severity of neuropathy and disability. Gain-of-function sensory abnormalities in general were rare in pDSPN in both the PiNS and our study. Dynamic mechanical allodynia was the only gain-of-function abnormality in the PiNS, whereas we also observed a high frequency of PHS, in concordance with the findings of Maier et al.24 Paradoxical heat sensations was more frequent in our patients with moderate to severe pain. This is plausible because PHS represent disturbances of thermal processing in the peripheral or central nervous system. Such disturbances were recently reported as a typical component of a “loss of function” QST sensory cluster in neuropathic pain syndromes including polyneuropathies.4 The IN pattern, recently described as a “thermal hyperalgesia” sensory cluster,4was only present in a minority of our patients with painful DSPN (13.6%), although not so rarely as in the PiNS study (6.3%).38 Similar to the PiNS study,38 the DA profile prevailed in our study. Patients with the IN profile showed lower neuropathy disability scores, shorter duration of pain, higher level of pain catastrophizing, and different characteristics of pain associated with lower GCPS and NSPI scores in comparison with most patients with pDSPN with the DA profile. Further research is needed to confirm whether or not the IN profile might represent a promising target for stratification of patients for analgesic treatment and future drug trials.
Correlations between NeuP and hyposensitivity to small-fiber–mediated thermal perception in both our and the PiNS study, and the absence of any correlation between NeuP and NCS underline the importance of small-fiber loss or dysfunction for the generation of pDSPN. The subpopulation of pDSPN subjects with less hyposensitivity to thermal and mechanical pain stimuli, either manifesting as the IN profile or displaying gain-of-function abnormalities like DMA or PHS, might represent a subgroup with specific mechanisms of NeuP.
Study participants with more severe NeuP reported higher scores for anxiety, depressive symptoms, and pain catastrophizing, and also more frequently reported other chronic pain compared with the study participants with no NeuP and mild NeuP. The association of emotional distress with the severity of NeuP in DSPN, although not indicating causal relationship, is in concordance with a previous study that reported a high prevalence of depression and anxiety in pDSPN, and which underlined the necessity to integrate these psychological factors into treatment of NeuP in DSPN.29
As for demographic factors, the proportion of women displaying severe NeuP was higher compared with men. The “risk factor” female sex was already reported several times.1,12,17,19 The influence of age, reported by some studies,18,40,45,46 was neither confirmed in our study nor in PiNS study.38
Unsatisfactory diabetes control, usually expressed as elevated HbA1c levels, and other factors associated with diabetes have been repeatedly associated with the development of DSPN, whereas association of factors related to diabetes with painful DSPN discussed is contradictory.32 Among factors related to diabetes reported as associated with increased risk of pain in some studies, but not confirmed in the others, were diabetes duration,17–19,31,40 diabetes type 119 and type 2,1 dyslipidemia (low high density lipoproteins,40 increased triglycerides40), obesity, increased weight, body mass index or waist circumference,33,40,45,46 peripheral arterial disease,45,46 and nephropathy.40 The PiNS study reported higher levels of HbA1c in painful DSPN38 as the only parameter related to pain, although the association was not as robust as in the other factors, such as severity of neuropathy. In our study, we were not able to confirm an association of painful neuropathy with any of these parameters with the exception of a higher proportion of increased creatinine serum levels or decreased estimated glomerular filtration rate as a sign of nephropathy in patients with pain. Increased serum level of methylgyoxal has been shown in painful diabetic neuropathy5 and knockdown of glyoxalase 1 in mice causes alterations in kidney morphology indistinguishable from those caused by diabetes,16 but the possible link between pain and nephropathy in diabetes is still unclear.
In our study, several factors were associated with the presence and severity of NeuP. We calculated a predictive model including the most important independent predictors of pDSPN. This model included neuropathy severity (mTCNS total score, motor function in the legs [mMRC]), pain catastrophizing and anxiety (PCS and STAI-T total scores), and loss of C-fiber function (WDT). The model was able to predict the presence of pain in DSPN with excellent sensitivity and moderate specificity.
