Abstract
Intracellular O2 is a key regulator of NO signaling, yet most in vitro studies are conducted in atmospheric O2 levels, hyperoxic with respect to the physiologic milieu. We investigated NO signaling in endothelial cells cultured in physiologic (5%) O2 and stimulated with histamine or shear stress. Culture of cells in 5% O2 (>5 d) decreased histamine- but not shear stress–stimulated endothelial (e)NOS activity. Unlike cells adapted to a hypoxic environment (1% O2), those cultured in 5% O2 still mobilized sufficient Ca2+ to activate AMPK. Enhanced expression and membrane targeting of PP2A-C was observed in 5% O2, resulting in greater interaction with eNOS in response to histamine. Moreover, increased dephosphorylation of eNOS in 5% O2 was Ca2+-sensitive and reversed by okadaic acid or PP2A-C siRNA. The present findings establish that Ca2+ mobilization stimulates both NO synthesis and PP2A-mediated eNOS dephosphorylation, thus constituting a novel negative feedback mechanism regulating eNOS activity not present in response to shear stress. This, coupled with enhanced NO bioavailability, underpins differences in NO signaling induced by inflammatory and physiologic stimuli that are apparent only in physiologic O2 levels. Furthermore, an explicit delineation between physiologic normoxia and genuine hypoxia is defined here, with implications for our understanding of pathophysiological hypoxia.—Keeley, T. P., Siow, R. C. M., Jacob, R., Mann, G. E. A PP2A-mediated feedback mechanism controls Ca2+-dependent NO synthesis under physiological oxygen.
Keywords: endothelial cells, normoxia, hypoxia
The majority of in vitro experiments are routinely conducted in atmospheric O2 (∼20%), whereas cells in vivo experience significantly lower levels. Such artifactual hyperoxic conditions create a pro-oxidized environment that reduces cellular lifespan (1, 2), alters adaptive antioxidant defenses (3, 4), and may thus influence the translational relevance of in vitro findings. The blood-dissolved O2 level in vivo ranges from 13% in the pulmonary circulation to ∼3–5% in most microvascular capillary beds (5) and ∼3.7% in venous umbilical blood (6). Thus, HUVECs cultured in standard conditions in vitro are normally exposed to >4 times the O2 level experienced in vivo. We have reported that culturing HUVECs in 5% ambient O2 results in a cytosolic O2 level of ∼3.5% (4), more accurately replicating levels measured in vivo and with important consequences for cellular antioxidant defense pathways.
NO synthesis and metabolism are O2 dependent, a relationship critical in determining normal tissue O2 homoeostasis (7, 8). Thus, we investigated whether standard culture in hyperoxic conditions affects endothelial NO signaling. Upon exposure to hypoxic (∼1–2% O2) conditions, endothelial (e)NOS expression follows a biphasic pattern of an acute increase in mRNA and protein levels, peaking at ∼12 h (9), followed by a gradual decline as hypoxia persists beyond 24 h (10). These biphasic changes in mRNA and protein levels are paralleled by changes in NO production. To date, there are no reports on the long-term (>72 h) effects of physiologic O2 levels on NO signaling induced by inflammatory mediators or shear stress. In comparison to phosphorylation, limited data are available on the role of dephosphorylation in the regulation of eNOS. Whereas dephosphorylation at the inhibitory residues T495 (11) and S114 (12) by protein phosphatase (PP)-1 or -2B (calcineurin) has been described, acute dephosphorylation at stimulatory residues, such as S1177 and S633, is less well understood. PP2A has been shown to dephosphorylate eNOS at S1177 in response to long-term treatment with ceramide (13), endostatin (14), vasoinhibins (15), and proteasome inhibition (16). However, the mechanisms by which phosphatases regulate acute, transient Ca2+-stimulated eNOS phosphorylation at S1177 and S633 remain to be elucidated.
We report the first evidence that long-term culture of human endothelial cells in physiologic (5%) O2 levels induces a phenotype distinct from that observed in atmospheric conditions. By select comparison with paired cells cultured in 1% O2 (hypoxia), we further differentiate the cellular phenotype in normoxia (5% O2) from that often observed in genuine hypoxia. Specifically, we demonstrate that enhanced extranuclear PP2A activity in physiologic O2 (5%) provides more stringent regulation of eNOS activity in response to Ca2+-dependent inflammatory mediators. In contrast, the responses to fluid shear stress, which are not subject to acute serine-threonine phosphatase regulation, appear to be potentiated in physiologic O2 levels, emphasizing the importance of shear stress as the predominant physiological mediator of NO production. Our study further highlights the importance of physiologically relevant O2 levels for in vitro research to better correlate such findings with studies in vivo.
MATERIALS AND METHODS
Reagents and antibodies
Fura-2 AM was purchased from Teflabs (Austin, TX, USA) and Cal-520 AM from Stratech (Newmarket, United Kingdom). eNOS, PP2A-A (α/β), and PR72/130 antibodies were from Santa Cruz Biotechnologies (Dallas, TX, USA). Anti-PP2A-C and α-tubulin were from Millipore-Sigma (Watford, United Kingdom). Anti-phospho-eNOS S1177, T495, and S633; anti-phospho-AMPK T172; and total α-AMPK, anti-phospho-protein kinase B (Akt) S473 and total Akt, anti-phospho-ERK1/2 (T204/202) and total ERK1/2 antibodies were from Cell Signaling Technology (Danvers, MA, USA). Anti-HIF1-α antibody was from Novus Biologicals (Littleton, CO, USA). cGMP ELISA was from Cayman Chemical (Ann Arbor, MI, USA). The anti-SERCA-2 antibody (2D8) was kindly provided by Dr. Kalwant Authi (Cardiovascular Division, King’s College London). On-Target Plus PP2A-C (human) and control scrambled siRNA were from Dharmacon (Lafayette, CO, USA). All other chemicals were purchased from Millipore-Sigma.
Culture of human primary endothelial cells
Umbilical cords from normal-term pregnancies were obtained from St. Thomas’ Hospital with informed participant consent and Research Ethics Committee approval (EC02/77, 15/EM/0290). HUVECs were isolated by collagenase digestion of cords from 73 participants to minimize potential variations in cell phenotype due to gender or ethnicity (17), and cultured in M199+20% fetal calf serum (FCS) (18). Human coronary artery endothelial cells (HCAECs) from a healthy 27-yr-old male donor (PromoCell, Heidelberg, Germany) were cultured in endothelial growth medium+5% FCS. Culture in physiologic (5%) or hypoxic (1%) O2 levels was achieved by transferring cells from a standard incubator (∼18% O2) into an O2-regulated workstation (Sci-tive; Baker-Ruskinn, Bridgend, United Kingdom) gassed to 5 or 1% O2. HUVECs were cultured in low O2 (10, 5, 3, or 1% O2) from passage 2 for a minimum of 5 d, to allow for cell proteome adaptation, and all subculture procedures performed in the appropriate percentage of O2. For siRNA transfection, cells were seeded at 35,000 cells per well and transfected for 48 h with scrambled or PP2A-C siRNA (50 nM). Cells at passage 3 were adapted to low serum (1% FCS) for 4 h before experimentation.
Application of shear stress to cultured endothelial cells
Confluent HUVECs were acutely exposed to fluid shear stress, with a parallel-plate flow system (Ibidi, Martinsried, Germany). In brief, HUVECs were seeded onto μ-slides and allowed to adhere for 24 h. The μ-slides were then connected to a fluidic unit to generate a unidirectional laminar shear stress of 15 dyn/cm2.
Intracellular Ca2+ measurements
Intracellular Ca2+ levels were measured in HUVECs loaded with Fura-2 AM (2 µM) and monitored in an O2 atmosphere-regulated plate reader (ClarioStar; BMG Labtech, Orteberg, Germany). Agonist or vehicle was injected with the integrated reagent injection system. Alternatively, for measurements of Ca2+ under shear stress, HUVECs were loaded with Cal-520 AM (2 µM) and fluorescence monitored at 480 excitation/520 emission on an inverted microscope (Lumascope; Etaluma, Carlsbad, CA, USA) within the Sci-tive workstation.
Modeling of NO bioavailability
Mathematical modeling of NO bioavailability after addition of the NO donor (Z)-1-[N-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate (DETA-NONOate) was performed using the method introduced in 1998 by Schmidt et al. (19), using previously published experimental parameters and accounting for the presence of cellular lipids (20) (see Supplemental Fig. S5 for details).
Measurement of eNOS activity and intracellular cGMP production
eNOS enzymatic activity was determined by measuring the conversion of l-[3H]-arginine (4 µCi/ml) to l-[3H]-citrulline, separated in formic acid digests on Dowex 50W8 columns. eNOS activity was expressed as l-NAME inhibitable l-[3H]-citrulline production. cGMP production was assessed using a chemiluminescent ELISA (Arbor Assays, Ann Arbor, MI, USA) in the presence of the phosphodiesterase inhibitor IBMX (0.5 mM), with or without the eNOS inhibitor l-NAME (100 µM).
