Abstract
Hepatocellular carcinoma (HCC) is the third leading form of cancer worldwide, and its incidence is increasing rapidly in the United States, tripling over the past 3 decades. The current chemotherapeutic strategies against localized and metastatic HCC are ineffective. Here we report that 6-methoxyethylamino-numonafide (MEAN) is a potent growth inhibitor of murine xenografts of 2 human HCC cell lines. At the same dose and with the same treatment strategies, MEAN was more efficacious in inhibiting tumor growth in mice than sorafenib, the only approved drug for HCC. Treatment by MEAN at an effective dose for 6 wk was well tolerated by animals. Combined therapy using both sorafenib and MEAN enhanced tumor growth inhibition over monotherapy with either agent. Additional experiments revealed that MEAN inhibited tumor growth through mechanisms distinct from those of either its parent compound, amonafide, or sorafenib. MEAN suppressed C-MYC expression and increased expression of several tumor suppressor genes, including Src homology region 2 domain-containing phosphatase-1 (SHP-1) and TXNIP (thioredoxin-interacting protein). As an encouraging feature for envisioned clinical application, the IC50 of MEAN was not significantly changed in several drug-resistant cell lines with activated P-glycoprotein drug efflux pumps compared to drug-sensitive parent cells, demonstrating the ability of MEAN to be effective in cells resistant to existing chemotherapy regimens. MEAN is a promising candidate for clinical development as a single-agent therapy or in combination with sorafenib for the management of HCC.—Liu, Y., Lou, G., Norton, J. T., Wang, C., Kandela, I., Tang, S., Shank, N. I., Gupta, P., Huang, M., Avram, M. J., Green, R., Mazar, A., Appella, D., Chen, Z., Huang, S. 6-Methoxyethylamino-numonafide inhibits hepatocellular carcinoma xenograft growth as a single agent and in combination with sorafenib.
Keywords: MEAN, HCC therapeutic, combination treatment, C-MYC inhibition
Hepatocellular carcinoma (HCC) is one of the most common causes of cancer deaths worldwide. In 2008, an estimated 750,000 new cases of liver cancer occurred, and ∼700,000 people died of this cancer worldwide (1). Although HCC was once a public health problem limited primarily to Asia and Africa, the incidence of HCC in the United States has been increasing rapidly, and will likely continue to increase over several decades. The age-adjusted incidence of HCC tripled in the United States between 1975 and 2014, increasing from 1.6 to 4.9 per 100,000 people (2). HCC is now the ninth leading cause of cancer deaths in the United States and the third leading cause of cancer deaths worldwide (3, 4). The incidence of HCC is rising in the United States, partly as a result of the hepatitis C epidemic. Once cirrhosis occurs in patients with hepatitis C, HCC develops at an annual rate of 1 to 5% (5–7). In addition, American’s obesity epidemic is resulting in a rapid increase in the incidence of nonalcoholic steatohepatitis/cryptogenic cirrhosis, which is also a major risk factor for the development of HCC (8, 9). Therefore, HCC is rapidly becoming a major health problem in the United States.
The primary curative therapy for HCC is surgical resection by either liver resection or liver transplantation (10, 11). In Western countries, only 5% of patients with HCC are candidates for surgical resection, and the only curative procedure for the remaining 95% of patients is liver transplantation, which is limited by the small number of available donor livers and the long wait times. Adjuvant therapies for HCC, such as percutaneous ablation, transcatheter arterial chemoembolization, or yttrium-90 microspheres (12), are often used as palliative therapies or as a bridge to liver transplantation; these treatments are typically not curative. Systemic chemotherapy is not recommended before liver transplantation or as a bridge to transplantation because of the poor efficacy of available chemotherapeutic agents. The only approved chemotherapy for HCC is sorafenib, a tyrosine and serine–threonine kinase inhibitor that has been shown to have efficacy in treating HCC (13, 14). However, the effectiveness of sorafenib appears to be limited: median overall survival was only extended from 7.9 to 10.7 mo and from 4.2 to 6.5 mo in 2 clinical trials in Asia (13–15). There was also significant drug toxicity in these studies (13–15). Attempts to identify combinatory partners for sorafenib have not been successful (16, 17). The lack of effective systemic chemotherapy for HCC is one of the major reasons for the poor 5 y survival rates for HCC patients in the United States (2).
Recently 6-methoxyethylamino-numonafide (MEAN) (18) was shown to be effective against HCC xenograft tumors in mice (19). MEAN was able to cause regression of established tumors over a 6-wk treatment period, and animals tolerated the treatment well (19). MEAN is a derivative of amonafide (AMN), an anticancer agent that is effective against a wide range of cancers with little risk of drug resistance as it is not a substrate for drug efflux pumps (20). AMN has a free aryl amine at the 5 position of the molecule that is acetylated by N-acetyltransferase 2 (NAT2), forming the toxic metabolite N-acetyl-AMN. The pharmacogenomic variability in the activity of NAT2 in human populations treated with AMN caused some patients to experience severe toxicities. As a result of its high and variable toxicity, AMN has not received U.S. Food and Drug Administration (FDA) approval (21, 22). MEAN is an analog of AMN, with the aryl amine at the sixth position blocked with an ethyl-methyoxy moiety. While MEAN has efficacy in inhibiting the growth of Huh7 and HepG2 HCC xenograft tumors similar to that of AMN, it has significantly less toxicity than AMN in murine studies (19).
In this study, we compared the antitumor efficacy and toxicities of sorafenib and MEAN monotherapy and combination therapy in murine xenograft models. We investigated the similarity and differences in the mechanisms of action between MEAN and AMN, as well as in sorafenib.
MATERIALS AND METHODS
Cells and culture conditions
Human HCC cell lines HepG2 and Huh7 were maintained in DMEM (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal bovine serum (Thermo Fisher Scientific), 100 U/ml penicillin, and 100 μg/ml streptomycin (Thermo Fisher Scientific). HepG2-luc and Huh7-luc cell lines were constructed as they were in on our previous studies with little modification (19), and they were also cultured in DMEM supplemented with 10% fetal bovine serum.
Mouse modeling
HepG2-luc and Huh7-luc xenograft tumor models were established as described (19), and mice were treated and cared for in accordance with the rules of the office of Laboratory Animal Welfare (23). Briefly, male nu/nu (nude) mice weighing 18 to 20 g at experiment initiation were maintained at the vivarium in a pathogen-free unit under a 12-h light/dark cycle. Mice were inoculated s.c. with HepG2-luc or with Huh7-luc (5 × 106 cells). Ten days after cell implantation, and before drug treatment, mice were randomized into groups of 5 mice. Sorafenib (LC Laboratories, Woburn, MA, USA) was dissolved in 1:1 ethanol and Cremophor EL (Sigma-Aldrich, St. Louis, MO, USA), then diluted in PBS to 5 mg/ml on the day of treatment. MEAN was also dissolved in 1:1 ethanol and Cremophor EL (Sigma-Aldrich) and then diluted in PBS to 2 mg/ml. The solutions were stored at −20°C between injections. Sorafenib (15 mg/kg) or MEAN (15 mg/kg) was administered by i.p. injection for 5 consecutive days, followed by 2 d without dosing. Vehicle control was 10% 1:1 ethanol and Cremophor EL (Sigma-Aldrich). In vivo bioluminescent imaging to determine tumor burden was performed with a Lumina imaging system (Nippon Roper, I.C.E., Tokyo, Japan). Ten minutes before imaging, mice were injected intraperitoneally with 150 mg/kg luciferin. Images were collected and analyzed by Living Image 4.1 (PerkinElmer, Waltham, MA, USA) and SlideBook 5 (Intelligent Imaging Innovations, Denver, CO, USA) software. Tumor volumes were determined twice weekly by mechanically measuring the length (a) and width (b), with volume = ab2/2. Tumor weights were measured at the experimental end point (d 42 from the start of treatment).
Alanine aminotransferase and aspartate aminotransferase measurement
Blood samples were collected from each mouse at the end of d 42. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured by Fuji Dri-Chem Slide GFP/ALT-PIII and GOT/AST- PIII, respectively, with an automated biochemical analyzer (Dri-Chem 4000ie; Fujifilm, Tokyo, Japan) according to the manufacturer’s instructions.
RNA array analyses in HepG2 cells
RNA was isolated from 107 HepG2 cells with Trizol 6 h after treatment with 20 μM AMN, MEAN, or vehicle (0.2% DMSO). RNA expression analysis was performed by GeneChip PrimeView Human Gene Expression Array (Thermo Fisher Scientific). Initially genes were identified as being differentially expressed on the basis of a statistically significant (P < 0.05) and 1.5-fold change (up or down) in expression levels in each comparison.
