Abstract
Exosomes are endosome-derived nanovesicles that are involved in cellular communication and signaling. Exosomes are produced by epithelial cells and are found in biologic fluids including blood and urine. The packaged material within exosomes includes proteins and lipids, but the molecular comparison within exosome subtypes is largely unknown. The purpose of this study was to investigate differences between exosomes derived from the apical plasma membrane and basolateral plasma membrane of polarized murine cortical collecting duct principal cells. Nanoparticle tracking analysis showed that the size and concentration of apical and basolateral exosomes remained relatively stable across 3 different temperatures (23, 37, and 42°C). Liquid chromatography–tandem mass spectrometry analysis revealed marked differences between the proteins packaged within the two types of exosomes from the same cells. Several proteins expressed at the inner leaflet of the plasma membrane, including α-actinin-1, moesin, 14-3-3 protein ζ/δ, annexin A1/A3/A4/A5/A6, clathrin heavy chain 1, glyceraldehyde-3-phosphate dehydrogenase, α-enolase, filamin-A, and heat shock protein 90, were identified in samples of apical plasma membrane–derived exosomes, but not in basolateral plasma membrane exosomes from mouse cortical collecting duct cells. In addition to differences at the protein level, mass spectrometry–based shotgun lipidomics analysis showed significant differences in the lipid classes and fatty acid composition of the two types of exosomes. We found higher levels of sphingomyelin and lower levels of cardiolipin, among other phospholipids in the apical plasma membrane compared to the basolateral plasma membrane exosomes. The molecular analyses of exosome subtypes presented herein will contribute to our understanding of exosome biogenesis, and the results may have potential implications for biomarker discovery.—Dang, V. D., Jella, K. K., Ragheb, R. R. T., Denslow, N. D., Alli, A. A. Lipidomic and proteomic analysis of exosomes from mouse cortical collecting duct cells.
Keywords: sphingomyelin, cardiolipin, mpkCCD, exosomal, NanoSight
The kidneys are essential for maintaining total body sodium balance and blood pressure control. The renal collecting duct plays an important role in salt and water transport. Polarized epithelial cells of the collecting duct express important transport proteins in the apical and basolateral plasma membranes. At the apical plasma membrane of principal cells of the collecting duct, the epithelial sodium channel (ENaC) allows sodium entry into the cell, whereas the renal outer medullary potassium channel regulates potassium secretion (1, 2). The sodium potassium ATPase at the basolateral plasma membrane provides the driving force for the transcellular ion transport. Osmotically driven water absorption in principal cells of the collecting duct is due to aquaporin (AQP)-2 water channels expressed in the apical plasma membrane (3, 4) and basolaterally expressed AQP3 and -4 (5–8).
Ion and water transport in principal cells of the collecting duct are sensitive to hormonal regulation (1, 9). Autocrine and paracrine factors modulate ion and water transport in these cells (1). Angiotensin II, aldosterone, and vasopressin are essential hormones for the regulation of body fluid homeostasis (1, 10). Multiple cytoskeletal, chaperone, and signaling proteins regulate the expression of these transporters in the collecting duct. Proteins that have been shown to regulate trafficking of AQP2 to the apical plasma membrane include F-actin, Hsc70 (heat shock constitutive), and A kinase anchoring proteins (11). ENaC directly interacts with the myristoylated alanine-rich C kinase substrate (12–14), filamin (13, 15), 14-3-3 proteins (16–18), actin (19–21), and actin binding proteins (22, 23). We recently showed ENaC activity in cultured mouse cortical collecting duct cells is regulated by exosomal GAPDH (24).
The apical and basolateral plasma membranes of polarized epithelial cells, such as collecting duct cells, both bud, and 2 types of nanosized vesicles are secreted across each cell surface domain. Exosomes are the most studied type of nanosized vesicles because of their physiologic roles in health and disease, potential source of biomarkers (25), and their use as therapeutic drug delivery vehicles (26). Although the protein and lipid compositions between the two cell surface domains of polarized epithelial cells in general have been shown to be different, the molecular composition of the exosomes that are secreted across each of these domains is largely unknown. It is important to identify the proteins and lipids that are highly enriched in these two types of exosomes to be able to perform studies aimed at investigating mechanisms involved in the uptake of exosomes by recipient cells and elucidating mechanisms of intercellular communication mediated by the packaged material within these exosomes.
There are important differences between exosomes and other nanosized vesicles, including microvesicles and apoptotic bodies. These extracellular vesicles differ in size, cargo, and biogenesis. Exosomes generally range from 30 to 150 nm in diameter (27), microvesicles are in the range 100–1000 nm in diameter (28) and apoptotic bodies are 1000–5000 nm in diameter (28).
The protein composition within the apical and basolateral plasma membranes of renal epithelial cells is essential for transport functions (29). The mouse cortical collecting duct (mpkCCD) principal cell line maintains important biochemical and physiologic properties of the cells from which they were derived (30). These cells are often used to investigate the expression, post-translational modification, trafficking and activity of transport proteins including ENaC, AQPs (31–35), and the Na,K-ATPase (36–38).
The stability of exosomes at temperatures around physiologic conditions is important to establish as a control for further studies. Although the mechanism for the degradation is not clearly understood, Sokolova et al. (39) showed that both size and concentration of exosomes at 37°C remained stable until 48 h when they then decreased by ∼36 and 60%, respectively. Here we show that these exosomes derived from mpKCCD cells remain stable at physiologic temperatures in the first 8 h.
The purpose of this study was to investigate the molecular differences between two types of exosomes produced by mouse cortical collecting duct cells. The significance and implications of the differences in the protein and lipid composition of exosomes derived from the apical and basolateral plasma membrane of kidney collecting duct cells is discussed.
MATERIALS AND METHODS
Cell culture
Immortalized mouse cortical collecting duct principal cells (mpkCCDcl4) were originally obtained from Dr. Alain Vandewalle (Institut National de la Santé et de la Recherche Médicale, Paris, France). Cells were cultured in permeable Transwell inserts (Costar Transwells, 0.4 μm pore, 24-mm diameter; Corning, Corning, NY, USA) in a 1:1 mixture of DMEM and Ham’s F-12 medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 2 mM l-glutamine, 20 mM HEPES (Millipore-Sigma, St. Louis, MO, USA), 1 nM triiodothyronine (Millipore-Sigma), 50 nM dexamethasone (Millipore-Sigma), 2% fetal bovine serum (Thermo Fisher Scientific), and 0.1% penicillin-streptomycin (Thermo Fisher Scientific) (14, 40). Cells were maintained in a 5% CO2-95% O2 atmosphere incubator at 37°C. Cells used for experiments had a passage number of <37.