Analysis of protein phosphorylation and expression by immunoblot analysis
HUVECs were grown to confluence and equilibrated to low serum (1% FCS) for 4 h. Cells were then equilibrated in Krebs buffer (mM: 131 NaCl2, 5.6 KCl, 20 HEPES, 25 NaHCO3, 5 d-glucose, 1 NaH2PO4, 0.1 l-arginine, 2 CaCl2, and 1 MgCl2) for a further 20 min before stimulation with histamine (10 µM), vehicle (double-distilled H2O), or laminar shear stress (15 dyn/cm2) for 2–30 min. Lysates were collected in SDS buffer containing phosphatase inhibitors. Equal amounts of protein (10–30 µg) were separated via SDS-PAGE. All immunoblot densities shown are relative to total protein (for phospho proteins) with β-actin used as an additional loading control.
Immunofluorescent imaging of PP2A
HUVECs were washed twice in ice-cold PBS and fixed with 4% paraformaldehyde for 10 min. The cells were permeabilized in 0.1% Triton-X, blocked in 5% bovine serum albumin, and incubated with PP2A-A (1:100 dilution) for 1 h. A secondary antibody conjugated to FITC was added for an additional 1 h, and cells were counterstained with DAPI for 5 min. Samples were visualized on a Diaphot microscope (Nikon, Tokyo, Japan) coupled to an ORCA-03G camera (Hamamatsu, Shizuoka, Japan). Nuclear distribution of PP2A was determined by measuring PP2A staining intensity in a DAPI mask and expressed as a percentage of total intensity. On average, 5–6 regions of interest, containing 2–5 cells, were imaged per condition. In total, 107 cells from 4 different donors were analyzed.
PP2A subcellular distribution and interactions with eNOS
Subcellular distribution of PP2A was assessed by immunofluorescence and coimmunoprecipitation (see below). PP2A–eNOS interactions were quantified by in situ proximity ligation (Duolink; Millipore-Sigma) (21). The cells were stained with PP2A and eNOS antibodies and incubated with PLA Plus and Minus probes, according to the manufacturer’s instructions. Nuclei were counterstained with DAPI and cells visualized with a ×40 objective. Six to 10 regions of interest were imaged per condition, with 1290 cells used for batch quantification with the analyze particles algorithm in ImageJ (National Institutes of Health, Bethesda, MD, USA). Results are expressed as a frequency distribution of PLA signals in individual cells and as mean PLA signals per cell for each condition and donor. Higher magnification (×60) images were used to visualize subcellular distribution of PLA signals. Coimmunoprecipitation of eNOS from 500 µg cell lysate was achieved by overnight incubation with protein A-labeled Dynabeads (Thermo Fisher Scientific, Loughborough, United Kingdom) conjugated to 1 µg eNOS antibody (Santa Cruz Biotechnology). SDS eluent was separated by PAGE and probed for PP2A-C content.
Preparation of cytosolic and particulate subcellular fractions
HUVECs were washed twice in ice-cold PBS and scraped into Eppendorf tubes (Hamburg, Germany) in homogenization buffer (in mM: HEPES 10, sorbitol 340, EDTA 1, DTT 2, and protease and phosphatase inhibitor cocktails). Lysates were separated into cytosolic (Cav1/SERCA−) and particulate (Cav1/SERCA+, α-tubulin−) fractions by ultracentrifugation (22).
Statistical analysis
Data are means ± sem of measurements in 3–11 different donors, with significance (P < 0.05) assessed with either a paired Student’s t test or 2-way ANOVA with Bonferroni post hoc analysis. Where appropriate, results were analyzed by regression analysis. Unless stated otherwise, the effects of both histamine and shear stress were significant (P < 0.05) vs. vehicle.
RESULTS
Maintenance of endothelial cells in 5% O2 does not stabilize HIF1α
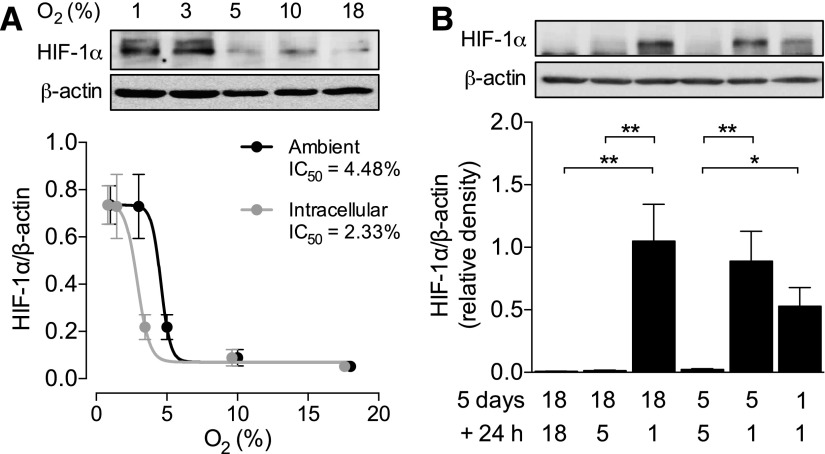
Culture facilitates the formation of a significant O2 gradient between atmospheric and intracellular O2 levels minimized in vivo because of the flow of blood. We have previously demonstrated that culture in 5% ambient O2 is necessary to compensate for this artifactual gradient in vitro, thus replicating intracellular O2 levels observed in HUVECs in vivo (∼3.7%) (4). We first sought to understand how culture in these conditions relates to traditionally hypoxic environments, using the stabilization of HIF-1α as a readout. When HUVECs were cultured in a range of different O2 levels (18, 10 5, 3, and 1% O2) for more than 5 d, HIF-1α stabilization was evident only when the ambient O2 level was ≤3–4% (Fig. 1A), with minimal expression evident at 5% O2 even when exposed acutely (Fig. 1B). When replotted against the corresponding mean intracellular O2 level [as determined previously (4)], there was a leftward shift in the sigmoidal curve with a reduction in IC50 from 4.5 to 2.3% O2. This shift is of particular physiologic significance, because blood oxygen (Po2) level of 4.5% is normoxic for most cell types in vivo, yet 2.3% O2 encroaches on hypoxia for endothelial cells. Hence intracellular, and not the ambient O2 level, is the important parameter when designing an in vitro model of physiologic normoxia. In the context of the current work, these data confirm that culture in 5% O2 does not induce a traditionally hypoxic phenotype in HUVECs.
Figure 1.
HIF-1α stabilization in HUVECs at various oxygen levels. A) HUVECs were cultured in 18, 10, 5, 3, or 1% O2 for at least 5 d, and lysates were immunoblotted for expression of HIF-1α. Means ± sem (n = 4) were plotted against either the ambient or intracellular O2 level [as determined previously (4)], and a sigmoidal curve was fitted for determination of the IC50. B) HUVECs cultured in 18, 5, or 1% O2 for 5 d were then cultured for a further 24 h in either the same or lower O2 levels, and expression of HIF-1α was determined as before. Representative immunoblots are provided above and densitometric analysis of HIF-1α expression relative to the loading control β-actin below. Data are means ± sem (n = 4–5 different donors). *P < 0.05, **P < 0.01.
Altered Ca2+ mobilization after culture in 5% O2
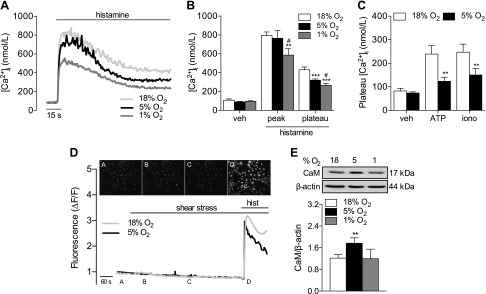
Acute NO production evoked by inflammatory mediators is mediated by an initial increase in intracellular Ca2+, and thus we measured mobilization of Ca2+ after stimulation of HUVECs with histamine (10 µM). Histamine induced a typical biphasic change in intracellular Ca2+ [Ca2+]i that was characterized by a sharp increase followed by a sustained plateau (Fig. 2A). Peak [Ca2+]i responses were similar in HUVECs cultured in 18 or 5% O2, but significantly lower in cells in 1% O2 (Fig. 2B), whereas the plateau phase was markedly reduced at both 5 and 1% O2. This effect was not due to differences in histamine- or inositol triphosphate–related signaling, as expression of the H1 histamine receptor was unaltered after long-term culture in 5% O2 (data not shown), and comparable results were obtained in HUVECs challenged with ATP (10 µM) or ionomycin (0.1 µM; Fig. 2C). Similar results were also observed in HCAECs (Supplemental Fig. S1A). Laminar shear stress (15 dyn/cm2) did not elicit an increase in mean intracellular Ca2+ over a 10 min period in HUVECs in either 18 or 5% O2 (Fig. 2D). As most endothelial cells experience only ≤1% O2 during periods of low- to no-flow ischemia, cells maintained at 1% O2 were not exposed to shear stress. We also observed significantly higher expression of calmodulin (CaM) in HUVECs cultured in 5% O2 compared with cells cultured in at either 18 or 1% O2 (Fig. 2E).
