Abstract
Cell production and death are tightly regulated in the rapidly renewing gut epithelium, with proliferation confined to crypts and apoptosis occurring in villi and crypts. This study sought to determine how stress alters these compartmentalized processes. Wild-type mice made septic via cecal ligation and puncture had decreased crypt proliferation and increased crypt and villus apoptosis. Fabpi-TAg mice expressing large T-antigen solely in villi had ectopic enterocyte proliferation with increased villus apoptosis in unmanipulated animals. Septic fabpi-TAg mice had an unexpected increase in villus proliferation compared with unmanipulated littermates, whereas crypt proliferation was decreased. Cell cycle regulators cyclin D1 and cyclin D2 were decreased in jejunal tissue in septic transgenic mice. In contrast, villus and crypt apoptosis were increased in septic fabpi-TAg mice. To examine the relationship between apoptosis and proliferation in a compartment-specific manner, fabpi-TAg mice were crossed with fabpl-Bcl-2 mice, resulting in expression of both genes in the villus but Bcl-2 alone in the crypt. Septic bi-transgenic animals had decreased crypt apoptosis but had a paradoxical increase in villus apoptosis compared with septic fabpi-TAg mice, associated with decreased proliferation in both compartments. Thus, sepsis unmasks compartment-specific proliferative and apoptotic regulation that is not present under homeostatic conditions.—Lyons, J. D., Klingensmith, N. J., Otani, S., Mittal, R., Liang, Z., Ford, M. L., Coopersmith, C. M. Sepsis reveals compartment-specific responses in intestinal proliferation and apoptosis in transgenic mice whose enterocytes re-enter the cell cycle.
Keywords: gut, critical illness, stress, cyclin, barrier
The small intestinal epithelium is a highly dynamic and tightly regulated structure, forming a vast single-cell boundary between the host and the ∼40 trillion microbes living within it (1–3). Cell proliferation occurs exclusively in the crypts, where pluripotent stem cells, which reside near the crypt base, divide. The majority of daughter cells migrate upward. Once they reach the villus—fingerlike projections that protrude into the bowel lumen, creating the vast surface area of the small intestine—intestinal cells differentiate into enterocytes, enteroendocrine cells, and goblet cells, which allow the intestine to absorb nutrients and play a first-line role in host defense from microbial invaders. A small number of daughter cells migrate to the crypt base, where they differentiate into defensin-producing Paneth cells. In addition, microfold cells, tuft cells, and cup cells make up a small fraction of intestinal epithelial cells, although their precise functions are less well understood (4). When cells reach the villus tip, they die by apoptosis or are exfoliated whole into the gut lumen (5). Under basal conditions, intestinal homeostasis is tightly controlled with cell birth in the crypts balanced by cell loss at the villus tip (with a small amount of apoptosis also occurring in the crypt). The entire journey of cell production, migration, differentiation, and death occurs every 3–7 d (6).
Sepsis is a life-threatening organ dysfunction secondary to a dysregulated host response to infection (7). The syndrome represents a significant public health problem, killing between 230,000 and 370,000 individuals in the United States each year, making it the third most common cause of death (8). The intestine is profoundly affected by sepsis (9, 10). Crypts have a marked decrease in proliferation, coinciding with a significant increase in apoptosis (11–13). Apoptosis is also up-regulated on the villus. The combined pathologic effects induced by sepsis on cell production and loss result in markedly decreased villus length. Simultaneously, gut barrier function is worsened by sepsis, with intestinal hyperpermeability associated with changes in multiple intestinal tight junction proteins (14, 15). These changes in gut epithelial integrity appear to be clinically important because when gut integrity can be maintained by decreasing apoptosis, increasing proliferation, or normalizing permeability, survival is improved in animal models of sepsis (16–19).
It is unknown whether the damage sustained by the crypts in sepsis occurs as a result of active cell cycling in a unique microenvironment. To answer this question and to determine the relationship between proliferation and apoptosis in the compartments of the small intestine, we induced sepsis in mice that have ectopic villus proliferation. The viral protein large T-antigen (TAg) is encoded by the DNA virus SV40 and is capable of transforming cell lines by binding and inhibiting p53 and pRb-family cell cycle regulators. When TAg is overexpressed solely in villus enterocytes in transgenic mice, differentiated villus cells re-enter the cell cycle by relieving pRb-mediated blockade of E2F transcription factors (20–22). Fabpi-TAg mice therefore have an epithelium with 2 distinct proliferative compartments: normal crypt proliferation and ectopic villus proliferation. Examining Fabpi-TAg mice after the onset of sepsis allows for the determination of whether sepsis-induced alterations in gut integrity are 1) uniquely and disparately hardwired into the crypt vs. villus microenvironments, 2) dependent upon the proliferative capacity of the gut epithelium independent of geographic location, and 3) affected after manipulating intrinsic proliferation and apoptosis mechanisms and how these modifications affect distinct geographic compartments.
MATERIALS AND METHODS
Mice
Male and female mice (8–12 wk old) with the TAg gene linked to nt −1178 to +28 of rat intestinal fatty acid binding protein (fabpi) and mice with human Bcl-2 linked to nt −596 to +21 of rat liver fatty acid binding protein (fabpl) have been previously described (20, 23). Colonies were maintained by breeding transgenic mice with wild-type (WT) mice. All animals were on an FVB/N genetic background. Transgene expression was confirmed by analysis of PCR amplification products with DNA gel electrophoresis (TAg primers: 5′-CTGTTTGGTTTGGTTTATCGCC-3′, 5′-CCATTCATCAGTTCCATAGGTTGG-3′; Bcl-2 primers: 5′-TGTTGACTTCACTTGTGGCC-3′, 5′-TGGATCCAGGATAACGGAGG-3′) (Integrated DNA Technologies, Coralville, IA, USA). Animals were housed in facilities managed by the Emory University Division of Animal Resources and had free access to food and water. All experiments were performed in accordance with the National Institutes of Health Guidelines for the Use of Laboratory Animals and received approval from the Institutional Animal Care and Use Committee at Emory University School of Medicine (Protocol DAR-2002717-042217GN).
Sepsis model
Animals were made septic via cecal ligation and puncture (CLP), a model of polymicrobial intra-abdominal sepsis (24). Under isoflurane anesthesia, a midline abdominal incision was made, and the cecum was identified and exteriorized. A silk ligature was placed near the base of the cecum without occluding stool flow from small to large bowel, and the ligated portion was punctured twice with a 25-gauge needle. A small amount of stool was then extruded from the puncture sites. The contents were then returned to the abdominal cavity, and the abdominal wall was closed in layers. Prior to incision, mice received buprenorphine, given subcutaneously at a dose of 0.1 mg/kg (McKesson Medical, San Francisco, CA, USA), to alleviate postoperative pain. Mice also received subcutaneous injections of normal saline (1 ml), ceftriaxone (50 mg/kg) (Sigma-Aldrich, St. Louis, MO, USA), and metronidazole (35 mg/kg) (Apotex, Weston, FL, USA) postoperatively to account for insensible fluid losses and to mimic the human condition where fluid resuscitation and antibiotics are a mainstay of sepsis management (25). Ceftriaxone and metronidazole were redosed a single time at 12 h. All animals were killed at 24 h for tissue collection.
