Abstract
Background:
The pharmacokinetics of long-acting intramuscular paliperidone in a naturalistic setting is not well documented. The objective of this study was to investigate the relationship between dose and serum concentrations of paliperidone using data from a routine therapeutic drug monitoring service.
Methods:
Serum concentration measurements in 310 samples from 110 male and 75 female patients receiving depot injections of paliperidone were retrospectively retrieved from the therapeutic drug monitoring database.
Results:
The median dose was 100 mg every 28 days. The median concentration/dose (C/D) ratio of paliperidone was 16.1 (nmol/L)/(mg/d), with a 10–90 percentile range of 7.8–31.0 (nmol/L)/(mg/d). Dose-adjusted serum concentrations were 33% higher in patients 65 years or older and more than 50% lower in patients taking the p-glycoprotein inducer carbamazepine. There were no significant effects of sex or dose on the C/D ratio. The median serum concentrations of paliperidone at the end of the dose interval were 31 nmol/L at an intramuscular dose of 50 mg/28 d, 53 nmol/L after a dose of 75 mg/28 d, 59 nmol/L after a dose of 100 mg/28 d, and 93 nmol/L after a dose of 150 mg/28 d. Forty-five percent of the measurements were lower than the suggested therapeutic range of 20–60 ng/mL (47–140 nmol/L).
Conclusions:
The data show a 4-fold interindividual difference in dose-adjusted serum concentrations within the 10–90 percentile range and illustrate the significant effects of age and p-glycoprotein induction on the pharmacokinetics of paliperidone. The study also indicates that at least in some patients, it might take longer time than anticipated to reach steady state.
Key Words: paliperidone, pharmacokinetics, intramuscular injections, therapeutic drug monitoring
INTRODUCTION
In schizophrenia, long-acting injectable antipsychotics may provide advantages over oral therapy, such as more stable serum concentrations and improved treatment adherence, especially in patients with limited insight into their own condition.1 Some patients also prefer depot injections over oral medication.2 Paliperidone (9-hydroxy-risperidone), the pharmacologically active main metabolite of risperidone, is approved for intramuscular depot administration in schizophrenia (Invega Sustenna and Xeplion, Janssen-Cilag International NV, Beerse, Belgium). After administration, the palmitic acid–bound drug is slowly dissolved and hydrolyzed to active paliperidone by a first-order process, after which it is absorbed into the circulation.3 The apparent elimination half-life is determined by the absorption rate from the administration site and is approximately 1 month (25–49 days), allowing for once-monthly administration.1,3 Initial loading doses are recommended to achieve therapeutic plasma concentrations rapidly. Otherwise, steady state may not be reached until 4–5 months after commencing treatment.4
Unlike most other antipsychotic agents, paliperidone undergoes only limited hepatic metabolism, mainly being excreted as unchanged drug in the urine.5,6 Consequently, paliperidone is less susceptible to pharmacokinetic drug interactions and cytochrome P-450 (CYP) polymorphisms compared with risperidone and most other antipsychotics. CYP2D6 genotype does not affect serum concentrations of paliperidone, in contrast to risperidone.7 However, impaired renal function necessitates dose adjustment.4 Moreover, paliperidone is a substrate for the p-glycoprotein efflux pump, which actively excretes drugs in the renal tubules.8 Accordingly, inhibitors of p-glycoprotein like verapamil and inducers like carbamazepine have been shown to increase and decrease paliperidone plasma concentrations, respectively.9–11 A report suggests that the pharmacokinetics of paliperidone long-acting injections may be significantly altered in certain patients due to obesity and erroneous injection technique.12
Therapeutic drug monitoring (TDM) of antipsychotics is often practiced, although its usefulness is not universally accepted.13 Few studies have investigated the relationship between serum or plasma concentrations of paliperidone and clinical response. A prospective, open-label naturalistic study from Japan with 15 patients found statistically significant correlations between serum concentrations and improved scores on several clinical rating scales.14 In a retrospective study, patients with the greatest improvement on the clinical global impression scale more often had concentrations within the suggested therapeutic range of 20–60 ng/mL (47–140 nmol/L).15 This concentration range is identical to the recommended therapeutic range of the active moiety of risperidone (risperidone + 9-OH-risperidone).16
The literature on the naturalistic use of paliperidone depot injections is sparse. The aim of this study was to investigate the pharmacokinetics of long-acting injectable paliperidone in a naturalistic setting, using data from our extensive routine TDM database.
