Supplemental Digital Content is available in the text
Keywords: cardiovascular risk, cholesterol, dolutegravir, HIV-1, lipids, protease inhibitors, randomized clinical trials
Abstract
Objective:
To compare the efficacy, safety, and impact on lipid fractions of switching from a ritonavir-boosted protease inhibitor (PI/r) to a dolutegravir (DTG) regimen.
Methods:
HIV type 1-infected adults more than 50 years or with a Framingham score more than 10% were eligible if plasma HIV RNA less than 50 copies per ml for at least 24 weeks while on a PI/r regimen. Patients were randomized to switch to DTG or to remain on PI/r. Primary endpoints were: proportion maintaining HIV RNA less than 50 copies per ml and percentage change from baseline of total cholesterol at week 48.
Results:
In total, 415 patients (32 sites in six European countries) were randomized: 205 to DTG and 210 to continue PI/r. About 89% were men, 87% more than 50 years, 74% had a Framingham score more than 10%, with a median CD4+ cell count of 617 cells per μl and suppressed viremia for a median of 5 years. At week 48, in the intent-to-treat analysis, treatment success rate was 93.1% in DTG group and 95.2% in PI/r group (difference −2.1%, 95% confidence interval −6.6 to 2.4, noninferiority demonstrated). There were four virological failures with DTG and one with PI/r with no emergent resistance mutations. There was no significant difference in severe adverse events or grade 3 or 4 adverse events or treatment modifying adverse events. Total cholesterol and other lipid fractions (except high-density lipoprotein cholesterol) improved significantly (P < 0.001) in the DTG group regardless of PI/r at baseline.
Conclusion:
Switching to a DTG regimen in virologically suppressed HIV type 1 patients with high cardiovascular disease risk was noninferior, and significantly improved lipid profiles.
Introduction
Dolutegravir (DTG) is an integrase strand transfer inhibitor (INSTI) of the HIV type 1 (HIV-1) [1–6]. DTG is a generally well tolerated [7] once daily drug, can be coformulated [8], has a low potential for drug–drug interactions [9], with infrequent emergence of resistance mutations when given as part of a combination regimen [10–13] and a neutral lipid profile [14]. In antiretroviral-naive patients, DTG has demonstrated noninferiority to raltegravir [15,16] and superiority to efavirenz [17] and the ritonavir-boosted protease inhibitors (PI/r) darunavir [18] and atazanavir [19].
Consensus guidelines recommend several treatment switch strategies in HIV-1-infected patients, who have achieved virological suppression with triple-drug treatment, to prevent or aid in the management of comorbidities, address adverse events or drug–drug interactions, to simplify the antiretroviral regimen, or to reduce costs [20–22]. HIV-1 infection may accentuate the risk of cardiovascular disease (CVD) regardless of control of viremia and after adjusting for established cardiovascular risk factors [23–25].
The most common switching strategy by far has been to focus on third agent switch by switching from a PI/r regimen to a new regimen with an unboosted protease inhibitor [26], to a more lipid friendly PI/r [27], a nonnucleoside reverse transcriptase inhibitor [28], and more recently to a INSTI [5,6,29,30]. The main objective of all these studies has been to improve plasma lipid profile and gastrointestinal symptoms in addition to avoiding potential drug–drug interactions and improving convenience for patients.
Raltegravir, the first INSTI to be investigated in switch studies, resulted in significant lipid improvements while maintaining virological suppression in the Switching Protease Inhibitors to Raltegravir (SPIRAL) [5] study but not in the SWITCHMRK 1 and 2 studies [6]. Elvitegravir requires boosting with cobicistat so the issue of drug–drug interactions remains [9,31]. In the STRIIVING study [30], an unselected population of virologically suppressed HIV-1 infected patients were randomized to switch from their current regimen to a single tablet of DTG/abacavir/lamivudine or to continue with the current regimen. Noninferiority criteria were met but the lipid profile did not improve probably because in 77% of the population the background regimen included tenofovir disoproxil fumarate [32] that was replaced by abacavir [30].
We performed a randomized, noninferiority, strategic trial to compare the efficacy, safety, and impact on lipid parameters of switching to DTG to that of remaining on a PI/r regimen in a targeted population with potential high CVD risk (HIV-a infection and age above 50 years and/or a Framingham [33,34] CVD risk score more than 10% at 10 years).
Methods
Study design and patients
NEAT022 was a randomized, open-label, noninferiority trial conducted in 32 clinical sites in six European countries (see supplementary Table 1 in Supplemental Digital Contents). Patients were recruited between May 2014 and November 2015. Eligible patients were HIV-1-infected adults older than 50 years or older than 18 years with a Framingham CVD risk score 10-year risk score more than 10% [33,34]. They had to be on a stable (>6 months) triple antiretroviral regimen consisting on a PI/r (that could be ritonavir-boosted lopinavir, darunavir, atazanavir, saquinavir, or fosamprenavir) and two nucleoside (tide) reverse transcriptase inhibitors (NtRTIs) and have a plasma HIV RNA less than 50 copies per ml for at least the previous 6 consecutive months. We excluded patients with prior evidence of primary viral resistance [35] to backbone nucleos(t)ides. We also excluded patients with previous episodes of documented virological failures. The full list of inclusion and exclusion criteria are in the supplementary Table 2 in Supplemental Digital Contents.
Ethics
The trial was conducted in accordance to the Good Clinical Practice and ethical principles of the declaration of Helsinki. The protocol was reviewed and approved by the ethics committees of all participating hospitals. All participants gave their written informed consent before undergoing study procedures. The study was registered on ClinicalTrials.gov NCT02098837 and EudraCT 2013-003704-39.
Randomization and masking
Eligible participants were randomly assigned (1 : 1) to either switch to DTG 50 mg per day and the same two NtRTIs or to continue with the same triple therapy regimen including a PI/r for 48 weeks after which all patients remaining on a PI/r were switched to DTG. We assigned patients to treatment groups by computer-generated permuted blocks of four and stratified by country. The study design was open label, so participants and investigators were not masked to group allocation but only the trial statistician had access to the entire randomization list during the trial.
Study procedures
Participants attended study centres at screening, baseline, weeks 4 (DTG group only), 12, 24, 36, and 48. All participants remained in the study up to the week 48 visit unless consent was withdrawn. Each visit included general assessment of vital signs, including arterial blood pressure (BP) and adverse events, physical examination, and collection of blood samples for full blood cell counts and serum chemistry, liver, renal function, and immunovirological measurements. CD4+ cell counts and plasma viral loads were measured at screening, baseline, week 24, and week 48. Fasting (overnight or >6 h) serum lipids were measured at all visits. Estimated glomerular filtration rate (eGFR) was calculated by the Chronic Kidney Disease Epidemilogy Collaboration method [36]. HIV RNA measurements in plasma and, if indicated, testing for antiretroviral resistance by genotype sequencing were done at local laboratories (the local laboratories were required to meet Clinical Laboratory Improvement Amendments regulations or the country's equivalent). Virological failure was defined as two consecutive measurements of plasma viral load above 50 copies per ml separated at least by 2 weeks during the assigned treatment. A viral blip was defined by a plasma viral load more than 50 copies of HIV RNA per ml followed by a second measurement less than 50 copies of HIV RNA per ml. Safety was assessed at all visits by monitoring of all adverse events and serious adverse events (SAEs), vital signs, and laboratory values. Adherence during the trial was monitored by participant questioning at each medical visit regarding missed tablets, at any moment during the trial or the prior week. Patients and investigators were advised not to change administration of lipid-lowering agents during the study period unless strictly necessary. Patients were also advised at each medical visit to give up smoking, to exercise daily to pay attention to their body weight, diet, and alcohol intake, and to control BP using a written predefined healthy life style guidance formulary. AIDS events and deaths, SAEs, adverse events grade 3 or above, adverse events leading to modification of study drugs, all protocol discontinuations, and all protocol-defined episodes of virological failures required confirmation by an independent endpoint review committee, whose members were unaware of individual patient's treatment regimens.