Intestinal proliferation
Mice were given intraperitoneal injections of the thymidine analog 5-bromo-2′-deoxyuridine (BRDU) (5 mg/ml in 0.9% saline) (Sigma-Aldrich) 90 min prior to being killed to label enterocytes in S-phase. The small intestine was removed en bloc and fixed overnight in 10% formalin before being embedded in paraffin. Slides were then deparaffinized and rehydrated. Samples were placed in 1% hydrogen peroxide in PBS for 10 min and then placed in citrate buffer in a pressure cooker for 45 min (Antigen Decloaker; Biocare Medical, Concord, CA, USA). Protein Block (Dako, Carpinteria, CA, USA) was applied for 30 min at room temperature, and then rat mAb-BRDU (Accurate Chemical and Scientific, Westbury, NY, USA) was applied overnight at 4°C. Slides were then incubated for 1 h at room temperature with goat anti-rat biotinylated secondary antibody (1:500) for 60 min (Accurate Chemical and Scientific) followed by streptavidin horseradish peroxidase (Dako). Slides were developed for 2–3 min in diaminobenzidine and counterstained with hematoxylin. BRDU staining was quantified by counting the number of positive cells at magnification ×40 in 100 contiguous, well-oriented intestinal crypts or in 10 contiguous villi by an examiner blinded to sample identity.
Intestinal apoptosis
Apoptotic cells were quantified using two complementary techniques: active caspase-3 staining and morphologic analysis of hematoxylin and eosin (H&E)-stained sections (26). For active caspase-3, slides were treated as above but placed in 3% hydrogen peroxide in methanol before antigen retrieval and blocked with 20% normal goat serum (Vector Laboratories, Burlingame, CA, USA). Incubation with rabbit polyclonal anti-active caspase-3 (1:100; Cell Signaling Technology, Beverly, MA, USA) was performed overnight at 4°C. Slides were then probed for 1 h at room temperature with goat anti-rabbit antibody (1:500; Vector Laboratories) followed by streptavidin horseradish peroxidase (Dako). Slides were developed in a manner similar to slides stained for proliferation. A separate set of slides for each group was analyzed for the presence of apoptotic cells on H&E-stained sections, where they were identified via characteristic morphologic changes. Apoptosis was quantified in 100 contiguous crypts or 20 contiguous villi by a single investigator blinded to sample identification.
Intestinal immunofluorescence
Jejunal tissue was formalin fixed and then paraffin embedded. Sections were deparaffinized in xylene, rehydrated in graded series of ethanol, and rinsed with distilled water. Heat-induced epitope retrieval was performed with Decloaker and then blocked with the SNIPER reagent (Biocare Medical, Walnut Creek, CA, USA) for 15 min at room temperature. Sections were then incubated with rabbit Anti-Bcl-2 (Sigma-Aldrich) and/or rabbit anti-T Ag (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 1 h at room temperature. Sections were subsequently incubated with secondary antibodies (1:500, Alexa Fluor 488/Alexa Fluor 546 conjugated donkey anti-rabbit; Abcam, Cambridge, United Kingdom) for 30 min at room temperature. Positive and negative controls were run in parallel. All images were acquired and analyzed with an Axio Imager Z1 microscope (Zeiss, Gottingen, Germany).
Intestinal epithelial permeability
Mice were orally gavaged 5 h prior to being killed with 0.5 ml of fluorescently labeled dextran (22 mg/ml) (FD4; Sigma-Aldrich). At time of death, blood was collected, and serum was diluted with an equivalent volume of PBS. Serum was assayed for FD4 by fluorospectrometry using an excitation/emission wavelength of 485/520 nm, with standard curve serial dilutions (BioTex Synergy HT, Winooski, VT, USA).
RNA analysis
RNA was extracted from 1-cm jejunal segments using RNeasy Mini-Kits according to the manufacturer’s instructions (Qiagen, Valencia, CA, USA). A total of 0.5 μg of RNA was used to generate cDNA with a Qiagen RT2 First Strand Kit, and products were analyzed using a Qiagen RT2 Profiler MAPk PCR array run on a real-time PCR system (Applied Biosystems, Carlsbad, CA, USA). Cycling times for genes of interest were compared with average cycling times for the housekeeping gene β-glucouronidase, and the ddCT method was used to determine fold-change in gene expression. Data were analyzed with web-based manufacturer software (http://www.qiagen.com/us/shop/genes-and-pathways/data-analysis-center-overview-page/#).
Western blots
Protein lysates from 1-cm jejunal segments were harvested and flash frozen in liquid nitrogen. Samples were subsequently thawed and immersed in lysis buffer [50 mM Tris-HCl (pH 7.4); 150 mM Triton X-100; 50 mM DTT 50 μg/ml leupeptin; 5 mM PMSF; 1 mM NaF; 1 mM Na3VO4] in an ice bath and homogenized using a sonicator. Samples were centrifuged at 4°C for 5 min, and the supernatant was analyzed for protein concentration using fluorometry. A total of 40 μg of protein was diluted with lysis buffer to a volume of 5 μl and combined with 5 μl Laemmli buffer, heated at 95°C for 5 min, loaded onto 4–20% gradient Stain-Free gels (Bio-Rad, Hercules, CA, USA), and run at 120 V for 60–90 min. After 5 min of UV activation, the proteins were transferred to polyvinyledene difluoride membranes (Bio-Rad) using Transblot Turbo equipment (Bio-Rad) and a semiwet method at 25 V for 10 min. Membranes were then blocked using 5% nonfat milk at room temperature for 1 h and probed with primary anti-rabbit antibodies at 4°C overnight. The following antibodies were used: cleaved caspase-3, Bcl-2, Bax, cytochrome C, AIF (Cell Signaling Technology); cyclin D1, cyclin D2, Cdk6 (Boster Biologic Technology, Pleasonton, CA, USA); Jun (Proteintech, Chicago, IL, USA); and HSP90ab1 (ProSci Incorporated, Poway, CA, USA). Membranes were then washed with Tris-buffered saline with 0.2% Tween 20 (Sigma-Aldrich) and incubated with horseradish peroxidase–conjugated goat anti-rabbit Ig for 1 h. Blots were developed with chemiluminescent detection reagent (GE Healthcare, Pittsburgh, PA, USA). Visualization was performed on a charged coupled device (ChemiDoc Touch; Bio-Rad). Protein bands and protein expression were analyzed and normalized to total lane protein using Image Lab v5.2 software (Bio-Rad) (27).
Statistics
All statistical analyses were performed with Prism 6.0 software (GraphPad, La Jolla, CA, USA). Data were tested for gaussian distribution using the D’Agostino-Pearson omnibus normality test. Comparison between two groups with a gaussian distribution was performed using the Student’s t test. Groups that did not have a gaussian distribution were compared using the Mann-Whitney test. For PCR arrays, P values for fold-change expression were based on a Student’s t test of the replicate 2^ΔCT for each gene in the unmanipulated and septic groups. A value of P < 0.05 was used to determine significance throughout.
RESULTS
Unmanipulated fabpi-TAg mice have ectopic villus proliferation and increased villus apoptosis
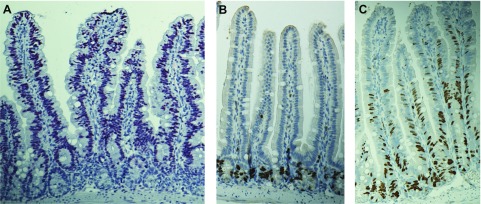
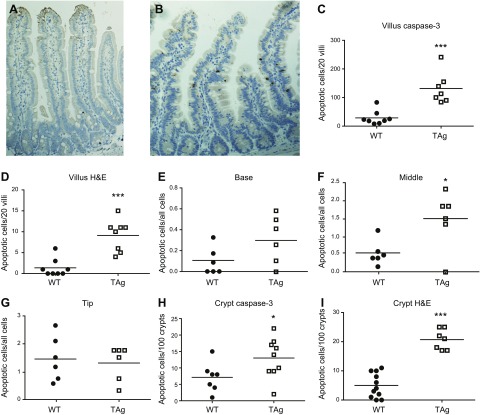
Immunostaining demonstrated that expression of the TAg protein was limited to villus enterocytes (Fig. 1A). Whereas intestinal proliferation was restricted to the crypts in WT mice (Fig. 1B), proliferation in transgenic mice was identified in both the crypts (where TAg is not expressed but proliferation normally occurs) and along the entire villus, with the majority of BRDU-positive cells identified in the lower third of the villus (Fig. 1C). Ectopic villus proliferation induced increased villus apoptosis in fabpi-TAg mice compared with WT mice (Fig. 2A–D). The increased apoptosis seen in fabpi-TAg villi primarily occurred in the mid villus, although a trend toward increased apoptosis was also seen at the villus base (Fig. 2E–G). Crypt apoptosis was also higher in fabpi-TAg than WT mice, despite the fact that the transgene is not expressed in the crypt (Fig. 2H, I).