Western blot analysis and antibodies
Cells or tumor chunks (tumors from 5 animals from each group pooled) were lysed with RIPA lysis buffer (Beyotime Biotechnology, Jiangsu, China) containing 1% halt protease and phosphatase inhibitor cocktail (100 times) (Thermo Fisher Scientific). Twenty milligrams of total protein per lane were separated by 10% SDS-PAGE gels, transferred to PVDF membranes (EMD Millipore, Billerica, MA, USA), and blocked with 1% bovine serum albumin in Tris-buffered saline–Tween 20 (0.05%, v/v) for 1 h at room temperature. The membrane was incubated with primary antibody overnight at 4°C. Antibodies for C-MYC and glyceraldehyde phosphate dehydrogenase were obtained from Cell Signaling Technology (Danvers, MA, USA), and thioredoxin-interacting protein (TXNIP), Src homology region 2 domain-containing phosphatase 1 (SHP-1), and sirtuin 1 (SIRT1) were obtained from Abcam (Cambridge, MA, USA). After washing, the membrane was incubated with the appropriate horseradish peroxidase–conjugated secondary antibody (1:3000; Eptmomics, Burlingame, CA, USA) for 1 h. Blots were visualized by ECL-associated fluorography (EMD Millipore). Relative band intensity was quantified by ImageJ software [Image Processing and Analysis in Java; National Institutes of Health (NIH), Bethesda, MD, USA; http://imagej.nih.gov/] to determine C-MYC, TXNIP, SHP-1, and SIRT1 concentrations (n = 3).
Statistical analysis
Statistical analysis was performed by Student’s t test to evaluate differences between groups. Each in vitro experiment was repeated at least 3 times, and data are provided as means ± se. A value of P < 0.05 was considered to be statistically significant.
Pharmacokinetic analyses of MEAN
A preliminary pharmacokinetic study of MEAN was conducted in female mice using a standard experimental design (24). The study included 3 female mice in the vehicle control group and 3 female mice in the treatment groups. CD1 mice, weighing 22 to 24 g, were purchased from Charles River Laboratories (Wilmington, MA, USA). They were housed 3 animals per cage with ad libitum access to water and standard chow (Teklad 7912; Harlan Industries, Indianapolis, IN, USA). Environmental controls for the animal room were set to maintain temperature between 68 and 75°F with relative humidity of 30 to 70% and a 12-h light/dark cycle. All animal experiments were conducted under protocols approved by the Animal Care and Use Committee of Northwestern University. For each time point in each treatment group, MEAN (60 mg/kg) was administered via i.v. bolus (0.1 ml), i.p. bolus (0.1 ml), and oral (p.o.) routes (0.1 ml) in separate studies. Mice were humanely killed by isoflurane at 2, 4, 6, 8, 12, and 24 h after injection, and blood samples for drug concentration measurement were collected from 3 mice in each dosing group by cardiac puncture using a syringe rinsed with an EDTA solution. Plasma was separated through centrifugation and stored at −80°C.
For drug concentration measurement, plasma MEAN concentrations were determined in duplicate by liquid chromatography–MS after sample preparation by solid-phase extraction of 100 µl plasma samples using atropine as an internal standard. Prepared samples were analyzed by an API 3000 liquid chromatography–tandem MS system (Thermo Fisher Scientific) equipped with an Agilent 1100 series HPLC system (Agilent Technologies, Santa Clara, CA, USA). The tandem mass spectrometer was operated with its electrospray source in the positive ionization mode. The mass-to-charge ratios of the precursor-to-product ion reactions monitored were 342.3 → 297.2 for MEAN and 290.2 → 124.2 for the internal standard. Fresh standard curves for plasma MEAN concentrations of 0.1 to 100 ng/ml were prepared in blank mouse plasma and run on the day of study sample analysis. Coefficients of variation of 10% or less were observed throughout the entire concentration range. Pharmacokinetic analysis–plasma drug concentration vs. time relationships were modeled by the SAAM II software system (SAAM Institute, Seattle, WA, USA) implemented on a Windows-based PC (25). Plasma drug concentrations were modeled with a compartmental pharmacokinetic model. Drug delivery (i.p. and p.o.) was modeled through a tanks-in-series delay element to characterize the noninstantaneous first appearance of the drug in the body (Supplemental Fig. 2A–D). In this model, the rate constants kin and kout represent the proportions of drug absorbed and not absorbed, respectively, rather than actual absorption rates, which are characterized by the delay element. The SAAM II objective function used was the extended least-squares maximum likelihood function using data weighted with the inverse of the model-based variance of the data at the observation times. Model misspecification was sought by inspection of the measured and predicted marker concentrations vs. time relationships. Data collected after i.v., i.p., and p.o. administration of a given dose were modeled simultaneously. Because bioavailability of the i.v. dose is 100%, simultaneous estimation of pharmacokinetic model parameters for i.v. and other routes of administration permit estimation of the bioavailability of the drug administered by a non-i.v. route (e.g., Fi.p.) as Fi.p. = kin/(kin + kout).
Toxicology (maximum tolerated dose) study with MEAN using CD1 mice
To evaluate the maximum tolerated dose of MEAN compound, 3 female and 3 male CD1 mice weighing 22 to 24 g [Crl:CD1(ICR); Charles River Laboratories] were assigned to receive 60, 75, and 90 mg/kg of mouse via tail vein injection (bolus). Assessment of adverse events was based on total body weight, clinical signs, complete blood count, and blood chemistry panels. All animals survived through their respective dosing schedule until they were humanely killed, except at 90 mg/kg. The dose at which no observed adverse event was observed was 75 mg/kg (Supplemental Fig. 1).
RESULTS
MEAN was more efficacious than sorafenib in inhibiting HCC xenograft tumor growth in nude mice under the same experimental conditions
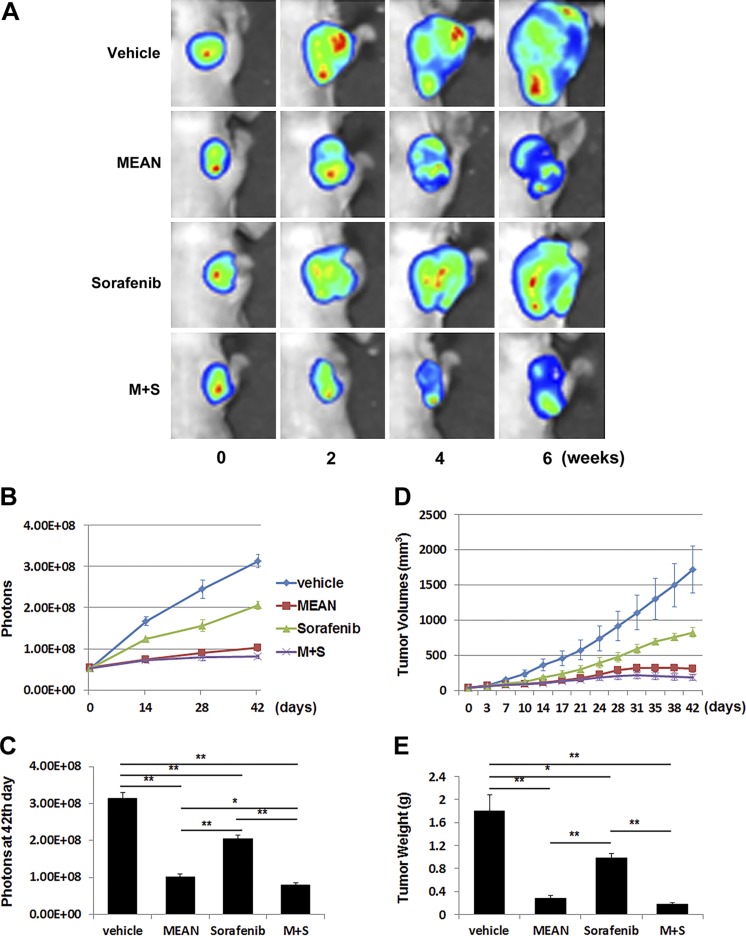
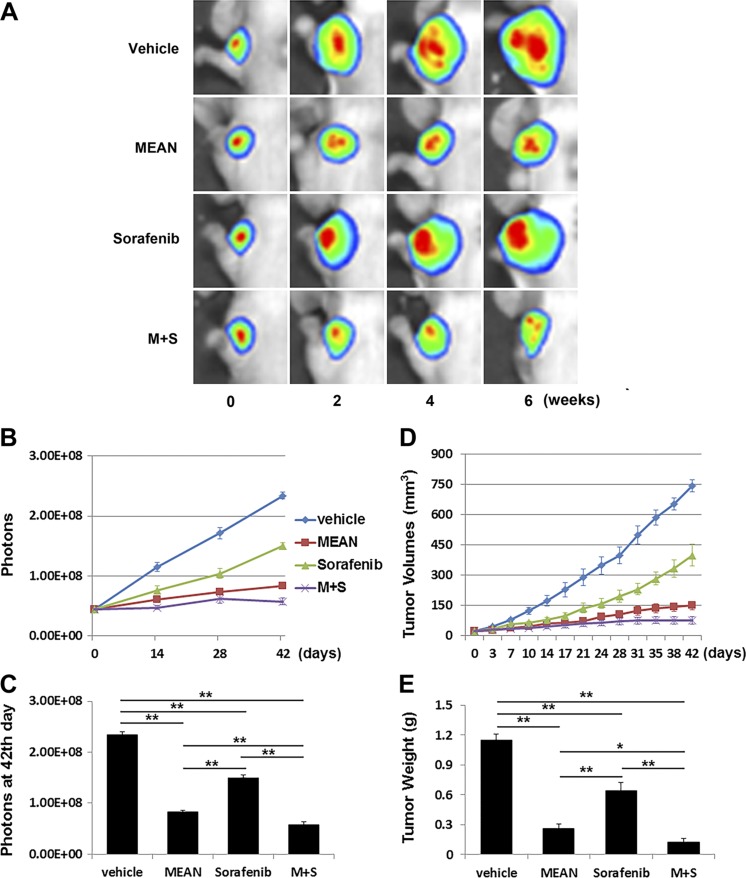
MEAN, at 100 μM/kg (34.2 mg/kg), regressed HCC xenograft tumor growth in mice (19). To directly compare the efficacy of MEAN with sorafenib, the same HCC xenograft models were treated in parallel with both agents using the same treatment strategy. Nude mice were inoculated s.c. with 5 × 106 Huh7-luc or HepG2-luc cells, and tumors were allowed to grow for 10 d. Treatment was initiated on d 11. Either MEAN or sorafenib at 15 mg/kg, at a half concentration of the established use, was injected i.p. into the peritoneal space with a schedule of 5 d on and 2 d off, for 42 d. Tumor growth was monitored through measurement of luciferase emission twice a week (Figs. 1A, B and 2A, B) and at the end of the experiments (Figs. 1C and 2C), tumor volume biweekly (Figs. 1D and 2D), and tumor weight at the end of the experiments (Figs. 1E and 2E). Treatment with both agents at the given dosages produced significant tumor growth retardation in treated mice compared to vehicle-only control mice (Figs. 1 and 2) (P < 0.05). Although the established tumors did not shrink, as shown previously with higher doses of MEAN (19), the 15 mg/kg MEAN treatment nearly flattened the tumor growth (Fig. 1). With the identical treatment doses and schedule, MEAN inhibited tumor growth more effectively than sorafenib (P < 0.05) by all measurements in both HCC xenograft tumor models (Figs. 1 and 2).