MATERIALS AND METHODS
A total of 622 serum concentration measurements from patients receiving the long-acting intramuscular paliperidone formulation Xeplion were retrieved from the routine TDM database. The data analysis was restricted to samples collected at the end of the dosing interval (±1 week) with complete information regarding dose and dosing interval. Thus, 310 serum concentration measurements analyzed from 185 patients (110 men and 75 women) were included in the study. The median age of the patients was 40 (range 19–78) years, and 12 patients were 65 years or older. The number of samples from each patient ranged from 1 to 30. For the concentration/dose ratio calculations, the last sample from each patient was used to reflect steady-state conditions most adequately. Most concomitantly used drugs were expected not to influence the pharmacokinetics of paliperidone; however, 3 patients used carbamazepine, a known inducer of p-glycoprotein.9
The serum concentration of paliperidone was analyzed with a liquid chromatography-mass spectrometry method developed and validated at our laboratory. In brief, 1-mL serum was mixed with 50-μL internal standard (CH3-risperidone 4 μmol/L), 200-μL Na2CO3 (1 mol/L), and 4-mL n-hexane:butanol:acetonitrile (93:5:2) solution. After centrifugation, the organic phase was evaporated to dryness under air, dissolved in 50-μL methanol, and transferred to vials. Samples were then inserted into an Agilent 1100 MSD LC-MS system (Agilent Hewlett Packard, Santa Clara, CA). Separation was performed on a Zorbax SB-C18 column 4.6 × 30 mm (Agilent) with methanol:ammonium acetate 50 mmol/L (65:35) as mobile phase. Paliperidone was monitored at m/z 427.0 and the internal standard at m/z 421.0. Interference with drugs often used concomitantly and with some drugs with chemical structure and molecular weight close to that of paliperidone was tested and found not to take place. The limit of quantification was 2.5 nmol/L. Linearity was achieved over the calibration range of 2.5–1000 nmol/L. The interassay coefficient of variation calculated from quality control samples at 10, 100, and 250 nmol/L was lower than 10%. The conversion factor from ng/mL to nmol/L is 2.34 (ie, a value in nmol/L should be divided by 2.34 to obtain the corresponding value in ng/mL).
The concentration/dose (C/D) ratio for paliperidone was calculated and compared between groups. Daily dose was calculated as the injected dose divided by the dosing interval in days—ie, 150-mg paliperidone every 28 days corresponds to a daily dose of 5.4 mg. When the dosing interval was reported to be 1 month, we based the calculations on a mean month length of 30.4 days. All doses are reported in paliperidone weight units (the Xeplion brand approved by the European Medicines Agency uses paliperidone weight units, whereas the Invega Sustenna brand approved by the U.S. Food and Drug Administration uses paliperidone palmitate weight units—ie, 150 mg of Xeplion equals 234 mg of Invega Sustenna).
Because the distribution of the C/D ratio was skewed, ratios are reported as medians and ranges. For the same reason, statistical comparisons of C/D ratio between groups were performed using Mann–Whitney U test (sex and age group) and Kruskal–Wallis test (dose category). Wilcoxon signed rank test was used to test whether the serum concentration increased or decreased over time in patients with multiple measurements. Two-sided P-values of less than 0.05 were considered significant. Statistical analyses were performed with SPSS version 21 (IBM Corporation, Armonk, NY).
RESULTS
In 145 of the 185 included samples (78%), the dosing interval was 28 days. The injected dose ranged from 50 to 200 mg, with the exception of 1 patient who received 12.5 mg. The average and median daily dose was 3.6 mg, which corresponds to 100 mg every 28 days. The individual serum concentrations of paliperidone as a function of dose are displayed in Figure 1, and aggregated data are presented in Table 1.
FIGURE 1.