Endpoints
The two coprimary endpoints were: the proportion of patients able to maintain treatment response (HIV RNA <50 copies per ml with no discontinuation of the study treatment) up to week 48; the percentage change from baseline in total cholesterol (TC) to week 48. Nonresponse was defined as any of the following: virological failure, death from any cause, loss to follow-up, consent withdrawal or permanent change or interruption of randomized treatment for any reason.
Main secondary endpoints were: frequency of all clinical and laboratory adverse events up to week 48; change in CD4+ cell count from baseline to week 48; percentage change from baseline to week 48 of other lipid fractions: non-high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, HDL cholesterol, triglycerides, and TC : HDL cholesterol ratio and changes from baseline to week 48 of Framingham CVD risk score at 10 years.
Statistical analyses
A total of 420 participants (210 per group) was estimated providing at least 90% power to exclude a noninferiority margin of 10% for the difference in proportion of participants reaching the primary endpoint, assuming 90% of participants have treatment success in the continuous PI/r therapy group and a one-sided α of 0.025 (two-sided α = 0.05). The study is powered for the first primary endpoint as this is the criterion that requires the larger sample size. However, with 210 patients per group, the study will have more than 99% power to detect a between treatment difference of 12% in the mean percentage change from baseline in TC, with a SD of 13.8%, a type I error of 0.05, and a two-tailed nonparametric test. No multiplicity adjustment is needed for having two coprimary endpoints.
All patients who underwent randomization were included in the intent-to-treat (ITT) population. In the primary, ITT analysis, the proportion of participants who had treatment success was estimated with Kaplan–Meier methods, censoring at week 48 or last follow-up date if missing viral load values at week 48. Treatment success was defined by the absence of virological failure and absence of a permanent discontinuation of study/study drugs (DTG or PI/r). Any discontinuation in the background NtRTIs for any reason with an undetectable viral load was not considered as failure. The difference in percentage of participants in treatment success (DTG – PI/r) was estimated and two-sided 95% confidence interval (CI) of the difference was obtained with bootstrap Standard error (SE) (1000 replicates) as proportions were estimated by time-to-event method. Log-rank test was also used to compare the two survival functions.
In the prespecified sensitivity analysis on the perprotocol population, individuals were ignored if they did not fulfil the eligibility criteria, withdrew consent, lost to follow-up or discontinued study medication for any reasons other than virological failure or adverse event. DTG containing regimens were considered noninferior to PI/r containing regimens if the lower bound of CI was below −10% for both ITT and per protocol analysis.
Subgroups analyses were performed stratified by participating country and by Framingham 10-year CVD risk score (<15%, ≥15%). The mean percentage change from baseline in lipid fractions: TC, non-HDL cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, and TC/HDL cholesterol ratio at week 48, the mean change from baseline in CD4+ cell counts, and eGFR to week 48 were analyzed with the ITT population, with the last observation carried forward approach. The nonparametric Mann–Whitney test was used to compare the changes from baseline between the two groups.
Post hoc analyses were also conducted to study the treatment effect by PI/r at screening (darunavir, atazanavir, other PI), Framingham 10-year CVD risk score [33,34] (<15% vs. ≥15%), and Framingham CVD 10-year risk score and age (age ≤50 year and CVD risk >10%, age >50 year and CVD risk>10%, age>50 year and CVD risk ≤10%) for all lipid fractions. Safety analysis was performed with randomized patients who received at least one-time any study treatment. Any adverse events, grade 3 and 4 adverse events, antiretroviral therapy-related adverse events (all grade), treatment-modifying adverse events (all grade), death, SAEs, and finally adverse events occurring in at least 5% of participants were described and compared by group, using Fisher's exact test.
Variables were summarized as proportions for categorical variables (based on the nonmissing sample size), the median and interquartile range for continuous baseline variables, and the mean and SD for continuous variables used as endpoints. All reported P values are two-tailed with a significant level of 0.05. Analyses were performed with International Business Machines SPSS Statistics version 24 (IBM, Armonk, New York, USA) and STATA SE version 13 (ATATA Corp, College Station, Texas, USA).
Results
Between May 2014 and November 2015, 455 patients from 32 sites in six European countries were screened and 415 randomized: 205 to switch to a DTG-based regimen and 210 to continue the PI/r-based regimen (ITT population; Fig. 1 and supplementary table 1 in Supplemental Digital Contents). At least one dose of study treatment was received by 412 patients: 204 in the DTG group and 208 in the PI/r-treatment group (Fig. 1). Baseline characteristics were balanced between study groups including the duration of previous virological suppression, the distribution of the baseline PI/r with tenofovir disoproxil fumarate or abacavir-based regimens and the percentage of patients receiving lipid-lowering agents (Table 1). A genotypic resistance test without mutations was available in 204 (49%) of the 415 patients.
Fig. 1.
Trial profile.
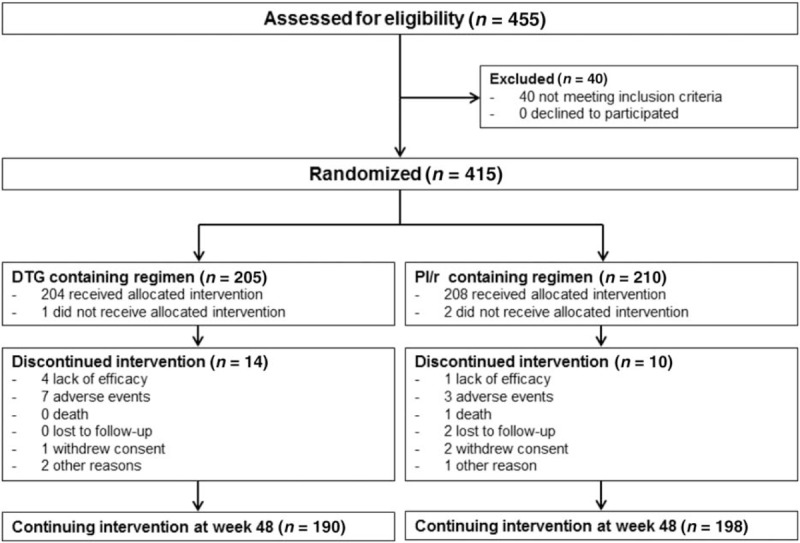
DTG, dolutegravir; PI/r, ritonavir-boosted protease inhibitors. A genotypic resistance test was available in 19 (47.5%) of the 40 patients assessed for eligibility but not randomized. Presence of resistance mutations was the reason in two (5%) of these 40 patients.
Table 1.