Figure 1.
MEAN inhibited Huh7-luc xenograft tumor growth more effectively than sorafenib under same treatment conditions in mice. Starting at d 11 after inoculation of tumor cells subcutaneously, mice were treated with MEAN (15 mg/kg), sorafenib (15 mg/kg), or MEAN and sorafenib in combination at 15 mg/kg each, or vehicle via i.p. administration at schedule of 5 d on and 2 d off for 42 d. Tumor growth was measured by whole-mount imaging of luciferin florescence (representative images) (A), photon counts twice a week (B), or photon count at end of experiment, d 42 (C), by volume (D), or by tumor weight at d 42 (E). *P < 0.5, **P < 0.01 (Student’s t test). Lack of black bar indicates P > 0.5 (n = 5).
Figure 2.
MEAN inhibited HepG2-luc xenograft tumor growth more effectively than sorafenib under same treatment conditions in mice. Starting at d 11 after inoculation of tumor cells s.c., mice were treated with MEAN (15 mg/kg), sorafenib (15 mg/kg), or MEAN and sorafenib in combination at 15 mg/kg each, or vehicle through i.p. administration at schedule of 5 d on and 2 d off for 42 d. Tumor growth was measured by whole-mount imaging of luciferin florescence (representative images) (A), photon counts twice a week (B), or photon count at end of experiment, d 42 (C), by volume (D), and by tumor weight at d 42 (E). *P < 0.5, **P < 0.01 (Student’s t test). Lack of black bar indicates P > 0.5 (n = 5).
MEAN and sorafenib combination enhanced efficacy in HCC xenograft tumor growth inhibition
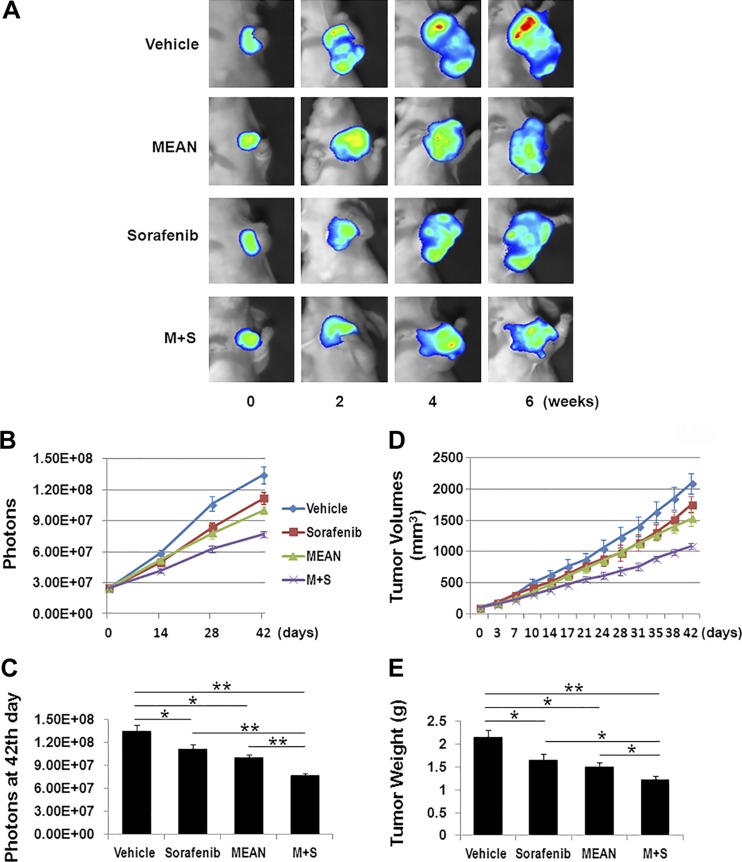
To determine whether treatment with both compounds could be more effective, the same doses of both MEAN and sorafenib (15 mg/kg) were administered together to treat Huh7 and HepG2 xenograft tumors in parallel with monotherapies of either agent. The combination of MEAN and sorafenib blocked tumor growth nearly completely, and it appeared to start reducing tumor size at approximately d 30 of the treatment (Figs. 1 and 2). While the combined treatment was significantly more efficacious than sorafenib monotherapy, the differences in efficacy between combined therapy and MEAN monotherapy were modest (Figs. 1C, E and 2C, E) in both tumor models, although with some statistical significance. The lack of enhancement of MEAN efficacy by addition of sorafenib could be because MEAN at a 15 mg/kg treatment concentration already produced near-maximal inhibition. To further evaluate whether the combination of the two agents could enhance tumor growth suppression, MEAN was used at 50% of the initial concentration at 7.5 mg/kg. Under this condition, combination treatment with MEAN and sorafenib significantly enhanced tumor growth inhibition in Huh7 xenograft model than monotherapies with either agent at the corresponding concentration (Fig. 3). This result encourages further dose optimization to increase the efficacy of the combination treatment.
Figure 3.
Combination treatment of sorafenib (15 mg/kg) and reduced MEAN (7.5 mg/kg) enhances tumor growth inhibition in Huh7-luc xenograft model. Starting at d 11 after inoculation of tumor cells subcutaneously, mice were treated with MEAN (7.5 mg/kg), sorafenib (15 mg/kg), or MEAN and sorafenib in combination at corresponding concentrations, or vehicle through i.p. administration at schedule of 5 d on and 2 d off for 42 d. Tumor growth was measured by whole-mount imaging of luciferin florescence (representative images) (A), photon counts twice a week (B), or photon count at end of experiment, d 42 (C), by volume (D), or by tumor weight at d 42 (E). *P < 0.5, **P < 0.01 (Student’s t test). Lack of black bar indicates P > 0.5 (n = 5).
