Abstract
Extensive liver resections are common, and bleeding is frequent in these operations. Impaired regeneration after partial hepatectomy (PHx) may contribute to liver failure. We thus assessed the impact of acute bleeding on the liver regeneration progress after PHx and explored possible contributing molecular mechanisms. In rats, the regeneration progress was delayed and attenuated with PHx and bleeding and was not restored with colloid resuscitation. Livers restored their initial volume by postoperative day (POD) 2 after PHx through hepatocyte proliferation vs. POD 4 in the PHx and bleeding group, primarily by hepatocyte hypertrophy. With bleeding, hepatocyte proliferation was hindered in two mechanisms: by inhibiting cells from starting proliferation and by causing hindrance in G1/S progression. Liver hypoxia was prominent, with significant prolonged up-regulation of hypoxia-inducible factors (HIF) and HIF-targeted genes only in the PHx and bleeding group. Gene expression profiling revealed alterations in numerous genes that belong to critical pathways, including cell cycle, DNA replication, PI3K-Akt, purine, and pyrimidine metabolism. Because liver surgery is frequently performed in patients with a predamaged liver, an improper regenerative process after PHx and bleeding might lead to decompensation. The results hint at specific pathways to target in order to improve liver regeneration during PHx and bleeding.—Matot, I., Nachmansson, N., Duev, O., Schulz, S., Schroeder-Stein, K., Frede, S., Abramovitch, R. Impaired liver regeneration after hepatectomy and bleeding is associated with a shift from hepatocyte proliferation to hypertrophy.
Keywords: hypoxia-inducible factor, liver failure, mTOR
Liver regeneration is a coordinated process that rapidly compensates for the acute loss of liver parenchyma (1, 2), allowing performance of extensive resections. The challenges imposed by the surgical and anesthesia teams during these extreme scenarios are remarkable; in certain cases, resection may lead to postoperative liver failure (3). Avoiding further damage to the remnant liver and the regenerating process is thus of utmost importance. One of the possible harmful events that are frequently encountered during extended liver operations is significant bleeding. The liver is well recognized as a target for injury in low flow states associated with acute and massive bleeding. Even after initial successful resuscitation, liver damage may persist for a prolonged period of time, which in turn is known to significantly increase morbidity and mortality (4–6). Previously we characterized in rats the effect of partial hepatectomy (PHx) on liver regeneration, perfusion, and oxygenation (7, 8). More recently, in an in vivo rat model of acute bleeding followed by either fluid or blood resuscitation, we observed significant liver injury induced by bleeding with marked differences in liver perfusion and oxygenation, as well as in indices of liver injury among the different resuscitation protocols (9, 10). The effect of bleeding on the regeneration process after PHx has been evaluated in only one previous study (11), which reported no significant differences in mitochondrial metabolic function during regeneration among rats having only PHx and rats in which hemorrhage was additionally induced. The focus of the current study was to further evaluate the effect of bleeding on liver regeneration kinetics after PHx. Additionally, potential contributing pathways for the adverse effects of bleeding on the regenerative course were assessed.
MATERIALS AND METHODS
Animal experiments
All experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Hebrew University [Approval OPRR-A01-5011; National Institutes of Health (NIH), Bethesda, MD, USA]. Adult male Sprague Dawley rats (n = 106; weight, 270 ± 20 g) were used for all experiments. Experiments were performed on spontaneously breathing anesthetized rats (100 mg/kg ketamine with 5 mg/kg xylazine, i.p.). Both femoral veins and femoral arteries were cannulated for measurements of blood pressure (small animal monitoring and gating system; SA Instruments, Stony Brook, NY, USA), for bleeding the animals, and for administration of fluids (resuscitation). Bleeding was achieved by withdrawing 3 ml of blood (1 ml/min). Arterial blood samples (0.2 ml) were taken at baseline and 10 min after bleeding and PHx for blood analysis (Cobas B221Omni-S; Roche Diagnostics, Indianapolis, IN, USA). PHx resection of 50% of the total liver mass was performed according to Higgins and Anderson (12) by removing the median and left lateral lobes (7, 8).
Rats were randomly divided into 6 experimental groups. The first group, the PHx group (n = 24), comprised animals that underwent 50% PHx. The second group, the PHx and bleeding group (n = 24), comprised animals that underwent 50% PHx and bleeding as previously described. The third group, the colloid resuscitation group (n = 24), comprised animals that, 10 min after 50% PHx and bleeding, received an infusion of 3 ml of colloids [hydroxyethyl starch (HAES) 10% 1 ml/min]. The fourth group was the bleeding-only group (n = 10), in which bleeding was achieved as previously described. The fifth group was the PHx and rapamycin group (n = 12), which was made up of animals that underwent 50% PHx while they were treated with rapamycin (Sirolimus; R-5000; LC Laboratories, Woburn, MA, USA) 0.4 mg/kg 3 h before surgery and daily until death. The sixth group was the PHx, bleeding, and rapamycin group (n = 12) and comprised animals that underwent 50% PHx and bleeding as previously described while treated with rapamycin (Sirolimus; R-5000; LC Laboratories) 0.4 mg/kg 3 h before surgery and daily until death.