Baseline characteristics.
| DTG (n = 205) | PI/r (n = 210) | Total (n = 415) | |
| Age (years) | 54 (51–58) | 53 (51–57) | 54 (51–58) |
| Age >50 years | 179 (87.3) | 184 (87.6) | 363 (87.5) |
| Framingham score at 10 years | |||
| <10% | 50 (24.4) | 59 (28.1) | 109 (26.3) |
| 10–15% | 62 (30.2) | 53 (25.2) | 115 (27.7) |
| 15–20% | 41 (20.0) | 48 (22.9) | 89 (21.4) |
| >20% | 52 (25.4) | 50 (23.8) | 102 (24.6) |
| Male sex | 181 (88.3) | 189 (90.0) | 370 (89.2) |
| White race | 173 (84.4) | 180 (85.7) | 353 (85.1) |
| Mode of HIV-1 transmission | |||
| Male homosexual sexual intercourse | 130 (63.4) | 131 (62.4) | 261 (62.9) |
| Heterosexual sexual intercourse | 43 (23.9) | 48 (22.9) | 97 (23.4) |
| Othera | 26 (12.7) | 31 (14.8) | 57 (13.7) |
| CD4+ cell count (cells per μl) | 635 (495–819) | 585 (471–830) | 617 (477–820) |
| HIV RNA >50 copies per ml | 7 (3.4) | 1 (0.5) | 8 (2) |
| Hepatitis C IgG antibodies | 27 (13.4) | 24 (11.6) | 51 (12.5) |
| Time since undetectable viral load (<50 copies per ml); years | 4.9 (2.5–9.1) | 5.3 (2.3–8.5) | 5 (2.4–8.8) |
| Backbone nucleos (t)ides | |||
| Tenofovir disoproxil fumarate/emtricitabine | 134 (65.4) | 135 (64.3) | 269 (64.8) |
| Abacavir /lamivudine | 63 (30.7) | 67 (31.9) | 130 (31.3) |
| Other | 8 (3.9) | 8 (3.8) | 16 (3.9) |
| PI/r at baseline | |||
| Lopinavir | 13 (6.4) | 23 (11.0) | 36 (8.7) |
| Darunavir | 105 (51.5) | 107 (51.0) | 212 (51.2) |
| Atazanavir | 77 (37.7) | 74 (35.2) | 151 (36.5) |
| Other | 9 (4.4) | 6 (2.9) | 15 (3.7) |
| Current smokers | 78 (38.0) | 79 (37.8) | 157 (37.9) |
| Diabetes mellitus | 11 (5.5) | 13 (6.3) | 24 (5.9) |
| Family history of cardiovascular disease | 87 (43.3) | 89 (43.4) | 176 (43.3) |
| Receiving lipid-lowering agents | 63 (30.7) | 60 (28.6) | 123 (29.6) |
| High blood pressureb | 72 (35.3) | 79 (37.6) | 151 (36.5) |
| Daily exercisec | 64 (31.2) | 59 (28.2) | 123 (29.7) |
| Cardiovascular risk factorsd | |||
| 0 | 54 (26.3) | 56 (26.7) | 110 (26.5) |
| 1 | 71 (34.6) | 63 (30.0) | 134 (32.3) |
| 2 | 49 (23.9) | 60 (28.6) | 109 (26.3) |
| ≥3 | 31 (15.1) | 31 (14.8) | 47 (11.3) |
| Fasting plasma lipids (mmol per l) | |||
| Total cholesterol | 5.2 (4.5–5.8) | 5.1 (4.5–5.6) | 5.1 (4.5–5.7) |
| Triglycerides | 1.6 (1.2–2.3) | 1.6 (1.2–2.2) | 1.6 (1.2–2.2) |
| Non-HDL cholesterol | 3.3 (2.9–4.0) | 3.8 (3.1–4.4) | 3.8 (3.2–4.5) |
| LDL-cholesterol | 3.1 (2.5–3.7) | 3.1 (2.5–3.6) | 3.1 (2.5–3.6) |
| HDL-cholesterol | 1.2 (1.0–1.5) | 1.2 (1.0–1.5) | 1.2 (1.0–1.5) |
| Total cholesterol/HDL cholesterol ratio | 4.2 (3.4–5.4) | 4.1 (3.4–5.2) | 4.1 (3.4–5.3) |
| eGFR (ml per min) | 90.8 (80.7–99.7) | 91.4 (78.3–101.8) | 91.1 (80–100.2) |
Data are n (%) or median (interquartile range).
HDL cholesterol levels above 1.5 mmol per l, implicates a subtraction of one risk factor.
DTG, dolutegravir; eGFR, estimated glomerular filtration rate; PI/r, ritonavir-boosted protease inhibitors.
aMode of HIV transmission was unknown in 22 (38.6%) of the 57 and 28 (49.1%) of the 57 were intravenous drugs users.
bDefined by SBP more than 140 mmHg or DBP more than 110 mmHg or receiving antihypertensive treatment addition.
cDefined as self-reported some exercise (duration not specified) every day.
dAn addition of male patients with age more than 50 years or female patients with age more than 60 years, current or past smoker within the last 3 years, HDL cholesterol less than 1 mmol per l, high blood pressure, diabetes mellitus, family history of cardiovascular diseases.
Adapted with permission [36].
Efficacy
At week 48, 14 patients in the DTG group and 10 in the PI/r group had experienced treatment failure; corresponding to a treatment success rate of 93.1 and 95.2%, respectively (difference −2.1%, 95% CI −6.6 to 2.4, noninferiority demonstrated); Fig. 2a and Supplementary Figure 1 in Supplemental Digital Contents. The perprotocol analysis gave a similar estimated difference of −3.0% (95% CI −6.8 to 0.8); Fig. 2b. Reasons for nonresponse were similar between groups. Approximately 90% of patients in both group reported 100% adherence at all-time points. There were four protocol-defined virological failures in the DTG group (plasma viral load at failures from 58 to 130 HIV RNA copies per ml) and one in the PI/r group (plasma viral load at failure 3373 HIV RNA copies per ml) with no emergent resistance mutations in the two of the five samples that could be amplified (supplementary Figure 2 in Supplemental Digital Contents). All these five patients but one reported 100% adherence at all-time points. Subgroup analysis for treatment response by country or by age and Framingham CVD risk score at baseline showed similar effect across all subgroups and can be seen in supplementary Figure 3 in Supplemental Digital Contents. Overall, 21 episodes of viral blips occurred in 19 participants: 12 in 10 participants in the DTG group and nine in nine participants in the PI/r group. (Supplementary Figure 2 in Supplemental Digital Contents). All these 19 patients but three reported 100% adherence at all-time points. Mean increases in CD4+ cell count from baseline to week 48 were 26 ± 151 cells per μl in the DTG group and −1 ± 156 cells per μl in the PI/r group (P = 0.028).
Fig. 2.
Outcomes at 48 weeks of primary efficacy endpoint (Kaplan–Meier estimates).
