Abstract
Purpose
Endoscopic submucosal dissection (ESD) is a minimally invasive treatment for early gastric cancer (EGC) with negligible risk of lymph node metastasis (LNM). When a patient is determined to have noncurative resection after ESD, additional surgical resection with lymph node dissection is recommended. Previous studies report that LNM is found in about 10% of these patients. It may be possible to avoid unnecessary surgical resection by selecting patients properly. We aimed to clarify the risk factors associated with LNM in EGC patients who underwent ESD and to develop a highly accurate diagnostic algorithm for LNM.
Patients and methods
Among 1005 patients with EGC who underwent ESD, 423 patients who could be followed up for more than 3 years after treatment or who underwent additional surgical resection were examined. We used the leave-one-out method to explore the combination of predictive factors of LNM and differentiated LNM by a unique classifier.
Results
Curative resection was achieved in 322 patients, whereas noncurative resection was achieved in 101 patients. In the noncurative resection group, LNM occurred in eight patients with additional surgical resection and one patient during follow-up. The combination of depth of invasion, lymphatic, and venous invasion showed the highest diagnostic performance and could differentiate LNM with 100% sensitivity, 86% specificity, and 86% diagnostic accuracy.
Conclusion
More than 500 μm submucosal invasion and lymphatic and venous invasion will be useful in assessing LNM after ESD for patients with EGC. When these three factors are not observed, follow-up alone might be appropriate and it may be possible to reduce unnecessary surgical resection.
Keywords: additional surgical resection, early gastric cancer, endoscopic submucosal dissection, leave-one-out method, lymph node metastasis
Introduction
Endoscopic submucosal dissection (ESD) for early gastric cancer has been developed as a minimally invasive treatment and lymph node dissection cannot be performed. Therefore, ESD is applied for early gastric cancer with negligible risk of lymph node metastasis (LNM). On the basis of the pathologic results of the patients with early gastric cancer treated by gastrectomy, Gotoda et al. 1 clarified the characteristics of gastric cancer with little risk of LNM, and thus the indications for ESD have been expanded carefully 2. There have been many reports of good outcomes of ESD treatment including for the expanded indications 3,4.
It is often difficult to correctly determine the indications for ESD before resection, and on the basis of the pathologic results after resection, additional gastrectomy with lymph node dissection is sometimes required. There are few reports in which the adequacy of surgical resection was validated in patients who received additional surgical resection after ESD. Although the prognoses of these patients were good, it was reported that LNM was actually identified in about 10% of them 5,6. It might be possible to avoid unnecessary surgical resection through proper patient selection. More accurate determination of the presence or absence of LNM could yield additional benefit.
There are many reports on the predictive factors for LNM. The aim of this study was to predict the presence of LNM with a high degree of accuracy by focusing solely on clinicopathologic data obtained from ESD and to examine criteria to determine whether patients should receive additional treatment after ESD or should be followed up. We selected predictive factors with 100% sensitivity to prevent a miss of LNM. We used the leave-one-out method 7 with high objectivity to explore LNM predictive markers and differentiated LNM by a unique classifier 8.
Patients and methods
Patients
Among 1005 patients with early gastric cancer who underwent ESD in Yamaguchi University Hospital between June 2004 and March 2015, 423 patients (343 men, 80 women) who could be followed up for more than 3 years after treatment or who had undergone additional surgical resection were examined (Fig. 1). The indication for treatment with ESD was early gastric cancer characterized by differentiated type according to the preoperative biopsy and no obvious submucosal invasion according to the endoscopic findings and endoscopic ultrasonography. ESD was performed by 10 specialists of the Japan Gastroenterological Endoscopy Society.
Fig. 1.