Isolation of exosomes
Exosomes were isolated from conditioned medium, as described (24, 41), but with the following modifications. A total of 3 exosome preparations were performed from consecutive passages of mpkCCD cells. The mpkCCD cells were grown to confluence on permeable Transwell inserts and then maintained in culture for 10 d before the conditioned media were collected from the top (apical) or the bottom (basolateral) compartments of 12 permeable inserts every other day. An epithelial voltmeter (EVOM2; World Precision Instruments, Sarasota, FL, USA) was used to measure transepithelial voltages and resistances across confluent cell monolayers before and after collecting the conditioned medium each day, to assure the permeable supports were not damaged during collections or between incubation times. The individual collections of conditioned media from the top of mpKCCD cells were pooled, and conditioned media collected from the bottom of these cells were also pooled. Both types of conditioned media were centrifuged at 1000 g for 10 min to remove dead cells and cellular debris. The supernatants were collected and then filtered with a 0.22 μm (220 nm) Nalgene filter (Thermo Fisher Scientific). The resulting supernatant was subject to centrifugation at 10,000 g for 30 min to remove any remaining debris and was then ultracentrifuged at 118,000 g for 70 min at 4°C with a fixed-angle rotor Ti-70 (Beckman Coulter, Brea, CA, USA). The pellets were washed with sterile 1× PBS and subjected to another cycle of ultracentrifugation at 118,000 g for 70 min at 4°C. The supernatant was discarded, and the pelleted exosomes were carefully reconstituted in sterile 1× PBS or lysed in RIPA buffer (both from Thermo Fisher Scientific).
Nanoparticle tracking analysis
Samples were analyzed by a NanoSight NS300 equipped with a high-sensitivity Hamamatsu sCMOS camera, ×20 objective lens, and a 50 mW green 532 nm laser. Samples were analyzed with Nanoparticle Tracking Analysis (NTA) 3.2 Build 16 software. NanoSight technology (Malvern Instruments, Malvern, United Kingdom) calculates size based on the relationship between Brownian motion and hydrodynamic diameter through the Stokes-Einstein equation. Concentration was calculated by particle observation on a frame-by-frame basis by the sCMOS camera (complementary metal oxide semiconductor; 25 frames/s). When recording the video, it averaged the concentration across all the frames, giving an absolute number average. Samples were processed across 3 × 60-s videos.
SDS-PAGE and Western blot analysis
Fifty micrograms of total exosomal protein was loaded and resolved on 4–20% Criterion Tris-HCl precast gels (Bio-Rad, Hercules, CA, USA) as described by our laboratory (12–14, 24, 40). The proteins were transferred to 0.45 μM nitrocellulose blotting membranes (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA), blocked in 5% nonfat milk 1× TBS for 1 h at room temperature and then incubated with anti-flotillin-1 antibody (Abcam, Cambridge, MA, USA) for 8 h at 4°C. The membranes were washed and then incubated with goat anti-rabbit peroxidase–conjugated secondary antibody (Bio-Rad) for 1 h at room temperature. The membranes were washed and subjected to incubation with SuperSignal West Dura Chemiluminescent Substrate (Thermo Fisher Scientific) for 5 min, and the blots were developed on a Bio-Rad imager.
Protein identification by liquid chromatography–tandem mass spectrometry
Exosomes were mixed in RIPA buffer, and the harvested proteins were resolved on 4–20% Criterion Tris-HCl precast gels (Bio-Rad). The gels were fixed and stained in colloidal Coomassie Blue stain, and the individual protein bands were excised. The proteins were identified by liquid chromatography–tandem mass spectrometry (LC-MS/MS; Proteomics Core Facility at the Moffitt Cancer Center, Tampa, FL, USA). In brief, the gel slices were destained and treated with Tris (2-carboxyethyl) phosphine and iodoacetamide to reduce and alkylate the proteins. In-gel digestion was performed with trypsin, and the peptides were extracted and concentrated by vacuum centrifugation. Tandem mass spectrometry peptide sequencing was performed on a Nanoflow liquid chromatograph (U3000; Dionex, Sunnyvale, CA, USA) connected to an electrospray Orbitrap mass spectrometer (LTQ-Orbitrap; Thermo Fisher Scientific). Samples were loaded onto a precolumn (5 mm × 300 µm internal diameter packed with C18 reversed-phase resin, 5 µm, and 100 Å) and then washed with aqueous 2% acetonitrile and 0.04% trifluoroacetic acid before eluting the trapped peptides onto an analytical column (C18, 75 µm internal diameter × 15 cm; Pepmap 100; Dionex) with a flow rate of 300 nl/min. A programmed 120 min gradient was used with the following steps: 95% solvent A (2% acetonitrile+0.1% formic acid) for 8 min, solvent B (90% acetonitrile+0.1% formic acid) from 5 to 50% for 90 min, followed by solvent B from 50 to 90% B for 7 min and holding at 90% for 5 min and then solvent B from 90 to 5% for 1 min and equilibration for 10 min, in addition to a 60-min gradient setup. A total of 5 tandem mass spectra were collected per ion and the MS/MS scans were performed in the linear ion trap with 60-s exclusion for sampled peptide peaks. Mascot searches were performed against the Swiss-Prot Mouse and Rat databases. The precursor mass tolerance was set at 1.08 Da, the MS/MS mass tolerance was set at 0.8 Da, and 2 trypsin missed cleavages were allowed. For target peptide quantification, the integrated peak areas were calculated from extracted ion chromatograms with QuanBrowser from Xcalibur 2.0 while restricting the values by m/z (±0.02) and retention time (120 s). The protein identification mass spectrometry data were uploaded to the PRIDE database. Functional enrichment and network analysis were performed for the proteomic data with the FunRich tool (42).
Lipid extraction
Lipid extraction was accomplished with the Bligh and Dyer method (43). Exosomes (2–6 × 1011) in 5 µl buffer were placed into 10-ml glass screw-capped tubes followed by addition of 2.9 ml of a mixture of methanol:dichloromethane (2:0.9 v/v). The sample was vortexed for 30 s and spiked with 5 µl of Splash Lipidomix internal standards (Avanti, Alabaster, AL, USA) including d7-phosphatidylchloline (PC) (15:0/18:1); d7-phosphatidylethanolamine (PE) (15:0/18:1); d7-phosphatidylserine (PS) (15:0/18:1); d7-phosphatidylglycerol (PG) (15:0/18:1); d7-phosphatidylinositol (PI) (15:0/18:1); d7-phosphatidic acid (PA) (15:0/18:1); d7-lysophosphatidylcholine (18:1); d7-lysophosphatidylethanolamine (LPE) (18:1); d7-cholesterol ester (CE) (18:1); d7-monoacylglycerol (MAG) (18:1); d7-diacylglycerol (DAG) (18:1); d7-triacylglycerol (TAG) (18:1); d9-sphingomyelin (SM) (18:1/18:1); and d7-cholesterol. The mixture was again vortexed for 30 s and incubated for 30 min at room temperature, followed by addition of 1 ml of HPLC-grade water and 900 µl of dichloromethane. The samples were gently inverted 10 times and then centrifuged at 1200 rpm for 10 min. The organic lower phase (dichloromethane) was collected, concentrated to dryness under an N2 stream and reconstituted into 200 µl of methanol:dichloromethane (1:1 v/v) containing 10 mM ammonium acetate before analysis.