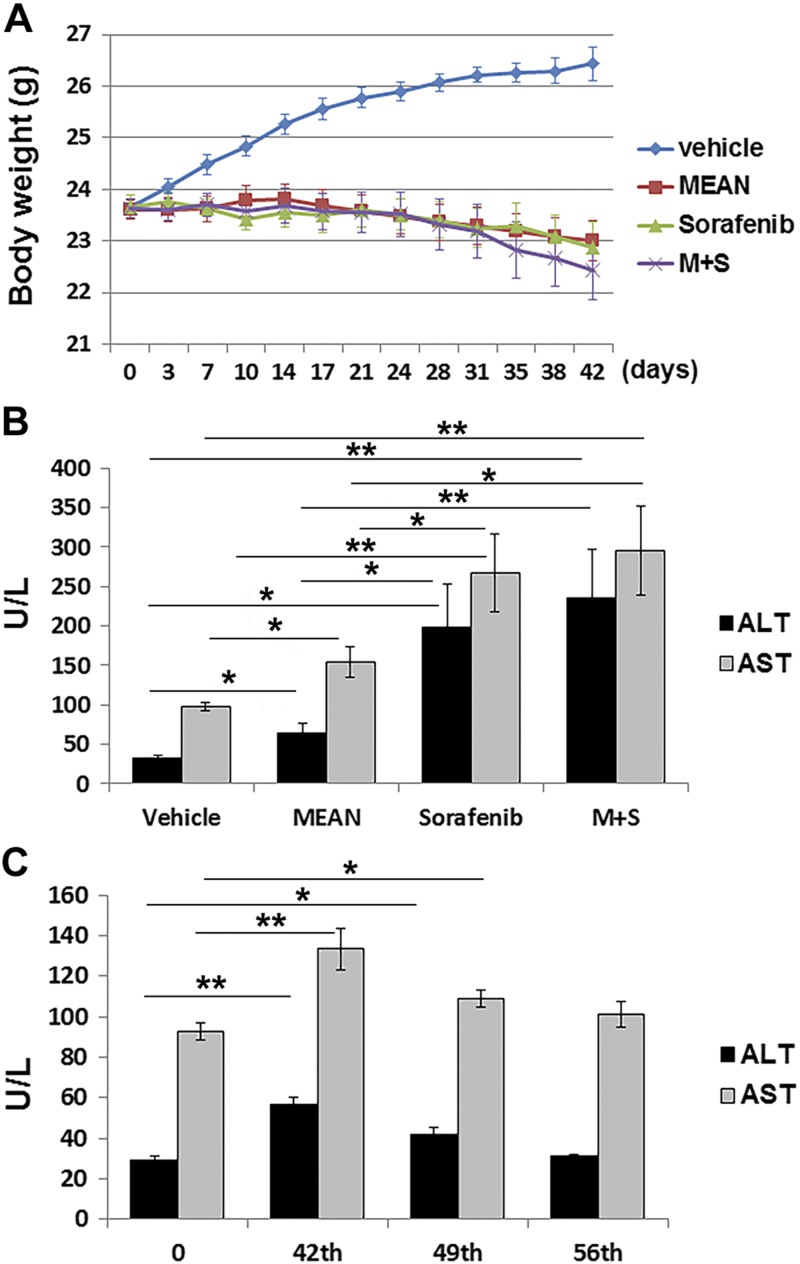
Combination of MEAN with sorafenib did not significantly increase toxicity
To determine whether the combined treatment of MEAN and sorafenib increased toxicity compared to monotherapy, the toxicity produced by treatment with both agents alone and in combination was evaluated. Toxicity was assessed biweekly by monitoring body weight, animal well-being (grooming, eating, stool consistency, and blood panel), and serum liver enzyme concentrations (ALT and AST) at the experimental end point (d 42). Through the 6 wk treatment with a schedule of 5 d on and 2 d off by i.p. injections, mice treated with MEAN at 15 mg/kg did not have significant changes in body weight (<3%) compared to their pretreatment weights (Fig. 4A). In comparison, vehicle-treated control mice had increased body weight at least partly attributable to tumor growth. Serum concentrations of liver enzymes at the experimental end point in the MEAN-treated group increased after 42 d of treatment (P < 0.05) (Fig. 4B). This increase was reversible within 2 wk of treatment withdrawal (Fig. 4C). Mice in the MEAN treatment and vehicle groups remained equally active, and they had normal stool consistency, food intake, and grooming behaviors (recorded twice a week). Furthermore, a single-dose toxicity evaluation in mice showed that up to 75 mg/kg MEAN i.p. injection did not produce significant toxicity 48 h after injection, as judged by body weight, organ weight, complete blood count, and liver enzymes (Supplemental Fig. 1). These results indicate that at the effective tumor inhibitory dose, MEAN does not produce obvious adverse effects in treated animals.
Figure 4.
Toxicity induced by treatment of MEAN, sorafenib, or combination of both was measured against vehicle-treated group through monitoring body weight changes throughout treatment course (A) and detecting serum levels of liver enzymes at experimental end point (B). Liver enzymes were recovered 2 wk after experimental end point (d 42) (C). *P < 0.5, **P < 0.01 (Student’s t test). Lack of black bar indicates P > 0.5 (n = 5).
In comparison, sorafenib treatment at the same concentration also did not alter animal body weight significantly (Fig. 4A). However, serum concentrations of ALT and AST were significantly higher in the sorafenib-treated group compared to vehicle- or MEAN-treated animals at d 42 of treatment (Fig. 4B). When mice were treated with the combination of MEAN (15 mg/kg) and sorafenib (15 mg/kg) at the same dosing schedule, their body weight did not differ significantly from monotherapy groups (Fig. 4A). However, the liver enzymes (ALT and AST) in combination-treatment mice were significantly higher than vehicle- or MEAN monotherapy–treated mice, although not significantly higher than in sorafenib-treated mice (Fig. 4B, P > 0.05). In summary, treatment with a combination of MEAN and sorafenib blocked tumor growth almost completely (Figs. 1 and 2) and did not significantly increase the hepatotoxicity more than sorafenib monotherapy (Fig. 4).
MEAN has different mechanisms than its parent compound, AMN
MEAN is a derivative AMN and has been shown to have topoisomerase II inhibitory activities, which are known to be a key mechanism of action for AMN (18). Additionally, like AMN, MEAN is not a substrate of activated P-glycoprotein, which is a common cause of drug resistance, allowing MEAN to remain effective in P-glycoprotein-mediated multidrug-resistant cell lines (Supplemental Fig. 3). However, differences in activities observed in vivo in previous studies suggest that MEAN may have additional molecular mechanisms of action distinct from those of AMN (18, 19). To examine this possibility, the COMPARE algorithm (26) was used to analyze the data of these compounds in NCI-60 cell line panel growth inhibition assays. The COMPARE algorithm gives a correlation value close to 1 when 2 drugs have a similar pattern of inhibition against the NCI-60 cancer cell line panel, a value of 0 when there is no correlation, and a value of −1 when there is an inverse correlation (range, −1 to 1). A similar inhibition pattern in the 60 cell assay is associated with a similar mode of action (26). The COMPARE algorithm analyses of AMN vs. the NIH National Cancer Institute’s standard compounds found 87 and 66 drugs with correlations of more than 0.5 based on median lethal concentrations (LC50) and median growth inhibitory concentrations (GI50), respectively (Table 1). In comparison, there were no compounds in the database with a correlation of more than 0.5 for MEAN based on LC50 and GI50 (Supplemental Table 1 and Table 1). When MEAN is compared to AMN directly, the correlation scores are <0.5 at both (LC50 and GI50) concentrations (Table 2), indicating that MEAN and AMN are likely to be different compounds with respect to their mechanisms of action in these cancer cells.
TABLE 1.
MEAN does not correlate with any tested compound in database at concentrations of LC50 and GI50 by COMPARE algorithm
| Compound | Total growth inhibition | LC50 | GI50 |
|---|---|---|---|
| AMN | 14 | 87 | 66 |
| MEAN | 3 | 0 | 0 |
Correlation (n > 0.5).
TABLE 2.
COMPARE algorithm shows that MEAN and AMN do not exhibit significant correlation in growth inhibition patterns
| Parameter | Correlation |
|---|---|
| GI50 | 0.400 |
| LC50 | 0.15 |
| GI50 | 0.327 |
Matrix COMPARE for MEAN vs. AMN.
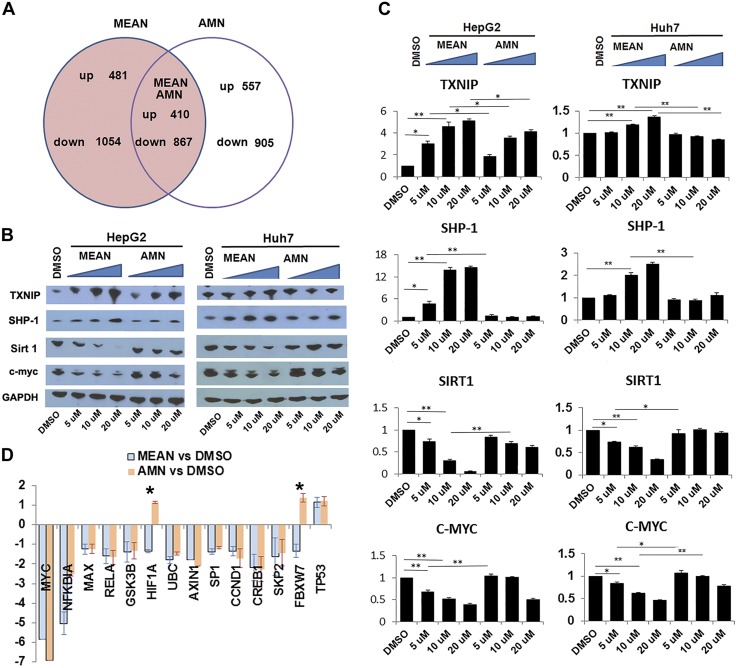
To identify the unique mechanisms by which MEAN inhibits HCC tumor growth, genome expression profiles at the RNA level in HepG2 cells treated with MEAN, AMN, or DMSO were examined and compared. HepG2 cells were treated by MEAN or AMN at a concentration of 20 μM or by DMSO for 6 h. The choice of the treatment concentration was based on the results of a pharmacokinetic study (Supplemental Fig. 2) that found the serum MEAN concentration was approximately 20 μM at ∼6 h after injection. In addition, a relatively short duration of treatment was designed to catch the closer-to-primary cellular responses at the early stage of the treatment. The GeneChip PrimeView Human Gene Expression Array was used to quantify RNA extracted from treated HepG2 cells in experimental triplicate. The results demonstrated that while MEAN and AMN shared about a third of affected genes, they also regulated distinct sets of genes (Fig. 5A), the number of genes that change more than ±1.5 log fold with statistical significance of P < 0.05. These findings further support the notion that MEAN has modes of action distinct from those of its parent compound, AMN.
Figure 5.