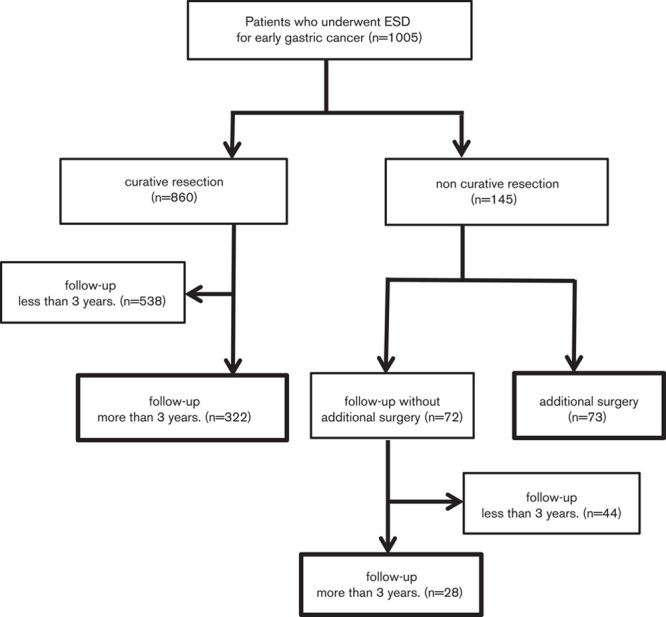
Clinical courses after endoscopic submucosal dissection (ESD).
Assessment of clinicopathological findings
Each patient was evaluated for the following clinicopathological factors: age, sex, tumor size, macroscopic type, differentiation type, depth of tumor invasion, horizontal/vertical margin, lymphatic invasion, venous invasion, and ulceration. Tumors were grouped according to size: up to 30 mm and more than 30 mm. Macroscopic type was classified as protruding type (types 0–I and 0–IIa) or flat or depressed type (types 0–IIb, 0–IIc, and 0–III). Lesions with a mixed protruding and depressed appearance were classified on the basis of whichever macroscopic type was dominant. Well-differentiated or moderately differentiated tubular adenocarcinoma and papillary adenocarcinoma were classified as differentiated carcinoma, whereas poorly differentiated tubular adenocarcinoma and signet-ring cell carcinoma were classified as undifferentiated carcinoma. Lesions containing both differentiated and undifferentiated carcinoma were classified on the basis of whichever macroscopic type was dominant. Depth of tumor invasion was classified as mucosal invasion (m), minute submucosal invasion within 500 μm of the lower margin of the muscularis mucosae (sm1), or submucosal invasion of more than 500 μm from the lower margin of the muscularis mucosae (sm2). Mucosal defect or convergence of mucous fold on endoscopy and deformation of the muscularis mucosae or fibrosis of the submucosal layer on histology were considered to be evidence of ulcer 2.
Definition of curative resection
Curative resection was defined as a lesion fulfilling the following criteria: en-bloc resection, differentiated type, mucosal cancer without ulcer, up to 20 mm in diameter, negative horizontal margin and negative vertical margin, and negative lymphovascular invasion. In addition, a case that fulfilled the following expanded criteria was eligible for curative resection: en-bloc resection, negative horizontal margin and negative vertical margin, negative lymphovascular invasion, and (i) differentiated type, mucosal cancer without ulcer, irrespective of size; (ii) differentiated type, mucosal cancer with ulcer, and up to 30 mm in diameter; (iii) undifferentiated type, mucosal cancer without ulcer, and up to 20 mm in diameter; and (iv) differentiated type cancer with microinvasion into the submucosal layer (≤500-μm penetration into the submucosa) without ulcer, and up to 30 mm in diameter. A case that did not meet any of these conditions was defined as a noncurative resection. The patients with curative resection were followed up, whereas those with noncurative resection were recommended to undergo additional surgical resection including D2 lymph node dissection. For these patients who underwent additional surgical resection, the presence or absence of LNM was confirmed at surgery. For those who rejected additional surgical resection, follow-up was performed. Computed tomography (CT) and esophagogastroduodenoscopy were performed once or more per year to evaluate metastasis and recurrence. A case of lymph node enlargement increased to 10 mm or more shown by CT was determined to be an LNM-positive case.
Selection method of the optimal combination of markers
The following eight clinicopathologic factors were used as predictive factors of LNM: sex, tumor size, gross morphology, differentiation type, depth of tumor invasion, lymphatic invasion, venous invasion, and presence or absence of ulcer. Of these eight markers, the combination of markers in which sensitivity to a diagnosis of LNM was 100% and specificity was maximal was selected and examined. We used the leave-one-out method 7 twice to find the optimal combination of markers and once to evaluate the marker combinations obtained.
Initially, to explore marker combinations, one case is selected as a test sample from the 423 cases on the basis of the leave-one-out method and the remaining 422 cases are assigned as training samples. A classifier is designed by 422 training samples, and this classifier distinguishes one test sample. This process is repeated until each sample is used only once as a test sample.