Sample analysis
Lipid analysis was conducted on a QTrap 6500 (AB Sciex, Redwood Shores, CA, USA) using flow infusion. Fifty microliters lipid extracts were injected into the mass spectrometer and semitargeted scans were used to focus on different phospholipid classes, including precursor ion scan and neutral loss scan modes (e.g., shotgun lipidomics). The sphingolipid and glyceride classes were also included in the analysis. For phospholipid analyses, different scan modes and ion polarities were used to monitor all 6 classes optimally in a multiplexed fashion, whereas sphingolipid and glyceride lipid classes were monitored only in the positive scan mode.
Lipid profile data were analyzed using LipidView software, v1.3 (AB Sciex). Data were normalized to internal standards followed by percentage composition (relative amounts of different lipid classes within a sample). Values are means of 3 replicates, and differences in relative amounts for each lipid class in different groups were analyzed for statistical significance. One-way ANOVA and Tukey post hoc t test were used to determine the significance (P < 0.05) using Prism 5.0 (GraphPad, La Jolla, CA, USA).
RESULTS
Characterization of exosomes from mpkCCD cells
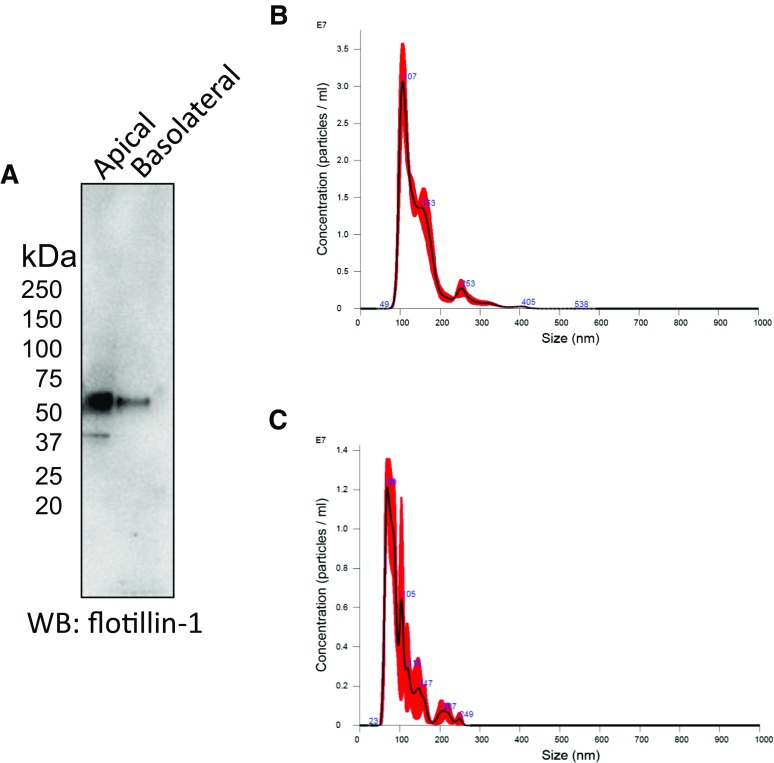
We cultured mpkCCD cells on permeable supports to maintain the polarization-dependent characteristics of endogenous proteins and investigate differences in exosomes produced by the budding of the apical and basolateral plasma membranes. Both types of exosomes were harvested for proteins and probed by Western blot for the exosomal marker flotillin-1 (Fig. 1A). Exosomes were subject to NanoSight analysis for size characterization and to determine the concentration of exosomes in the samples (Fig. 1B, C). The concentration of exosomes isolated from 40 ml of conditioned medium collected on the apical side of mpkCCD cells was 1.82e+012 ± 1.24e+011 particles/ml (Fig. 1B), whereas the concentration of exosomes isolated from the same volume of conditioned medium collected on the basolateral side of mpkCCD cells was 5.67e+011 ± 2.36e+010 particles/ml (Fig. 1C). Apical exosomes from mpkCCD cells were significantly larger (147.9 ± 55.9 nm) than the basolateral exosomes (100 ± 39.2 nm). The conditioned media from which the two types of exosomes were isolated were similar in osmolality (Table 2).
Figure 1.
Identification of 2 types of exosomes from mpkCCD cells. A) Western blot showing the exosomal marker flotillin-1 in exosomes isolated from the conditioned medium collected from the apical side or the basolateral side of mpkCCD cells. B, C) NanoSight analysis showing the concentration and size of apical (B) and basolateral (C) exosomes from mpkCCD cells.
TABLE 2.
Osmolality values of the conditioned medium from mpkCCD cells used to isolate each of the 3 batches of exosomes
| Solution | Osmolality (mM/kg) |
|---|---|
| Basolateral compartment conditioned medium | 457.8 ± 1.1 |
| Apical compartment conditioned medium | 476.2 ± 6.2 |
| RIPA buffer | 351.0 ± 2.0 |
| PBS | 321.3 ± 5.4 |
Data are expressed as means ± sem. The osmolalities of the RIPA buffer used to lyse the mpkCCD cells and the PBS used to resuspend the exosomal pellet is also given.
Both apical and basolateral plasma membrane exosomes maintain stability at different temperatures
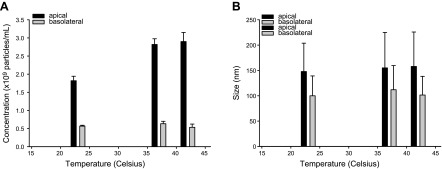
To determine whether the stability of the two types of exosomes produced by mpkCCD cells was affected by temperature, we subjected the apical and basolateral plasma exosomes to 23, 37, and 42°C for 8 h before performing NanoSight analysis. The concentration and size of the two types of exosomes were unaffected at these temperatures (Fig. 2). Basolateral plasma exosomes showed an sd in size of 6.2% and in concentration of 8.7%. Apical membranes showed an sd in size of 3.3% and an initial climb in concentration from 23 to 37–42°C, potentially because of kinetic energy generated at elevated temperatures. Between 37 and 42°C, there was only a 2% deviation. Across the 3 temperatures, there is a significant difference in concentration between the apical exosomes (2.53e+011 ± 6.02e+010 particles/ml) and the basolateral exosomes (5.78e+010 ± 5.04e+009 particles/ml).
Figure 2.
Stability of apical and basolateral exosomes derived from mpkCCD cells. Both types of exosomes were held at 3 different temperatures (23, 37, and 42°C) for 8 h and then measured for stability. A) Concentration maintained relatively stable with a slight increase related to the kinetic energy at elevated temperatures. B) Size remained unaffected by temperature changes.
FunRich analysis of molecular function and biologic pathway and process
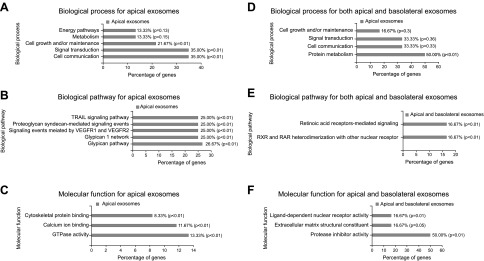
To provide a graphic illustration of the exosomal proteomic dataset, we used the FunRich analysis tool (42). A Venn diagram was generated with this open-access enrichment and interaction network tool and it revealed 142 proteins expressed only in the apical exosomes, 2 proteins expressed only in the basolateral exosomes, and 33 proteins expressed in both types of exosomes (data not shown). Figure 3A shows the percentage of genes encoding specific proteins identified in apical exosomes that are involved in biologic processes, including energy pathways, metabolism, cell growth, signal transduction, and cell communication. The percentage of these genes involved in various biologic pathways is shown in Fig. 3B. The percentage of genes encoding specific proteins identified in apical exosomes that are involved in molecular function, including cytoskeletal protein binding, calcium ion binding, and GTPase activity is shown in Fig. 3C. Figure 3D–F shows a similar analysis for genes encoding proteins identified in both apical and basolateral exosomes.