Figure 2.
Attenuated Ca2+ mobilization in human endothelial cells cultured in various levels of O2. HUVECs were maintained at 18% O2 or cultured in 5 or 1% O2 for at least 5 d. Cells were stimulated with histamine (10 µM), and [Ca2+]i was monitored. A) Ca2+ transients in HUVECs stimulated with histamine (10 µM) in 18, 5, or 1% O2. B) Average peak and plateau [Ca2+]i values in response to histamine. C) Plateau [Ca2+]i levels in response to ATP (10 µM) or ionomycin (0.1 µM). D) HUVECs were loaded with Cal520-AM and then exposed to 15 dyn/cm2 unidirectional shear stress for 10 min, followed by an acute stimulation with histamine. Representative images are provided from HUVECs at 18% O2. E) Calmodulin (CaM) expression in HUVECs in 18, 5, or 1% O2. Representative immunoblots and analysis of CaM expression relative to β-actin. Treatment with histamine was significant (P < 0.05 vs. respective control) in relevant panels. Data are means ± sem (n = 4–11 different donors). **P < 0.01, ***P < 0.001 vs. 18% O2; #P < 0.05 vs. 5% O2.
Activation of kinases in endothelial cells in physiologic O2 levels
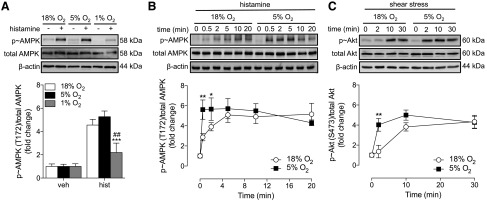
Because AMPK acts downstream of the H1 receptor in a Ca2+-dependent manner (23), we investigated whether alterations in Ca2+ mobilization were paralleled by activation of AMPK. Culturing HUVECs in 5% O2 had no significant effect on basal kinase expression or phosphorylation (Supplemental Fig. S2A). When cells were cultured in 1% O2, no significant phosphorylation of AMPK (Fig. 3A) or ERK1/2 (data not shown) was detected in response to histamine. In contrast, in both 18 and 5% O2 histamine (0–20 min) caused rapid AMPK phosphorylation, reaching maximum levels after 5 min (Fig. 3B). HUVECs cultured in 5% O2 responded more rapidly to histamine, with significantly higher AMPK phosphorylation detected after 0.5 and 2 min. Although histamine also induced phosphorylation of ERK1/2, no differences were detected between cells cultured in 18 or 5% O2 (Supplemental Fig. S2B). Akt is largely responsible for mediating eNOS activation at basal Ca2+ levels, especially in response to physiologic stimuli, such as shear stress (24). In this study, shear stress (0–30 min) elicited a marked and sustained phosphorylation of Akt in both 18 and 5% O2, and HUVECs cultured in 5% O2 responded more rapidly with significantly higher Akt phosphorylation detected at 2 min (Fig. 3C).
Figure 3.
Activation of kinases in HUVECs cultured in various O2 levels. HUVECs were maintained at 18% O2 or cultured in 5 or 1% O2 for at least 5 d. Cells were then stimulated with histamine (10 µM) for 2 min (A) or 0–20 min (B), or laminar shear stress (15 dyn/cm2) for 0–30 min (C). Lysates were immunoblotted for p-AMPK or p-Akt and expressed relative to total kinase levels and β-actin as loading controls. Data are means ± sem (n = 5–6 different donors). Treatment with histamine or shear stress was significant (P < 0.05 vs. respective control) in relevant panels. *P < 0.05, **P < 0.01, ***P < 0.001 vs. 18% O2; ##P < 0.01 vs. 5% O2.
eNOS phosphorylation in physiologic O2 levels
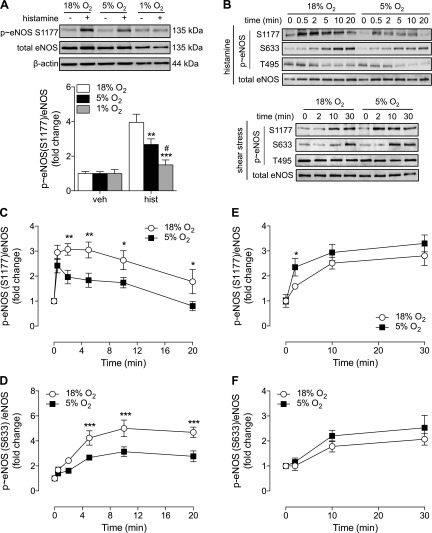
Differential Ca2+ mobilization did not appear to affect the extent of AMPK or ERK1/2 activation in HUVECs cultured in 5% O2, and thus we examined whether eNOS phosphorylation would be similarly unaffected. eNOS protein expression (Supplemental Fig. S2C) and basal phosphorylation at 2 key stimulatory residues, S1177 and S633, (Supplemental Fig. S2A), were unaltered during culture in 5% O2. Treatment with histamine (2 min) led to rapid eNOS-S1177 phosphorylation in HUVECs at both 18 and 5% O2, but not at 1% O2 (Fig. 4A). Notably, phosphorylation of eNOS-S1177 in response to histamine was similar in cells in 18 or 5% O2 after 30 s but then declined more rapidly over 2–15 min in cells cultured in 5% O2 (Fig. 4C, and at 2 min in HCAECs; Supplemental Fig. S1B). S633 was also phosphorylated in response to histamine, again significantly less in cells in 5% O2 (Fig. 4D). Dephosphorylation of eNOS at the inhibitory site T495 is known to enhance enzyme activity (11), but no difference in histamine-stimulated T495 dephosphorylation was observed in HUVECs at either 18 or 5% O2 (data not shown). Exposing HUVECs to shear stress (0–30 min) resulted in a time-dependent increase in eNOS-S1177 phosphorylation, with significantly higher phosphorylation detected after 2 min in cells cultured in 5% O2 (Fig. 4E). As with S1177, shear stress also led to rapid phosphorylation of S633, which was negligibly affected by the ambient O2 level (Fig. 4F). Furthermore, shear stress did not affect eNOS-T495 phosphorylation (data not shown).
Figure 4.
eNOS phosphorylation in HUVECs cultured in various O2 levels. HUVECs were maintained at 18% O2 or cultured in 5 or 1% O2 for at least 5 d. Cells were then stimulated with histamine (10 µM) for 2 min (A) or 0.5–20 min (B–D), or shear stress for 0–30 min (B, E, F), and phosphorylation of eNOS at S1177 (A–C, E) and S633 (B, D, F) (human sequence) assessed by immunoblot analysis relative to total eNOS, a β-actin loading control, or both. Data are expressed as fold change and are means ± sem (n = 4–6 different donors). Treatment with histamine or shear stress was significant (P < 0.05 vs. respective control) in relevant panels, except at 1% O2 in A. *P < 0.05, **P < 0.01; ***P < 0.001 vs. 18% O2; #P < 0.05 vs. 5% O2.
Consequences for endothelial NO and cGMP production
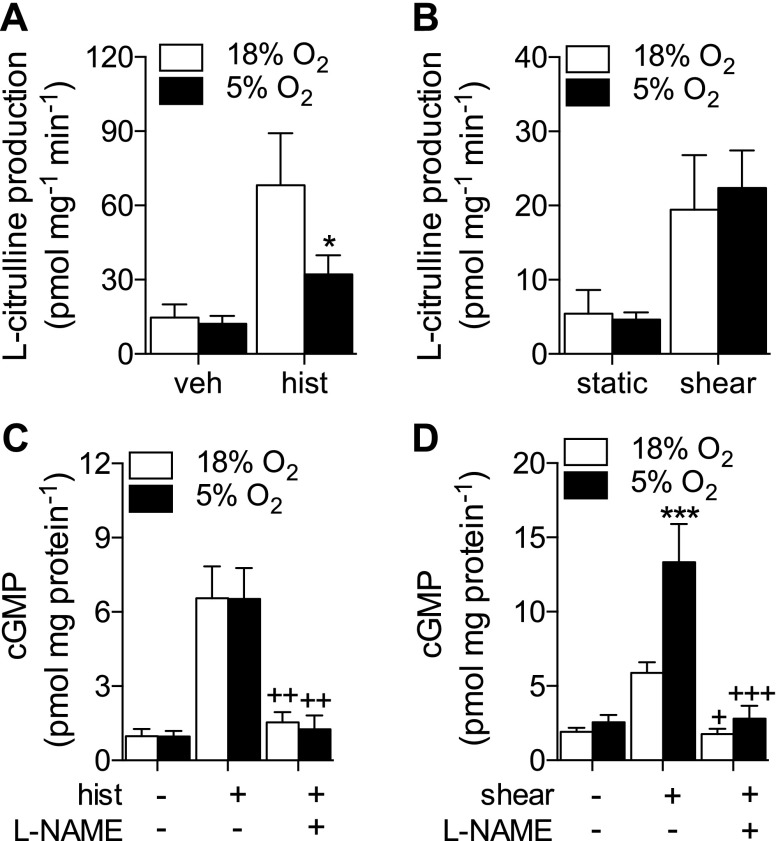
To evaluate whether alterations in eNOS phosphorylation translated to enzymatic activity and thus NO production, histamine- and shear stress–stimulated eNOS activity and cGMP production were assessed in HUVECs cultured in 5% O2. Histamine stimulated an increase in eNOS activity that was significantly attenuated in HUVECs at 5% O2 (Fig. 5A). In contrast, the increase in eNOS activity upon shear stress stimulation was similar at the 2 O2 levels (Fig. 5B). l-NAME inhibitable cGMP generation increased significantly after stimulation with either histamine or shear stress (Fig. 5C, D, respectively). Although histamine-stimulated eNOS activity was lower in 5% O2, cGMP production remained similar. In comparison, shear stress–stimulated cGMP production was significantly higher in cells at 5% O2 (Fig. 5D), despite similar levels of eNOS activity. It is well established that chronic (>24 h) hypoxia reduces eNOS activity and subsequent cGMP production in endothelial cells (9, 25). In agreement with this, we observed negligible increases in eNOS activity and significantly lower cGMP generation in response to histamine at 1% O2 (Supplemental Fig. S3A, B). Furthermore, expression of soluble guanylate cyclase was unchanged after long-term culture in 5 or 1% O2 (Supplemental Fig. S3C).