The design method of the classifier with the use of training samples is explained below. Then, one marker combination is selected and using the leave-one-out method once again, one case from the training samples is again selected as a subtest sample and the remaining 421 cases are used as subtraining samples. A classifier is designed that distinguishes the subtest sample. The procedure is repeated independently 422 times, and the sensitivity and specificity to the subtest samples are calculated. This process is repeated until all candidate marker combinations are evaluated. Specifically, the number of markers is changed between one and all eight of the markers. Among all combinations, one marker combination with 100% sensitivity and maximal specificity for a diagnosis of LNM for a subtest sample is selected. For the selected marker combination, another classifier is designed using the above-mentioned 422 training samples, and this classifier distinguishes the remaining one test sample. The above differentiation test is repeated independently 423 times. As a result, 423 different marker combinations are selected. The combination of markers selected most frequently was considered to contain the essential factors to predict LNM and this is the optimal combination of markers.
Identification of lymph node metastasis
The leave-one-out method is used once more to evaluate the optimal combination of markers. The optimal combination of markers is fixed and a classifier is designed using 423 cases according to the leave-one-out method. Then, the sensitivity, specificity, and rate of diagnostic accuracy for the test samples are estimated. Because the data used for a classifier included categorical data, a novel discrete Bayes classifier 8 was used instead of the conventional Bayes classifier. This classifier calculates the posterior probabilities of two classes using categorical data and distinguishes patients into a class with a maximal posterior probability.
Results
Table 1 shows the clinicopathologic characteristics of the 423 patients with early gastric cancer in whom ESD was performed. Of these patients, 322 underwent curative resection and 101 underwent noncurative resection, among whom 73 patients underwent additional surgical resection. The median follow-up periods in the curative resection patients and noncurative resection patients were 59 (36–124) and 61 (36–112) months, respectively. Local recurrence and LNM were not observed in the curative resection patients during follow-up, whereas in the noncurative resection patients, LNM was observed in eight of the 73 patients in whom additional surgical resection was performed. Among the 28 patients who had been followed up after noncurative resection, lymph node enlargement was observed by CT in one patient.
Table 1.
Clinicopathological characteristics of the 423 cases treated by endoscopic submucosal dissection
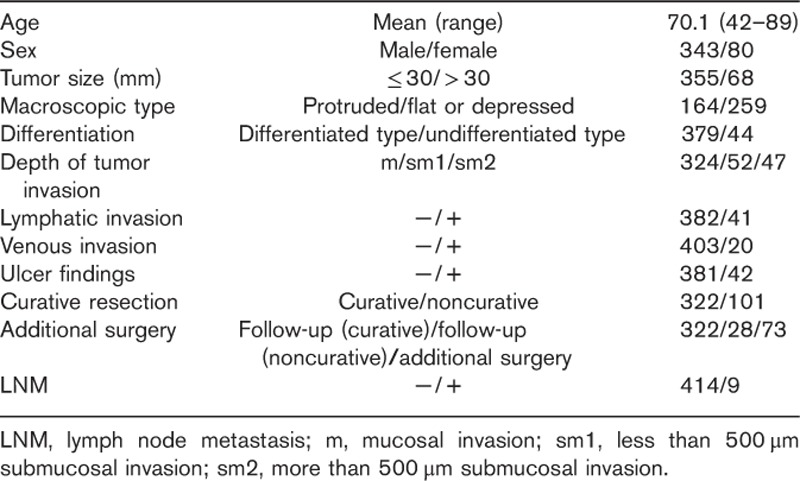
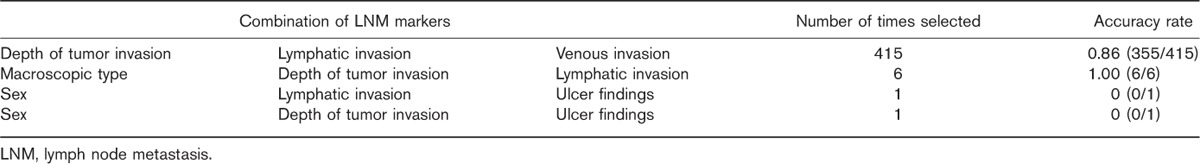
Table 2 shows the relationship between the clinicopathologic findings and LNM. On the basis of the results of 423 independent discrimination tests in the test samples, the most frequently selected combination of markers (in 415 tests) included the three factors of depth of tumor invasion, lymphatic invasion, and venous invasion (Table 3), and these were determined as the optimal combination of markers.