Figure 1.
TAg induces ectopic villus proliferation. A) TAg is expressed solely in villus enterocytes without crypt expression in fabpi-TAg transgenic mice (TAg stains red). B) In WT mice, proliferation as measured by BRDU staining is confined to the crypt epithelium (BRDU stains brown). C) Transgenic mice that overexpress TAg in their villus have proliferation in both the crypt and the villus.
Figure 2.
TAg induces crypt and villus apoptosis in unmanipulated transgenic mice. A, B) Representative histomicrographs demonstrate increased apoptosis in unmanipulated fabpi-TAg mice stained for active caspase 3 (B) (apoptotic cells stain brown) compared with WT mice (A). C, D) Quantification of cell death verifies that apoptosis is barely detectable in WT mice in the villi [(C) n = 7–8/group, P = 0.0003; (D) n = 8/group, P = 0.0008] whether assayed by active caspase 3 or H&E staining. In contrast, apoptosis is increased in unmanipulated fabpi-TAg transgenic mice. E–G) Quantification demonstrated a nonsignificant trend toward increased villus apoptosis in the proximal third of the villus (E) (n = 6/group, P = 0.11), increased villus apoptosis in the middle third of the villus (F) (n = 6/group, P = 0.049), and no apparent change in the distal third of the villus (G) (n = 6/group, P = 0.90). H, I) Similarly, crypt apoptosis is barely detectable in WT mice but is increased in unmanipulated fabpi-TAg transgenic mice [(H) n = 7–9/group, P = 0.03; (I) n = 7–11/group, P < 0.0001] whether assayed by active caspase 3 or H&E staining.
Sepsis reduces crypt proliferation but increases villus proliferation in fabpi-TAg mice
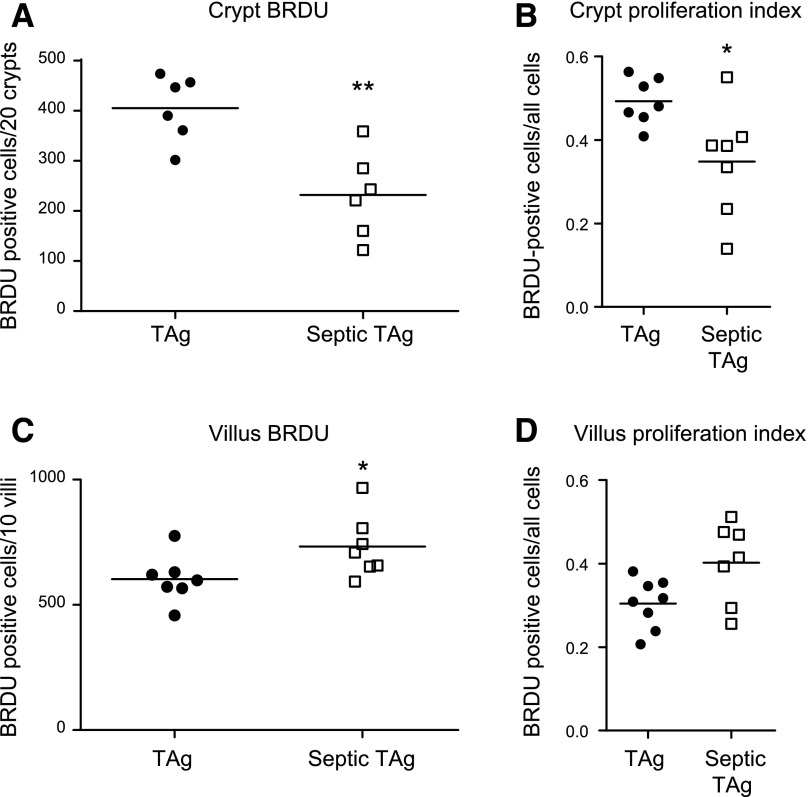
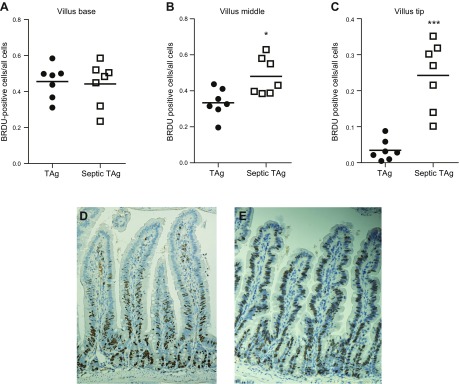
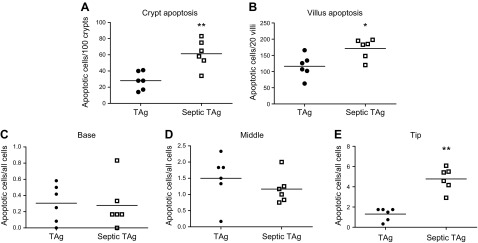
In WT mice, sepsis decreases crypt proliferation and induces both crypt and villus apoptosis (data not shown) (11–13). To determine whether ectopic villus proliferation alters this stress-induced compartmentalized crypt/villus response, transgenic mice were made septic via CLP. In the crypts (where TAg is not expressed), proliferation was decreased in sepsis by both absolute number and percentage of BRDU-positive cells, consistent with findings in WT mice (Fig. 3A, B). In contrast, villus proliferation unexpectedly increased after sepsis with higher absolute numbers of S-phase cells (Fig. 3C), associated with a trend (P = 0.05) toward increased percentage of BRDU-positive villus cells (Fig. 3D). The hyperproliferation observed in septic fabpi-TAg villi did not occur uniformly but rather resulted from increased BRDU labeling in the middle and distal thirds of the villus. In unmanipulated transgenic animals, BRDU was detected in 46% of cells in the proximal third of the villus, 33% of cells in the middle third of the villus, and only 3% of cells in the distal third of the villus. After CLP, however, the BRDU index increased 1.5-fold in the middle third of the villus and 8-fold at the villus tip (Fig. 4). No comparison can be made to WT animals because proliferation does not occur in villi of WT mice. Of note, unlike the compartment-specific differential proliferative response, sepsis induced an increase in apoptosis in both the crypt and villus of fabpi-TAg mice compared with unmanipulated animals of the same genotype (Fig. 5A, B). The increased villus apoptosis is not uniformly distributed but is exclusively seen in the distal third of the villus (Fig. 5C–E).
Figure 3.
Crypt proliferation is decreased, whereas villus proliferation is increased in septic fabpi-TAg mice. A) The number of BRDU-positive cells in fabpi-TAg mice decreased in the crypt epithelium (where TAg is not expressed) after CLP compared with unmanipulated genetically identical mice (n = 6/group, P = 0.004). B) To verify this was not a result of the number of cells in the gut epithelium (because sepsis alters proliferation, migration, and apoptosis), the proliferative index was calculated, and the number of BRDU-positive cells/all cells was also decreased in septic fabpi-TAg mice (n = 7/group, P = 0.02). C, D) In contrast, the number of BRDU-positive cells increased in the villus epithelium after CLP in septic fabpi-TAg mice (C) (n = 7/group, P = 0.04), with a similar trend in the villus proliferative index (D) (n = 7–8/group, P = 0.05).