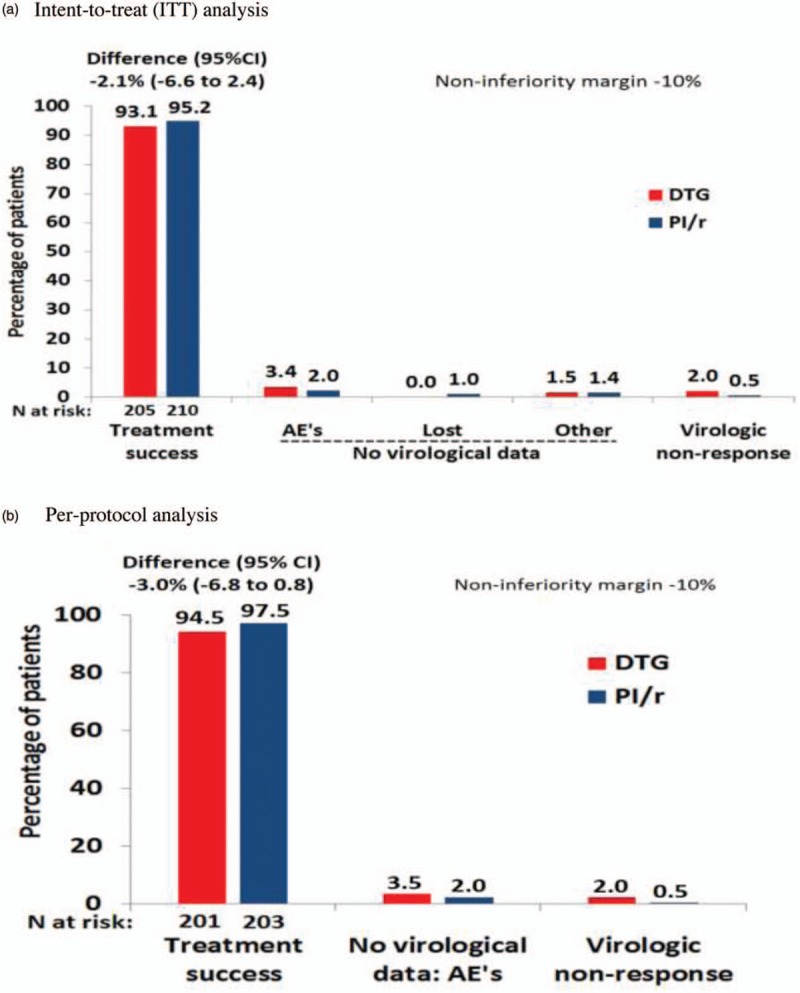
CI, confidence interval; DTG, dolutegravir; PI/r, ritonavir-boosted protease inhibitors. (a) Intent-to-treat analysis. (b) Perprotocol analysis.
Changes in lipids and in other cardiovascular disease risk factors
TC and other proatherogenic lipid fractions significantly (P < 0.001) decreased in the DTG group: TC −8.7 ± 13.8% vs. 0.7 ± 15.6%, LDL cholesterol −7.7 ± 22.3% vs. 2.0 ± 23.9%, non-HDL cholesterol −11.3 ± 17.4% vs. 0.5 ± 20.9%, TC/HDL cholesterol ratio −7.0 ± 23.7% vs. 0.4 ± 23.1% and triglycerides −18.4 ± 40.7% vs. 4.2 ± 41.4% (Fig. 3). Similar significant improvements were detected in the DTG group when analysis of lipid changes were stratified by baseline age and Framingham CVD risk score, by baseline PI/r, and also by backbone administration of tenofovir or abacavir (supplementary Figures 4a, 4b, 4c, 4d and 4e in Supplemental Digital Contents). The change from baseline to week 48 in the percentage of patients receiving lipid-lowering agents, receiving or requiring [37] lipid-lowering agents, currently smoking, taking daily exercise, and with high BP was 0, −3.9, −0.9, 6.4, and −4.5%, respectively, in the DTG group and 2.8, 4.3, −1, 5.8, and −2.4%, respectively, in the PI/r group. None of these changes were statistically significant. No statistically significant changes from baseline to week 48 occurred in the Framingham CVD risk score. More than 95% of patients, in both groups, reported having received healthy life style guidance at all clinical visits.
Fig. 3.
Changes in fasting lipid concentration from baseline to week 48 (N = 415).

DTG, dolutegravir; PI/r, ritonavir-boosted protease inhibitors; TC, total cholesterol.
Safety
Adverse events, all grades and causalities, were reported in 153 patients (75%) of 204 in the DTG group and in 132 patients (63.5%) of 208 in the PI/r group (P = 0.01) of whom 12 (5.9%) in the DTG group and 16 (7.7%) in the PI/r group (P = 0.56) were considered SAEs (Table 2). Seven (3.4%) patients in the DTG group (six because of mood disturbances or insomnia) and three (1.4%) in the PI/r group discontinued the study drug because of adverse events (P = 0.22). The six cases in the DTG group who discontinued because of mood disturbances or insomnia occurred between weeks 0 and 18 after switching to DTG (between September 2014 and February 2016). The most frequent adverse events occurring in at least 5% of patients were digestive, muscular, or skeletal, respiratory, neuropsychiatric, or dermatological and were comparable between groups except genitourinary which were slightly more frequent (P = 0.02) in the DTG group (Table 2). A major cardiovascular event occurred in one patient in the DTG group and in two patients in the PI/r group (Table 2). One death event occurred during the trial in the PI/r group because of an accidental fall with a temporal bone fracture and a subdural hematoma.
Table 2.
Adverse events in 412 patients who received either dolutegravir (n = 204) or ritonavir boosted protease inhibitor (n = 208).
| DTG (n = 204) | PI/r (n = 208) | ||||
| Patients n (%) | Adverse events (n) | Patients n (%) | Adverse events (n) | P value | |
| Summary of adverse events | |||||
| Any adverse event | 153 (75.0) | 395 | 132 (63.5) | 352 | 0.01 |
| Grade 3 or 4 adverse events | 12 (5.9) | 17 | 19 (9.1) | 32 | 0.26 |
| Serious adverse events | 12 (5.9) | 14 | 16 (7.7) | 27 | 0.56 |
| Discontinuation because of adverse event | 7 (3.4)a | 7 | 3 (1.4)b | 3 | 0.22 |
| Any adverse event related to antiretroviral therapy | 26 (12.8) | 41c | 15 (7.2) | 21c | 0.07 |
| Death | 0 | 1 (0.5)d | 1.00 | ||
| Adverse events, any grade, occurring in at least 5% of patients in either group | |||||
| Digestive | 42 (20.6) | 52 | 38 (18.3) | 54 | 0.62 |
| Muscular or skeletal | 51 (25.0) | 66 | 39 (18.8) | 56 | 0.15 |
| Cardiovascular | 11 (5.4) | 13e | 21 (10.1) | 23e | 0.10 |
| Respiratory | 64 (31.4) | 94 | 49 (23.6) | 66 | 0.08 |
| Dermatological | 36 (17.6) | 43 | 27 (13.0) | 38 | 0.22 |
| Genitourinary | 28 (13.7) | 33 | 14 (6.7) | 26 | 0.02 |
| Systemic | 27 (13.2) | 28 | 31 (14.9) | 38 | 0.67 |
| Neuropsychiatric | 44 (21.6) | 64 | 36 (17.3) | 47 | 0.32 |
| Grade 3 or 4 laboratory adverse events | |||||
| Any grade 3 or 4 laboratory adverse event | 5 (2.5) | 8 | 29 (13.9) | 46 | <0.01 |
| Alanine aminotransferase concentration >5 × ULN | 1 (0.5) | 1 | 1 (0.5) | 1 | 1.00 |
| Bilirubin >2.5 × ULN | 2 (1.0) | 4 | 16 (7.7) | 28 | <0.01 |
| LDL cholesterol >4.9 mmol per l | 0 (0.0) | 0 | 10 (4.8) | 13 | <0.01 |
Data are number of patients (%) or number of events.
P value: comparison of proportion of patients with at least one adverse event between the two groups.
DTG, dolutegravir group; LDL, low-density lipoprotein; PI/r, ritonavir-boosted protease inhibitor; ULN, upper limit of normality.
aOne case of acute hepatitis C and six cases of mood and/or sleep disorders.
bOne case of hepatitis C, one case of dyspepsia, and one case of declining renal function.
c15/41 and 6/21 were episodes of mood, sleep, or central nervous system disorders.
dAccidental fall with a temporal bone fracture and subdural hematoma.
e1/13 and 2/23 were a major cardiovascular event.