Table 2.
Association of clinicopathological characteristics of the 423 early gastric cancer lesions with lymph node metastasis
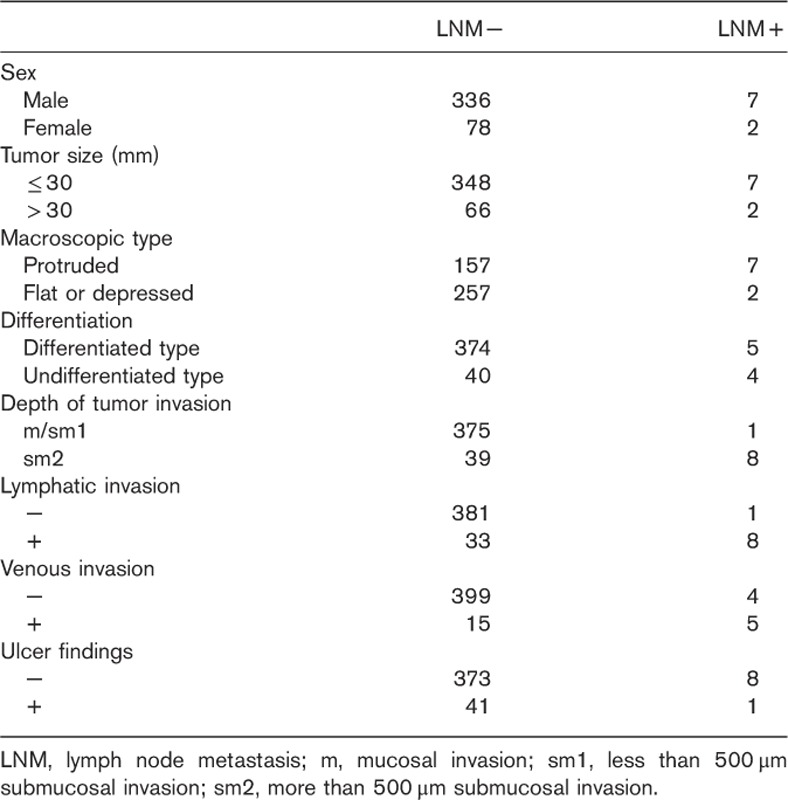
Table 3.
Number of times each marker was selected as a predictive factor of lymph node metastasis
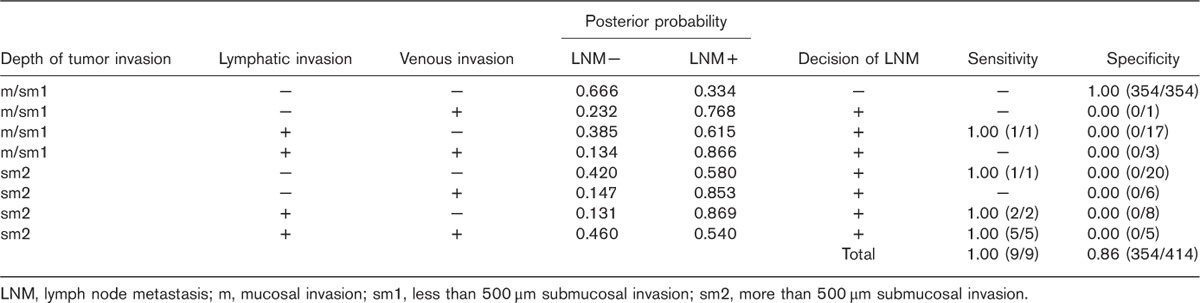
We then designed a classifier by fixing the combination of depth of tumor invasion, lymphatic invasion, and venous invasion using 423 cases according to the leave-one-out method. Table 4 shows the diagnostic capability of LNM by our original discrete Bayes classifier. Assuming that a patient is classified as depth of tumor invasion sm2, lymphatic invasion +, and venous invasion +, the posterior probabilities, P (LNM− | x)=0.46<P (LNM+ | x)=0.54, are obtained. In this pattern, the classifier distinguishes LNM+, which results in 100% sensitivity (5/5) and 0% specificity (0/5). After all 423 cases were examined, we could thus differentiate LNM with a sensitivity of 100%, a specificity of 86%, and a rate of diagnostic accuracy of 86%.
Table 4.