Figure 4.
Increased villus proliferation in septic fabpi-TAg mice disproportionately occurs in the upper half of the villus. A) Sepsis has no impact on the number of BRDU-positive cells in the lower third of the villus in transgenic mice (n = 7/group, P = 0.87). B, C) In contrast, there was a significant increase in BRDU-positive cells in transgenic mice in the middle third and distal third of the villus after CLP [(B) n = 7/group, P = 0.03; (C) n = 7/group, P = 0.0006]. D, E) Representative micrographs (BRDU stains brown) demonstrate almost all BRDU staining is present in the bottom half of the villus in unmanipulated fabpi-TAg mice (D), but the zone of proliferation is greatly expanded after the onset of sepsis (E).
Figure 5.
Sepsis induces apoptosis in both the crypt and villus epithelium in transgenic mice. A, B) Whereas the proliferative response is compartment specific after CLP in fabpi-TAg mice, apoptosis (as assayed by active caspase 3) is increased in both crypts and villi [(A) n = 6/group, P = 0.009; (B) n = 6/group, P = 0.03]. C–E) Quantification showed no difference in apoptosis in the proximal (C) (n = 6/group, P = 0.74) or middle third of the villus (D) (n = 6/group, P = 0.22) but showed a significant increase only in the distal third of the villus (E) (n = 6/group, P = 0.002).
Sepsis decreases cyclin D1 and D2 in intestinal cells
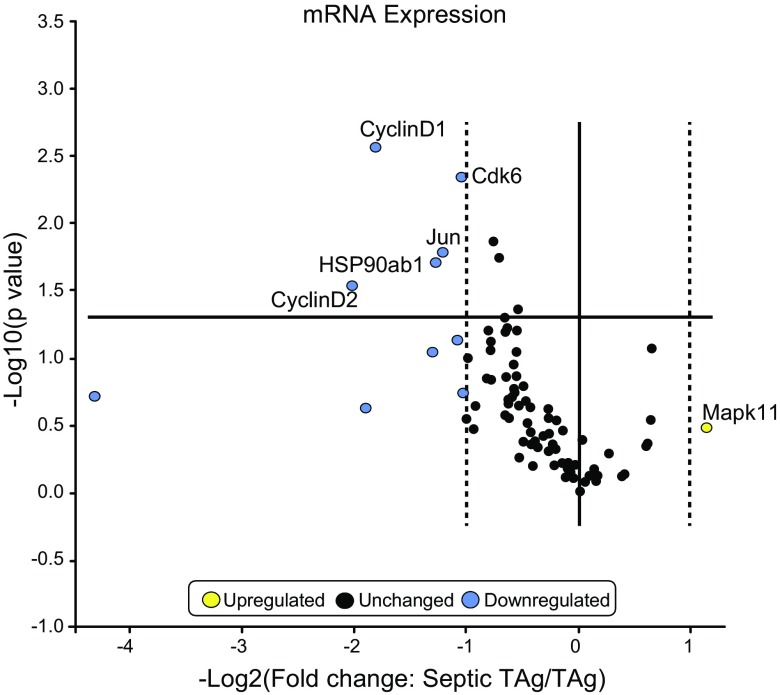
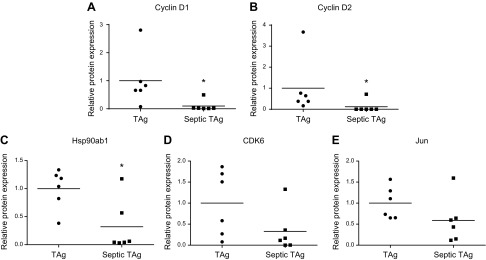
To determine potential mechanisms through which villus proliferation increased in fabpi-TAg animals, expression of 96 genes in the MAPk pathway was compared between unmanipulated and septic fabpi-TAg animals. Significant decreases were detected in cyclin D1, cyclin D2, CDK6, Jun, and HSP90ab1. In contrast, no genes were identified as being statistically significantly up-regulated (Fig. 6). To validate these changes at the protein level, Western blots were performed and demonstrated statistically significant differences in cyclin D1, cyclin D2, and HSP90ab1 as well as nonsignificant trend toward decreases in CDK6 and Jun (Fig. 7).
Figure 6.
mRNA expression in the gut epithelium of fabpi-TAg mice after sepsis (n = 3/group). CLP induces a statistically significant decrease in intestinal cyclin D1 (−3.5 fold), cyclin D2 (−4.1 fold), CDK6 (−2.1 fold), Jun (−2.3 fold), and HSP90ab1 (−2.4 fold). Although other genes had a >2-fold decrease (shown in blue), these were not statistically significant. There were no statistically significant increases in any of the 96 genes assayed after sepsis (the closest was a 2.2-fold increase in Mapk11).
Figure 7.
Protein expression in jejunal tissue of fabpi-TAg mice after sepsis. To validate the mRNA levels in Fig. 6, Western blots were performed on intestinal homogenate from a different group of mice 24 h after CLP. A–C) A statistically significant decrease was seen in cyclin D1 (A) (n = 6/group, P = 0.04), cyclin D2 (B) (n = 6/group, P = 0.04), and HSP90ab1 (C) (n = 6/group, P = 0.02). D, E) A trend toward decreases that was not statistically significant was seen in CDK6 (D) (n = 6/group, P = 0.10) and Jun (E) (n = 6/group, P = 0.15).