Figure 5.
NO and cGMP production in HUVECs cultured in 5% O2. HUVECs were maintained at 18% O2 or cultured in 5% O2 for at least 5 d. Cells were pretreated in the absence or presence of the eNOS inhibitor l-NAME (100 µM) for 30 min before stimulation with histamine or shear stress. A, B) eNOS activity after stimulation with histamine (10 µM, 5 min) (A) or shear stress (15 dyn/cm2, 10 min) (B), plotted as l-NAME inhibitable l-[3H]citrulline production. C, D) cGMP production in response to histamine (10 µM, 5 min) (C) or shear stress (15 dyn/cm2, 10 min) (D). Data are means ± sem (n = 5–9 different donors). Treatment with histamine or shear stress was significant (P < 0.05 vs. respective control) in relevant panels. *P < 0.05, ***P < 0.001 vs. 18% O2; +P < 0.05, ++P < 0.01, +++P < 0.001 vs. histamine/shear stress alone.
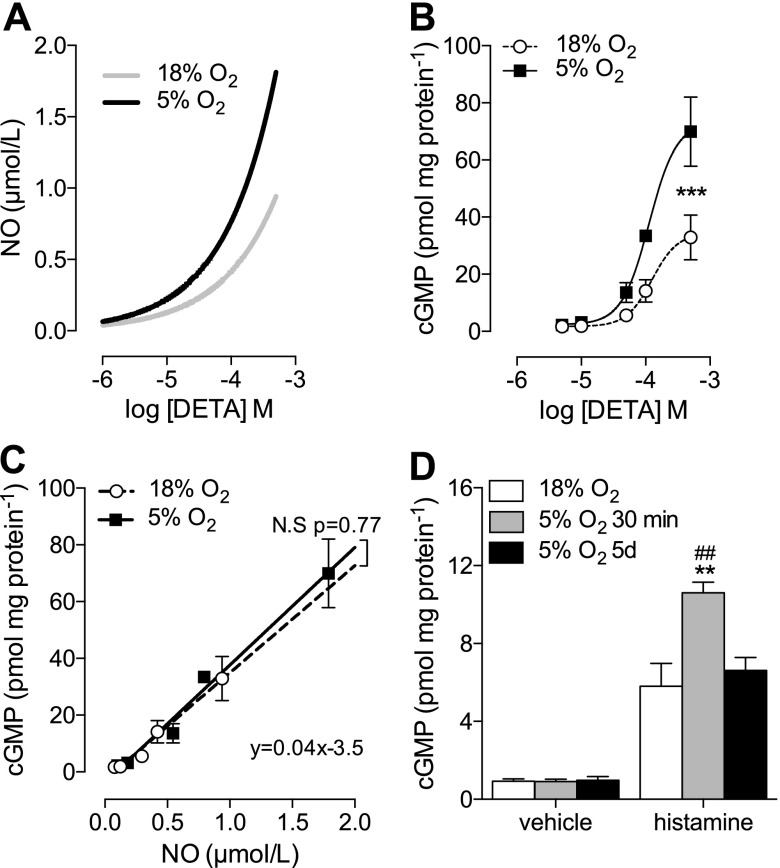
O2 levels have been shown to be a key determinant of NO bioavailability (7, 8, 20), given that NO radicals react rapidly with molecular O2 to produce NO2−. A mathematical model of NO generation by DETA and removal by reaction with O2 (19) shows that the resulting NO concentration is higher in 5% O2 at any given concentration of DETA (Fig. 6A). In addition, cGMP production in HUVECs after a 10-min stimulation with DETA (0–500 µM) was significantly higher in cells at 5% O2 (Fig. 6B). When cGMP production was plotted against calculated [NO] over a range of DETA concentrations (Fig. 6C), there was a strong linear relationship that did not vary significantly between O2 levels. To frame this in a physiological context, we took HUVECs cultured in 18% O2 and exposed them to 5% O2 for only 20 min, long enough for intracellular O2 to re-equilibrate (4) without evoking changes in eNOS activity. We hypothesized that an increase in NO bioavailability at 5% O2 levels without a change in eNOS activity would result in increased cGMP production, which was indeed observed (Fig. 6D).
Figure 6.
Impact of O2 on NO bioavailability in endothelial cells. A) Modeling of NO release from different levels of DETA after 10 min of decomposition. B) HUVECs were maintained at 18% O2 or cultured in 5% O2 for at least 5 d. Cells were treated with DETA (0–500 µM, 10 min), and cGMP production was assayed. Vehicle (NaOH 0.05%) had no effect on cGMP production (not shown). C) Correlation of predicted [NO] with measured cGMP levels. Differences in neither slope nor intercept were significant when 18 and 5% O2 were fitted separately, so one equation represents both O2 levels. D) Histamine (10 µM, 5 min) stimulated cGMP levels in HUVECs cultured in 18% O2 and then exposed acutely to 5% O2 for 30 min. Treatment with histamine was significant (P < 0.05 vs. respective control). Data are means ± sem (n = 3–9 different donors). **P < 0.01, ***P < 0.001 vs. 18% O2; ##P < 0.01 vs. 5% O2 (5 d).
PP2A is responsible for enhanced eNOS dephosphorylation in physiologic O2
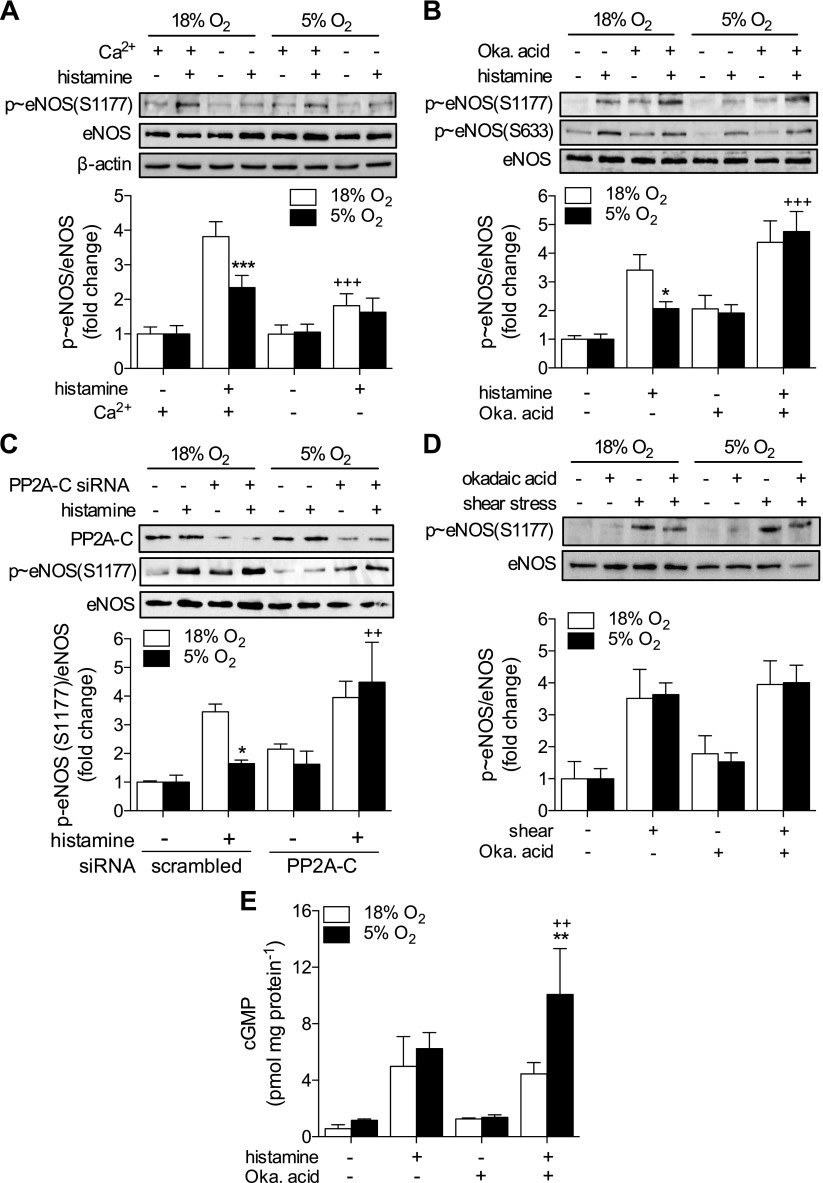
The discrepancy between kinase activation (Fig. 3) and eNOS phosphorylation (Fig. 4) in response to histamine in 5% O2 suggests a role for increased dephosphorylation. Most dephosphorylation of serine/threonine is catalyzed by PP1, -2A, or -2B (26). Although all 3 have been demonstrated to target eNOS, only PP2A has been shown to dephosphorylate S1177, whereas residues T495 and S114 are strongly targeted by PP2B and possibly PP1 (11, 27). The lack of upstream kinase activation at 1% O2 in response to histamine (Fig. 3A) likely explains the absence of eNOS phosphorylation (Fig. 4A), and therefore the role of phosphatases was investigated only in cells cultured in 18 and 5% O2. The effect of culture at 5% O2 was Ca2+-dependent, because in nominally Ca2+-free conditions, the dependence of S1177 phosphorylation on O2 was no longer apparent (Fig. 7A), indicating the involvement of either PP2A or -2B. To investigate this further, HUVECs, pretreated with the PP2A inhibitor okadaic acid (100 nM, 30 min), were stimulated with histamine for a further 5 min. This concentration is the lowest possible dose that demonstrates phosphatase inhibition in cells, thereby limiting nonspecific effects on other phosphatases (PP1, -4, and -6) (28). Treatment with okadaic acid increased basal S1177 phosphorylation and rescued histamine-stimulated S1177 phosphorylation in cells in 5% O2 (Fig. 7B). Similar effects were observed for eNOS-S633 phosphorylation and after siRNA knockdown of PP2A–C (Fig. 7C). In contrast, pretreatment with the PP2B inhibitor FK506 had no effect on eNOS S1177/S633 phosphorylation (data not shown). Pretreatment with okadaic acid had no significant effect on shear stress–stimulated S1177 phosphorylation (Fig. 7D). cGMP production in HUVECs treated with histamine+okadaic acid was significantly higher in 5% O2 compared to 18% O2 (Fig. 7E).