Figure 3.
FunRich functional enrichment and interaction network analysis of exosomal proteins from mpkCCD cells. Biologic process (A), biologic pathway (B), and molecular function (C) of proteins enriched in apical exosomes from mpkCCD cells (CLH1, ACTN1, MOES, 1433Z, ANXA5, ANXA6, G3P, ENOA, FLNA, ANXA1, ANXA3, ANXA4, and HSP90A). Biologic process (D), biologic pathway (E), and molecular function (F) of proteins enriched in both apical and basolateral exosomes from mpkCCD cells.
Enrichment of scaffolding and cytoskeletal proteins in exosomes from mpkCCD cells
The protein composition of kidney exosomes is dependent on the biogenesis of exosomes that originate from cells within the nephron (24). These polarized renal epithelial cells form a tight phenotype with the apical plasma membrane facing the tubular lumen and the basolateral plasma membrane facing the peritubular capillaries. Renal epithelial cells maintain many of their native characteristics when cultured in vitro on permeable supports. In this study, we investigated the protein composition of exosomes derived from apical and basolateral plasma membranes of cultured mpkCCD cells. As shown in Tables 1 and 2, α-actinin-1, moesin, 14-3-3 protein ζ/δ, annexin A1/A3/A4/A5/A6, clathrin heavy chain 1, glyceraldehyde-3-phosphate dehydrogenase, α-enolase, filamin-A, and heat shock protein (HSP)-90 are enriched in apical but not basolateral plasma membrane exosomes isolated from the conditioned medium of mpkCCD cells.
TABLE 1.
Identification of proteins isolated from apical or basolateral plasma membrane exosomes
| Identified proteins | Gene | Accession no. | Molecular mass (kDa) | AMPKEXO | BMPKEXO |
|---|---|---|---|---|---|
| Cluster of Myosin-9 | Myh9 | MYH9_MOUSE [3] | 226 | 58 | 0 |
| Clathrin heavy chain-1 | Cltc | CLH1_MOUSE | 192 | 36 | 0 |
| Cluster of α-actinin-1 | Actn1 | ACTN1_MOUSE [2] | 103 | 24 | 0 |
| Cluster of actin, cytoplasmic 2 | Actg1 | ACTG_MOUSE [4] | 42 | 16 | 2 |
| Cluster of moesin | Msn | MOES_MOUSE [3] | 68 | 26 | 0 |
| Cluster of tubulin β-5 chain | Tubb5 | TBB5_MOUSE [5] | 50 | 15 | 1 |
| Cluster of heat shock cognate 71 kDa protein | Hspa8 | HSP7C_MOUSE [3] | 71 | 14 | 1 |
| Cluster of tubulin α-1C chain | Tuba1c | TBA1C_MOUSE [2] | 50 | 10 | 5 |
| Cluster of 14-3-3 protein ζ/δ | Ywhaz | 1433Z_MOUSE [6] | 28 | 20 | 0 |
| Cluster of pyruvate kinase PKM | Pkm | KPYM_MOUSE | 58 | 12 | 3 |
| Neprilysin | Mme | NEP_MOUSE | 86 | 13 | 0 |
| Gelsolin | Gsn | GELS_MOUSE | 86 | 12 | 5 |
| Annexin A2 | Anxa2 | ANXA2_MOUSE | 39 | 13 | 3 |
| Programmed cell death 6-interacting protein | Pdcd6ip | PDC6I_MOUSE | 96 | 11 | 2 |
| Annexin A5 | Anxa5 | ANXA5_MOUSE | 36 | 13 | 0 |
| Cluster of sodium/potassium-transporting ATPase subunit α-1 | Atp1a1 | AT1A1_MOUSE | 113 | 9 | 4 |
| Elongation factor 2 | Eef2 | EF2_MOUSE | 95 | 6 | 1 |
| T-complex protein 1 subunit α | Tcp1 | TCPA_MOUSE | 60 | 11 | 0 |
| Annexin A6 | Anxa6 | ANXA6_MOUSE | 76 | 13 | 0 |
| Lactadherin | Mfge8 | MFGM_MOUSE | 51 | 7 | 3 |
| Phosphoglycerate kinase 1 | Pgk1 | PGK1_MOUSE | 45 | 9 | 0 |
| Glyceraldehyde-3-phosphate dehydrogenase | Gapdh | G3P_MOUSE | 36 | 7 | 0 |
| Major vault protein | Mvp | MVP_MOUSE | 96 | 9 | 0 |
| Cluster of α-enolase | Eno1 | ENOA_MOUSE | 47 | 9 | 0 |
| Cluster of Filamin-A | Flna | FLNA_MOUSE | 281 | 10 | 0 |
| Annexin A1 | Anxa1 | ANXA1_MOUSE | 39 | 9 | 0 |
| Annexin A3 | Anxa3 | ANXA3_MOUSE | 36 | 9 | 0 |
| Ras GTPase-activating-like protein IQGAP1 | Iqgap1 | IQGA1_MOUSE | 189 | 10 | 0 |
| Cluster of EH domain-containing protein 1 | Ehd1 | EHD1_MOUSE [2] | 61 | 6 | 0 |
| Elongation factor 1-α 1 | Eef1a1 | EF1A1_MOUSE | 50 | 8 | 0 |
| Integrin β-1 | Itgb1 | ITB1_MOUSE | 88 | 5 | 2 |
| Transforming protein ρA | Rhoa | RHOA_MOUSE | 22 | 5 | 0 |
| T-complex protein 1 subunit-β | Cct2 | TCPB_MOUSE | 57 | 10 | 0 |
| Heat shock protein HSP 90-β | Hsp90ab1 | HS90B_MOUSE | 83 | 7 | 1 |
| Unconventional myosin-Ic | Myo1c | MYO1C_MOUSE | 122 | 6 | 0 |
| Heat shock protein HSP 90-α | Hsp90aa1 | HS90A_MOUSE | 85 | 9 | 0 |
| Prominin-1 | Prom1 | PROM1_MOUSE | 97 | 8 | 0 |
| T-complex protein 1 subunit θ | Cct8 | TCPQ_MOUSE | 60 | 8 | 0 |
| Annexin A4 | Anxa4 | ANXA4_MOUSE | 36 | 7 | 0 |
| Myoferlin | Myof | MYOF_MOUSE | 233 | 7 | 0 |
| T-complex protein 1 subunit γ | Cct3 | TCPG_MOUSE | 61 | 9 | 0 |
| Cluster of guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit β-2 | Gnb2 | GBB2_MOUSE [2] | 37 | 8 | 0 |
| T-complex protein 1 subunit δ | Cct4 | TCPD_MOUSE | 58 | 7 | 0 |
| T-complex protein 1 subunit ζ | Cct6a | TCPZ_MOUSE | 58 | 6 | 0 |
| Chloride intracellular channel protein 1 | Clic1 | CLIC1_MOUSE | 27 | 6 | 0 |
| l-lactate dehydrogenase A chain | Ldha | LDHA_MOUSE | 36 | 5 | 0 |
| T-complex protein 1 subunit η | Cct7 | TCPH_MOUSE | 60 | 6 | 0 |
| Elongation factor 1-γ | Eef1g | EF1G_MOUSE | 50 | 5 | 0 |
| Heat shock 70 kDa protein 1A | Hspa1a | HS71A_MOUSE (+1) | 70 | 5 | 0 |
| G-protein coupled receptor family C group 5 member C | Gprc5c | GPC5C_MOUSE | 48 | 5 | 0 |
| Adenosylhomocysteinase | Ahcy | SAHH_MOUSE | 48 | 5 | 0 |
We identified 254 proteins. Only those with <5 signature peptides are listed. AMPKEXO, apical exosomes from mpkCCD cells; BMPKEXO, to basolateral exosomes from mpkCCD cells.