Bcl-2 overexpression paradoxically increases enterocyte apoptosis in fabpi-TAg mice
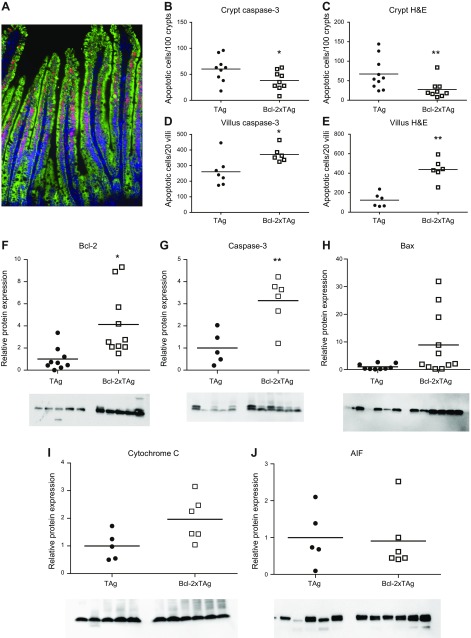
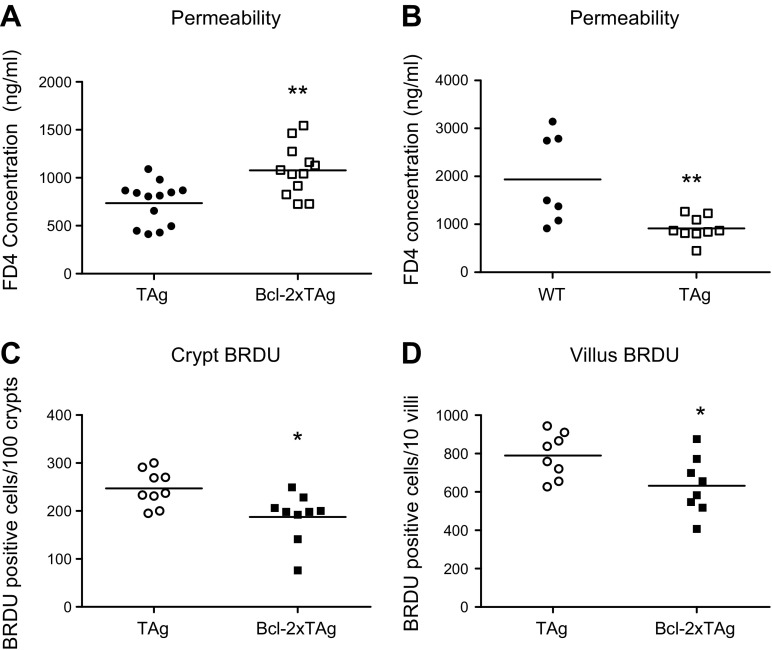
To determine whether the augmented villus proliferation observed in septic fabpi-TAg animals was dependent on apoptosis, bi-transgenic animals were created by crossing fabpi-TAg to fabpl-Bcl-2 mice. Overexpression of the antiapoptotic protein Bcl-2 in the crypts and villi of these transgenic mice has previously been shown to prevent sepsis-induced apoptosis in both compartments and partially restore crypt proliferative capacity (11–13). Based on the cellular expression induced by each promoter, the resultant bi-transgenic animals overexpressed both TAg and Bcl-2 in their villus enterocytes but overexpressed Bcl-2 alone in the crypts (Fig. 8A). As expected, Bcl-2 overexpression decreased sepsis-induced apoptosis in crypts (where TAg is not expressed) (Fig. 8B, C). Paradoxically, coexpression of Bcl-2 and TAg increased villus apoptosis after CLP compared with septic mice solely expressing TAg (Fig. 8D, E). In light of the unexpected histologic findings in Bcl-2xTAg mice, protein immunoblots were performed on jejunal isolates and revealed increased concentrations of Bcl-2 (verifying protein expression) and the proapoptotic active caspase-3 as well as a trend toward increases in Bax and cytochrome C (P = 0.05 for both) (Fig. 8F–I). No differences were detected in AIF (Fig. 8J). Notably, intestinal permeability was also increased in septic Bcl-2xTAg mice compared with septic fabpi-TAg mice (Fig. 9A). This is due to the combination of both transgenes because unmanipulated fabpi-TAg mice have lower intestinal permeability than unmanipulated WT mice (data not shown) and because septic fabpi-TAg mice have lower intestinal permeability than septic WT mice (Fig. 9B).
Figure 8.
Bcl-2xTAg mice have a paradoxical increase in villus apoptosis after CLP. A) Bi-transgenic mice overexpress TAg (red) in the nucleus of villus enterocytes and Bcl-2 (green) in the cytoplasm along the entire crypt–villus axis. Crypt and villus cells are also stained blue with DAPI. B, C) Crypt apoptosis was decreased in bi-transgenic mice (which overexpress Bcl-2 in their crypts) after CLP compared with fabpi-TAg mice (which do not have transgene expression in their crypts) whether assayed by active caspase 3 (B) (n = 8–9/group, P = 0.046) or H&E (C) (n = 9–10/group, P = 0.02). D, E) In contrast, bi-transgenic Bcl-2xTAg mice (which express both transgenes in their villi) have a paradoxical increase in villus apoptosis compared with fabpi-TAg mice (which express TAg in their villi) after CLP by both active caspase 3 (D) (n = 6–7/group, P = 0.02) and H&E (E) (n = 6/group, P = 0.002). F–I) This was associated with an increase in Bcl-2, consistent with its overexpression in bi-transgenic mice (F) (n = 9–10/group, P = 0.001) and the final common executioner of apoptosis active caspase 3 (G) (n = 5–6/group, P = 0.02), with trends toward increases in Bax (H) (n = 9–11/group, P = 0.05) and cytochrome C (I) (n = 5–6/group, P = 0.05). J) No difference was noted in AIF (n = 5–6/group, P = 0.65).
Figure 9.
Bi-transgenic mice have increased permeability and decreased crypt proliferation after CLP. A) The increased apoptosis in septic Bcl-2xTAg mice was associated with increased intestinal permeability to FD4 (n = 12–13/group, P = 0.002). B) In contrast, permeability was lower in septic fabpi-TAg mice than septic WT mice (n = 7–9, P = 0.005). C, D) Whereas apoptosis was compartment-specific in bi-transgenic mice, proliferation was decreased in septic Bcl-2xTAg mice compared with fabpi-TAg mice in both crypts (C) (n = 9/group, P = 0.01) and villi (D) (n = 7–8/group, P = 0.03).
Sepsis-induced changes in proliferation are not due to alterations in apoptosis
Proliferation was lower in both the crypt and villi of septic Bcl-2xTAg mice compared with septic fabpi-TAg mice (Fig. 9C, D). Thus, proliferation was decreased regardless of whether apoptosis was increased (villus) or decreased (crypt), arguing against a causal relationship.
DISCUSSION
Sepsis decreases crypt proliferation but unexpectedly increases villus proliferation in fabpi-TAg mice compared with unmanipulated genetically identical animals. This contrasts with apoptosis, which increases in both the crypt and villus compartments after CLP. Bi-transgenic mice that overexpress TAg in the villus and Bcl-2 in both the crypt and the villus have the expected decrease in crypt apoptosis but a paradoxical increase in villus apoptosis. Proliferation is decreased in both the crypt and villus environments in bi-transgenic animals, suggesting that apoptotic and proliferative changes are not causally related.
The results presented herein yield new insights into the importance of microenvironment in mediating the proliferative and apoptotic response in the intestine. The compartments of the intestine responded very differently to sepsis-induced stress: crypt proliferation decreased after CLP, whereas villus proliferation increased. This suggests that it is not merely a state of cell cycling that determines how a cell responds to an inflammatory insult. Rather, the proliferative response seems to depend on cell intrinsic or local environment factors. It is notable that the increase in villus proliferation seen in septic transgenic mice disproportionately affects the upper villus, as opposed to proliferation in unmanipulated transgenic mice, which occurs predominantly in the lower to mid third of the villus. Given that critical illness markedly slows enterocyte migration in young mice (28), it seems unlikely that BRDU-positive cells at the distal aspect of the intestinal villus migrated there from the proliferation-rich proximal villus third in just 24 h. Further, the presence of interspersed BRDU-negative cells argues that positive cells at the tip were not “pushed” by continuously dividing cells lower on the villus but rather actively began replicating DNA only after the septic insult.
These results contrast with findings in fabpi-TAg mice subjected to ionizing radiation, which have decreases in both villus and crypt proliferation (29). This suggests that the molecular mechanisms responsible for villus proliferation are differentially altered by the type of stress an animal is subjected to. It has previously been demonstrated that, under basal conditions, cell cycle kinetics are different in villus enterocytes of fabpi-TAg mice compared with crypt cells, with an S-phase to M-phase ratio 3-fold higher in the former (21). This is associated with an induction of cyclin D1 and cdk2. To determine how sepsis induced villus hyperproliferation in fabpi-TAg mice, we examined 96 genes related to the MAP-kinase signaling families because there is evidence that progrowth signaling cascades mitigate the damage induced by epithelial ischemia/reperfusion (30). Interestingly, there was no statistically significant up-regulation of any gene (the closest was a 2.2-fold increase in MAPK11). However, there were significant decreases detected in jejunal cyclin D1 and cyclin D2, as well as in CDK6, Jun, and HSP90ab1, when comparing mRNA levels of septic transgenic mice with unmanipulated transgenic mice.