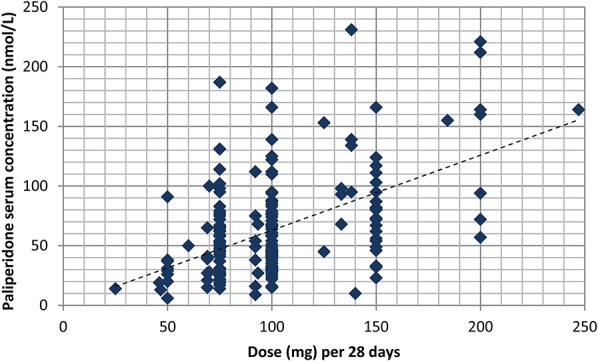
Serum concentration of paliperidone in relation to dose (normalized to a dose interval of 28 days) at the end of the dosing interval in 185 patients treated with Xeplion, an intramuscular depot formulation of paliperidone.
TABLE 1.
Doses, Serum Concentrations, and Concentration/Dose (C/D) Ratios of Paliperidone at the End of the Dosing Interval in 185 Patients Receiving Paliperidone Depot Injections
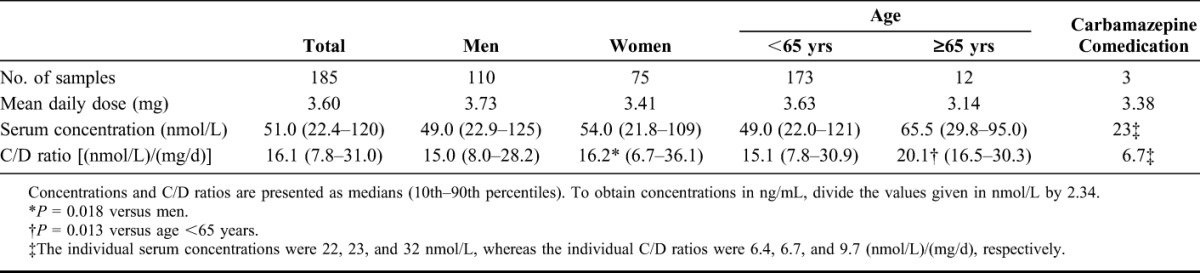
Overall median C/D ratio was 16.1 (nmol/L)/(mg/d). There was a large interindividual variation, with a total range of 2.0–70 and a 10–90 percentile range of 7.8–31 (nmol/L)/(mg/d). Median C/D ratio was 33% higher in the group aged 65 years or above (P = 0.013). The sex difference in median C/D ratio was small and not statistically significant (P = 0.18). Median C/D ratios were similar across the doses 50, 75, 100, and 150 mg [17.3, 19.9, 16.6, and 17.3 (nmol/L)/(mg/d), respectively; P = 0.45].
Three samples were analyzed from patients concomitantly receiving carbamazepine. These patients all had low C/D ratios, 6.4, 6.7, and 9.7 (nmol/L)/(mg/d), respectively, which is below the 10th percentile of the general distribution. None of these patients had measurements without carbamazepine.
Over a period of just more than 2 years, our laboratory received a total of 30 samples from the same male patient, aged 29 years at the beginning of the period. His C/D ratios over time are displayed in Figure 2. The dose varied in the beginning of the period, but from week 26 onward, the dose was constant at 150 mg every 17 days. All samples were taken at the end of the dosing interval. The patient was committed to a high-security psychiatric ward for the whole period; apart from this, the authors do not have any clinical information about the rationale for the frequent serum concentration analyses or the unusual dosing.
FIGURE 2.
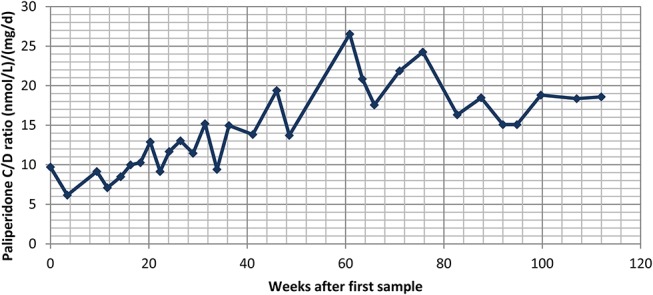
Concentration/dose (C/D) ratios of paliperidone calculated from 30 consecutive serum concentration measurements in a 29-year old male patient treated with Xeplion, an intramuscular depot formulation of paliperidone, over a period of just more than 2 years. Dosing was constant at 150 mg every 17 days from week 26 onward. In the 12 samples drawn after assumed steady state (from week 56 onward), the mean C/D ratio was 19.3 ± 3.6 (nmol/L)/(mg/d).