One of the limitations of our study was that the pain scoring was not based on a diary, but on a one-time assessment. As analgesic therapy was not stopped before assessment of pain severity, it might have influenced subclassification of painful cases into pDSPN-m and pDSPN-s subgroups. In fact, 21 painful DSPN cases who reported mild pain in the last week before assessment (the mean NRS during the last week between 1 and 3 points), reported pain of higher severity (NRS >4) before administration of analgesic treatment. Numerical rating scale values reported retrospectively with a delay of several months or years are, however, less reliable for subclassification of painful cases.
Despite the fact that patients were included consecutively into the study, the proportion of painful cases is higher than expected. This might be due to a higher motivation of patients with painful DSPN to participate.
The presence of foot pain that per definition must not be present in the painless cases was one of the items of mTCNS that were used to compare severity of neuropathy between painless and painful DSPN subgroups. This fact might slightly influence the differences in mTCNS symptoms and total scores, but not the mTCNS sensory tests subscore or the conclusion concerning the influence of severity of neuropathy on pain presence and severity in DSPN.
In conclusion, NeuP presence and severity is related to neuropathy severity, predominant thermal sensory loss, female sex, and nephropathy. It is also associated with levels of anxiety, depressive symptoms, and pain catastrophizing. A minority of pDSPN subjects display gain QST abnormalities. Different sensory profiles might represent distinctive pathophysiological mechanisms of NeuP in diabetes and new target populations for future pain trials.
Conflict of interest statement
J. Raputova, I. Srotova, E. Vlckova, I. Kovalova, B. Adamova, J. Bednarik, H. Rittner, C. Rebhorn, L. Forer, and F. Birklein report grants from FP7 EU Grant during the conduct of the study. N. Üçeyler reports grants from FP7 EU Grant during the conduct of the study; grants from Genzyme, grants from Shire, other from Daiichi Sankyo, personal fees from Baxalta, outside the submitted work. C. Sommer reports grants from FP7 EU Grant during the conduct of the study; personal fees from Air Liquide, personal fees from Astellas, personal fees from Baxalta, personal fees from CSL Behring, personal fees from Genzyme, personal fees from Pfizer, personal fees from UCB, grants from Kedrion, outside the submitted work. The remaining authors have no conflicts of interest to declare.
Supported by the European Commission (602133—ncRNAPain), and by the project “CEITEC—Central European Institute of Technology” (CZ.1.05/1.1.00/02.0068) from European Regional Development Fund.
Supplementary Material
Acknowledgements
The authors thank all patients for their participation and the research support team and medical and nursing staff at University Hospital in Würzburg, Mainz, and Brno for assistance with recruitment (Jana Novohradska, Barbara Broll, Barbara Dekant, Mathias Leinders, Sonja Mildner).
Author contributions: J. Raputova and I. Srotova were involved in the literature review, data collection including QST, data interpretation, data analysis, prepared the first draft of the manuscript and contributed equally to the article. I. Kovalova and C. Rebhorn contributed to data collection and data interpretation. E. Kralickova contributed to data interpretation and analysis (neuropsychological tests). B. Adamova contributed to collection and interpretation of electrophysiological data. J. Belobradkova, J. Olsovsky and P. Weber contributed to recruitment and pre-screening of diabetic patients in 2 diabetologic centers in Brno. L. Dusek contributed to planning of study design and power analysis, performed statistical analysis and contributed to manuscript preparation including figures. J. Jarkovsky contributed to interpretation of data (statistical analysis). L. Forer contributed to design and administration of online database used for data management. E. Vlckova was involved in literature review, contributed to study design, data collection (electrophysiological data), data interpretation, data analysis, prepared draft of the manuscript. N. Üçeyler contributed to study design, was involved in data collection, data interpretation, and reviewed/edited manuscript. H. Rittner contributed to data interpretation, data analysis, and reviewed/edited manuscript. C. Sommer and F. Birklein contributed to study design and data interpretation, to discussion and reviewed/edited manuscript. J. Bednarik assumed overall responsibility for the study, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. He contributed to study design, data analysis, data interpretation, manuscript preparation including figures, and writing, and prepared the first draft of the manuscript. All authors approved the final manuscript.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/A474 and http://links.lww.com/PAIN/A475.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
References
- [1].Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care 2011;34:2220–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].American Association of Electrodiagnostic Medicine. Guidelines in electrodiagnostic medicine. Muscle Nerve 1999;22(suppl 8):S3–300. [Google Scholar]
- [3].Baron R, Forster M, Binder A. Subgrouping of patients with neuropathic pain according to pain-related sensory abnormalities: a first step to a stratified treatment approach. Lancet Neurol 2012;11:999–1005. [DOI] [PubMed] [Google Scholar]
- [4].Baron R, Maier C, Attal N, Binder A, Bouhassira D, Cruccu G, Finnerup NB, Haanpää M, Hansson P, Hüllemann P, Jensen TS, Freynhagen R, Kennedy JD, Magerl W, Mainka T, Reimer M, Rice AS, Segerdahl M, Serra J, Sindrup S, Sommer C, Tölle T, Vollert J, Treede RD; German Neuropathic Pain Research Network (DFNS), and the EUROPAIN and NEUROPAIN consortia. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. PAIN 2017;158:261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bierhaus A, Fleming T, Stoyanov S, Leffler A, Babes A, Neacsu C, Sauer SK, Eberhardt M, Schnölzer M, Lasitschka F, Neuhuber WL, Kichko TI, Konrade I, Elvert R, Mier W, Pirags V, Lukic IK, Morcos M, Dehmer T, Rabbani N, Thornalley PJ, Edelstein D, Nau C, Forbes J, Humpert PM, Schwaninger M, Ziegler D, Stern DM, Cooper ME, Haberkorn U, Brownlee M, Reeh PW, Nawroth PP. Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nat Med 2012;18:926–33. [DOI] [PubMed] [Google Scholar]
- [6].Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, Cunin G, Fermanian J, Ginies P, Grun-Overdyking A, Jafari-Schluep H, Lantéri-Minet M, Laurent B, Mick G, Serrie A, Valade D, Vicaut E. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). PAIN 2005;114:29–36. [DOI] [PubMed] [Google Scholar]
- [7].Bril V, Tomioka S, Buchanan RA, Perkins BA; the mTCNS Study Group. Reliability and validity of the modified Toronto clinical neuropathy score in diabetic sensorimotor polyneuropathy. Diabetic Med 2009;26:240–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care 2006;29:1518–22. [DOI] [PubMed] [Google Scholar]
- [9].Demant DT, Lund K, Vollert J, Maier C, Segerdahl M, Finnerup NB, Jensen TS, Sindrup SH. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: a randomised, double-blind, placebo controlled phenotype-stratified study. PAIN 2014;155:2263–73. [DOI] [PubMed] [Google Scholar]
- [10].Eng J. ROC analysis: web-based calculator for ROC curves. Baltimore: Johns Hopkins University; Available at: http://www.jrocfit.org. Accessed March 19, 2014. [Google Scholar]
- [11].England JD, Gronseth GS, Franklin G, Carter GT, Kinsella LJ, Cohen JA, Asbury AK, Szigeti K, Lupski JR, Latov N, Lewis RA, Low PA, Fisher MA, Herrmann D, Howard JF, Lauria G, Miller RG, Polydefkis M, Sumner AJ; American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Evaluation of distal symmetric polyneuropathy: the role of autonomic testing, nerve biopsy, and skin biopsy (an evidence-based review). Muscle Nerve 2009;39:106–15. [DOI] [PubMed] [Google Scholar]