Figure 7.
PP2A is responsible for histamine-stimulated eNOS dephosphorylation. HUVECs were maintained at 18% O2 or cultured in 5% O2 for at least 5 d. A) Cells were stimulated with histamine (10 µM, 2 min), with or without external Ca2+, and eNOS-S1177 phosphorylation was assessed. B) HUVECs were pretreated with the PP2A inhibitor okadaic acid (100 nM, 30 min), and phosphorylation of eNOS-S1177/S633 was assessed in response to histamine (10 µM, 5 min). C) HUVECs were transfected with scrambled (−) or PP2A-C siRNA and then stimulated with histamine. D) eNOS phosphorylation at S1177 in HUVECs pretreated with okadaic acid and then subjected to shear stress (15 dyn/cm2, 10 min). E) cGMP production in response to histamine (10 µM, 5 min) after pretreatment with okadaic acid. Representative immunoblots and densitometric analyses for p-eNOS expression relative to total eNOS and a β-actin loading control (data not shown in all panels). Data are expressed as fold change and are means ± sem (n = 4–6 different donors). Treatment with histamine or shear stress was significant (P < 0.05 vs. respective control) in relevant panels. *P < 0.05, **P < 0.01, ***P < 0.001 vs. 18% O2; +P < 0.05, ++P < 0.01, +++P < 0.001 vs. histamine alone/+Ca2+.
PP2A expression and subcellular location in endothelial cells in physiologic O2 levels
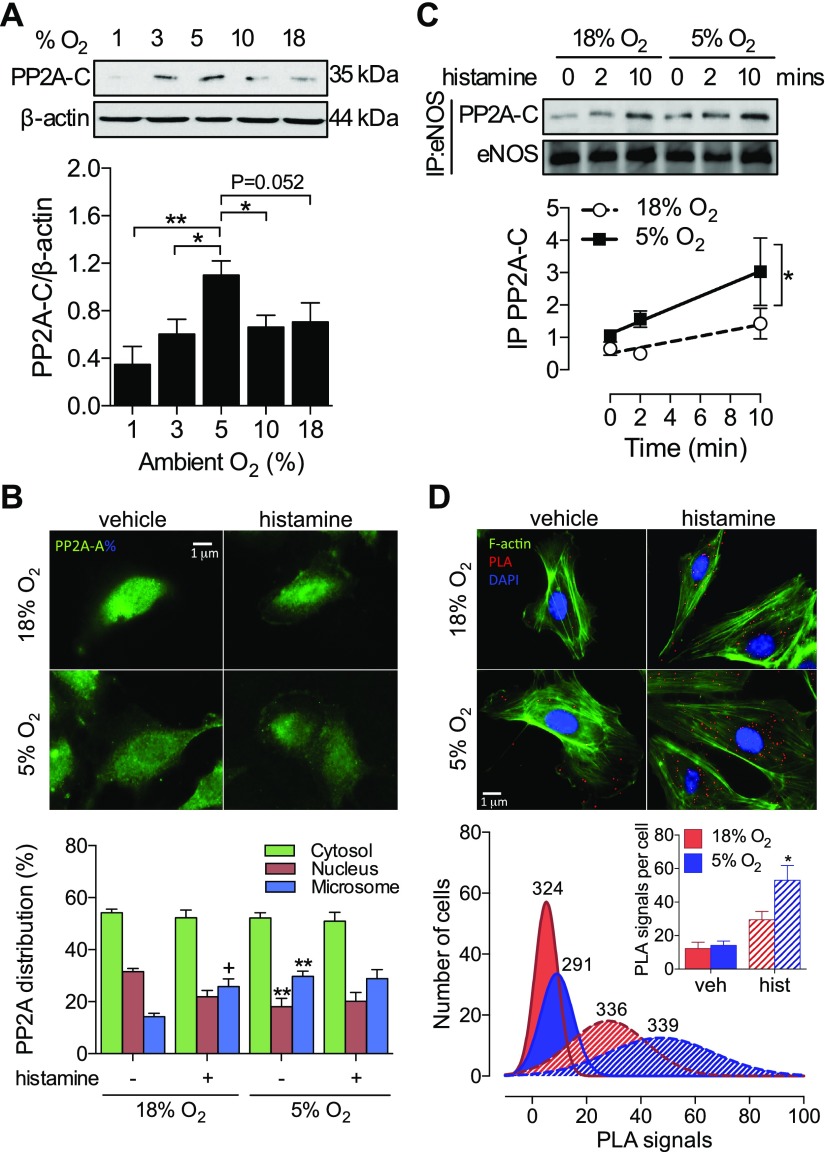
A scaffold (A) and catalytic (C) subunit form the basal PP2A heterodimer, with substrate specificity and activity conferred by the choice of regulatory (B) subunit association (26). Notably, the expression of PP2A-C exhibited a bell-shaped distribution across a range of ambient O2 levels (1–18% O2), peaking at 5% and falling steeply as intracellular O2 levels became hypoxic (Fig. 8A). The expression of PP2A-A and a Ca2+-sensitive B subunit (PR72) were unchanged, as was PP2B-C (data not shown). Previous studies have revealed translocation of PP2A to the plasma membrane, where it dephosphorylates eNOS (13, 16). Thus, we investigated whether PP2A-mediated eNOS dephosphorylation in cells cultured in 5% O2 could be explained by increased membrane localization. Because the PP2A-C subunit was induced by culture in 5% O2, we examined the localization of its dimer-forming partner PP2A-A (Fig. 8B). eNOS was found almost exclusively within the microsomal fraction (Supplemental Fig. S4), reflecting its known distribution near the plasma membrane or Golgi apparatus (29). In 18% O2, PP2A was predominantly (∼50%) found within the cytosolic fraction, with a smaller percentage associated with the nucleus, consistent with its role in cell cycle progression (30, 31). Moreover, histamine induced a redistribution from the nucleus to the microsome (Fig. 8B). In cells cultured in 5% O2, a similar redistribution was already apparent in unstimulated cells, and histamine did not induce a further redistribution. This basal redistribution in 5% O2 resulted in a more rapid interaction with eNOS, as reflected by an increased coimmunoprecipitation of PP2A-C with eNOS (Fig. 8C) and direct interaction assessed via in situ proximity ligation (21) (Fig. 8D).
Figure 8.