Six rats per time point were humanely killed at postoperative days (POD) 1, 2, 4, and 7, and liver samples were either snap-frozen in liquid nitrogen and stored at −80°C for RNA extraction, or fixed in formalin for histologic evaluation. Blood samples were also collected for blood cell count and for analyses of liver enzymes, bilirubin levels, and serum cytokine levels. Liver enzyme levels in serum [alanine aminotransferase (ALT) and aspartate aminotransferase] were determined in duplicate using Reflotron (Roche Diagnostics). For measurement of serum prothrombin time, sodium citrate (0.105 M) was added immediately to the blood sample at a ratio of 1:10 (v/v), and values were recorded with an ACL 9000 coagulation analyzer (Beckman Coulter, Brea, CA, USA) according to the manufacturer’s instructions.
Cytokine levels
Serum cytokine levels of IL-6 were measured using a flow cytometry bead array (BD Biosciences, San Jose, CA, USA) according to the manufacturer’s protocol. The analysis was conducted using a conventional flow cytometer (FACScalibur; Becton Dickinson, San Diego, CA, USA).
Histology, immunohistochemistry, and Western blot analyses
Liver samples for histologic evaluation were routinely processed by formalin fixation and paraffin embedding, followed by hematoxylin and eosin staining. Apoptosis was assessed by immunostaining using TUNEL (Roche Diagnostics). Bromodeoxyuridine (BrdU) immunostaining was performed to assess cell proliferation; BrdU (Sigma-Aldrich, St. Louis, MO, USA) was injected (100 mg/kg, i.p.) 3 h before rats were humanely killed. After target retrieval, sections were incubated with primary monoclonal anti-BrdU antibody (1:200; Neomarker, Fremont, CA, USA). Proliferation cell nuclear antigen (PCNA) immunostaining was performed using monoclonal antibody (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Ki-67 immunostaining was performed using polyclonal anti-Ki-67 antibody (1:100; AB9260; EMD Millipore, Billerica, MA, USA), and for cyclin D1, we used anti–cyclin D1 antibody (1:125; Diagnostic Biosystems, Pleasanton, CA, USA). Mapping of hepatic hypoxic regions (pO2 < 10 mmHg) was performed with pimonidazole (Hypoxyprobe; HPI, Burlington, MA, USA), which was injected (60 mg/kg, i.p.) 1 h before rats were humanely killed. After target retrieval, sections were incubated with anti-pimonidazole (1:1000 Hypoxyprobe; Natural Pharmacia International, Belmont, MA, USA). Histologic slides of the livers were analyzed in a blinded manner by a professional pathologist. For each immunostaining, the number of positive cells per high-power field (magnification, ×400) was counted in 10 randomly selected fields per liver (6 rats/group/time point), and mean values ± sd were determined.
Measurement of cell size
Digital images of liver thin sections (5 μm thick) prepared from formalin-fixed, paraffin-embedded samples stained by immunofluorescence for β-catenin (BD Biosciences) were photographed at a magnification of ×400. Hepatocyte size was measured as area in pixels using ImageJ software (Image Processing and Analysis in Java; NIH; http://imagej.nih.gov/) in 100 hepatocytes per rat by an observer who was blinded to the treatment groups.
Gene expression profiling
RNA was isolated from liver samples of rats obtained 24 h after PHx (n = 3/group). Total RNA was isolated from frozen liver tissues with Trizol reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Amplified and biotinylated sense-strand DNA were prepared according to the standard Affymetrix protocol from 100 ng total RNA (Expression Analysis WT Plus Technical Manual 2013; Affymetrix, Santa Clara, CA, USA). After fragmentation, 2.3 µg of biotinylated sense-strand DNA was hybridized for 16 h at 45°C on the GeneChip Rat Gene 2.0 ST Array. GeneChips were washed and stained in the Affymetrix Fluidic Station 450. GeneChips were scanned using the Affymetrix Gene Chip Scanner 3000. Preprocessing was performed with Affymetrix tools, and 2 algorithms for summarizing microarrays probes (robust multichip average algorithm and probe logarithmic intensity error) were deployed with the aim of increasing the statistical power of the gene differentiation analysis. Gene and gene ontology annotations were determined for each probe set according to the annotation files published on the Affymetrix website (http://www.affymetrix.com/). Log2-normalized expression values, after thresholding and filtering, were submitted to Kyoto Encyclopedia of Genes and Genomes (KEGG)-based pathway search and whole-gene GO-term analysis.