- [12].Erbas T, Ertas M, Yucel A, Keskinaslan A, Senocak M; TURNEP Study Group. Prevalence of peripheral neuropathy and painful peripheral neuropathy in Turkish diabetic patients. J Clin Neurophysiol 2011;28:51–5. [DOI] [PubMed] [Google Scholar]
- [13].Fields HL, Rowbotham M, Baron R. Postherpetic neuralgia: irritable nociceptors and deafferentation. Neurobiol Dis 1998;5:209–27. [DOI] [PubMed] [Google Scholar]
- [14].Finnerup NB, Haroutounian S, Kamerman P, Baron R, Bennett DLH, Bouhassira D, Cruccu G, Freeman R, Hansson P, Nurmikko T, Raja SN, Rice ASC, Serra J, Smith BH, Treeder RD, Jensen TS. Neuropathic pain: an updated grading system for research and clinical practice. PAIN 2016;157:1599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22:1911–20. [DOI] [PubMed] [Google Scholar]
- [16].Giacco F, Du X, D'Agati VD, Milne R, Sui G, Geoffrion M, Brownlee M. Knockdown of glyoxalase 1 mimics diabetic nephropathy in Nondiabetic mice. Diabetes 2014;63:291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Halawa MR, Karawagh A, Zeidan A, Mahmoud AE, Sakr M, Hegazy A. Prevalence of painful diabetic peripheral neuropathy among patients suffering from diabetes mellitus in Saudi Arabia. Curr Med Res Opin 2010;26:337–43. [DOI] [PubMed] [Google Scholar]
- [18].Harris M, Eastman R, Cowie C. Symptoms of sensory neuropathy in adults with NIDDM in the U.S. population. Diabetes Care 1993;16:1446–52. [DOI] [PubMed] [Google Scholar]
- [19].Jambart S, Ammache Z, Haddad F, Younes A, Hassoun A, Abdalla K, Selwan CA, Sunna N, Wajsbrot D, Youseif E. Prevalence of painful diabetic peripheral neuropathy among patients with diabetes mellitus in the Middle East region. J Int Med Res 2011;39:366–77. [DOI] [PubMed] [Google Scholar]
- [20].Kimura J. Electrodiagnosis in diseases of nerve and muscles. 4th ed New York: Oxford University Press, 2013. [Google Scholar]
- [21].Krämer HH, Rolke R, Bickel A, Birklein F. Thermal thresholds predict painfulness of diabetic neuropathies. Diabetes Care 2004;27:2386–91. [DOI] [PubMed] [Google Scholar]
- [22].Lauria G, Hsieh ST, Johansson O, Kennedy WR, Leger JM, Mellgren SI, Nolano M, Merkies IS, Polydefkis M, Smith AG, Sommer C, Valls-Solé J; European Federation of Neurological Societies; Peripheral Nerve Society. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol 2010;17:903–12. [DOI] [PubMed] [Google Scholar]
- [23].Magerl W, Krumova EK, Baron R, Tölle T, Treede RD, Maier C. Reference data for quantitative sensory testing (QST): refined stratification for age and a novel method for statistical comparison of group data. PAIN 2010;151:598–605. [DOI] [PubMed] [Google Scholar]
- [24].Maier C, Baron R, Tölle TR, Binder A, Birbaumer N, Birklein F, Gierthmühlen J, Flor H, Geber C, Huge V, Krumova EK, Landwehrmeyer GB, Magerl W, Maihöfner C, Richter H, Rolke R, Scherens A, Schwarz A, Sommer C, Tronnier V, Uçeyler N, Valet M, Wasner G, Treede RD. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. PAIN 2010;150:439–50. [DOI] [PubMed] [Google Scholar]
- [25].Merkies ISJ, Schmitz PIM, van der Meché FGA, Samijn JPA, van Doorn PA; Inflammatory Neuropathy Cause and Treatment (INCAT) group. Clinimetric evaluation of a new overall disability scale in immune mediated polyneuropathies. J Neurol Neurosurg Psychiatry 2002;72:596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Osman A, Barrios F, Kopper B, Hauptmann W, Jones J, O'Neill E. Factor structure, reliability, and validity of the pain catastrophizing scale. J Behav Med 1997;20:589–605. [DOI] [PubMed] [Google Scholar]
- [27].Rolke R, Baron R, Maier C, Tölle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Bötefür IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihöfner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. PAIN 2006;123:231–43. [DOI] [PubMed] [Google Scholar]