Twenty-eight patients with more than 3 samples were identified. After excluding the first sample to avoid possible bias due to sampling during the initial loading phase, significantly more patients showed an increase in C/D ratio from the second to the last sample received. The mean C/D ratio increase was 51.6%, with an increase in 20 patients, a decrease in 7, and no change in 1 patient (P = 0.021).
DISCUSSION
The principal finding in this study is that the C/D ratio in patients using paliperidone depot injections varies considerably, with a 4-fold variation from the 10th to the 90th percentile. This is similar to previously published TDM data on risperidone depot injections.17 This variability may be explained by differences in renal function4 and differences in p-glycoprotein activity, both due to genetic and environmental factors.11,18 When applied to the standard maintenance dose of 75 mg every 28 days, the 10th and 90th C/D ratio percentiles correspond to 80% of the patients falling within a serum concentration range of approx. 21–83 nmol/L. This is considerably lower than the suggested therapeutic range of 47–140 nmol/L15,16; however, the therapeutic range is not well established.
Increased C/D ratio in patients 65 years or older is to be expected and is most likely due to reduced renal function with age.4 The age effect was not very pronounced in our material, with only a 33% increase in median C/D ratio. However, no patients in our material were older than 78 years, and larger increases in C/D ratio could be anticipated in very old subjects. Thus, serum concentration measurements should be considered in elderly patients treated with standard doses of paliperidone.
Low C/D ratios in patients using carbamazepine are in agreement with earlier reports of the profound lowering effect of carbamazepine on paliperidone serum concentrations.9,11 No patients in the present material used potent p-glycoprotein inhibitors concomitantly, so we could not extend the previous findings reported, for example, for the powerful p-glycoprotein inhibitor verapamil on the levels of paliperidone (9-hydroxy-risperidone) in a patient treated with risperidone.10 Concomitant use of drugs known to affect p-glycoprotein activity should nevertheless be followed up by clinical assessment, preferably guided by TDM.
Although paliperidone is commonly administered in higher doses at the beginning of treatment to achieve effective serum concentrations more rapidly, the specific case presented suggests that the serum concentration may continue to increase long after the initial loading regime. A previous report suggested that erroneous injection technique, depositing the drug in adipose tissue, may slow absorption and thus greatly increase the depot effect.12 As many patients with schizophrenia are overweight,19,20 this may be a possible explanation.
The limited clinical information available on the requisition forms precludes a full account of patient-related factors influencing the pharmacokinetics of paliperidone. Notably, we had no data on the kidney function of the patients. Although we aimed to include only steady-state samples, we cannot exclude that some samples did not reflect steady-state conditions. However, the naturalistic study setting provides a good indication of the expected variability in real-life TDM conditions. We cannot exclude the possibility that our sample is selected toward patients with either lack of effect or adverse effects, which may exaggerate the variability because such patients could be expected to have especially low and high plasma concentrations, respectively. Because paliperidone is available exclusively for intramuscular administration, medication adherence is not expected to contribute to the variability seen in this study.