PP2A is responsible for histamine-stimulated eNOS dephosphorylation. HUVECs were maintained at 18% O2 or cultured in 10, 5, 3, or 1% O2 for at least 5 d. A) Representative immunoblot and densitometric analysis of PP2A-C expression relative to β-actin. B) Analysis of PP2A subcellular distribution in response to histamine stimulation (10 µM, 5 min) using immunofluorescence and ultracentrifugation (Supplemental Fig. S4). DAPI costaining not shown for clarity. C, D) Colocalization of eNOS and PP2A-C assessed by coimmunoprecipitation (C) and proximity ligation assay (D). Red dots indicate physical protein interaction. The total number of cells is indicated above each frequency distribution; data are means ± sem. PLA signals per cell shown in the inset. All other data represent means ± sem from 3 to 9 different donors. *P < 0.05, **P < 0.01 vs. 18% O2; +P < 0.05 vs. vehicle.
DISCUSSION
We have presented compelling evidence that in vitro data obtained at 5% O2 reveal functionally different NO signaling in endothelial cells from that observed in atmospheric O2. Long-term (>5 d) culture in physiologic normoxia highlights a novel negative-feedback mechanism regulating Ca2+-dependent eNOS activity, in which PP2A rapidly targets eNOS after Ca2+ mobilization to initiate dephosphorylation. Such feedback was not apparent during exposure to shear stress, providing clear delineation between physiologic and inflammatory pathways of NO production in endothelial cells. Genuine hypoxia (1% O2), in contrast to physiologic normoxia (5% O2), diminished responses to histamine at every stage of eNOS signaling from initial Ca2+ mobilization to cGMP production, consistent with previous reports (9, 10, 32–35).
The concept of physiologic normoxic cell culture has received increasing attention in recent years, with reports in primary lymphocytes (3, 36), neurons (37, 38), and stem cells (39–41) directly addressing the artifactual conditions in which cells are routinely cultured. In such studies, it is often demonstrated that cells exhibit less spontaneous or stimulated apoptosis (38), have less phenotypic differentiation during prolonged culture ex vivo (3), and have greater viability and regenerative capacity when transplanted into damaged organs in vivo (39, 40). Our previous findings in human endothelial cells (4) and in the present study illustrate a significant functional impact of culture at physiologic O2, with important implications for understanding endothelial cell responses to endogenous and pharmacological agents, as well as biomechanical forces. A picture is beginning to emerge in which long-term readaptation to physiologically relevant O2 levels in vitro dramatically alters cellular phenotype, with significant impact for translation of findings to animal models and the clinic.
In the present study, histamine and shear stress were used as classic endothelial cell stimuli to investigate Ca2+-sensitive and -insensitive pathways of NO generation in different O2 levels. Although a transient minor increase in [Ca2+]i after flow initiation is sometimes observed in endothelial cells (42, 43), the Ca2+-insensitive nature of eNOS activation by this stimulus is defined by the lack of effect of removing external Ca2+ (44–46). The transient nature of histamine-stimulated eNOS activation contrasts with the gradual yet sustained activation observed during exposure to shear stress, indicating divergent signaling pathways. Because kinase activation in response to histamine was sustained (Fig. 3), the transience of eNOS phosphorylation implies a delayed dephosphorylation in cells cultured in 18% O2.
The finding that the culturing cells in 5% O2 significantly lowers plateau [Ca2+]i, whereas leaving peak responses largely unaffected is similar to findings reported by Østergaard et al. (10). A reduced plateau [Ca2+]i was also observed in response to ATP or ionomycin treatment, indicating that the effect of culture in 5% O2 is not confined to H1-receptor activation and instead lies at a point of convergence downstream of inositol metabolism and receptor-mediated Ca2+ mobilization. CaM expression was found to be significantly increased after culture in 5% O2 (Fig. 2E), in line with previously published evidence (47). Cytosolic CaM concentrations are a key rate-limiting step in Ca2+-mediated signal transduction (48) and eNOS activation (49), and hence increased CaM expression may partially compensate for reductions in Ca2+ mobilization. Similarly, a lack of such a compensatory increase in CaM expression in 1% O2 may in part explain why hypoxic cells show little activation upon histamine stimulation. Increases in CaM may also provide an explanation for the enhanced AMPK phosphorylation in HUVECs at 5% O2, with Ca2+/CaM-dependent kinase II known to be an AMPK kinase (50).
Our experiments in human endothelial cells during physiologic normoxia have revealed a novel Ca2+-sensitive negative-feedback mechanism in regulating eNOS activation orchestrated by PP2A. In response to histamine, but not to shear stress, PP2A is rapidly recruited to eNOS and mediates the dephosphorylation at S1177 and, as demonstrated in this study for the first time, also at S633. This finding provides a mechanistic explanation of why Ca2+-dependent stimuli result in transient eNOS phosphorylation. In cells cultured in 1% O2, no upregulation of PP2A-C (Fig. 8A) or histamine-stimulated dephosphorylation was observed (data not shown), so this mechanism cannot explain the hypoxic reduction in eNOS phosphorylation, which is most likely due to severe global cellular dysfunction. Several members of the PP2A-B subunit family have been identified as Ca2+-sensitive, including the CaM-associated striatin (51) and the B family (PR70/72/130), which contain 2 EF-hand motifs and therefore can directly bind free Ca2+ (52). As B subunits regulate PP2A substrate specificity (53), the recruitment of one of these subunits likely mediates the relatively selective targeting of eNOS over other proposed PP2A substrates. Furthermore, members of the PP2A-B subunit family have been observed within the microsomal domain (54), providing an explanation for the similar distribution of active PP2A:eNOS complexes observed in the present study (Fig. 8). The enhanced basal microsomal distribution of PP2A in conjunction with increased expression of the catalytic subunit observed at 5% O2 may facilitate more rapid interaction with eNOS, which itself migrates toward the Golgi after stimulation (29).
To our knowledge, we report the first evidence of a relationship between cytosolic O2 levels and PP2A-C expression. Typically, PP2A subunit expression is regulated by translational autoregulation (55), although the absence of a change in basal phosphatase activity observed in our study suggests a different regulatory mechanism. It is unlikely that the PPPC2A mRNA promoter sequence contains a consensus hypoxia–response element (predicted based on sequence analysis; UCSC Genome Browser, University of California, Santa Cruz, CA, USA), and our observation that the expression of PP2A-C and HIF-1α show generally opposing responses to ambient O2 (Fig. 1A vs. Fig. 8A) suggest that PP2A-C expression is not regulated by traditional hypoxia signaling pathways. Rather, it seems likely that upregulation of PP2A-C specifically in physiologic normoxia (5% O2) may be a reflection of the cellular redox state, with both hyperoxia (18% O2) and hypoxia (1% O2) associated with higher levels of oxidative stress (56, 57). Further work is warranted to better understand the relationship between cellular redox state and PP2AC protein expression.
Using a combination of mathematical modeling and cell-based in vitro experiments, we highlight an inverse relationship between [O2] and [NO], first reported by Lancaster and colleagues (7, 20). In the present study, this relationship is framed within a dynamic physiologic context, examining its role in modulating the vascular response to stimulation. Accordingly, endothelial cells cultured in 5% O2 still generated an equivalent cGMP in response to histamine, despite reduced eNOS activity, whereas equivalent eNOS activity in response to shear stress generated considerably higher cGMP levels in 5% O2. Endothelial cells cultured in 1% O2 exhibited significant dysfunction upstream of soluble guanylate cyclase (Figs. 2, 3, and 4) and therefore did not capitalize fully on the reduced O2 conditions. Although other factors also contribute to the half-life of NO, such as the oxidation state of cytochrome c oxidase (58) and reactive oxygen species generation (59), these generally have a higher contribution in hyperoxic conditions (18% O2) and would therefore exaggerate only the existing predictions.
The data presented here highlight a clear disparity between the cellular responses to normoxia and hypoxia that extend beyond the specific focus of the current work. In this context, the following important points may be concluded based on our findings: 1) when examined under the auspice of adaptation (>5 d) and not acute exposure (<24 h), the cellular response to reduced O2 levels is not, as previously thought, proportional to the magnitude of reduction in oxygen levels; 2) changes in the expression of a protein during genuine hypoxia (1% O2) may be more appropriately determined when compared to physiologic normoxia rather than room air, as we demonstrated for CaM (Fig. 2E) and PP2A-C (Fig. 8A) expression; and 3) effects of reducing cytosolic O2 levels within the physiologic range can still be readily observed in the absence of significant HIF-1α stabilization (Fig. 1).
In summary, our findings highlight several physiologic readouts significantly altered when cells are cultured in physiologic O2. Specific to this study, culture in 5% O2 facilitated the identification a novel negative feedback mechanism regulating Ca2+-sensitive NO production. Given its central role, changes in the physiology of PP2A in 5% O2, from subunit expression to subcellular distribution, have widespread implications for cell physiology. Advances in our understanding of physiology must be accompanied by adaptations in the way we study it, and thus we propose considering physiologic O2 levels as a significant advancement in routine cell culture.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Kalwant Authi for assistance with ultracentrifugation, Paraskevi-Maria Psefteli for experimental assistance, and Prof. Jeremy Pearson and Dr. Sarah J. Chapple (all from King’s College) for helpful discussions, and the midwives of St. Thomas’ Hospital (London, United Kingdom) for assistance in the collection of umbilical cords. This work was supported by Grant FS/13/66/30445 from the British Heart Foundation and NET01/13 from Heart Research UK. The authors declare no conflicts of interest.