MEAN has distinct mechanisms of action compared to parent compound AMN. RNA array analyses show that 2 of 3 gene expression changes in treated HepG2 cells are not shared between MEAN and AMN (A). MEAN and AMN differentially altered gene expression in treated HepG2 and Huh7 cell lines in culture. MEAN significantly reduced C-MYC protein levels when using <10 μM, while AMN did not significantly reduce C-MYC expression at same concentration (B, C). MEAN also reduced SIRT1, and increased SHP-1 and TXNIP protein levels, while AMN did not significantly affect levels of these proteins. Intensity of vehicle treatment is set at 1, and ratios of treatment to vehicle are plotted in each quantitative graph (C). Expression levels of several downstream target genes of C-MYC were reduced in MEAN- and AMN-treated cells, while 2 genes are differentially regulated (D). Y axis represents log fold differences in RNA levels of each indicated gene between agents and DMSO (D). *P < 0.5, **P < 0.01 (Student’s t test). Lack of black bar indicates P > 0.5.
MEAN significantly changed gene expressions
On the basis of results of the array analyses, the expression changes of some of the genes in cell lines or in tumors were confirmed experimentally. While MEAN and AMN both significantly reduce C-MYC RNA expression in treated HCC cells, MEAN suppressed C-MYC protein expression in treated HepG2 more than AMN (Fig. 5B, C). Because C-MYC is well known for its oncogenic role in HCC (27) and in carcinogenesis in general (28–30), reduction of C-MYC could significantly contribute to tumor growth suppression. To determine the downstream effect of C-MYC in treated cells, the RNA levels of some of the downstream targets in HepG2 cells were evaluated. As shown in Fig. 5D, while most of the downstream elements of C-MYC were also reduced similarly by the treatment with MEAN and AMN, 2 genes (HIF1A and FBXW7) were exceptions, suggesting that some of these factors might be regulated by other mechanisms, which could respond differently to the treatment of the 2 agents. To determine the upstream elements that mediate the reduction of C-MYC by MEAN, a kinase array (43 kinases, ARY003B; R&D Systems, Minneapolis, MN, USA) were examined quantitatively for possible changes of kinase functions in MEAN-treated cells. The results did not show significant alterations in this panel (data not shown), further supporting the notion that several kinases known to regulate C-MYC, including ERK, AKT, and MAPK, were apparently not involved in MEAN-induced C-MYC reduction.
To determine whether gene expression changes in response to MEAN is cell type specific, another HCC cell line, Huh7, was also examined for changes in expression of the corresponding genes on the treatment with MEAN. The changes of the concentrations of C-MYC and other factors in these cells were similar to those observed in HepG2 cells (Fig. 5B, C). To determine whether similar gene expression is also found in tumors that were treated with MEAN, the resected tumor tissues from animals were examined for protein concentrations of the corresponding genes. MEAN reduced C-MYC significantly in both Huh7 and HepG2 xenograft tumors at the end of a 42 d treatment course compared to those treated with vehicle alone (Fig. 6A, B). These data thus showed that MEAN reduced C-MYC concentrations both in vitro and in vivo.
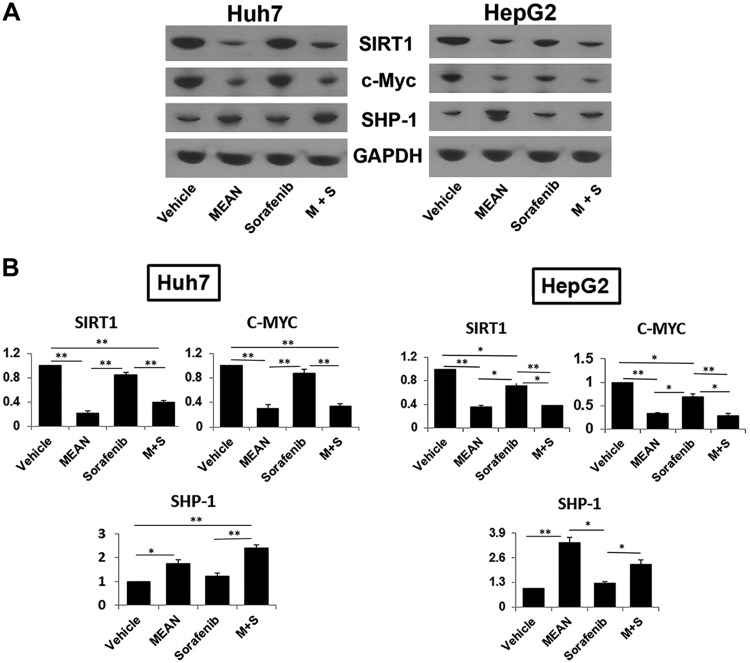
Figure 6.
Sorafenib and MEAN differentially affected gene expression in xenograft tumors. Western blot analysis of tumor tissue treated by MEAN, sorafenib, combination of both, or vehicle demonstrated that MEAN suppressed proteins levels of C-MYC and SIRT1, and increased protein levels of SHP-1, similar to findings in culture cells (A, B). Sorafenib did not significantly affect expression of these genes (A, B). Intensity of vehicle is set at 1, and ratios of treatment to vehicle are plotted in each quantitative graph (B). *P < 0.5, **P < 0.01 (Student’s t test). Lack of black bar indicates P > 0.5.
In addition to C-MYC, the expressions of a few other genes were also reduced. SIRT1, a highly expressed gene in HCC tumors (31, 32), was one of them. Western blot analyses of HepG2 and Huh7 cells showed that SIRT1 protein concentrations were significantly reduced in MEAN-treated cells but were unchanged in AMN-treated cells (Fig. 5B, C). Thus, SIRT1 is specifically reduced in MEAN-treated HCC cells and tumors but not in AMN-treated HCC cells. To determine whether C-MYC or SIRT1 reduction resulted from general transcriptional decrease or from more specific mechanisms targeted by MEAN, gene expressions across the genome were evaluated through RNA array analyses. These analyses found that many RNAs were up-regulated in response to MEAN treatment compared to controls (Fig. 5A), supporting the idea that the reduction of a subset of gene expression was not due to a global inhibition of transcription. These findings, taken together, demonstrate that MEAN has mechanisms of action distinct from those of its parent compound, AMN. The expression of genes affected by MEAN treatment is not all via the same cellular pathways or implicated in similar functions, suggesting the likelihood of multiple targets and multiple modes of action for MEAN in tumor growth inhibition.
MEAN and sorafenib do not share common mechanisms of action in tumor growth inhibition
Combination treatment with MEAN and sorafenib resulted in enhanced efficacy, and addition of MEAN did not significantly increase sorafenib hepatotoxicity; this suggests that the two compounds may not share the same mechanisms. Sorafenib is a well-interrogated and FDA-approved drug that was identified from screens against a protein tyrosine kinase (33, 34). However, sorafenib could have additional mechanisms of action. The COMPARE algorithm (26) was used to analyze NCI-60 cell line data for MEAN and sorafenib. The results yielded negative correlation scores at all concentrations, including LC50 and GI50 (Table 3), suggesting that it is unlikely that MEAN and sorafenib have common mechanisms of action. To further assess this possibility, the protein levels in tumors treated by MEAN, sorafenib, and their combination were evaluated. As shown in Fig. 6, sorafenib treatment did not significantly change the concentrations of proteins, including those of C-MYC, SIRT1, or SHP-1, as much as MEAN treatment (Fig. 6B, C). Furthermore, the combination of MEAN and sorafenib did not significantly change the levels of these proteins from those treated by MEAN alone (Fig. 6B, C). The combined treatment also did not produce significantly more toxicity than sorafenib monotherapy. In addition, MEAN did not directly interfere with the activities of a panel of kinases (data not shown). Our observations thus support the notion that MEAN and sorafenib do not share common mechanisms of action in tumor inhibition.
TABLE 3.
Analyses using COMPARE algorithm with NCI-60 cell panel show that MEAN is unlikely to share mechanisms of action with sorafenib
| Parameter | Correlation |
|---|---|
| GI50 | −0.048 |
| LC50 | −0.044 |
| GI50 | −0.173 |
Matrix COMPARE for MEAN vs. sorafenib.
DISCUSSION
Human HCC is a major health problem in the United States and worldwide. The lack of effective chemotherapy significantly contributes to the high mortality from the disease, particularly because donor livers for curative liver transplantations are limited by supply. The tyrosine kinase inhibitors sorafenib and its analog are the only chemotherapeutic agents approved for HCC. However, sorafenib treatment in patients with HCC only results in a limited increase in life expectancy at the cost of significant toxicities, and approved combination chemotherapy is lacking (15, 35). The benefit of the drug is further diminished by intrinsic and secondary drug resistance problems (36–38). Thus, new monotherapy or combined therapy is in an urgent need for the majority of HCC patients. A small molecule, MEAN, has been shown to be effective in inhibiting tumor growth in 2 HCC xenograft tumor models without significant toxicity. This report explored the direct comparisons of HCC xenograft tumor growth efficacy between MEAN and sorafenib under identical treatment conditions in mice, as well as the potential for the combination treatment of the two compounds. Furthermore, it reports the possible mechanisms of action for MEAN in tumor growth inhibition against HCC xenograft models.