CONCLUSIONS
This study illustrates the considerable serum concentration variability of paliperidone in a naturalistic TDM setting and confirms the significant impact of age and p-glycoprotein induction on the serum concentration of paliperidone. Our data also indicate that at least in some patients, it might take longer time than anticipated to reach steady state.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Park EJ, Amatya S, Kim MS, et al. Long-acting injectable formulations of antipsychotic drugs for the treatment of schizophrenia. Arch Pharm Res. 2013;36:651–659. [DOI] [PubMed] [Google Scholar]
- 2.Walburn J, Gray R, Gournay K, et al. Systematic review of patient and nurse attitudes to depot antipsychotic medication. Br J Psychiatry. 2001;179:300–307. [DOI] [PubMed] [Google Scholar]
- 3.Samtani MN, Vermeulen A, Stuyckens K. Population pharmacokinetics of intramuscular paliperidone palmitate in patients with schizophrenia: a novel once-monthly, long-acting formulation of an atypical antipsychotic. Clin Pharmacokinet. 2009;48:585–600. [DOI] [PubMed] [Google Scholar]
- 4.Samtani MN, Gopal S, Gassmann-Mayer C, et al. Dosing and switching strategies for paliperidone palmitate: based on population pharmacokinetic modelling and clinical trial data. CNS Drugs. 2011;25:829–845. [DOI] [PubMed] [Google Scholar]
- 5.Vermeir M, Naessens I, Remmerie B, et al. Absorption, metabolism, and excretion of paliperidone, a new monoaminergic antagonist, in humans. Drug Metab Dispos. 2008;36:769–779. [DOI] [PubMed] [Google Scholar]
- 6.Boom S, Talluri K, Janssens L, et al. Single- and multiple-dose pharmacokinetics and dose proportionality of the psychotropic agent paliperidone extended release. J Clin Pharmacol. 2009;49:1318–1330. [DOI] [PubMed] [Google Scholar]
- 7.Patteet L, Haufroid V, Morrens K, et al. Genotype and co-medication dependent CYP2D6 metabolic activity: effects on serum concentrations of aripiprazole, haloperidol, risperidone, paliperidone and zuclopenthixol. Eur J Clin Pharmacol. 2016;72:175–184. [DOI] [PubMed] [Google Scholar]
- 8.Wang JS, Ruan Y, Taylor RM, et al. The brain entry of risperidone and 9-hydroxyrisperidone is greatly limited by P-glycoprotein. Int J Neuropsychopharmacol. 2004;7:415–419. [DOI] [PubMed] [Google Scholar]
- 9.Akamine Y, Uehara H, Miura M, et al. Multiple inductive effects of carbamazepine on combined therapy with paliperidone and amlodipine. J Clin Pharm Ther. 2015;40:480–482. [DOI] [PubMed] [Google Scholar]
- 10.Nakagami T, Yasui-Furukori N, Saito M, et al. Effect of verapamil on pharmacokinetics and pharmacodynamics of risperidone: in vivo evidence of involvement of P-glycoprotein in risperidone disposition. Clin Pharmacol Ther. 2005;78:43–51. [DOI] [PubMed] [Google Scholar]
- 11.Yasui-Furukori N, Kubo K, Ishioka M, et al. Interaction between paliperidone and carbamazepine. Ther Drug Monit. 2013;35:649–652. [DOI] [PubMed] [Google Scholar]
- 12.Helland A, Syrstad VE, Spigset O. Prolonged elimination of paliperidone after administration of paliperidone palmitate depot injections. J Clin Psychopharmacol. 2015;35:95–96. [DOI] [PubMed] [Google Scholar]
- 13.Lopez LV, Kane JM. Plasma levels of second-generation antipsychotics and clinical response in acute psychosis: a review of the literature. Schizophr Res. 2013;147:368–374. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki H, Gen K, Otomo M, et al. Relationship between the plasma concentration of paliperidone and the clinical and drug-induced extrapyramidal symptoms in elderly patients with schizophrenia. Hum Psychopharmacol. 2014;29:244–250. [DOI] [PubMed] [Google Scholar]
- 15.Nazirizadeh Y, Vogel F, Bader W, et al. Serum concentrations of paliperidone versus risperidone and clinical effects. Eur J Clin Pharmacol. 2010;66:797–803. [DOI] [PubMed] [Google Scholar]
- 16.Hiemke C, Baumann P, Bergemann N, et al. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. pharmaco-psychiatry. 2011;44:195–235. [DOI] [PubMed] [Google Scholar]
- 17.Castberg I, Spigset O. Serum concentrations of risperidone and 9-hydroxyrisperidone after administration of the long-acting injectable form of risperidone: evidence from a routine therapeutic drug monitoring service. Ther Drug Monit. 2005;27:103–106. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki Y, Tsuneyama N, Fukui N, et al. Impact of the ABCB1 gene polymorphism on plasma 9-hydroxyrisperidone and active moiety levels in Japanese patients with schizophrenia. J Clin Psychopharmacol. 2013;33:411–414. [DOI] [PubMed] [Google Scholar]
- 19.Homel P, Casey D, Allison DB. Changes in body mass index for individuals with and without schizophrenia, 1987-1996. Schizophr Res. 2002;55:277–284. [DOI] [PubMed] [Google Scholar]
- 20.Saarni SE, Saarni SI, Fogelholm M, et al. Body composition in psychotic disorders: a general population survey. Psychol Med. 2009;39:801–810. [DOI] [PubMed] [Google Scholar]