Western blot validated a statistically significant decrease in protein levels of cyclin D1, cyclin D2, and HSP90ab1, as well as a nonsignificant trend toward decreases in CDK6 and Jun. The majority of these genes are positive regulators of the cell cycle. In cooperation with cyclin-dependent kinases, cyclins phosphorylate target proteins and are required for orderly progression through the G1/S transition (31, 32). Further, Jun binds directly to an AP1 site in the cyclin D1 promotor and plays a critical role in cell cycle progression (33). Because these genes were measured on whole intestinal tissue, it is possible that the decreases detected underlie the decrease in crypt proliferation rather than the increase in villus proliferation in sepsis in transgenic animals; however, the mechanisms through which proliferation is regulated in a compartment-specific manner after sepsis remain to be fully determined.
Under basal conditions, fabpi-TAg mice also have an increase in villus apoptosis. It is not clear whether this cell death increase reflects a compensatory reaction to maintain villus homeostasis or whether villus enterocytes expressing the TAg protein are simply more prone to apoptosis. However, it is notable that WT mice have increased villus apoptosis in multiple models of sepsis, even though there is cell cycle arrest in the villus, without any proliferating cells (34). We found here that fabpi-TAg mice also had an increase in sepsis-induced villus apoptosis after CLP. In contrast, the villi in fabpi-TAg mice subjected to ionizing radiation are entirely radio resistant, with no alterations in villus apoptosis despite their enterocytes re-entering the cell cycle (29). This suggests that the augmented villus apoptosis seen in septic fabpi-TAg mice is at least partially independent of villus cycling and may be related to the same mechanisms in both the mitochondrial and receptor-mediated pathways of apoptosis that drive gut epithelial apoptosis in sepsis (34).
There is significant evidence that changes in proliferation or apoptosis can produce a compensatory change in the reciprocal process under select circumstances (35–37). To determine whether increased sepsis-induced villus apoptosis played a role in increasing villus proliferation in transgenic mice, we created bi-transgenic Bcl-2xTAg mice. These animals express TAg solely in villus enterocytes and the antiapoptotic protein Bcl-2 in both crypt and villus epithelial cells. Fabpl-Bcl-2 mice have no detectable phenotype under basal conditions but prevent stress-induced crypt and villus apoptosis in multiple animal models of sepsis and ischemia/reperfusion (11, 12, 23). In theory, the bi-transgenic mice should have allowed us to decrease apoptosis in villus cells with ectopic increased proliferation while examining the crypt as a control where Bcl-2 should decrease apoptosis without TAg being expressed. However, although we observed the expected decrease in crypt apoptosis after CLP, we unexpectedly found that villus apoptosis was increased in bi-transgenic mice by multiple different techniques. This was associated with increases in multiple apoptotic mediators in the mitochondrial pathways of cell death in septic bi-transgenic mice. There is no clear explanation for these results. TAg is known to be capable of binding a Bcl-2 homology domain 3–containing proapoptosis protein (38). Further, TAg induces poly(ADP-ribose) polymerase 1–mediated apoptotic signaling, and activation of this molecule and caspases is required for TAg infection in nondividing cells (39). It is theoretically possible that Bcl-2 overexpression leads to a paradoxical displacement of proapoptotic factors in the setting of the complex interactions between TAg and the host cell, which not only directly binds multiple molecules but also alters apoptosis, survival, and stress pathways (39–41). Although studying bi-transgenic mice did not answer the initial question of whether decreasing villus apoptosis would alter proliferation, it did allow us to examine the relationship between proliferation and apoptosis on the villus. Here we found that, despite the increased villus apoptosis in bi-transgenic mice, villus proliferation decreased in septic bi-transgenic animals compared with septic fabpi-TAg mice. The decreased villus proliferation in the setting of increased villus apoptosis suggests that the regulators of intestinal apoptosis and proliferation are mostly or entirely independent after a septic insult.
Although the crypt and villus exist on a geographic continuum, we did not find evidence that alterations in the villus affect the crypt in sepsis because changes in villus proliferation and apoptosis after CLP were not accompanied by changes in the crypt in the experiments described herein. However, ectopic villus proliferation increases crypt (as well as villus) apoptosis in unmanipulated fabpi-TAg mice. Further, the presence of proliferating enterocytes in fabpi-TAg animals rendered crypts more susceptible to ionizing radiation–induced apoptosis, despite the fact that the transgene is not expressed in the crypts (29). This suggests that control of the well-described crosstalk between the components of the intestine (42) is complex and involves factors independent of an animal’s genetics and proliferative/apoptotic capacity.
This study has a number of limitations. The first relates to the initial conception of the experimental design because villus cells do not proliferate, either under basal conditions or in most disease states. As such, there is no direct linkage of our findings to health or a disease state (the closest would likely be cancer where transformed cells in the intestine can proliferate without checks on the cell cycle). Despite this, we believe there is significant merit to our experiments. This is due to our belief that an enhanced understanding of the complex inner working of the intestine has intrinsic interest in understanding host biology. The intestinal environment is extremely complex with considerable variability depending upon: 1) proximal to distal location (esophagus to rectum), 2) base to tip location (crypt to villus), 3) cell types within the epithelium (4 main cell types within the small intestine that come from a single progenitor stem cell), and 4) different types of cells (epithelial, immune, microbial). Further, the intestinal epithelium is constantly regenerating, with the host growing a new intestine every week or sooner. Understanding the interplay between the components of the intestine and elements of how a host can continuously renew its intestine has inherent interest from a discovery point of view. In addition, there is currently no treatment for sepsis beyond fluid resuscitation and antibiotics (25). Manipulating the gut for therapeutic gain has been proposed as a treatment for sepsis and a variety of other diseases (9, 10, 43–45). Understanding the interplay between the crypt (where proliferation occurs) and the villus (where the differentiated cells perform the majority of the function of the intestine, including host defense, food absorption, and hormone production) would be critical in optimizing the beneficial effects on gut integrity without inducing unwanted and unexpected side effects.
All experiments on septic animals were performed at a single timepoint. Although the rationale for looking at 24 h is based upon prior experience that this is the timepoint that both apoptosis and proliferation are most affected by sepsis, there is no a priori way to know how sepsis would affect cells with ectopic villus proliferation, and examining mice at additional times could have led to a more comprehensive understanding. The precise mechanism underlying the unexpected increase in villus proliferation in septic fabpi-TAg mice is not clear (nor is the mechanism for the paradoxical increase in villus apoptosis in bi-transgenic mice), and, although our data strongly suggest that apoptotic and proliferative changes in the intestinal epithelium of septic mice are at least partially dependent on cell-intrinsic or local environmental effects, the array experiments examining intestinal tissue were done on whole jejunal tissue as opposed to laser capture microdissection to separate villus from crypt cells. Further, although intestinal permeability was increased in bi-transgenic mice, the tight junctions responsible for altered permeability in sepsis are complex, and how this is altered in the setting of TAg and Bcl-2 was not examined in this study.
Additionally, there is a small variance in the number of animals examined in different experiments between groups due to technical limitations or premature mortality in animals that died prior to 24 h, and this could have affected the quantification of proliferation and apoptosis. Finally, we did not assay the microbiome of any of the animals used for experiments. Although it is possible that there were similarities between the microbiome because all animals were bred, born, and lived their entire life in the same barrier facility, the impact of the microbiome on all of our results (and, in turn, the impact of changes in the epithelium on the microbiome) was not examined. The microbiome has been shown to be of tremendous importance toward host health, and there are significant data to suggest that the microbiome is altered in critical illness in both patients and preclinical studies as early as 6 h after the onset of sepsis (46–48). It is likely that luminal environment parameters (including, but not limited, to bacterial metabolite composition, virulence, pH, etc.) are likely to play an important role in the process of intestinal epithelium renewal, and this should be a focus of future experiments (49, 50).