Because sorafenib is the only drug approved by the FDA for treatment of HCC, it serves as a standard against which to compare the efficacy and toxicity of MEAN in treating HCC xenograft tumors in mice. In these studies, animals bearing HepG2 and Huh7 xenograft tumors were treated with MEAN or sorafenib in parallel at the same doses and with the same treatment strategies. MEAN was more effective in inhibiting HCC xenograft growth than sorafenib (Figs. 1 and 2). When MEAN and sorafenib were used in combination at the same concentration, tumor growth inhibition was significantly enhanced compared to sorafenib monotherapy, but not as significantly compared to MEAN monotherapy. The modest enhancement of the combined therapy over MEAN monotherapy could be due to the dose of MEAN used in this study. At 15 mg/kg, MEAN inhibited more than 80% of tumor growth and practically haltered tumor growth completely after 30 d of treatment, making the enhancement of efficacy by addition of sorafenib less obvious. However, reduction of the concentration of MEAN by an additional 50% in the combination treatment showed significant enhancement of tumor growth inhibition than either agent alone (Fig. 3). These observations encourage future experiments to optimize doses of both compounds to maximize efficacy and reduce toxicity by decreasing the concentrations.
While monotherapy with either MEAN or sorafenib did not produce significant toxicity and animals tolerated both drugs well, as judged by body weight maintenance and biweekly wellness checks in behaviors and basic physiology, both drugs increased serum concentrations of liver enzymes at the end of treatment (d 42), with sorafenib resulting in greater increases than MEAN (Fig. 4). A single-dose (75 mg/kg) toxicity study found that MEAN did not produce significant changes in body weight, major organ weight, serum liver enzyme concentrations, and hematopoietic panels (Supplemental Fig. 1) at 48 h after i.p. injection of MEAN. These findings indicate the need for optimization of doses for MEAN and sorafenib for combined therapy in order to identify the best combined treatment strategy that minimizes toxicity. The results of pharmacokinetic studies (Supplemental Fig. 2) will help guide the dosing optimization.
The lack of correlation between MEAN and sorafenib using the COMPARE algorithm at any treatment concentration, and the lack of direct impact on activities of 43 well-known kinases support the idea that they inhibit tumor growth by different mechanisms. While sorafenib also reduces C-MYC in treated esophageal adenocarcinoma cancer cells (39), MEAN reduced C-MYC more significantly than sorafenib in tumor tissues under identical experimental conditions (Fig. 6), indicating that the two may regulate C-MYC expression by different mechanisms. Sorafenib is an inhibitor of the RAF/MEK/ERK pathway in tumors, which is upstream of C-MYC expression and the tyrosine kinases VEGFR/PDRFR in the vasculature (40, 41). In comparison, MEAN has not been shown to affect the function of these kinases in kinase panel analyses (data not shown). Furthermore, sorafenib did not affect the levels of proteins known to be regulated by MEAN in treated tumors (Fig. 6), providing further evidence that the two have different modes of action. The differences in the mechanisms of action can be beneficial for the combined use of the two compounds, leading to enhanced efficacy without increased toxicity.
MEAN is a derivative of the well-interrogated anticancer compound AMN. AMN is effective against a wide range of cancers without the risk of multidrug resistance. Despite a large number of clinical trials, the severe and variable toxicity of AMN has prevented its further development (21, 22, 42, 43). It is believed that acetylation of AMN at the fifth position by liver NAT2 is responsible for the toxicity. MEAN is an analog of AMN with the nitrogen moved from the fifth to the sixth position (Fig. 4A) (18), thereby preventing it from being acetylated by NAT2 (18). While MEAN was similarly effective in inhibiting the growth of Huh7 and HepG2 HCC xenograft tumors, it had significantly less toxicity than AMN in vivo (19). In addition, similar to AMN, MEAN is also effective against xenograft tumor growth in other models, including gastric (19) and breast cancers (data not shown), suggesting a broad-spectrum antitumor activity. The genome profiling studies of HepG2 cells treated by either MEAN or AMN found that approximately two-thirds of genes that changed more than 1.5-fold are regulated differently by the two. While both MEAN and AMN significantly reduced C-MYC expression, MEAN reduced it to a greater extent than did AMN (Fig. 5) in cultured cells. The well-known oncoprotein has been shown to be a key player in oncogenesis in general (28–30). Overexpression of C-MYC has been linked to human HCC (27, 44, 45). Additionally, MEAN, but not AMN, inhibited SIRT1 expression. SIRT1 is a member of the sirtuin deacetylase family, which has substrates ranging from histones to enzymes involved in glucose metabolism (46). SIRT1 plays diverse roles in the homeostasis of many cellular processes, including energy metabolism, oxidation, Wnt, TGF-β, and NF-κB signaling and pathways (46, 47), and it is found overexpressed in cancer cells (48). SIRT1 may regulate TERT and promote C-MYC activities in HCC cells (49), and knockdown of SIRT1 induced arrest of HCC cell growth (31). These findings suggest that the reduction of SIRT1 expression by MEAN could play a role in C-MYC expression inhibition and tumor growth suppression in treated HCC cells and tumors.
Treatment with MEAN, but not with AMN, significantly increased the expression of TXNIP (Fig. 5), a multifunction tumor suppressor (50–53) implicated in reactive oxygen species metabolism (54–56), glucose homeostasis (57), p53 stabilization (58, 59), and tumor suppression through inhibition of mTORC1 (mechanistic target of rapamycin complex 1) (60). TXNIP has been shown to be involved in HCC development (61, 62). TXNIP-deficient mice are prone to HCC development (61), and induction of TXNIP suppresses the growth of HCC tumors (62). Furthermore, a recent report of an antitumor small molecule also has the effect of increasing TXNIP (63). In addition to TXNIP, MEAN treatment enhanced the expression of SHP-1, a protein tyrosine phosphatase, which has been shown to be both a tumor suppressor and enhancer (64–66). SHP-1 is a multifunctional protein involved in glucose metabolism and autophage activation (67–69), the reduction of which was associated with HCC (67). Activation of SHP-1 significantly represses HCC colony formation in vitro and inhibits xenograft HCC tumor growth in vivo (67). Thus, the activation of SHP-1 by MEAN could also contribute to tumor growth inhibition. Taken together, the observations from this report support the idea that chemical modification of AMN into MEAN resulted in a novel molecule with unique mechanisms of action; that MEAN affects more than one pathway and has multifaceted functions in cells; and that the sum of these effects could be responsible for tumor growth inhibition both in vitro and in vivo.
In summary, MEAN is a small molecule with novel modes of action, and is more effective and has less toxicity than sorafenib in HCC xenograft tumor growth inhibition. Combined treatment with the two enhances the efficacy over sorafenib monotherapy without significant increases in toxicity. Mechanistically, MEAN inhibits the expression of the oncoprotein C-MYC and other prooncogenic proteins while enhancing protein levels of known tumor suppressors, including TXNIP and SHP-1. MEAN shows different mechanisms of action from its parent compound (AMN) and from sorafenib. MEAN was not a P-glycoprotein substrate, a common cause for drug resistance. These findings indicate that MEAN is an excellent candidate to be further developed as a monotherapy or a combination therapy with sorafenib for human HCC treatment.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported, in part, by the International Science and Technology Cooperation Project (Re-Innovation Industrialization; Gramt 2012C14028 to Z.C.); the H. Foundation and the Rosenberg Foundation of the Robert H. Lurie Comprehensive Cancer Center; the U.S. National Institutes of Health (NIH) National Institute of General Medical Sciences (Grant 2 R01 GM078555-05 to S.H.); NIH Intramural Research Program funding to D.A.; and intramural funding to M.H. The authors declare no conflicts of interest.
Glossary
- ALT
alanine aminotransferase
- AMN
amonafide
- AST
aspartate aminotransferase
- FDA
U.S. Food and Drug Administration
- GI50
median growth inhibitory concentration
- HCC
hepatocellular carcinoma
- LC50
median lethal concentration
- MEAN
6-methoxyethylamino-numonafide
- NAT2
N-acetyltransferase 2
- p.o.
oral route
- SHP-1
Src homology region 2 domain-containing phosphatase 1
- SIRT1
sirtuin 1
- TXNIP
thioredoxin-interacting protein
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
Y. Liu, R. Green, A. Mazar, D. Appella, Z. Chen, and S. Huang initiated the project, designed it, and performed most of the experiments; Y. Liu, G. Lou, J. T. Norton, C. Wang, I. Kandela, S. Tang, N. I. Shank, P. Gupta, M. Huang, and M. J. Avram were involved in detailed experimental design and execution; Y. Liu, R. Green, and S. Huang were mainly responsible for writing the article; and all authors were involved in data interpretation and were invited to comment.
REFERENCES
- 1.Ferlay J., Shin H. R., Bray F., Forman D., Mathers C., Parkin D. M. (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 127, 2893–2917 [DOI] [PubMed] [Google Scholar]
- 2. American Cancer Society. (2016) Cancer facts and figures. Accessed August 15, 2016 at: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2016.html.
- 3. American Cancer Society. (2017) Liver and bile duct cancer—patient version. Accessed August 15, 2016 at: https://seer.cancer.gov/statfacts/html/livibd.html.
- 4. Centers for Disease Control and Prevention. (2016) Viral hepatitis and liver cancer. Accessed August 15, 2016 at: https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/viral-hep-liver-cancer.pdf.