Grade 3 or 4 laboratory adverse events were observed in 2.5% of the patients in the DTG group and 13.9% in the PI/r group (P < 0.01; Table 2). There was also a small but significant (P < 0.001) decrease in the calculated eGFR in the DTG group compared with the PI/r group (supplementary Figure 5 in Supplemental Digital Contents).
Discussion
This is the first study, to specifically examine switching from a regimen containing two NtRTIs and a PI/r to a regimen with the same backbone and DTG in virologically stable patients with high CVD risk (87% of the patients were both older than 50 years and with a Framingham risk score >10%). The study demonstrated noninferiority for maintenance of control of HIV RNA in the switch group and maintaining the CD4+ cell response and without an overall significant increase in SAEs or in any grade adverse events related with antiretroviral therapy.
In the DTG group, a reduction of the LDL cholesterol of 7.7% (approximately 0.3 mmol per l) from baseline values was achieved. This level of reduction in the general population is associated with a significant reduction in the relative risk of major cardiovascular events in all baseline strata of cardiovascular risk [38]. As 60% of study participants switched away from PI/r regimens containing ritonavir-boosted lopinavir [39] or darunavir [40] both independently associated in the Data Collection on Adverse Events of Anti-HIV Drugs study with an increased CVD risk there may be an additional favourable impact on estimated CVD risk. CVD is a major cause of morbidity and mortality in persons with HIV-1 infection with an estimated risk of approximately 1.5–2.0-fold higher among HIV-1-infected individuals compared with the general population [41]. Data from the Data Collection on Adverse Events of Anti-HIV Drugs study showed that CVD accounts for approximately 11% of deaths among HIV-1-infected persons [42] and, the EuroSIDA study showed that cardiovascular events account for about one-third of non-AIDS-defining clinical events in the HIV-1-infected population [43].
Proatherogenic lipid fractions are important risk factors for CVD. The US National Lipid Association [44] suggests that lipid goals be based on the number of risk factors present which include LDL cholesterol and non-HDL cholesterol and that HIV-1 infection status may be counted as a risk factor. The effect of antiretroviral therapy per se[39,40,45] or through its effect on lipids should also be considered as contributing to risk of CVD [46]. HIV-1-treatment guidelines recommend evaluating and managing serum lipids according to specific goals; for patients on antiretroviral therapy, in addition to lifestyle changes and lipid-lowering therapy, modifications of antiretroviral regimen can be an important part of overall CVD risk reduction through improvement of proatherogenic lipid fractions [47,48]. Although there are no clinical trial data to demonstrate that interventions to modify plasma lipids reduces CVD risk in the context of HIV-1 disease, there is good evidence from the general population that reducing TC and LDL cholesterol reduces CVD risk [38]. There is an ongoing Aids Clinical Trials Group study in HIV-1 disease to examine the long-term cardiovascular impact of adding pitavastatin [49].
Few studies have compared the effects of switching antiretrovirals or treating dyslipidemia in HIV-1 infected individuals with statins and most are limited to small, mostly nonrandomized or nonplacebo-controlled trials with a limited follow-up [50–52]. Switching antiretrovirals to improve lipid profiles is a supplemental strategy to the use of lipid-lowering agents and may also have the advantage of reducing the daily pill burden. Switching from PI/r to nonnucleoside reverse transcriptase inhibitor or to INSTI in virologically suppressed patients usually maintains antiviral activity, may improve gastrointestinal symptoms, may offer more convenient dosing, may reduce pill burden, and result in fewer potentially serious drug–drug interactions; however, impact on lipid profile is variable and largely nonsignificant [5,6,27,29,53]. For those with a high CVD risk, the INSTI DTG, may have advantages as a switch choice because of its neutral effect on plasma lipids and our study with patients with high CVD risk showed that virological suppression could be maintained. TC and other proatherogenic lipid fractions significantly (P < 0.001) improved in the DTG group even when stratified by baseline age and Framingham risk score and also by baseline PI/r. Most switching studies have not included in the analysis non-HDL cholesterol fractions an important risk factor for CVD that has been recently incorporated into the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) guidelines [37]. Switching to DTG significantly decreased the TC : HDL cholesterol ratio, a factor used in some CVD risk equations, which is usually unaffected in other antiretroviral switch studies. The study was not powered for differences in cardiovascular events and only three major cardiovascular events were observed. Another limitation of the study can be that most (85%) of the study population were White men all of them coming from developed western European countries.
There are some risks associated with switching to a new regimen in virologically suppressed patients. In our study, protocol defined virological failures were numerically more common in the DTG group (four vs. one in the PI/r group) albeit all at low level and not associated with emergent resistance mutations. Although there were few discontinuations, there were more in the DTG group which is often seen when patients are switching from a regimen that they have been tolerating from a long period of time. Of note, six out of seven discontinued because of mood disturbances or insomnia which have been recently highlighted [54–56] as side-effects of DTG. Moreover, switching from a PI/r regimen to an INSTI regimen may have additional potential benefits in reducing inflammation [5,57,58], immune activation [59], and residual viral replication [60,61].
In conclusion, compared with continuing a PI/r-based regimen switching to a DTG regimen in virologically suppressed HIV-1-infected patients with high CVD risk was noninferior, well tolerated, and significantly improved lipid profiles.
Acknowledgements
We thank the patients who have participated and their families. NEAT022 trial was supported by NEAT-ID Foundation, a not for profit private foundation to promote research and education projects in the HIV field. NEAT022 trial was also supported by SSAT and ViiV Healthcare. We thank the NEAT022 study participants and their partners, families, care givers, and the staff of all the centres taking part in the study. We also thank the European AIDS Treatment Group for collaboration. Spanish centres and Spanish investigators were partially supported by the project RD12/0017 integrated in the Plan Nacional I+D+i and cofunded by ISCIII – Subdirección General de Evaluación and European Regional Development Fund (ERDF). We also acknowledge the Spanish HIV HGM biobank supported by the Spanish Instituto de Salud Carlos III, which is integrated in the Spanish AIDS research network (RIS).
(∗) NEAT 022 study investigators (32 sites from six European countries): Belgium: Linos Vandekerckhove, Els Caluwé, Stephane De Wit, Coca Necsoi, Eric Florence, and Maartje Van Frankenhuijsen; France: Francois Raffi, Clotilde Allavena, Véronique Reliquet, Morane Cavellec, Audrey Rodallec, Thierry Le Tourneau, Jérôme Connault, Jean-Michel Molina, Samuel Ferret, Miresta Previlon, Yazdan Yazdanpanah, Roland Landman, Véronique Joly, Adriana Pinto Martinez, Christine Katlama, Fabienne Caby, Nadine Ktorza and Luminita Schneider; Germany: Christoph Stephan, Timo Wolf, Gundolf Schüttfort, Juergen Rockstroh, Jan-Christian Wasmuth, Carolynne Schwarze-Zander, Christoph Boesecke, Hans-Jurgen Stellbrink, Christian Hoffmann, Michael Sabranski, Stephan Esser, Robert Jablonka, Heidi Wiehler, Georg Behrens, Matthias Stoll, and Gerrit Ahrenstorf; Italy: Giovanni Guaraldi, Giulia Nardini, Barbara Beghetto, Antonella D’Arminio Montforte, Teresa Bini, Viola Cogliandro, Massimo Di Pietro, Francesco Maria Fusco, Massimo Galli, Stefano Rusconi, Andrea Giacomelli, and Paola Meraviglia; Spain: Esteban Martinez, Ana González-Cordón, Berta Torres, Pere Domingo, Gracia Mateo, Mar Gutierrez, Joaquin Portillo, Esperanza Merino, Sergio Reus, Vicente Boix, Mar Masia, Félix Gutiérrez, Sergio Padilla, Bonaventura Clotet, Eugenia Negredo, Anna Bonjoch, José L. Casado, Sara Bañón-Escandell, Jose Saban, Africa Duque, Daniel Podzamczer, Maria Saumoy, Laura Acerete, Juan Gonzalez-Garcia, José Ignacio Bernardino, José Ramón Arribas, and Victor Hontañón; United Kingdom: Graeme Moyle, Nicole Pagani, Margherita Bracchi, Jaime Vera, Amanda Clarke, Tanya Adams, Celia Richardson, Alan Winston, Borja Mora-Peris, Scott Mullaney, Laura Waters, Nahum de Esteban, Ana Milinkovic, Sarah Pett, Julie Fox, Juan Manuel Tiraboschi, Margaret Johnson, Mike Youle, Chloe Orkin, Simon Rackstraw, James Hand, Mark Gompels, Louise Jennings, Jane Nicholls and Sarah Johnston.