Despite these limitations, the results of this study illustrate the complexity of cell regulation in the intestine. Proliferation and apoptosis are regulated in a manner that is partially dependent on microenvironment (crypt vs. villus), the state of the host (homeostatic vs. stress), and the type of stress (sepsis vs. previous publications from us on ionizing radiation). Although alterations in proliferation or apoptosis can lead to compensatory changes in the other process, this relationship is not uniform, and these basic functions appear to be distinct when villus proliferation is ectopically induced. Given the intestine’s role as the “motor” of critical illness, these insights should spark further investigation into the fundamental mechanisms controlling intestinal regeneration under conditions of acute stress.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health, National Institute of General Medical Sciences Grants GM072808, GM095442, GM104323, GM109779, GM113228, and GM117895. The authors declare no conflicts of interest.
Glossary
- BRDU
5-bromo-2′-deoxyuridine
- CLP
cecal ligation and puncture
- Fabpi
intestinal fatty acid binding protein
- Fabpl
liver fatty acid binding protein
- H&E
hematoxylin and eosin
- TAg
T antigen
- WT
wild type
AUTHOR CONTRIBUTIONS
J. D. Lyons and C. M. Coopersmith designed the research; J. D. Lyons, N. J. Klingensmith, S. Otani, R. Mittal, and Z. Liang performed the research; M. L. Ford and C. M. Coopersmith contributed new reagents or analytic tools; J. D. Lyons, N. J. Klingensmith, S. Otani, R. Mittal, Z. Liang, M. L. Ford, and C. M. Coopersmith analyzed the data; J. D. Lyons and C. M. Coopersmith wrote the paper.
REFERENCES
- 1.Peterson L. W., Artis D. (2014) Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 14, 141–153 [DOI] [PubMed] [Google Scholar]
- 2.Sender R., Fuchs S., Milo R. (2016) Are we really vastly outnumbered? revisiting the ratio of bacterial to host cells in humans. Cell 164, 337–340 [DOI] [PubMed] [Google Scholar]
- 3.Odenwald M. A., Turner J. R. (2017) The intestinal epithelial barrier: a therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 14, 9–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerbe F., Legraverend C., Jay P. (2012) The intestinal epithelium tuft cells: specification and function. Cell. Mol. Life Sci. 69, 2907–2917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall P. A., Coates P. J., Ansari B., Hopwood D. (1994) Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J. Cell Sci. 107, 3569–3577 [DOI] [PubMed] [Google Scholar]
- 6.Helander H. F., Fändriks L. (2014) Surface area of the digestive tract: revisited. Scand. J. Gastroenterol. 49, 681–689 [DOI] [PubMed] [Google Scholar]
- 7.Singer M., Deutschman C. S., Seymour C. W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G. R., Chiche J. D., Coopersmith C. M., Hotchkiss R. S., Levy M. M., Marshall J. C., Martin G. S., Opal S. M., Rubenfeld G. D., van der Poll T., Vincent J. L., Angus D. C. (2016) The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315, 801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaieski D. F., Edwards J. M., Kallan M. J., Carr B. G. (2013) Benchmarking the incidence and mortality of severe sepsis in the United States. Crit. Care Med. 41, 1167–1174 [DOI] [PubMed] [Google Scholar]
- 9.Klingensmith N. J., Coopersmith C. M. (2016) The gut as the motor of multiple organ dysfunction in critical illness. Crit. Care Clin. 32, 203–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mittal R., Coopersmith C. M. (2014) Redefining the gut as the motor of critical illness. Trends Mol. Med. 20, 214–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coopersmith C. M., Stromberg P. E., Dunne W. M., Davis C. G., Amiot D. M. II, Buchman T. G., Karl I. E., Hotchkiss R. S. (2002) Inhibition of intestinal epithelial apoptosis and survival in a murine model of pneumonia-induced sepsis. JAMA 287, 1716–1721 [DOI] [PubMed] [Google Scholar]
- 12.Coopersmith C. M., Chang K. C., Swanson P. E., Tinsley K. W., Stromberg P. E., Buchman T. G., Karl I. E., Hotchkiss R. S. (2002) Overexpression of Bcl-2 in the intestinal epithelium improves survival in septic mice. Crit. Care Med. 30, 195–201 [DOI] [PubMed] [Google Scholar]
- 13.Coopersmith C. M., Stromberg P. E., Davis C. G., Dunne W. M., Amiot D. M. II, Karl I. E., Hotchkiss R. S., Buchman T. G. (2003) Sepsis from Pseudomonas aeruginosa pneumonia decreases intestinal proliferation and induces gut epithelial cell cycle arrest. Crit. Care Med. 31, 1630–1637 [DOI] [PubMed] [Google Scholar]
- 14.Yoseph B. P., Klingensmith N. J., Liang Z., Breed E. R., Burd E. M., Mittal R., Dominguez J. A., Petrie B., Ford M. L., Coopersmith C. M. (2016) Mechanisms of intestinal barrier dysfunction in sepsis. Shock 46, 52–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Q., Zhang Q., Wang C., Liu X., Li N., Li J. (2009) Disruption of tight junctions during polymicrobial sepsis in vivo. J. Pathol. 218, 210–221 [DOI] [PubMed] [Google Scholar]
- 16.Clark J. A., Clark A. T., Hotchkiss R. S., Buchman T. G., Coopersmith C. M. (2008) Epidermal growth factor treatment decreases mortality and is associated with improved gut integrity in sepsis. Shock 30, 36–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark J. A., Gan H., Samocha A. J., Fox A. C., Buchman T. G., Coopersmith C. M. (2009) Enterocyte-specific epidermal growth factor prevents barrier dysfunction and improves mortality in murine peritonitis. Am. J. Physiol. Gastrointest. Liver Physiol. 297, G471–G479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dominguez J. A., Vithayathil P. J., Khailova L., Lawrance C. P., Samocha A. J., Jung E., Leathersich A. M., Dunne W. M., Coopersmith C. M. (2011) Epidermal growth factor improves survival and prevents intestinal injury in a murine model of Pseudomonas aeruginosa pneumonia. Shock 36, 381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klingensmith N. J., Yoseph B. P., Liang Z., Lyons J. D., Burd E. M., Margoles L. M., Koval M., Ford M. L., Coopersmith C. M. (2017) Epidermal growth factor improves intestinal integrity and survival in murine sepsis following chronic alcohol ingestion. Shock 47, 184–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hauft S. M., Kim S. H., Schmidt G. H., Pease S., Rees S., Harris S., Roth K. A., Hansbrough J. R., Cohn S. M., Ahnen D. J., et al. (1992) Expression of SV-40 T antigen in the small intestinal epithelium of transgenic mice results in proliferative changes in the crypt and reentry of villus-associated enterocytes into the cell cycle but has no apparent effect on cellular differentiation programs and does not cause neoplastic transformation. J. Cell Biol. 117, 825–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandrasekaran C., Coopersmith C. M., Gordon J. I. (1996) Use of normal and transgenic mice to examine the relationship between terminal differentiation of intestinal epithelial cells and accumulation of their cell cycle regulators. J. Biol. Chem. 271, 28414–28421 [DOI] [PubMed] [Google Scholar]
- 22.Ahuja D., Sáenz-Robles M. T., Pipas J. M. (2005) SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene 24, 7729–7745 [DOI] [PubMed] [Google Scholar]
- 23.Coopersmith C. M., O’Donnell D., Gordon J. I. (1999) Bcl-2 inhibits ischemia-reperfusion-induced apoptosis in the intestinal epithelium of transgenic mice. Am. J. Physiol. 276, G677–G686 [DOI] [PubMed] [Google Scholar]