- 5.Yang J. D., Roberts L. R. (2010) Hepatocellular carcinoma: a global view. Nat. Rev. Gastroenterol. Hepatol. 7, 448–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Serag H. B., Rudolph K. L. (2007) Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 132, 2557–2576 [DOI] [PubMed] [Google Scholar]
- 7.Nordenstedt H., White D. L., El-Serag H. B. (2010) The changing pattern of epidemiology in hepatocellular carcinoma. Dig. Liver Dis. 42(Suppl 3), S206–S214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liou I., Kowdley K. V. (2006) Natural history of nonalcoholic steatohepatitis. J. Clin. Gastroenterol. 40(Suppl 1), S11–S16 [DOI] [PubMed] [Google Scholar]
- 9.Clark J. M. (2006) The epidemiology of nonalcoholic fatty liver disease in adults. J. Clin. Gastroenterol. 40(Suppl 1), S5–S10 [DOI] [PubMed] [Google Scholar]
- 10.Clavien P. A., Lesurtel M., Bossuyt P. M., Gores G. J., Langer B., Perrier A.; OLT for HCC Consensus Group (2012) Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 13, e11–e22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanish S. I., Knechtle S. J. (2011) Liver transplantation for the treatment of hepatocellular carcinoma. Oncology (Williston Park) 25, 752–757 [PubMed] [Google Scholar]
- 12.Memon K., Lewandowski R. J., Riaz A., Salem R. (2013) Yttrium 90 microspheres for the treatment of hepatocellular carcinoma. Recent Results Cancer Res. 190, 207–224 [DOI] [PubMed] [Google Scholar]
- 13.Cheng A. L., Kang Y. K., Chen Z., Tsao C. J., Qin S., Kim J. S., Luo R., Feng J., Ye S., Yang T. S., Xu J., Sun Y., Liang H., Liu J., Wang J., Tak W. Y., Pan H., Burock K., Zou J., Voliotis D., Guan Z. (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 10, 25–34 [DOI] [PubMed] [Google Scholar]
- 14.Llovet J. M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J. F., de Oliveira A. C., Santoro A., Raoul J. L., Forner A., Schwartz M., Porta C., Zeuzem S., Bolondi L., Greten T. F., Galle P. R., Seitz J. F., Borbath I., Häussinger D., Giannaris T., Shan M., Moscovici M., Voliotis D., Bruix J.; SHARP Investigators Study Group (2008) Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359, 378–390 [DOI] [PubMed] [Google Scholar]
- 15.Razumilava N., Gores G. J. (2011) Sorafenib for HCC: a pragmatic perspective. Oncology (Williston Park) 25, 300, 302. [PubMed] [Google Scholar]
- 16.Abdel-Rahman O., Fouad M. (2014) Risk of cardiovascular toxicities in patients with solid tumors treated with sunitinib, axitinib, cediranib or regorafenib: an updated systematic review and comparative meta-analysis. Crit. Rev. Oncol. Hematol. 92, 194–207 [DOI] [PubMed] [Google Scholar]
- 17.Wörns M. A., Koch S., Niederle I. M., Marquardt J. U., Nguyen-Tat M., Gamstätter T., Schuchmann M., Schulze-Bergkamen H., Galle P. R., Weinmann A. (2013) The impact of patient and tumour baseline characteristics on the overall survival of patients with advanced hepatocellular carcinoma treated with sorafenib. Dig. Liver Dis. 45, 408–413 [DOI] [PubMed] [Google Scholar]
- 18.Norton J. T., Witschi M. A., Luong L., Kawamura A., Ghosh S., Stack M. S., Sim E., Avram M. J., Appella D. H., Huang S. (2008) Synthesis and anticancer activities of 6-amino amonafide derivatives. Anticancer Drugs 19, 23–36 [DOI] [PubMed] [Google Scholar]
- 19.Liu Y., Norton J. T., Witschi M. A., Xu Q., Lou G., Wang C., Appella D. H., Chen Z., Huang S. (2011) Methoxyethylamino-numonafide is an efficacious and minimally toxic amonafide derivative in murine models of human cancer. Neoplasia 13, 453–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chau M., Christensen J. L., Ajami A. M., Capizzi R. L. (2008) Amonafide, a topoisomerase II inhibitor, is unaffected by P-glycoprotein-mediated efflux. Leuk. Res. 32, 465–473 [DOI] [PubMed] [Google Scholar]
- 21.Ingrassia L., Lefranc F., Kiss R., Mijatovic T. (2009) Naphthalimides and azonafides as promising anti-cancer agents. Curr. Med. Chem. 16, 1192–1213 [DOI] [PubMed] [Google Scholar]
- 22.Costanza M. E., Weiss R. B., Henderson I. C., Norton L., Berry D. A., Cirrincione C., Winer E., Wood W. C., Frei E. III, McIntyre O. R., Schilsky R. L. (1999) Safety and efficacy of using a single agent or a phase II agent before instituting standard combination chemotherapy in previously untreated metastatic breast cancer patients: report of a randomized study—Cancer and Leukemia Group B 8642. J. Clin. Oncol. 17, 1397–1406 [DOI] [PubMed] [Google Scholar]
- 23. U.S. Department of Health and Human Services; National Institutes of Health Office of Laboratory Animal Welfare. (2015) Public health service policy on humane care and use of laboratory animals. Accessed August 15, 2016 at: https://grants.nih.gov/grants/olaw/references/phspolicylabanimals.pdf.
- 24.Stecklair K. P., Hamburger D. R., Egorin M. J., Parise R. A., Covey J. M., Eiseman J. L. (2001) Pharmacokinetics and tissue distribution of halofuginone (NSC 713205) in CD2F1 mice and fischer 344 rats. Cancer Chemother. Pharmacol. 48, 375–382 [DOI] [PubMed] [Google Scholar]
- 25.Avram M. J., Henthorn T. K., Spyker D. A., Krejcie T. C., Lloyd P. M., Cassella J. V., Rabinowitz J. D. (2007) Recirculatory pharmacokinetic model of the uptake, distribution, and bioavailability of prochlorperazine administered as a thermally generated aerosol in a single breath to dogs. Drug Metab. Dispos. 35, 262–267 [DOI] [PubMed] [Google Scholar]
- 26.Paull K. D., Shoemaker R. H., Hodes L., Monks A., Scudiero D. A., Rubinstein L., Plowman J., Boyd M. R. (1989) Display and analysis of patterns of differential activity of drugs against human tumor cell lines: development of mean graph and COMPARE algorithm. J. Natl. Cancer Inst. 81, 1088–1092 [DOI] [PubMed] [Google Scholar]
- 27.Shiraha H., Yamamoto K., Namba M. (2013) Human hepatocyte carcinogenesis (review). Int. J. Oncol. 42, 1133–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Deng K., Guo X., Wang H., Xia J. (2014) The lncRNA-MYC regulatory network in cancer. Tumour Biol. 35, 9497–9503 [DOI] [PubMed] [Google Scholar]
- 29. Gabay M., Li Y., Felsher D. W. (2014) MYC activation is a hallmark of cancer initiation and maintenance. Cold Spring Harb. Perspect. Med. 4, a014241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sewastianik T., Prochorec-Sobieszek M., Chapuy B., Juszczyński P. (2014) MYC deregulation in lymphoid tumors: molecular mechanisms, clinical consequences and therapeutic implications. Biochim. Biophys. Acta 1846, 457–467 [DOI] [PubMed] [Google Scholar]
- 31.Choi H. N., Bae J. S., Jamiyandorj U., Noh S. J., Park H. S., Jang K. Y., Chung M. J., Kang M. J., Lee D. G., Moon W. S. (2011) Expression and role of SIRT1 in hepatocellular carcinoma. Oncol. Rep. 26, 503–510 [DOI] [PubMed] [Google Scholar]
- 32.Knight J. R., Milner J. (2012) SIRT1, metabolism and cancer. Curr. Opin. Oncol. 24, 68–75 [DOI] [PubMed] [Google Scholar]
- 33.Liu L., Cao Y., Chen C., Zhang X., McNabola A., Wilkie D., Wilhelm S., Lynch M., Carter C. (2006) Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 66, 11851–11858 [DOI] [PubMed] [Google Scholar]
- 34.Cervello M., Bachvarov D., Lampiasi N., Cusimano A., Azzolina A., McCubrey J. A., Montalto G. (2012) Molecular mechanisms of sorafenib action in liver cancer cells. Cell Cycle 11, 2843–2855 [DOI] [PubMed] [Google Scholar]
- 35.Kim R., Byrne M. T., Tan A., Aucejo F. (2011) What is the indication for sorafenib in hepatocellular carcinoma? a clinical challenge. Oncology (Williston Park) 25, 283–291, 295 [PubMed] [Google Scholar]
- 36.Bellmunt J., Eisen T., Fishman M., Quinn D. (2011) Experience with sorafenib and adverse event management. Crit. Rev. Oncol. Hematol. 78, 24–32 [DOI] [PubMed] [Google Scholar]