Glossary
- Akt
protein kinase B
- [Ca2+]i
intracellular Ca2+
- CaM
calmodulin
- DETA NONOate
(Z)-1-[N-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate
- eNOS
endothelial NOS
- FCS
fetal calf serum
- HCAEC
human coronary artery endothelial cell
- HIF
hypoxia-inducible factor
- IBMX
3-isobutyl-1-methylxanthine
- NAME
NG-nitro-l-arginine methyl ester
- PLA
proximity ligation assay
- PP
protein phosphatase
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
T. P. Keeley and G. E. Mann conceived of the study; T. P. Keeley, R. Jacob, and G. E. Mann devised the methodology; T. P. Keeley performed the investigation; T. P. Keeley wrote the original draft; T. P. Keeley, R. C. M. Siow, R. Jacob, and G. E. Mann reviewed and edited the draft; and R. C. M. Siow and G. E. Mann were responsible for acquisition of funding.
REFERENCES
- 1.Balin A. K., Goodman D. B., Rasmussen H., Cristofalo V. J. (1977) The effect of oxygen and vitamin E on the lifespan of human diploid cells in vitro. J. Cell Biol. 74, 58–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parrinello S., Samper E., Krtolica A., Goldstein J., Melov S., Campisi J. (2003) Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat. Cell Biol. 5, 741–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkuri K. R., Herzenberg L. A., Niemi A.-K., Cowan T., Herzenberg L. A. (2007) Importance of culturing primary lymphocytes at physiological oxygen levels. Proc. Natl. Acad. Sci. USA 104, 4547–4552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapple S. J., Keeley T. P., Mastronicola D., Arno M., Vizcay-Barrena G., Fleck R., Siow R. C. M., Mann G. E. (2016) Bach1 differentially regulates distinct Nrf2-dependent genes in human venous and coronary artery endothelial cells adapted to physiological oxygen levels. Free Radic. Biol. Med. 92, 152–162 [DOI] [PubMed] [Google Scholar]
- 5.Tsai A. G., Johnson P. C., Intaglietta M. (2003) Oxygen gradients in the microcirculation. Physiol. Rev. 83, 933–963 [DOI] [PubMed] [Google Scholar]
- 6.Riley R. J., Johnson J. W. (1993) Collecting and analyzing cord blood gases. Clin. Obstet. Gynecol. 36, 13–23 [DOI] [PubMed] [Google Scholar]
- 7.Thomas D. D., Liu X., Kantrow S. P., Lancaster J. R. Jr (2001) The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proc. Natl. Acad. Sci. USA 98, 355–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Victor V. M., Nuñez C., D’Ocón P., Taylor C. T., Esplugues J. V., Moncada S. (2009) Regulation of oxygen distribution in tissues by endothelial nitric oxide. Circ. Res. 104, 1178–1183 [DOI] [PubMed] [Google Scholar]
- 9.Takemoto M., Sun J., Hiroki J., Shimokawa H., Liao J. K. (2002) Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation 106, 57–62 [DOI] [PubMed] [Google Scholar]
- 10.Østergaard L., Stankevicius E., Andersen M. R., Eskildsen-Helmond Y., Ledet T., Mulvany M. J., Simonsen U. (2007) Diminished NO release in chronic hypoxic human endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 293, H2894–H2903 [DOI] [PubMed] [Google Scholar]
- 11.Fleming I., Fisslthaler B., Dimmeler S., Kemp B. E., Busse R. (2001) Phosphorylation of Thr(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circ. Res. 88, E68–E75 [DOI] [PubMed] [Google Scholar]
- 12.Kou R., Greif D., Michel T. (2002) Dephosphorylation of endothelial nitric-oxide synthase by vascular endothelial growth factor: implications for the vascular responses to cyclosporin A. J. Biol. Chem. 277, 29669–29673 [DOI] [PubMed] [Google Scholar]
- 13.Zhang Q.-J., Holland W. L., Wilson L., Tanner J. M., Kearns D., Cahoon J. M., Pettey D., Losee J., Duncan B., Gale D., Kowalski C. A., Deeter N., Nichols A., Deesing M., Arrant C., Ruan T., Boehme C., McCamey D. R., Rou J., Ambal K., Narra K. K., Summers S. A., Abel E. D., Symons J. D. (2012) Ceramide mediates vascular dysfunction in diet-induced obesity by PP2A-mediated dephosphorylation of the eNOS-Akt complex. Diabetes 61, 1848–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urbich C., Reissner A., Chavakis E., Dernbach E., Haendeler J., Fleming I., Zeiher A. M., Kaszkin M., Dimmeler S. (2002) Dephosphorylation of endothelial nitric oxide synthase contributes to the anti-angiogenic effects of endostatin. FASEB J. 16, 706–708 [DOI] [PubMed] [Google Scholar]
- 15.García C., Aranda J., Arnold E., Thébault S., Macotela Y., López-Casillas F., Mendoza V., Quiroz-Mercado H., Hernández-Montiel H. L., Lin S.-H., de la Escalera G. M., Clapp C. (2008) Vasoinhibins prevent retinal vasopermeability associated with diabetic retinopathy in rats via protein phosphatase 2A-dependent eNOS inactivation. J. Clin. Invest. 118, 2291–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei Q., Xia Y. (2006) Proteasome inhibition down-regulates endothelial nitric-oxide synthase phosphorylation and function. J. Biol. Chem. 281, 21652–21659 [DOI] [PubMed] [Google Scholar]
- 17.Lorenz M., Koschate J., Kaufmann K., Kreye C., Mertens M., Kuebler W. M., Baumann G., Gossing G., Marki A., Zakrzewicz A., Miéville C., Benn A., Horbelt D., Wratil P. R., Stangl K., Stangl V. (2015) Does cellular sex matter? Dimorphic transcriptional differences between female and male endothelial cells. Atherosclerosis 240, 61–72 [DOI] [PubMed] [Google Scholar]
- 18.Rowlands D. J., Chapple S., Siow R. C. M., Mann G. E. (2011) Equol-stimulated mitochondrial reactive oxygen species activate endothelial nitric oxide synthase and redox signaling in endothelial cells: roles for F-actin and GPR30. Hypertension 57, 833–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt K., Desch W., Klatt P., Kukovetz W. R., Mayer B. (1998) Release of NO from donor compounds: a mathematical model for calculation of NO concentrations in the presence of oxygen. Methods Mol. Biol. 100, 281–289 [DOI] [PubMed] [Google Scholar]
- 20.Liu X., Miller M. J., Joshi M. S., Thomas D. D., Lancaster J. R. Jr (1998) Accelerated reaction of nitric oxide with O2 within the hydrophobic interior of biological membranes. Proc. Natl. Acad. Sci. USA 95, 2175–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Söderberg O., Gullberg M., Jarvius M., Ridderstråle K., Leuchowius K.-J., Jarvius J., Wester K., Hydbring P., Bahram F., Larsson L.-G., Landegren U. (2006) Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods 3, 995–1000 [DOI] [PubMed] [Google Scholar]
- 22.Bokkala S., el-Daher S. S., Kakkar V. V., Wuytack F., Authi K. S. (1995) Localization and identification of Ca2+ATPases in highly purified human platelet plasma and intracellular membranes. Evidence that the monoclonal antibody PL/IM 430 recognizes the SERCA 3 Ca2+ATPase in human platelets. Biochem. J. 306, 837–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thors B., Halldórsson H., Clarke G. D., Thorgeirsson G. (2003) Inhibition of Akt phosphorylation by thrombin, histamine and lysophosphatidylcholine in endothelial cells: differential role of protein kinase C. Atherosclerosis 168, 245–253 [DOI] [PubMed] [Google Scholar]
- 24.Dimmeler S., Fleming I., Fisslthaler B., Hermann C., Busse R., Zeiher A. M. (1999) Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399, 601–605 [DOI] [PubMed] [Google Scholar]
- 25.Kourembanas S., McQuillan L. P., Leung G. K., Faller D. V. (1993) Nitric oxide regulates the expression of vasoconstrictors and growth factors by vascular endothelium under both normoxia and hypoxia. J. Clin. Invest. 92, 99–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi Y. (2009) Serine/threonine phosphatases: mechanism through structure. Cell 139, 468–484 [DOI] [PubMed] [Google Scholar]
- 27.Harris M. B., Ju H., Venema V. J., Liang H., Zou R., Michell B. J., Chen Z. P., Kemp B. E., Venema R. C. (2001) Reciprocal phosphorylation and regulation of endothelial nitric-oxide synthase in response to bradykinin stimulation. J. Biol. Chem. 276, 16587–16591 [DOI] [PubMed] [Google Scholar]
- 28.Favre B., Turowski P., Hemmings B. A. (1997) Differential inhibition and posttranslational modification of protein phosphatase 1 and 2A in MCF7 cells treated with calyculin-A, okadaic acid, and tautomycin. J. Biol. Chem. 272, 13856–13863 [DOI] [PubMed] [Google Scholar]