- 24.Rittirsch D., Huber-Lang M. S., Flierl M. A., Ward P. A. (2009) Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 4, 31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhodes A., Evans L. E., Alhazzani W., Levy M. M., Antonelli M., Ferrer R., Kumar A., Sevransky J. E., Sprung C. L., Nunnally M. E., Rochwerg B., Rubenfeld G. D., Angus D. C., Annane D., Beale R. J., Bellinghan G. J., Bernard G. R., Chiche J. D., Coopersmith C., De Backer D. P., French C. J., Fujishima S., Gerlach H., Hidalgo J. L., Hollenberg S. M., Jones A. E., Karnad D. R., Kleinpell R. M., Koh Y., Lisboa T. C., Machado F. R., Marini J. J., Marshall J. C., Mazuski J. E., McIntyre L. A., McLean A. S., Mehta S., Moreno R. P., Myburgh J., Navalesi P., Nishida O., Osborn T. M., Perner A., Plunkett C. M., Ranieri M., Schorr C. A., Seckel M. A., Seymour C. W., Shieh L., Shukri K. A., Simpson S. Q., Singer M., Thompson B. T., Townsend S. R., Van der Poll T., Vincent J. L., Wiersinga W. J., Zimmerman J. L., Dellinger R. P. (2017) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit. Care Med. 45, 486–552 [DOI] [PubMed] [Google Scholar]
- 26.Vyas D., Robertson C. M., Stromberg P. E., Martin J. R., Dunne W. M., Houchen C. W., Barrett T. A., Ayala A., Perl M., Buchman T. G., Coopersmith C. M. (2007) Epithelial apoptosis in mechanistically distinct methods of injury in the murine small intestine. Histol. Histopathol. 22, 623–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor S. C., Berkelman T., Yadav G., Hammond M. (2013) A defined methodology for reliable quantification of Western blot data. Mol. Biotechnol. 55, 217–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neal M. D., Sodhi C. P., Dyer M., Craig B. T., Good M., Jia H., Yazji I., Afrazi A., Richardson W. M., Beer-Stolz D., Ma C., Prindle T., Grant Z., Branca M. F., Ozolek J., Hackam D. J. (2013) A critical role for TLR4 induction of autophagy in the regulation of enterocyte migration and the pathogenesis of necrotizing enterocolitis. J. Immunol. 190, 3541–3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coopersmith C. M., Gordon J. I. (1997) gamma-Ray-induced apoptosis in transgenic mice with proliferative abnormalities in their intestinal epithelium: re-entry of villus enterocytes into the cell cycle does not affect their radioresistance but enhances the radiosensitivity of the crypt by inducing p53. Oncogene 15, 131–141 [DOI] [PubMed] [Google Scholar]
- 30.Ban K., Peng Z., Kozar R. A. (2013) Inhibition of ERK1/2 worsens intestinal ischemia/reperfusion injury. PLoS One 8, e76790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Darzynkiewicz Z., Zhao H., Zhang S., Lee M. Y., Lee E. Y., Zhang Z. (2015) Initiation and termination of DNA replication during S phase in relation to cyclins D1, E and A, p21WAF1, Cdt1 and the p12 subunit of DNA polymerase δ revealed in individual cells by cytometry. Oncotarget 6, 11735–11750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duronio R. J., Xiong Y. (2013) Signaling pathways that control cell proliferation. Cold Spring Harb. Perspect. Biol. 5, a008904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wisdom R., Johnson R. S., Moore C. (1999) c-Jun regulates cell cycle progression and apoptosis by distinct mechanisms. EMBO J. 18, 188–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perrone E. E., Jung E., Breed E., Dominguez J. A., Liang Z., Clark A. T., Dunne W. M., Burd E. M., Coopersmith C. M. (2012) Mechanisms of methicillin-resistant Staphylococcus aureus pneumonia-induced intestinal epithelial apoptosis. Shock 38, 68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryoo H. D., Gorenc T., Steller H. (2004) Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev. Cell 7, 491–501 [DOI] [PubMed] [Google Scholar]
- 36.Fan Y., Bergmann A. (2008) Apoptosis-induced compensatory proliferation. The cell is dead. Long live the cell! Trends Cell Biol. 18, 467–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mollereau B., Perez-Garijo A., Bergmann A., Miura M., Gerlitz O., Ryoo H. D., Steller H., Morata G. (2013) Compensatory proliferation and apoptosis-induced proliferation: a need for clarification. Cell Death Differ. 20, 181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai S. C., Pasumarthi K. B., Pajak L., Franklin M., Patton B., Wang H., Henzel W. J., Stults J. T., Field L. J. (2000) Simian virus 40 large T antigen binds a novel Bcl-2 homology domain 3-containing proapoptosis protein in the cytoplasm. J. Biol. Chem. 275, 3239–3246 [DOI] [PubMed] [Google Scholar]
- 39.Butin-Israeli V., Drayman N., Oppenheim A. (2010) Simian virus 40 infection triggers a balanced network that includes apoptotic, survival, and stress pathways. J. Virol. 84, 3431–3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drayman N., Ben-Nun-Shaul O., Butin-Israeli V., Srivastava R., Rubinstein A. M., Mock C. S., Elyada E., Ben-Neriah Y., Lahav G., Oppenheim A. (2016) p53 elevation in human cells halt SV40 infection by inhibiting T-ag expression. Oncotarget 7, 52643–52660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sáenz Robles M. T., Pipas J. M. (2009) T antigen transgenic mouse models. Semin. Cancer Biol. 19, 229–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clark J. A., Coopersmith C. M. (2007) Intestinal crosstalk: a new paradigm for understanding the gut as the “motor” of critical illness. Shock 28, 384–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meng M., Klingensmith N. J., Coopersmith C. M. (2017) New insights into the gut as the driver of critical illness and organ failure. Curr. Opin. Crit. Care 23, 143–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lyons J. D., Coopersmith C. M. (2017) Pathophysiology of the gut and the microbiome in the host response. Pediatr. Crit. Care Med. 18, S46–S49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fay K. T., Ford M. L., Coopersmith C. M. (2017) The intestinal microenvironment in sepsis. [E-pub ahead of print] Biochim. Biophys. Acta doi:10.1016/j.bbadis.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alverdy J. C., Krezalek M. A. (2017) Collapse of the microbiome, emergence of the pathobiome, and the immunopathology of sepsis. Crit. Care Med. 45, 337–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McDonald D., Ackermann G., Khailova L., Baird C., Heyland D., Kozar R., Lemieux M., Derenski K., King J., Vis-Kampen C., Knight R., Wischmeyer P. E. (2016) Extreme dysbiosis of the microbiome in critical illness. mSphere 1, e00199–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayakawa M., Asahara T., Henzan N., Murakami H., Yamamoto H., Mukai N., Minami Y., Sugano M., Kubota N., Uegaki S., Kamoshida H., Sawamura A., Nomoto K., Gando S. (2011) Dramatic changes of the gut flora immediately after severe and sudden insults. Dig. Dis. Sci. 56, 2361–2365 [DOI] [PubMed] [Google Scholar]
- 49.Nyangale E. P., Mottram D. S., Gibson G. R. (2012) Gut microbial activity, implications for health and disease: the potential role of metabolite analysis. J. Proteome Res. 11, 5573–5585 [DOI] [PubMed] [Google Scholar]
- 50.Blachier F., Beaumont M., Andriamihaja M., Davila A. M., Lan A., Grauso M., Armand L., Benamouzig R., Tomé D. (2017) Changes in the luminal environment of the colonic epithelial cells and physiopathological consequences. Am. J. Pathol. 187, 476–486 [DOI] [PubMed] [Google Scholar]