Characterization of the lipid composition of mpkCCD exosomes
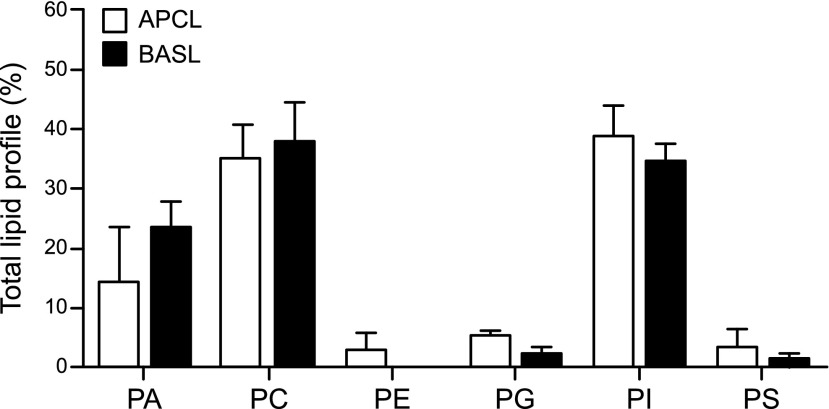
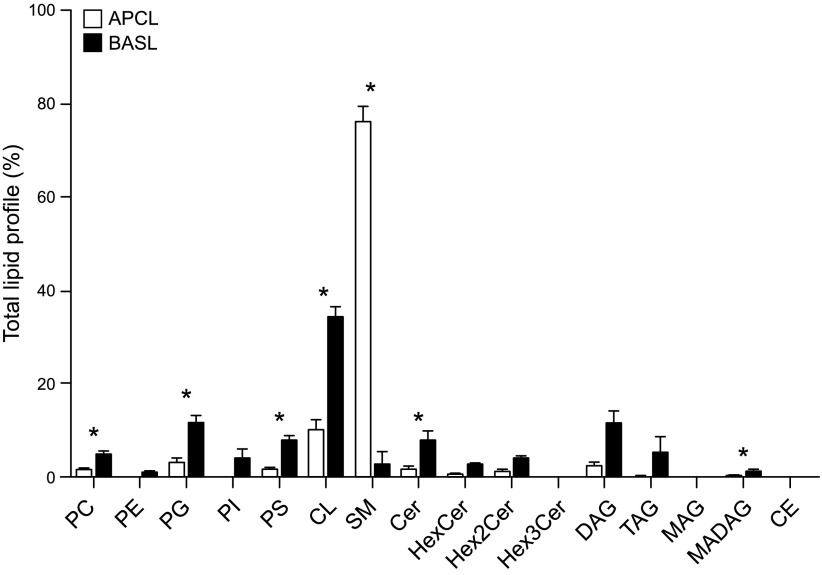
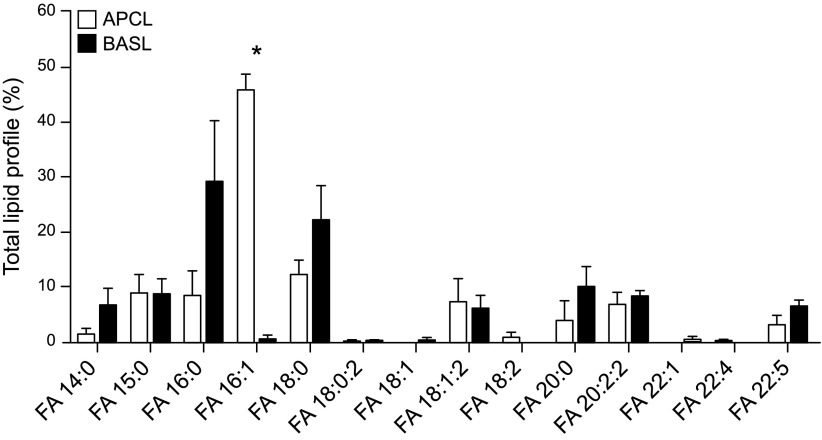
We investigated differences in lipid classes between the two types of exosomes from mpkCCD cells. Under negative ionization mode, no significant differences in phospholipid classes were observed between the apical and basolateral plasma membrane exosomes, and both groups appeared to be enriched in PA, PC, and PI and showed lower amounts of PE, PG, and PS (Fig. 4). However, lipid class profiles were significantly different for the two types of exosomes when analyzed in positive scan mode (Fig. 5). Contributions of specific lipids within the PC, PG, and PS classes to total lipid profiles were much higher in basolateral exosomes than in apical exosomes. Two important classes of lipids appeared to distinguish apical membrane exosomes from basolateral plasma membrane exosomes, with SM in greater relative amounts in the former and cardiolipin (CL) in the latter (Fig. 5). Ceramides (Cer) and hexosyl ceramides including HexCer, Hex2Cer, and Hex3Cer, and glycerides were measured, but only Cer and monoalkyldiacylglycerol were significantly different between the two membrane exosomes. In addition, fatty acids (FAs) of various lengths from 14 to 22 carbons and various saturation levels were identified (Fig. 6). FA 16:0, FA 16:1, and FA 18:0 were the most abundant species for both the apical and basolateral plasma membrane exosomes. In comparison, apical exosomes contained more FA 16:1 than the basolateral exosomes from the same cells (Fig. 6).
Figure 4.
Comparative lipid profile analysis of 6 phospholipid classes in mpkCCD exosomes in negative scan mode. Three independent samples of exosomes isolated from the apical (APCL) side of mpkCCD cells or the basolateral (BASL) side of the same cells were examined for their lipid content by LC-MS/MS. Values were corrected with internal standards spiked into samples before extraction and then were normalized to total lipid and thus represent relative concentrations of lipids. Means ± sem.
Figure 5.