NEAT-ID Foundation, St Stephen AIDS Trust (SSAT), ViiV Healthcare, and by the project RD12/0017 integrated in the Plan Nacional I+D+i and co-funded by ISCIII- Subdirección General de Evaluación and European Regional Development Fund (ERDF) for the Spanish participating centers.
J.M.G., A.L.P., S.D.W., E.M., G.G., and F.R. designed the study in consultation with the trial steering committee. G.M., L.W., M.J., P.D., J.F., E.M., G.G., M.M., M.G., S.D.W., E.F., and S.E. enrolled participants into the study. J.M.G., G.M., L.W., M.J., P.D., J.F., A.L.P., F.R., G.G., M.M., M.G., S.D.W., E.M., E.F., and S.E. contributed to the coordination and oversight of the study. L.A. did the statistical analysis. All authors participated in data interpretation. The manuscript was drafted by J.M.G., G.M., L.W., A.L.P., L.A., and P.D. All authors provided input to the report and approved the final version of the manuscript.
Conflicts of interest
J.M.G. has received honoraria for lectures or advisory boards and his institution research grant from ViiV Healthcare, Gilead Sciences, MSD, and Janssen.
G.M. has received honoraria for lectures or advisory boards from Gilead Sciences, MSD, Tobira Therapeutics, and Thera Technologies.
L.W. has received support for attending conferences or honoraria for lectures or advisory boards from Gilead Sciences, ViiV Healthcare, MSD, and Janssen.
M.J. has received consultancy fees from Gilead Sciences, ViiV Healthcare, and MSD.
P.D. has received honoraria for lectures or advisory boards and his institution research grant from ViiV Healthcare, Gilead Sciences, MSD, and Janssen.
J.F. has received research grant from ViiV Healthcare and Gilead Sciences.
E.M. has received honoraria for lectures or advisory boards from ViiV Healthcare, MSD, and Janssen and his institution research grant from ViiV Healthcare, Gilead Sciences, MSD, and Janssen.
H-J.S. has received honoraria for lectures or advisory boards from AbbVie, MSD, Janssen, Gilead Sciences, Teva, and Bristol-Myers Squibb, as well as documentation fees for clinical trials from Gilead, ViiV Healthcare, Janssen, and MSD.
G.G. has received honoraria for lectures or advisory boards and his institution research grant from ViiV Healthcare, Gilead Sciences, MSD, and Janssen.
M.M. has received honoraria for lectures or advisory boards and his institution research grant from ViiV Healthcare, Gilead Sciences, MSD, and Janssen.
M.G. has received educational support to attend CROI from BMS and is undertaking clinical trial work for Merck Sharp & Dohme, Gilead Sciences, and Janssen.
S.D.W. has received honoraria for lecture from Janssen and his institution has received research grants from BMS, Gilead Sciences, Janssen, MSD, and ViiV Healthcare.
E.F. and his institution have received research grants and honoraria for advisory boards participation from BMS, Gilead Sciences, Janssen, MSD, and ViiV Healthcare.
S.E. has received honoraria for lectures or advisory boards and his institution research grants from ViiV Healthcare, Gilead Sciences, MSD, and Janssen.
F.R. has received honoraria for lectures or advisory boards and his institution research grants from, Gilead Sciences, Janssen, Merck, and MSD.
A.L.P. has received honoraria for lectures or advisory boards, and his institution research grants from ViiV Healthcare, Gilead Sciences, MSD, and Janssen.
Supplementary Material
Contributor Information
Collaborators: NEAT022 Study Group∗
References
- 1.Llibre JM, Walmsley S, Gatell JM. Backbones versus core agents in initial ART regimens: one game, two players. J Antimicrob Chemother 2016; 71:856–861. [DOI] [PubMed] [Google Scholar]
- 2.Hare S, Smith SJ, Metifiot M, Jaxa-Chamiec A, Pommier Y, Hughes SH, Cherepanov P. Structural and functional analyses of the second-generation integrase strand transfer inhibitor dolutegravir (S/GSK1349572). Mol Pharmacol 2011; 80:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohn J, Bekker LG, Bygrave H, Calmy A. Hit me with your best shot: dolutegravir: a space in the next WHO guidelines?. AIDS 2015; 29:2067–2070. [DOI] [PubMed] [Google Scholar]
- 4.Kandel CE, Walmsley SL. Dolutegravir: a review of the pharmacology, efficacy, and safety in the treatment of HIV. Drug Des Devel Ther 2015; 9:3547–3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez E, Larrousse M, Llibre JM, Gutierrez F, Saumoy M, Antela A, et al. SPIRAL Study Group. Substitution of raltegravir for ritonavir-boosted protease inhibitors in HIV-infected patients: the SPIRAL study. AIDS 2010; 24:1697–1707. [DOI] [PubMed] [Google Scholar]
- 6.Eron JJ, Young B, Cooper DA, Youle M, Dejesus E, Andrade-Villanueva J, et al. SWITCHMRK 1 and 2 investigators. Switch to a raltegravir-based regimen versus continuation of a lopinavir-ritonavir-based regimen in stable HIV-infected patients with suppressed viraemia (SWITCHMRK 1 and 2): two multicentre, double-blind, randomised controlled trials. Lancet 2010; 375:396–407. [DOI] [PubMed] [Google Scholar]
- 7.Lee FJ, Carr A. Tolerability of HIV integrase inhibitors. Curr Opin HIV AIDS 2012; 7:422–428. [DOI] [PubMed] [Google Scholar]
- 8.Greig SL, Deeks ED. Abacavir/dolutegravir/lamivudine single-tablet regimen: a review of its use in HIV-1 infection. Drugs 2015; 75:503–514. [DOI] [PubMed] [Google Scholar]
- 9.Boffito M, Back D, Gatell JM. Twenty years of boosting antiretroviral agents: where are we today?. AIDS 2015; 29:2229–2233. [DOI] [PubMed] [Google Scholar]
- 10.Wainberg MA, Han YS. Will drug resistance against dolutegravir in initial therapy ever occur?. Front Pharmacol 2015; 6:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wainberg MA, Mesplede T, Raffi F. What if HIV were unable to develop resistance against a new therapeutic agent?. BMC Med 2013; 11:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anstett K, Fusco R, Cutillas V, Mesplede T, Wainberg MA. Dolutegravir-selected HIV-1 containing the N155H and R263K resistance substitutions does not acquire additional compensatory mutations under drug pressure that lead to higher-level resistance and increased replicative capacity. J Virol 2015; 89:10482–10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brenner BG, Wainberg MA. Clinical benefit of dolutegravir in HIV-1 management related to the high genetic barrier to drug resistance. Virus Res 2016; 239:1–9. [DOI] [PubMed] [Google Scholar]