- 37.Kuczynski E. A., Lee C. R., Man S., Chen E., Kerbel R. S. (2015) Effects of sorafenib dose on acquired reversible resistance and toxicity in hepatocellular carcinoma. Cancer Res. 75, 2510–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riechelmann R. P., Chin S., Wang L., Tannock I. F., Berthold D. R., Moore M. J., Knox J. J. (2008) Sorafenib for metastatic renal cancer: the Princess Margaret experience. Am. J. Clin. Oncol. 31, 182–187 [DOI] [PubMed] [Google Scholar]
- 39.Delgado J. S., Mustafi R., Yee J., Cerda S., Chumsangsri A., Dougherty U., Lichtenstein L., Fichera A., Bissonnette M. (2008) Sorafenib triggers antiproliferative and pro-apoptotic signals in human esophageal adenocarcinoma cells. Dig. Dis. Sci. 53, 3055–3064 [DOI] [PubMed] [Google Scholar]
- 40.Gotink K. J., Verheul H. M. (2010) Anti-angiogenic tyrosine kinase inhibitors: what is their mechanism of action? Angiogenesis 13, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iyer R., Fetterly G., Lugade A., Thanavala Y. (2010) Sorafenib: a clinical and pharmacologic review. Expert Opin. Pharmacother. 11, 1943–1955 [DOI] [PubMed] [Google Scholar]
- 42.Innocenti F., Iyer L., Ratain M. J. (2001) Pharmacogenetics of anticancer agents: lessons from amonafide and irinotecan. Drug Metab. Dispos. 29, 596–600 [PubMed] [Google Scholar]
- 43.Savaraj N., Liang J., Lu K., Feun L. G., Hsu T. C. (1989) Genotoxicity of [1H]benz[de]isoquinoline-1,3[2H]dione,5 amino-2-,[2-dimethylamino) ethyl] (BIDA) in human lymphocytes. Cancer Invest. 7, 117–121 [DOI] [PubMed] [Google Scholar]
- 44.Cao Z., Fan-Minogue H., Bellovin D. I., Yevtodiyenko A., Arzeno J., Yang Q., Gambhir S. S., Felsher D. W. (2011) MYC phosphorylation, activation, and tumorigenic potential in hepatocellular carcinoma are regulated by HMG-CoA reductase. Cancer Res. 71, 2286–2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu G., Lu X., Wang Y., He H., Meng X., Xia S., Zhen K., Liu Y. (2014) Epigenetic high regulation of ATAD2 regulates the Hh pathway in human hepatocellular carcinoma. Int. J. Oncol. 45, 351–361 [DOI] [PubMed] [Google Scholar]
- 46.Simmons G. E. Jr., Pruitt W. M., Pruitt K. (2015) Diverse roles of SIRT1 in cancer biology and lipid metabolism. Int. J. Mol. Sci. 16, 950–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu T. F., McCall C. E. (2013) Deacetylation by SIRT1 reprograms inflammation and cancer. Genes Cancer 4, 135–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uhlen M. Human protein atlas, SIRT1. (2015) Available at: http://www.proteinatlas.org/ENSG00000096717-SIRT1/cancer. Accessed August 15, 2016
- 49.Mao B., Zhao G., Lv X., Chen H. Z., Xue Z., Yang B., Liu D. P., Liang C. C. (2011) Sirt1 deacetylates c-Myc and promotes c-Myc/Max association. Int. J. Biochem. Cell Biol. 43, 1573–1581 [DOI] [PubMed] [Google Scholar]
- 50.Dunn L. L., Buckle A. M., Cooke J. P., Ng M. K. (2010) The emerging role of the thioredoxin system in angiogenesis. Arterioscler. Thromb. Vasc. Biol. 30, 2089–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watanabe R., Nakamura H., Masutani H., Yodoi J. (2010) Anti-oxidative, anti-cancer and anti-inflammatory actions by thioredoxin 1 and thioredoxin-binding protein-2. Pharmacol. Ther. 127, 261–270 Erratum in: Pharmacol. Ther. 2011;129:239 [DOI] [PubMed] [Google Scholar]
- 52.Morrison J. A., Pike L. A., Sams S. B., Sharma V., Zhou Q., Severson J. J., Tan A. C., Wood W. M., Haugen B. R. (2014) Thioredoxin interacting protein (TXNIP) is a novel tumor suppressor in thyroid cancer. Mol. Cancer 13, 62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peterson C. W., Ayer D. E. (2011) An extended Myc network contributes to glucose homeostasis in cancer and diabetes. Front. Biosci. (Landmark Ed.) 16, 2206–2223 [DOI] [PubMed] [Google Scholar]
- 54.Zhou J., Yu Q., Chng W. J. (2011) TXNIP (VDUP-1, TBP-2): a major redox regulator commonly suppressed in cancer by epigenetic mechanisms. Int. J. Biochem. Cell Biol. 43, 1668–1673 [DOI] [PubMed] [Google Scholar]
- 55.Lane T., Flam B., Lockey R., Kolliputi N. (2013) TXNIP shuttling: missing link between oxidative stress and inflammasome activation. Front. Physiol. 4, 50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu J., Holmgren A. (2014) The thioredoxin antioxidant system. Free Radic. Biol. Med. 66, 75–87 [DOI] [PubMed] [Google Scholar]
- 57.Chutkow W. A., Patwari P., Yoshioka J., Lee R. T. (2008) Thioredoxin-interacting protein (Txnip) is a critical regulator of hepatic glucose production. J. Biol. Chem. 283, 2397–2406 [DOI] [PubMed] [Google Scholar]
- 58.Suh H. W., Yun S., Song H., Jung H., Park Y. J., Kim T. D., Yoon S. R., Choi I. (2013) TXNIP interacts with hEcd to increase p53 stability and activity. Biochem. Biophys. Res. Commun. 438, 264–269 [DOI] [PubMed] [Google Scholar]
- 59.Jung H., Kim M. J., Kim D. O., Kim W. S., Yoon S. J., Park Y. J., Yoon S. R., Kim T. D., Suh H. W., Yun S., Min J. K., Lee H. G., Lee Y. H., Na H. J., Lee D. C., Kim H. C., Choi I. (2013) TXNIP maintains the hematopoietic cell pool by switching the function of p53 under oxidative stress. Cell Metab. 18, 75–85 [DOI] [PubMed] [Google Scholar]
- 60.Jin H. O., Seo S. K., Kim Y. S., Woo S. H., Lee K. H., Yi J. Y., Lee S. J., Choe T. B., Lee J. H., An S., Hong S. I., Park I. C. (2011) TXNIP potentiates Redd1-induced mTOR suppression through stabilization of Redd1. Oncogene 30, 3792–3801 [DOI] [PubMed] [Google Scholar]
- 61.Sheth S. S., Bodnar J. S., Ghazalpour A., Thipphavong C. K., Tsutsumi S., Tward A. D., Demant P., Kodama T., Aburatani H., Lusis A. J. (2006) Hepatocellular carcinoma in Txnip-deficient mice. Oncogene 25, 3528–3536 [DOI] [PubMed] [Google Scholar]
- 62.Yamaguchi F., Hirata Y., Akram H., Kamitori K., Dong Y., Sui L., Tokuda M. (2013) FOXO/TXNIP pathway is involved in the suppression of hepatocellular carcinoma growth by glutamate antagonist MK-801. BMC Cancer 13, 468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Santagata S., Mendillo M. L., Tang Y. C., Subramanian A., Perley C. C., Roche S. P., Wong B., Narayan R., Kwon H., Koeva M., Amon A., Golub T. R., Porco J. A. Jr., Whitesell L., Lindquist S. (2013) Tight coordination of protein translation and HSF1 activation supports the anabolic malignant state. Science 341, 1238303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu C., Sun M., Liu L., Zhou G. W. (2003) The function of the protein tyrosine phosphatase SHP-1 in cancer. Gene 306, 1–12 [DOI] [PubMed] [Google Scholar]
- 65. Schmitz R., Ceribelli M., Pittaluga S., Wright G., Staudt L. M. (2014) Oncogenic mechanisms in Burkitt lymphoma. Cold Spring Harb. Perspect. Med. 4, a014282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rhee Y. H., Jeong S. J., Lee H. J., Lee H. J., Koh W., Jung J. H., Kim S. H., Sung-Hoon K. (2012) Inhibition of STAT3 signaling and induction of SHP1 mediate antiangiogenic and antitumor activities of ergosterol peroxide in U266 multiple myeloma cells. BMC Cancer 12, 28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Su J. C., Chiang H. C., Tseng P. H., Tai W. T., Hsu C. Y., Li Y. S., Huang J. W., Ko C. H., Lin M. W., Chu P. Y., Liu C. Y., Chen K. F., Shiau C. W. (2014) RFX-1-dependent activation of SHP-1 inhibits STAT3 signaling in hepatocellular carcinoma cells. Carcinogenesis 35, 2807–2814 [DOI] [PubMed] [Google Scholar]
- 68.Bergeron S., Dubois M. J., Bellmann K., Schwab M., Larochelle N., Nalbantoglu J., Marette A. (2011) Inhibition of the protein tyrosine phosphatase SHP-1 increases glucose uptake in skeletal muscle cells by augmenting insulin receptor signaling and GLUT4 expression. Endocrinology 152, 4581–4588 [DOI] [PubMed] [Google Scholar]
- 69.Dubois M. J., Bergeron S., Kim H. J., Dombrowski L., Perreault M., Fournès B., Faure R., Olivier M., Beauchemin N., Shulman G. I., Siminovitch K. A., Kim J. K., Marette A. (2006) The SHP-1 protein tyrosine phosphatase negatively modulates glucose homeostasis. Nat. Med. 12, 549–556 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.