Lipid profile analysis of exosomes in positive scan mode. Values are means of 3 replicates ± sem. Data were corrected for the amount of internal standards spiked into the samples and then normalized to total lipid. HexCer, hexosyl ceremide; TAG, triacylglycerol; DAG, diacylglycerol; MAG, monoacylglycerol; CE, cholesterol ester. *P ≤ 0.05 (FA abundances that are significantly different between the samples).
Figure 6.
MS/MS spectra of the relative abundance of FAs within mpkCCD exosomes. Product ion spectra of FAs 14:0, 15:0, 16:0, 16:1, 18:0, 18:1, 18:1;2, 18:2, 20:0, 20:2;2, 22:1, 22:4, 22:5, respectively (x axis). *P ≤ 0.05, FA abundances that are significantly different between the samples.
DISCUSSION
Lipid rafts are cell membrane platforms that enhance the assembly and function of signaling molecules (44). A number of transmembrane proteins that are expressed in kidney collecting duct cells have been shown to be enriched in lipid rafts. For example, the phosphorylated form of AQP-2 was shown to be enriched in lipid rafts (45, 46), and expression of AQP-2 in lipid rafts within the endoplasmic reticulum membranes indicates this complex occurs early in the biosynthesis pathway (47). Similarly, ENaC was shown to traffic to the apical plasma membrane in lipid rafts (48). Consistent with other published studies, our group had shown a relationship between the fraction of the total population of ENaC with lipid rafts (12). In addition to their role in mediating intracellular signaling, lipid rafts are involved in the uptake of exosomes. Lipid raft–mediated endocytosis plays a role in exosome formation and uptake in recipient cells. Tan et al. (49) showed that mesenchymal stem cell exosomes originate from endocytosed lipid rafts. Svensson et al. (50) showed that the uptake of exosomes is negatively regulated by the lipid raft–associated protein caveolin-1. The studies performed by this group showed that the uptake of exosomes is inhibited by depletion of membrane cholesterol. Moreover, ERK1/2 activation of HSP27 was shown to enhance exosome uptake whereas caveolin-1 suppressed ERK1/2 signaling and inhibited exosome uptake (50).
There were some limitations in the identification of proteins within the two types of exosomes in our study. The proteins listed in Table 1 do not reflect a comprehensive list of proteins present in the exosome samples or expressed in mpkCCD cells. These are the most abundant proteins present in the exosome samples that were identified by LC-MS/MS. The abundantly expressed proteins may mask less abundantly expressed proteins in the sample. For example, we were able to detect rare and less abundant proteins including ENaCα, -β, and -γ subunits from apical plasma membrane exosomes derived from mpkCCD cells by Western blot analysis with our validated antibodies (data not shown). Newer generation mass spectrometers are capable of detecting rare and small amounts of proteins. Alternatively, an antibody enrichment step can be performed to increase detection. Nevertheless, the proteins we identified by LC-MS/MS represent putative regulators of biologic processes, biologic function, and function in kidney collecting duct cells (Fig. 3).
There has been much research to identify the biogenesis of exosomes and to understand the specific cargo they carry as they communicate from cell to cell [reviewed in Record et al. (51)]. Exosomes originate from the late endosomal compartment of live cells and transport their cargo out of cells to communicate with receptive cells. It appears that their lipid composition is unique and does not reflect the composition of the plasma membrane. Exosomes are enriched in lipids such as PCs and PEs and bioactive lipids involved in signaling such as SM, Cers, hexceramides, cholesterol, lysophosphatidylcholine, prostaglandins, and leukotrienes, among others (52). In our study, we found significant differences in the lipids of exosomes secreted from the apical and basolateral membranes of the same cell, suggesting that the exosomes originated from separate biogenesis pathways from within the same cell.
Although our study did not involve a comprehensive lipidomics analysis as might be performed with an Orbitrap high-resolution instrument (Thermo Fisher Scientific), we nevertheless had excellent reproducibility with triplicate exosome samples from the two membranes, and because we normalized results to deuterated lipidomics standards, we are confident of our results. Exosomes isolated from the apical membrane contain significantly higher levels of SM than those derived from the basolateral membrane. Conversely, the exosomes isolated from the basolateral membrane were enriched in CLs, Cers, and other phospholipids, suggesting different biogenic pathways. CLs are major components of mitochondria (53), suggesting that mitochondria are somehow involved in the biogenesis of exosomes for the basolateral side but not the apical side, an interesting idea that should be further investigated. The differences we observed in lipid composition of the two types of exosomes may play a role in their ability to communicate with other cells and their function.
In summary, exosomes released from the apical and basolateral sides of the same mpkCCD cells have different protein and lipid composition, possibly reflecting on their specific biogenic pathways and also on their projected function as they communicate with target cells. The lipid profiles are not comprehensive, as we used direct infusion into the mass spectrometer for these studies. In future studies, we will perform additional column separation steps using hydrophilic interaction liquid chromatography and C18 columns to get better resolution of the lipid compositions. We will also cross-verify lipid identities with a high-resolution Orbitrap mass spectrometer. These studies are currently ongoing.
ACKNOWLEDGMENTS
The authors thank Wei Guan and Bin Fang, Ph.D., (both from Proteomics Core Facility, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA) for assistance with proteomics. This project was made possible by U.S. National Institutes of Health (NIH) Shared Instrumentation (Grant 1S10OD018141-01A1); NIH, National Institute of Diabetes and Digestive and Kidney Diseases (Grant K01 DK099617; to A.A.A.); the University of Florida College of Medicine; in part, by the Proteomics Core Facility at the H. Lee Moffitt Cancer Center and Research Institute; and by the NIH, National Cancer Institute designated Comprehensive Cancer Center (Grant P30-CA076292). The authors declare no conflicts of interest.
Glossary
- AQP
aquaporin
- Cer
ceramide
- CL
cardiolipin
- ENaC
epithelial sodium channel
- FA
fatty acid
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- PA
phosphatidic acid
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PG
phosphatidylglycerol
- PI
phosphatidylinositol
- PS
phosphatidylserine
- SM
sphingomyelin
AUTHOR CONTRIBUTIONS
V. D. Dang, K. K. Jella, R. R. T. Ragheb, N. D. Denslow, and A. A. Alli designed the research, analyzed the data, wrote the paper, and approved the final version of the paper; and V. D. Dang, R. R. T. Ragheb, and A. A. Alli performed the experiments.