- 29.Fulton D., Fontana J., Sowa G., Gratton J.-P., Lin M., Li K.-X., Michell B., Kemp B. E., Rodman D., Sessa W. C. (2002) Localization of endothelial nitric-oxide synthase phosphorylated on serine 1179 and nitric oxide in Golgi and plasma membrane defines the existence of two pools of active enzyme. J. Biol. Chem. 277, 4277–4284 [DOI] [PubMed] [Google Scholar]
- 30.Schmitz M. H. A., Held M., Janssens V., Hutchins J. R. A., Hudecz O., Ivanova E., Goris J., Trinkle-Mulcahy L., Lamond A. I., Poser I., Hyman A. A., Mechtler K., Peters J.-M., Gerlich D. W. (2010) Live-cell imaging RNAi screen identifies PP2A-B55alpha and importin-beta1 as key mitotic exit regulators in human cells. Nat. Cell Biol. 12, 886–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grallert A., Boke E., Hagting A., Hodgson B., Connolly Y., Griffiths J. R., Smith D. L., Pines J., Hagan I. M. (2015) A PP1-PP2A phosphatase relay controls mitotic progression. Nature 517, 94–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olszewska-Pazdrak B., Hein T. W., Olszewska P., Carney D. H. (2009) Chronic hypoxia attenuates VEGF signaling and angiogenic responses by downregulation of KDR in human endothelial cells. Am. J. Physiol. Cell Physiol. 296, C1162–C1170 [DOI] [PubMed] [Google Scholar]
- 33.Toporsian M., Govindaraju K., Nagi M., Eidelman D., Thibault G., Ward M. E. (2000) Downregulation of endothelial nitric oxide synthase in rat aorta after prolonged hypoxia in vivo. Circ. Res. 86, 671–675 [DOI] [PubMed] [Google Scholar]
- 34.Murata T., Sato K., Hori M., Ozaki H., Karaki H. (2002) Decreased endothelial nitric-oxide synthase (eNOS) activity resulting from abnormal interaction between eNOS and its regulatory proteins in hypoxia-induced pulmonary hypertension. J. Biol. Chem. 277, 44085–44092 [DOI] [PubMed] [Google Scholar]
- 35.McQuillan L. P., Leung G. K., Marsden P. A., Kostyk S. K., Kourembanas S. (1994) Hypoxia inhibits expression of eNOS via transcriptional and posttranscriptional mechanisms. Am. J. Physiol. 267, H1921–H1927 [DOI] [PubMed] [Google Scholar]
- 36.Atkuri K. R., Herzenberg L. A., Herzenberg L. A. (2005) Culturing at atmospheric oxygen levels impacts lymphocyte function. Proc. Natl. Acad. Sci. USA 102, 3756–3759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tiede L. M., Cook E. A., Morsey B., Fox H. S. (2011) Oxygen matters: tissue culture oxygen levels affect mitochondrial function and structure as well as responses to HIV viroproteins. Cell Death Dis. 2, e246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stacpoole S. R. L., Bilican B., Webber D. J., Luzhynskaya A., He X. L., Compston A., Karadottir R., Franklin R. J. M., Chandran S. (2011) Derivation of neural precursor cells from human ES cells at 3% O(2) is efficient, enhances survival and presents no barrier to regional specification and functional differentiation. Cell Death Differ. 18, 1016–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li T.-S., Cheng K., Malliaras K., Matsushita N., Sun B., Marbán L., Zhang Y., Marbán E. (2011) Expansion of human cardiac stem cells in physiological oxygen improves cell production efficiency and potency for myocardial repair. Cardiovasc. Res. 89, 157–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stacpoole S. R. L., Webber D. J., Bilican B., Compston A., Chandran S., Franklin R. J. M. (2013) Neural precursor cells cultured at physiologically relevant oxygen tensions have a survival advantage following transplantation. Stem Cells Transl. Med. 2, 464–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Alami M., Viña-Almunia J., Gambini J., Mas-Bargues C., Siow R. C. M., Peñarrocha M., Mann G. E., Borrás C., Viña J. (2014) Activation of p38, p21, and NRF-2 mediates decreased proliferation of human dental pulp stem cells cultured under 21% O2. Stem Cell Rep. 3, 566–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamamoto K., Korenaga R., Kamiya A., Qi Z., Sokabe M., Ando J. (2000) P2X(4) receptors mediate ATP-induced calcium influx in human vascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 279, H285–H292 [DOI] [PubMed] [Google Scholar]
- 43.Ando J., Komatsuda T., Kamiya A. (1988) Cytoplasmic calcium response to fluid shear stress in cultured vascular endothelial cells. In Vitro Cell. Dev. Biol. 24, 871–877 [DOI] [PubMed] [Google Scholar]
- 44.Ayajiki K., Kindermann M., Hecker M., Fleming I., Busse R. (1996) Intracellular pH and tyrosine phosphorylation but not calcium determine shear stress-induced nitric oxide production in native endothelial cells. Circ. Res. 78, 750–758 [DOI] [PubMed] [Google Scholar]
- 45.Fleming I., Bauersachs J., Fisslthaler B., Busse R. (1998) Ca2+-independent activation of the endothelial nitric oxide synthase in response to tyrosine phosphatase inhibitors and fluid shear stress. Circ. Res. 82, 686–695 [DOI] [PubMed] [Google Scholar]
- 46.Lantoine F., Iouzalen L., Devynck M. A., Millanvoye-Van Brussel E., David-Dufilho M. (1998) Nitric oxide production in human endothelial cells stimulated by histamine requires Ca2+ influx. Biochem. J. 330, 695–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Østergaard L., Simonsen U., Eskildsen-Helmond Y., Vorum H., Uldbjerg N., Honoré B., Mulvany M. J. (2009) Proteomics reveals lowering oxygen alters cytoskeletal and endoplasmatic stress proteins in human endothelial cells. Proteomics 9, 4457–4467 [DOI] [PubMed] [Google Scholar]
- 48.Persechini A., Stemmer P. M. (2002) Calmodulin is a limiting factor in the cell. Trends Cardiovasc. Med. 12, 32–37 [DOI] [PubMed] [Google Scholar]
- 49.Busse R., Mülsch A. (1990) Calcium-dependent nitric oxide synthesis in endothelial cytosol is mediated by calmodulin. FEBS Lett. 265, 133–136 [DOI] [PubMed] [Google Scholar]
- 50.Woods A., Dickerson K., Heath R., Hong S.-P., Momcilovic M., Johnstone S. R., Carlson M., Carling D. (2005) Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2, 21–33 [DOI] [PubMed] [Google Scholar]
- 51.Moreno C. S., Park S., Nelson K., Ashby D., Hubalek F., Lane W. S., Pallas D. C. (2000) WD40 repeat proteins striatin and S/G(2) nuclear autoantigen are members of a novel family of calmodulin-binding proteins that associate with protein phosphatase 2A. J. Biol. Chem. 275, 5257–5263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Janssens V., Jordens J., Stevens I., Van Hoof C., Martens E., De Smedt H., Engelborghs Y., Waelkens E., Goris J. (2003) Identification and functional analysis of two Ca2+-binding EF-hand motifs in the B"/PR72 subunit of protein phosphatase 2A. J. Biol. Chem. 278, 10697–10706 [DOI] [PubMed] [Google Scholar]
- 53.Davis A. J., Yan Z., Martinez B., Mumby M. C. (2008) Protein phosphatase 2A is targeted to cell division control protein 6 by a calcium-binding regulatory subunit. J. Biol. Chem. 283, 16104–16114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu Q., Pallas D. C., Surks H. K., Baur W. E., Mendelsohn M. E., Karas R. H. (2004) Striatin assembles a membrane signaling complex necessary for rapid, nongenomic activation of endothelial NO synthase by estrogen receptor alpha. Proc. Natl. Acad. Sci. USA 101, 17126–17131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baharians Z., Schönthal A. H. (1998) Autoregulation of protein phosphatase type 2A expression. J. Biol. Chem. 273, 19019–19024 [DOI] [PubMed] [Google Scholar]
- 56.Sanders S. P., Zweier J. L., Kuppusamy P., Harrison S. J., Bassett D. J., Gabrielson E. W., Sylvester J. T. (1993) Hyperoxic sheep pulmonary microvascular endothelial cells generate free radicals via mitochondrial electron transport. J. Clin. Invest. 91, 46–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chandel N. S., Maltepe E., Goldwasser E., Mathieu C. E., Simon M. C., Schumacker P. T. (1998) Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl. Acad. Sci. USA 95, 11715–11720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palacios-Callender M., Hollis V., Mitchison M., Frakich N., Unitt D., Moncada S. (2007) Cytochrome c oxidase regulates endogenous nitric oxide availability in respiring cells: a possible explanation for hypoxic vasodilation. Proc. Natl. Acad. Sci. USA 104, 18508–18513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. (1990) Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 87, 1620–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.