- 14.Quercia R, Roberts J, Martin-Carpenter L, Zala C. Comparative changes of lipid levels in treatment-naive, HIV-1-infected adults treated with dolutegravir vs. efavirenz, raltegravir, and ritonavir-boosted darunavir-based regimens over 48 weeks. Clin Drug Investig 2015; 35:211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raffi F, Rachlis A, Brinson C, Arasteh K, Gorgolas M, Brennan C, et al. Dolutegravir efficacy at 48 weeks in key subgroups of treatment-naive HIV-infected individuals in three randomized trials. AIDS 2015; 29:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raffi F, Rachlis A, Stellbrink HJ, Hardy WD, Torti C, Orkin C, et al. SPRING-2 Study Group. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, noninferiority SPRING-2 study. Lancet 2013; 381:735–743. [DOI] [PubMed] [Google Scholar]
- 17.Walmsley SL, Antela A, Clumeck N, Duiculescu D, Eberhard A, Gutierrez F, et al. SINGLE Investigators. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med 2013; 369:1807–1818. [DOI] [PubMed] [Google Scholar]
- 18.Clotet B, Feinberg J, van Lunzen J, Khuong-Josses MA, Antinori A, Dumitru I, et al. ING114915 Study Team. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet 2014; 383:2222–2231. [DOI] [PubMed] [Google Scholar]
- 19.Orrell C, Hagins D, Belonosova E, Porteiro N, Walmsley S, Falco V, et al. Superior efficacy of dolutegravir/abacavir/lamivudine FDC compared with ritonavir-boosted atazanavir plus tenofovir disoproxil fumarate/emtricitabine FDC in treatment-naive women with HIV-1 infection: ARIA study. 21st International AIDS Conference. Durban, South Africa; 18–22 July 2016. [Google Scholar]
- 20.Gunthard HF, Saag MS, Benson CA, Del RC, Eron JJ, Gallant JE, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the international antiviral society-USA panel. JAMA 2016; 316:191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. July 2016. Department of Health and Human Services USA, Washington, D.C., USA: https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv-guidelines/0 [Accessed 13 February 2017]. [Google Scholar]
- 22.Panels on EACS Guidelines. Guidelines V 8.1 (October 2016). European AIDS Clinical Society (EACS). Brussels: http://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html [Accessed 13 February 2017]. [Google Scholar]
- 23.Armah KA, Chang CC, Baker JV, Ramachandran VS, Budoff MJ, Crane HM, et al. Veterans Aging Cohort Study (VACS) Project Tea. Prehypertension, hypertension, and the risk of acute myocardial infarction in HIV-infected and -uninfected veterans. Clin Infect Dis 2014; 58:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Althoff KN, McGinnis KA, Wyatt CM, Freiberg MS, Gilbert C, Oursler KK, et al. Veterans Aging Cohort Study (VACS). Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer in HIV-infected vs uninfected adults. Clin Infect Dis 2014; 60:627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calvo-Sanchez M, Perello R, Perez I, Mateo MG, Junyent M, Laguno M, et al. Differences between HIV-infected and uninfected adults in the contributions of smoking, diabetes and hypertension to acute coronary syndrome: two parallel case-control studies. HIV Med 2013; 14:40–48. [DOI] [PubMed] [Google Scholar]
- 26.Gatell J, Salmon-Ceron D, Lazzarin A, Van Wijngaerden E, Antunes F, Leen C, et al. SWAN Study Group. Efficacy and safety of atazanavir-based highly active antiretroviral therapy in patients with virologic suppression switched from a stable, boosted or unboosted protease inhibitor treatment regimen: the SWAN study (AI424-097) 48-week results. Clin Infect Dis 2007; 44:1484–1492. [DOI] [PubMed] [Google Scholar]
- 27.Mallolas J, Podzamczer D, Milinkovic A, Domingo P, Clotet B, Ribera E, et al. ATAZIP Study Group. Efficacy and safety of switching from boosted lopinavir to boosted atazanavir in patients with virological suppression receiving a LPV/r-containing HAART: the ATAZIP study. J Acquir Immune Defic Syndr 2009; 51:29–36. [DOI] [PubMed] [Google Scholar]
- 28.Martinez E, Arnaiz JA, Podzamczer D, Dalmau D, Ribera E, Domingo P, et al. Nevirapine, Efavirenz, and Abacavir (NEFA) Study Team. Substitution of nevirapine, efavirenz, or abacavir for protease inhibitors in patients with human immunodeficiency virus infection. N Engl J Med 2003; 349:1036–1046. [DOI] [PubMed] [Google Scholar]
- 29.Arribas JR, Pialoux G, Gathe J, Di Perri G, Reynes J, Tebas P, et al. Simplification to coformulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus continuation of ritonavir-boosted protease inhibitor with emtricitabine and tenofovir in adults with virologically suppressed HIV (STRATEGY-PI): 48 week results of a randomised, open-label, phase 3b, noninferiority trial. Lancet Infect Dis 2014; 14:581–589. [DOI] [PubMed] [Google Scholar]
- 30.Trottier B, Lake J, Logue K, Brinson C, Santiago L, Brennan C, et al. Switching to Abacavir/Dolutegravir/Lamivudine Fixed Dose Combination (ABC/DTG/3TC FDC) from a PI, INI or NNRTI Based Regimen Maintains HIV Suppression. 55th Interscience Conference on Antimicrobials Agents and Chemotherapy, San Diego, California, USA; 17–21 September 2015. [Google Scholar]
- 31.Marzolini C, Gibbons S, Khoo S, Back D. Cobicistat versus ritonavir boosting and differences in the drug-drug interaction profiles with co-medications. J Antimicrob Chemother 2016; 71:1755–1758. [DOI] [PubMed] [Google Scholar]
- 32.Randell PA, Jackson AG, Zhong L, Yale K, Moyle GJ. The effect of tenofovir disoproxil fumarate on whole-body insulin sensitivity, lipids and adipokines in healthy volunteers. Antivir Ther 2010; 15:227–233. [DOI] [PubMed] [Google Scholar]
- 33.Copenhagen HIV Program (CHIP). Risk Assesment Tool System (RATS). http://www.chip.dk/TOOLS [Accessed 13 February 2017]. [Google Scholar]
- 34.Framinghan Heart Study. A project of the National Heart Lung and Blood Institute and Boston University. General Cardiovascular Disease (10-year risk) Prediction Using Lipids. https://www.framinghamheartstudy.org/risk-functions/cardiovascular-disease/10-year-risk.php# [Accessed 13 February 2017]. [Google Scholar]
- 35.Johnson VA, Calvez V, Gunthard HF, Paredes R, Pillay D, Shafer RW, et al. Update of the drug resistance mutations in HIV-1: March 2013. Top Antivir Med 2013; 21:6–14. [PMC free article] [PubMed] [Google Scholar]