Diagnostic capability of lymph node metastasis
Discussion
In patients with early gastric cancer who underwent surgical resection, the respective incidences of LNM from intramucosal carcinoma and submucosal invasive cancer were reportedly 3.2 and 19.2% 9. It has been reported that a rate of LNM of ~10% was observed in additional surgical resection after ESD 5,6, and this resection was considered to be excessive invasion in many cases. However, even in elderly patients older than 75 years of age, it is reported that those who underwent additional surgical resection had a better prognosis than those who were only followed up 10. Therefore, those patients who may have LNM should not be left untreated. We believe that there are more essential factors to determine the incidence of LNM in the condition of noncurative resection and that by evaluating these factors appropriately, unnecessary surgical resection can be avoided. Especially in Japan, with its rapidly aging population, many patients have comorbidities for which surgical resection causes high risk; therefore, more accurate determination of the presence or absence of LNM could yield additional benefits.
There may be slight differences in the intended population between the cases of ESD and gastrectomy examined by Gotoda et al. 1. Thus, to investigate results more suited to the actual circumstances, we examined cases limited to ESD in this study.
The rate of curative resection for all 1005 patients was 85.6% (860/1005). With respect to curative resection, neither LNM nor distant metastasis was observed. Generally, in the case of expanded indications, there are few reports of patients who have died a tragic death by recurrence. This indicates that if we adhere to the guidelines with expanded indications, almost no recurrence occurs. However, among the 73 patients undergoing additional surgical resection, LNM was observed in only eight (11.0%). In other words, in the current guidelines, the sensitivity for detecting LNM is very high, but the specificity is insufficient. It is important to maintain high sensitivity so as not to cause deaths by the recurrence of early gastric cancer. The present study aimed to identify the predictive factors of LNM by which sensitivity was 100% and specificity was maximal.
We differentiated LNM using an original discrete Bayes classifier. This classifier is unique and can deal not only with non-numerical data but also with numerical data on the basis of Bayesian discrimination theory using posterior probability. To explore the optimal combination of markers and evaluate distinguishability, the leave-one-out method was adopted. By this estimation, we can eliminate arbitrariness in the determination of the LNM and obtain a very objective probability distribution.
Our results indicated that a combination of three factors, depth of invasion sm2, lymphatic invasion +, and venous invasion +, could differentiate LNM most significantly. If a physician recognizes even one of these three factors and determines the patient as being LNM+, the physician can differentiate LNM with a sensitivity of 100%, a specificity of 86%, and a rate of diagnostic accuracy of 86%. Even when we increased the number of factors, the diagnostic accuracy did not improve. Kim et al. 5 reported that lymphovascular invasion is the most important risk factor for LNM (odds ratio: 21.41). Lymphovascular invasion is also a prognostic factor for patients with noncurative resection who did not undergo additional surgical resection 11. If these three factors alone are applied as the indications of noncurative resection to the patients in this study, 14 of the 73 patients with additional surgical resection could have avoided surgery. In fact, LNM was not recognized in 14 patients. Narrowing the indications of noncurative resection to these three factors has the advantages that unnecessary surgery can be avoided, as discussed above, and there is no cumbersome procedure for the assessment of expanded indications as in the present guidelines. In particular, elements such as a mixture of differentiated and undifferentiated types and presence or absence of ulcer, which tend to produce ambiguity in the pathological determination, can be excluded.
In terms of the positive rate of LNM, there was a considerable difference between the follow-up group (1/28) and the additional surgical resection group (8/73). This was probably because the attending physician tended to strongly recommend additional resection for patients whose risk of LNM was particularly high as reported previously 5,11, such as in patients positive for lymphovascular invasion. Actually, the number of patients positive for lymphovascular invasion in the additional surgical resection group was 39 of 73, whereas that in the follow-up group was nine of 28.