- [28].Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction 1993;88:791–804. [DOI] [PubMed] [Google Scholar]
- [29].Selvarajah D, Cash T, Sankar A, Thomas L, Davies J, Cachia E, Gandhi R, Wilkinson ID, Wilkinson N, Emery CJ, Tesfaye S. The contributors of emotional distress in painful diabetic neuropathy. Diab Vasc Dis Res 2014;11:218–25. [DOI] [PubMed] [Google Scholar]
- [30].Sommer C, Richter H, Rogausch JP, Frettlöh J, Lungenhausen M, Maier Ch. A modified score to identify and discriminate neuropathic pain: a study on the German version of the neuropathic pain symptom inventory (NPSI). BMC Neurol 2011;11:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sorensen L, Molyneaux L, Yue DK. Insensate vs painful diabetic neuropathy: the effects of height, gender, ethnicity and glycemic control. Diabetes Res Clin Pract 2002;57:45–51. [DOI] [PubMed] [Google Scholar]
- [32].Spallone V, Greco C. Painful and painless diabetic neuropathy: one disease or two? Curr Diab Rep 2013;13:533–49. [DOI] [PubMed] [Google Scholar]
- [33].Spallone V, Morganti R, D'Amato C, Cacciotti L, Fedele T, Maiello MR, Marfia G. Clinical correlates of painful diabetic neuropathy and relationship of neuropathic pain with sensorimotor and autonomic nerve function. Eur J Pain 2011;15:153–60. [DOI] [PubMed] [Google Scholar]
- [34].Spielberger CD. Manual for the State-Trait Anxiety Inventory: STAI (Form Y). Palo Alto: Consulting Psychologists Press, 1983. [Google Scholar]
- [35].Srotova I, Vlckova E, Strakova J, Kincova S, Ryba L, Dusek L, Adamova B, Bednarik J. Validation of the Czech version of the Neuropathic Pain Symptom Inventory (NPSIcz). Cesk Slov Neurol N 2015;78/111:45–56. [Google Scholar]
- [36].Srotova I, Vlckova E, Strakova J, Kincova S, Adamova B, Dusek L, Jarkovsky J, Bednarik J. Validation of Czech version of DFNS protocol for quantitative sensory testing. Cesk Slov Neurol N 2015;78/111:442–52. [Google Scholar]
- [37].Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010;33:2285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Themistocleous AC, Ramirez JD, Shillo PR, Lees JG, Selvarajah D, Orengo C, Tesfaye S, Rice AS, Bennett DL. The Pain in Neuropathy Study (PiNS): a cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. PAIN 2016;157:1132–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, Hansson P, Hughes R, Nurmikko T, Serra J. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 2008;70:1630–5. [DOI] [PubMed] [Google Scholar]
- [40].Van Acker K, Bouhassira D, De Bacquer D, Weiss S, Matthys K, Raemen H, Mathieu C, Colin IM. Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes Metab 2009;35:206–13. [DOI] [PubMed] [Google Scholar]
- [41].Veves A, Manes C, Murray HJ, Young MJ, Boulton AJ. Painful neuropathy and foot ulceration in diabetic patients. Diabetes Care 1993;16:1187–9. [DOI] [PubMed] [Google Scholar]
- [42].Vlckova-Moravcova E, Bednarik J, Belobradkova J, Sommer C. Small-fiber involvement in diabetic patients with neuropathic foot pain. Diabet Med 2008;25:692–9. [DOI] [PubMed] [Google Scholar]
- [43].Vlckova-Moravcova E, Bednarik J, Dušek L, Toyka KV, Sommer C. Diagnostic validity of epidermal nerve fiber densities in painful sensory neuropathies. Muscle Nerve 2008;37:50–60. [DOI] [PubMed] [Google Scholar]
- [44].Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. PAIN 1992;50:133–49. [DOI] [PubMed] [Google Scholar]
- [45].Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A; KORA Study Group. Neuropathic pain in diabetes, prediabetes and normal glucose tolerance: the MONICA/KORA Augsburg Surveys S2 and S3. Pain Med 2009;10:393–400. [DOI] [PubMed] [Google Scholar]
- [46].Ziegler D, Rathmann W, Meisinger C, Dickhaus T, Mielck A; KORA Study Group. Prevalence and risk factors of neuropathic pain in survivors of myocardial infarction with pre-diabetes and diabetes. The KORA Myocardial Infarction Registry. Eur J Pain 2009;13:582–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