REFERENCES
- 1.Pearce D., Soundararajan R., Trimpert C., Kashlan O. B., Deen P. M., Kohan D. E. (2015) Collecting duct principal cell transport processes and their regulation. Clin. J. Am. Soc. Nephrol. 10, 135–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang W. H., Giebisch G. (2009) Regulation of potassium (K) handling in the renal collecting duct. Pflugers Arch. 458, 157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fenton R. A., Moeller H. B. (2008) Recent discoveries in vasopressin-regulated aquaporin-2 trafficking. Prog. Brain Res. 170, 571–579 [DOI] [PubMed] [Google Scholar]
- 4.Hasler U. (2009) Controlled aquaporin-2 expression in the hypertonic environment. Am. J. Physiol. Cell Physiol. 296, C641–C653 [DOI] [PubMed] [Google Scholar]
- 5.Klussmann E., Maric K., Rosenthal W. (2000) The mechanisms of aquaporin control in the renal collecting duct. Rev. Physiol. Biochem. Pharmacol. 141, 33–95 [DOI] [PubMed] [Google Scholar]
- 6.Xing L., Wen J. G., Frøkiær J., Djurhuus J. C., Nørregaard R. (2014) Ontogeny of the mammalian kidney: expression of aquaporins 1, 2, 3, and 4. World J. Pediatr. 10, 306–312 [DOI] [PubMed] [Google Scholar]
- 7.Ecelbarger C. A., Terris J., Frindt G., Echevarria M., Marples D., Nielsen S., Knepper M. A. (1995) Aquaporin-3 water channel localization and regulation in rat kidney. Am. J. Physiol. 269, F663–F672 [DOI] [PubMed] [Google Scholar]
- 8.Terris J., Ecelbarger C. A., Marples D., Knepper M. A., Nielsen S. (1995) Distribution of aquaporin-4 water channel expression within rat kidney. Am. J. Physiol. 269, F775–F785 [DOI] [PubMed] [Google Scholar]
- 9.Chou C. L., Yip K. P., Michea L., Kador K., Ferraris J. D., Wade J. B., Knepper M. A. (2000) Regulation of aquaporin-2 trafficking by vasopressin in the renal collecting duct: roles of ryanodine-sensitive Ca2+ stores and calmodulin. J. Biol. Chem. 275, 36839–36846 [DOI] [PubMed] [Google Scholar]
- 10.Lee B. H., Kwon T. H. (2007) Regulation of AQP2 in collecting duct: an emphasis on the effects of angiotensin II or aldosterone. Electrolyte Blood Press. 5, 15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nedvetsky P. I., Tamma G., Beulshausen S., Valenti G., Rosenthal W., Klussmann E. (2009) Regulation of aquaporin-2 trafficking. Handb. Exp. Pharmacol. 190, 133–157 [DOI] [PubMed] [Google Scholar]
- 12.Alli A. A., Bao H. F., Alli A. A., Aldrugh Y., Song J. Z., Ma H. P., Yu L., Al-Khalili O., Eaton D. C. (2012) Phosphatidylinositol phosphate-dependent regulation of Xenopus ENaC by MARCKS protein. Am. J. Physiol. Renal Physiol. 303, F800–F811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alli A. A., Bao H. F., Liu B. C., Yu L., Aldrugh S., Montgomery D. S., Ma H. P., Eaton D. C. (2015) Calmodulin and CaMKII modulate ENaC activity by regulating the association of MARCKS and the cytoskeleton with the apical membrane. Am. J. Physiol. Renal Physiol. 309, F456–F463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reifenberger M. S., Yu L., Bao H. F., Duke B. J., Liu B. C., Ma H. P., Alli A. A., Eaton D. C., Alli A. A. (2014) Cytochalasin E alters the cytoskeleton and decreases ENaC activity in Xenopus 2F3 cells. Am. J. Physiol. Renal Physiol. 307, F86–F95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q., Dai X. Q., Li Q., Tuli J., Liang G., Li S. S., Chen X. Z. (2013) Filamin interacts with epithelial sodium channel and inhibits its channel function. J. Biol. Chem. 288, 264–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ichimura T., Yamamura H., Sasamoto K., Tominaga Y., Taoka M., Kakiuchi K., Shinkawa T., Takahashi N., Shimada S., Isobe T. (2005) 14-3-3 proteins modulate the expression of epithelial Na+ channels by phosphorylation-dependent interaction with Nedd4-2 ubiquitin ligase. J. Biol. Chem. 280, 13187–13194 [DOI] [PubMed] [Google Scholar]
- 17.Liang X., Peters K. W., Butterworth M. B., Frizzell R. A. (2006) 14-3-3 isoforms are induced by aldosterone and participate in its regulation of epithelial sodium channels. J. Biol. Chem. 281, 16323–16332 [DOI] [PubMed] [Google Scholar]
- 18.Nagaki K., Yamamura H., Shimada S., Saito T., Hisanaga S., Taoka M., Isobe T., Ichimura T. (2006) 14-3-3 Mediates phosphorylation-dependent inhibition of the interaction between the ubiquitin E3 ligase Nedd4-2 and epithelial Na+ channels. Biochemistry 45, 6733–6740 [DOI] [PubMed] [Google Scholar]
- 19.Mazzochi C., Bubien J. K., Smith P. R., Benos D. J. (2006) The carboxyl terminus of the alpha-subunit of the amiloride-sensitive epithelial sodium channel binds to F-actin. J. Biol. Chem. 281, 6528–6538 [DOI] [PubMed] [Google Scholar]
- 20.Berdiev B. K., Latorre R., Benos D. J., Ismailov I. I. (2001) Actin modifies Ca2+ block of epithelial Na+ channels in planar lipid bilayers. Biophys. J. 80, 2176–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jovov B., Tousson A., Ji H. L., Keeton D., Shlyonsky V., Ripoll P. J., Fuller C. M., Benos D. J. (1999) Regulation of epithelial Na(+) channels by actin in planar lipid bilayers and in the Xenopus oocyte expression system. J. Biol. Chem. 274, 37845–37854 [DOI] [PubMed] [Google Scholar]
- 22.Ilatovskaia D. V., Pavlov T. S., Neguliaev IuA., Starushchenko A. V. (2011) Mechanism of epithelial sodium channel (ENaC) regulation by cortactin: involvement of dynamin. Tsitologiia 53, 903–910 [PubMed] [Google Scholar]
- 23.Ilatovskaya D. V., Pavlov T. S., Levchenko V., Negulyaev Y. A., Staruschenko A. (2011) Cortical actin binding protein cortactin mediates ENaC activity via Arp2/3 complex. FASEB J. 25, 2688–2699 [DOI] [PubMed] [Google Scholar]
- 24.Jella K. K., Yu L., Yue Q., Friedman D., Duke B. J., Alli A. A. (2016) Exosomal GAPDH from proximal tubule cells regulate ENaC activity. PLoS One 11, e0165763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee M. J., Park D. H., Kang J. H. (2016) Exosomes as the source of biomarkers of metabolic diseases. Ann. Pediatr. Endocrinol. Metab. 21, 119–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferguson S. W., Nguyen J. (2016) Exosomes as therapeutics: the implications of molecular composition and exosomal heterogeneity. J. Control. Release 228, 179–190 [DOI] [PubMed] [Google Scholar]
- 27.Zeringer E., Barta T., Li M., Vlassov A. V. (2015) Strategies for isolation of exosomes. Cold Spring Harb. Protoc. 2015, 319–323 [DOI] [PubMed] [Google Scholar]