Our study has several limitations because of its retrospective design and insufficient number of only nine LNM+ cases. To obtain highly accurate results even with a small number of samples, the leave-one-out method was used in this study. Because each sample is used as a training sample and as a test sample, but never as a training sample and a test sample, at the same time, the leave-one-out method is excellent in use efficiency and independence, and therefore, high estimated accuracy can be expected. Because this study deals with early gastric cancer that was believed to have fulfilled the indication of the guidelines preoperatively, cases clearly out of the indication were not included. This suggests that the study may not cover all patterns of early gastric cancer that can produce LNM. Previous examination by our department showed that the factors of tumor size of more than 30 mm, undifferentiated type, and ulcer +, which are included in the expanded indications, can become risk factors for incomplete resection with a positive margin 12, and thus these factors are still important in terms of the ESD procedure. Toyokawa et al. 13 reported that lymphovascular invasion and undifferentiated type are independent risk factors of LNM and Son et al. 6 reported that patients with a tumor size of more than 20 mm are at the highest risk for LNM. Some literature has described factors for LNM other than the three in our study, and there is room for the development of more accurate criteria by assessing more cases in multiple institutions. In the future, we will add the data from multiple institutions to our data and establish more precise criteria, and subsequently, we aim to carry out a prospective study to verify the validity of the findings of the present study.
Conclusion
Three factors, depth of invasion sm2, lymphatic invasion +, and venous invasion +, were suggested to be useful for the determination of LNM after ESD in patients with early gastric cancer. When these three factors are not observed, follow-up alone may be appropriate and it may be possible to reduce unnecessary surgical resection. For the purpose of clinical application, an additional prospective study carried out in multiple institutions is required.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- 1.Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000; 3:219–225. [DOI] [PubMed] [Google Scholar]
- 2.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (Ver. 3). Gastric Cancer 2011; 14:113–123. [DOI] [PubMed] [Google Scholar]
- 3.Peng LJ, Tian SN, Lu L, Chen H, Ouyang YY, Wu YJ. Outcome of endoscopic submucosal dissection for early gastric cancer of conventional and expanded indications: systematic review and meta-analysis. J Dig Dis 2015; 16:67–74. [DOI] [PubMed] [Google Scholar]
- 4.Oda I, Oyama T, Abe S, Ohnita K, Kosaka T, Hirasawa K, et al. Preliminary results of multicenter questionnaire study on long-term outcomes of curative endoscopic submucosal dissection for early gastric cancer. Dig Endosc 2014; 26:214–219. [DOI] [PubMed] [Google Scholar]
- 5.Kim H, Kim JH, Park JC, Lee YC, Noh SH, Kim H. Lymphovascular invasion is an important predictor of lymph node metastasis in endoscopically resected early gastric cancers. Oncol Rep 2011; 25:1589–1595. [DOI] [PubMed] [Google Scholar]
- 6.Son SY, Park JY, Ryu KW, Eom BW, Yoon HM, Cho SJ, et al. The risk factors for lymph node metastasis in early gastric cancer patients who underwent endoscopic resection: is the minimal lymph node dissection applicable? A retrospective study. Surg Endosc 2013; 27:3247–3253. [DOI] [PubMed] [Google Scholar]
- 7.Lachenbruch PA. Estimation of error rates in discriminant analysis [PhD Dissertation], University of California at Los Angeles; 1965.
- 8.Ogihara H, Iizuka N, Hamamoto Y. Prediction of early recurrence of liver cancer by a novel discrete bayes decision rule for personalized medicine. Biomed Res Int 2016; 2016:8567479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwee RM, Kwee TC. Predicting lymph node status in early gastric cancer. Gastric Cancer 2008; 11:134–148. [DOI] [PubMed] [Google Scholar]
- 10.Kusano C, Iwasaki M, Kaltenbach T, Conlin A, Oda I, Gotoda T. Should elderly patients undergo additional surgery after non-curative endoscopic resection for early gastric cancer? Long-term comparative outcomes. Am J Gastroenterol 2011; 106:1064–1069. [DOI] [PubMed] [Google Scholar]
- 11.Ahn JY, Jung HY, Choi JY, Kim MY, Lee JH, Choi KS, et al. Natural course of noncurative endoscopic resection of differentiated early gastric cancer. Endoscopy 2012; 44:1114–1120. [DOI] [PubMed] [Google Scholar]
- 12.Goto A, Nishikawa J, Okamoto T, Hamabe K, Nishimura J, Nakamura M, et al. Outcomes of endoscopic submucosal dissection for early gastric cancer and factors associated with incomplete resection. Hepatogastroenterology 2013; 60:46–53. [DOI] [PubMed] [Google Scholar]
- 13.Toyokawa T, Ohira M, Tanaka H, Minamino H, Sakurai K, Nagami Y, et al. Optimal management for patients not meeting the inclusion criteria after endoscopic submucosal dissection for gastric cancer. Surg Endosc 2016; 30:2404–2414. [DOI] [PubMed] [Google Scholar]