- 36.Mocroft A, Ryom L, Reiss P, Furrer H, d’Arminio MA, Gatell J, et al. EuroSIDA in EuroCOORD. A comparison of estimated glomerular filtration rates using Cockcroft-Gault and the Chronic Kidney Disease Epidemiology Collaboration estimating equations in HIV infection. HIV Med 2014; 15:144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Cholesterol Education Program. National Heart Lung and Blood Institute. National Institutes of Health. ATP III Guidelines At-A-Glance. Quick Desk Reference. National Cholesterol Education Program: National Heart Lung and Blood Institute. National Institutes of Health. https://www.nhlbi.nih.gov/files/docs/guidelines/atglance.pdf [Accessed 4 April 2017]. [Google Scholar]
- 38.Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, et al. Cholesterol Treatment Trialists CTT. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012; 380:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Worm SW, Sabin C, Weber R, Reiss P, El-Sadr W, Dabis F, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis 2010; 201:318–330. [DOI] [PubMed] [Google Scholar]
- 40.Ryom L, Lundgren J, El-Sadr WM, Reiss P, Phillips A, Kirk O, et al. Association between cardiovascular disease & contemporarely used protease inhibitors. Conference Retrovirus Opportunistic Infections 2017 (CROI 2017). Seattle, Washington, USA; 13–16 February 2017. [Google Scholar]
- 41.Islam F, Wu J, Jansson J, Wilson D. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV Med 2012; 13:453–468. [DOI] [PubMed] [Google Scholar]
- 42.Smith C, Sabin CA, Lundgren JD, Thiebaut R, Weber R, Law M, et al. Data Collection on Adverse Events of Anti-HIV drugs (D:A:D) Study Group. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS 2010; 24:1537–1548. [DOI] [PubMed] [Google Scholar]
- 43.Mocroft A, Reiss P, Gasiorowski J, Ledergerber B, Kowalska J, Chiesi A, et al. EuroSIDA Study Group. Serious fatal and nonfatal non-AIDS-defining illnesses in Europe. J Acquir Immune Defic Syndr 2010; 55:262–270. [DOI] [PubMed] [Google Scholar]
- 44.Bays HE, Jones PH, Orringer CE, Brown WV, Jacobson TA. National lipid association annual summary of clinical lipidology 2016. J Clin Lipidol 2016; 10 1 Suppl:S1–S43. [DOI] [PubMed] [Google Scholar]
- 45.Friis-Moller N, Sabin CA, Weber R, D’Arminio MA, El Sadr WM, Reiss P, et al. Data Collection on Adverse Events of Anti-HIV Drugs (DAD) Study Group. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 2003; 349:1993–2003.14627784 [Google Scholar]
- 46.Estrada V, Geijo P, Fuentes-Ferrer M, Garcia Alcalde ML, Rodrigo M, Galindo MJ, et al. Dyslipidemia in HIV-infected women on antiretroviral therapy. Analysis of 922 patients from the Spanish VACH cohort. BMC Womens Health 2011; 11:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polo Rodriguez R, Galindo Puerto MJ, Duenas C, Gomez Candela C, Estrada V, Villar NG, et al. Panel of experts from the Metabolic Disorders Study Group (GEAM), National Aids Plan (SPNS), Aids Study Group (GeSIDA). Executive summary of the consensus document on metabolic disorders and cardiovascular risk in patients with HIV infection. Enferm Infecc Microbiol Clin 2015; 33:41–47. [DOI] [PubMed] [Google Scholar]
- 48.British HIV Association. British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2015. http://www.bhiva.org/HIV-1-treatment-guidelines.aspx [Accessed 13 February 2017]. [Google Scholar]
- 49.Evaluating the Use of Pitavastatin to Reduce the Risk of Cardiovascular Disease in HIV-Infected Adults (REPRIEVE). https://clinicaltrials.gov/ct2/results?cond=&term=reprieve&cntry1=&state1=&recrs= [Accessed 23 October 2017]. [Google Scholar]
- 50.Calza L, Manfredi R, Chiodo F. Statins and fibrates for the treatment of hyperlipidaemia in HIV-infected patients receiving HAART. AIDS 2003; 17:851–859. [DOI] [PubMed] [Google Scholar]
- 51.Visnegarwala F, Maldonado M, Sajja P, Minihan JL, Rodriguez-Barradas MC, Ong O, et al. Lipid lowering effects of statins and fibrates in the management of HIV dyslipidemias associated with antiretroviral therapy in HIV clinical practice. J Infect 2004; 49:283–290. [DOI] [PubMed] [Google Scholar]
- 52.Lee FJ, Monteiro P, Baker D, Bloch M, Roth N, Finlayson R, et al. Rosuvastatin vs. protease inhibitor switching for hypercholesterolaemia: a randomized trial. HIV Med 2016; 17:605–614. [DOI] [PubMed] [Google Scholar]
- 53.Palella FJ, Jr, Fisher M, Tebas P, Gazzard B, Ruane P, Van Lunzen J, et al. Simplification to rilpivirine/emtricitabine/tenofovir disoproxil fumarate from ritonavir-boosted protease inhibitor antiretroviral therapy in a randomized trial of HIV-1 RNA-suppressed participants. AIDS 2014; 28:335–344. [DOI] [PubMed] [Google Scholar]
- 54.de Boer MG, van den Berk GE, van HN, Oryszcyn JE, Dorama W, Moha DA, Brinkman K. Intolerance of dolutegravir-containing combination antiretroviral therapy regimens in real-life clinical practice. AIDS 2016; 30:2831–2834. [DOI] [PubMed] [Google Scholar]
- 55.Fettiplace A, Stainsby C, Winston A, Givens N, Puccini S, Vannappagari V, et al. Psychiatric symptoms in patients receiving dolutegravir. J Acquir Immune Defic Syndr 2017; 74:423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Penafiel J, de Lazzari E, Padilla M, Rojas J, Gonzalez-Cordon A, Blanco JL, et al. Tolerability of integrase inhibitors in a real-life setting. J Antimicrob Chemother 2017; 72:1752–1759. [DOI] [PubMed] [Google Scholar]
- 57.Martinez E, D’Albuquerque PM, Llibre JM, Gutierrez F, Podzamczer D, Antela A, et al. Changes in cardiovascular biomarkers in HIV-infected patients switching from ritonavir-boosted protease inhibitors to raltegravir. AIDS 2012; 26:2315–2326. [DOI] [PubMed] [Google Scholar]
- 58.Silva EF, Charreau I, Gourmel B, Mourah S, Kalidi I, Guillon B, et al. ANRS 138 EASIER Study Group. Decreases in inflammatory and coagulation biomarkers levels in HIV-infected patients switching from enfuvirtide to raltegravir: ANRS 138 substudy. J Infect Dis 2013; 208:892–897. [DOI] [PubMed] [Google Scholar]
- 59.Hileman CO, Kinley B, Scharen-Guivel V, Melbourne K, Szwarcberg J, Robinson J, et al. Differential reduction in monocyte activation and vascular inflammation with integrase inhibitor-based initial antiretroviral therapy among HIV-infected individuals. J Infect Dis 2015; 212:345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buzon MJ, Massanella M, Llibre JM, Esteve A, Dahl V, Puertas MC, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med 2010; 16:460–465. [DOI] [PubMed] [Google Scholar]
- 61.Hatano H, Strain MC, Scherzer R, Bacchetti P, Wentworth D, Hoh R, et al. Increase in 2-long terminal repeat circles and decrease in D-dimer after raltegravir intensification in patients with treated HIV infection: a randomized, placebo-controlled trial. J Infect Dis 2013; 208:1436–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