- 28.Perez-Hernandez J., Cortes R. (2015) Extracellular vesicles as biomarkers of systemic lupus erythematosus. Dis. Markers 2015, 613536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoops E. H., Caplan M. J. (2014) Trafficking to the apical and basolateral membranes in polarized epithelial cells. J. Am. Soc. Nephrol. 25, 1375–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chassin C., Bens M., Vandewalle A. (2007) Transimmortalized proximal tubule and collecting duct cell lines derived from the kidneys of transgenic mice. Cell Biol. Toxicol. 23, 257–266 [DOI] [PubMed] [Google Scholar]
- 31.Yip K. P., Cha B. J., Tse C. M., Amin M. E., Amin J. (2015) Functional expression of aquaporin-2 tagged with photoconvertible fluorescent protein in mpkCCD cells. Cell. Physiol. Biochem. 36, 670–682 [DOI] [PubMed] [Google Scholar]
- 32.Cheema M. U., Irsik D. L., Wang Y., Miller-Little W., Hyndman K. A., Marks E. S., Frøkiær J., Boesen E. I., Norregaard R. (2015) Estradiol regulates AQP2 expression in the collecting duct: a novel inhibitory role for estrogen receptor α. Am. J. Physiol. Renal Physiol. 309, F305–F317 [DOI] [PubMed] [Google Scholar]
- 33.Hasler U., Vinciguerra M., Vandewalle A., Martin P. Y., Féraille E. (2005) Dual effects of hypertonicity on aquaporin-2 expression in cultured renal collecting duct principal cells. J. Am. Soc. Nephrol. 16, 1571–1582 [DOI] [PubMed] [Google Scholar]
- 34.Kortenoeven M. L., Trimpert C., van den Brand M., Li Y., Wetzels J. F., Deen P. M. (2012) In mpkCCD cells, long-term regulation of aquaporin-2 by vasopressin occurs independent of protein kinase A and CREB but may involve Epac. Am. J. Physiol. Renal Physiol. 302, F1395–F1401 [DOI] [PubMed] [Google Scholar]
- 35.Li C., Wang W., Rivard C. J., Lanaspa M. A., Summer S., Schrier R. W. (2011) Molecular mechanisms of angiotensin II stimulation on aquaporin-2 expression and trafficking. Am. J. Physiol. Renal Physiol. 300, F1255–F1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verrey F., Summa V., Heitzmann D., Mordasini D., Vandewalle A., Féraille E., Zecevic M. (2003) Short-term aldosterone action on Na,K-ATPase surface expression: role of aldosterone-induced SGK1? Ann. N. Y. Acad. Sci. 986, 554–561 [DOI] [PubMed] [Google Scholar]
- 37.Féraille E., Mordasini D., Gonin S., Deschênes G., Vinciguerra M., Doucet A., Vandewalle A., Summa V., Verrey F., Martin P. Y. (2003) Mechanism of control of Na,K-ATPase in principal cells of the mammalian collecting duct. Ann. N. Y. Acad. Sci. 986, 570–578 [DOI] [PubMed] [Google Scholar]
- 38.Xie L., Hoffert J. D., Chou C. L., Yu M. J., Pisitkun T., Knepper M. A., Fenton R. A. (2010) Quantitative analysis of aquaporin-2 phosphorylation. Am. J. Physiol. Renal Physiol. 298, F1018–F1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sokolova V., Ludwig A. K., Hornung S., Rotan O., Horn P. A., Epple M., Giebel B. (2011) Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf. B Biointerfaces 87, 146–150 [DOI] [PubMed] [Google Scholar]
- 40.Montgomery D. S., Yu L., Ghazi Z. M., Thai T. L., Al-Khalili O., Ma H. P., Eaton D. C., Alli A. A. (2017) ENaC activity is regulated by calpain-2 proteolysis of MARCKS proteins. Am. J. Physiol. Cell Physiol. 313, C42–C53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jella K. K., Rani S., O’Driscoll L., McClean B., Byrne H. J., Lyng F. M. (2014) Exosomes are involved in mediating radiation induced bystander signaling in human keratinocyte cells. Radiat. Res. 181, 138–145 [DOI] [PubMed] [Google Scholar]
- 42.Pathan M., Keerthikumar S., Ang C. S., Gangoda L., Quek C. Y., Williamson N. A., Mouradov D., Sieber O. M., Simpson R. J., Salim A., Bacic A., Hill A. F., Stroud D. A., Ryan M. T., Agbinya J. I., Mariadason J. M., Burgess A. W., Mathivanan S. (2015) FunRich: an open access standalone functional enrichment and interaction network analysis tool. Proteomics 15, 2597–2601 [DOI] [PubMed] [Google Scholar]
- 43.Bligh E. G., Dyer W. J. (1959) A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 44.Suzuki K. G. (2012) Lipid rafts generate digital-like signal transduction in cell plasma membranes. Biotechnol. J. 7, 753–761 [DOI] [PubMed] [Google Scholar]
- 45.Yu M. J., Pisitkun T., Wang G., Aranda J. F., Gonzales P. A., Tchapyjnikov D., Shen R. F., Alonso M. A., Knepper M. A. (2008) Large-scale quantitative LC-MS/MS analysis of detergent-resistant membrane proteins from rat renal collecting duct. Am. J. Physiol. Cell Physiol. 295, C661–C678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fenton R. A., Pedersen C. N., Moeller H. B. (2013) New insights into regulated aquaporin-2 function. Curr. Opin. Nephrol. Hypertens. 22, 551–558 [DOI] [PubMed] [Google Scholar]
- 47.Procino G., Barbieri C., Carmosino M., Rizzo F., Valenti G., Svelto M. (2010) Lovastatin-induced cholesterol depletion affects both apical sorting and endocytosis of aquaporin-2 in renal cells. Am. J. Physiol. Renal Physiol. 298, F266–F278 [DOI] [PubMed] [Google Scholar]
- 48.Hill W. G., Butterworth M. B., Wang H., Edinger R. S., Lebowitz J., Peters K. W., Frizzell R. A., Johnson J. P. (2007) The epithelial sodium channel (ENaC) traffics to apical membrane in lipid rafts in mouse cortical collecting duct cells. J. Biol. Chem. 282, 37402–37411 [DOI] [PubMed] [Google Scholar]
- 49.Tan S. S., Yin Y., Lee T., Lai R. C., Yeo R. W., Zhang B., Choo A., Lim S. K. (2013) Therapeutic MSC exosomes are derived from lipid raft microdomains in the plasma membrane. J. Extracell. Vesicles 2, 22614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Svensson K. J., Christianson H. C., Wittrup A., Bourseau-Guilmain E., Lindqvist E., Svensson L. M., Mörgelin M., Belting M. (2013) Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. J. Biol. Chem. 288, 17713–17724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Record M., Subra C., Silvente-Poirot S., Poirot M. (2011) Exosomes as intercellular signalosomes and pharmacological effectors. Biochem. Pharmacol. 81, 1171–1182 [DOI] [PubMed] [Google Scholar]
- 52.Laulagnier K., Motta C., Hamdi S., Roy S., Fauvelle F., Pageaux J. F., Kobayashi T., Salles J. P., Perret B., Bonnerot C., Record M. (2004) Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem. J. 380, 161–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schlame M., Ren M., Xu Y., Greenberg M. L., Haller I. (2005) Molecular symmetry in mitochondrial cardiolipins. Chem. Phys. Lipids 138, 38–49 [DOI] [PubMed] [Google Scholar]