Gene expression analysis by real-time PCR
Total RNA was extracted from frozen liver section obtained 1, 2, 4, and 7 d after PHx with or without bleeding. Tissue was homogenized in Trizol (Thermo Fisher Scientific) and extracted according to the manufacturer’s instructions. cDNA synthesis of 2 µg total RNA was performed using the high-capacity cDNA synthesis kit (Thermo Fisher Scientific). cDNA expression was detected by real-time PCR using commercially available TaqMan probes specific for the detection of the mRNA of hypoxia-inducible factor (HIF)-1α, HIF-2α, aldolase, phosphoglycerate kinase 1 (PGK-1), erythropoietin, and TGF-β. Amounts of specific cDNA were normalized to the housekeeping gene 18S, and cDNA expression was calculated as relative expression to naive control (∆∆Ct method).
Isolation and preparation of whole-cell extracts from liver tissue
Whole-cell extract proteins were isolated from liver tissues. The livers were homogenated with lysis buffer (buffer A) containing 20 mM HEPES (pH 7.5), 1.5 mM MgCl2, 0.2 mM EDTA, 100 mM NaCl, 2 mM DTT, 0.4 mM PMSF, 1 mM Na3VO4, and a protease inhibitor cocktail (Roche Diagnostics). The lysates were incubated on ice for 15 min, followed by centrifugation at 10,000g for 30 min at 4°C. The supernatants were collected, and buffer B was added in v/v (1:1) with buffer A. Buffer B contained 20 mM HEPES (pH 7.5), 1.5 mM MgCl2, 0.2 mM EDTA, 40% glycerol, 2 mM DTT, 0.4 mM PMSF, 1 mM Na3VO4, and the protease inhibitor cocktails previously listed. After vortex mixing, total protein concentration in each lysate was determined using the Bradford assay (Bio-Rad, Hercules, CA, USA).
Western blot analysis
Equal amounts of lysates containing 50 µg of total protein were separated on 7.5 or 8.5% SDS-PAGE gels and electrotransferred onto nitrocellulose membranes (Schleicher and Schuell, Keene, NH, USA). The membranes were blocked in 2% (w/v) bovine serum albumin in a solution of 1× PBS and 0.05% Tween 20. The membranes were incubated at 4°C overnight with a 1:1000 dilution of a primary polyclonal rabbit anti-phospho–mammalian target of rapamycin (mTOR) antibody (Cell Signaling Technology, Danvers, MA, USA), with a 1:1000 dilution of a primary polyclonal rabbit antibody directed against total mTOR (Cell Signaling Technology) or for detection of pS6 using a 1:1000 diluted primary polyclonal rabbit antibody directed against pS6 (Cell Signaling Technology). Loading control was detected using 1:15,000 diluted primary mouse monoclonal anti-β-actin antibody (MP Biomedicals, Solon, OH, USA). The secondary antibodies were either horseradish peroxidase–conjugated goat anti-rabbit IgG or horseradish peroxidase–conjugated goat anti-mouse IgG (Dako, Glostrup, Denmark). Immunoblot signals were detected using enhanced chemiluminescence and quantified by scanning densitometry (Image Lab 5.2; Bio-Rad). Protein loading and images exposure were uniform throughout all experiments.
Statistical analysis
One-way ANOVA for repeated measurements was used to determine statistical significance between the groups, followed by the Student–Newman–Keuls post hoc test. Whenever data were distributed unevenly, the Mann-Whitney U test was used to determine differences between groups. For multiple comparisons, the significance value was subsequently adjusted by the Bonferroni method. A paired, 2-tailed Student’s t test was used for comparisons within groups. We considered a 2-tailed value of P < 0.05 to be statistically significant. Results are presented as means ± sd. Data were analyzed by SigmaStat software (Jandel, San Rafael, CA, USA).
RESULTS
In order to evaluate the consequences of blood loss on liver regeneration, we evaluated the kinetics of this process in rats after PHx and controlled bleeding. Bleeding of 3 ml blood concomitant with 50% liver resection caused significant reductions in mean arterial pressure from 99 ± 6 to 74 ± 14 mmHg and in hemoglobin levels from 12.8 ± 1.0 to 8.3 ± 1.0 Gr% (granulocyte percentage) (Table 1). Platelet count also decreased significantly, from 560 ± 13 to 295 ± 8 or to 130 ± 4 × 103/µl for PHx and for PHx and bleeding, respectively, on the first day after liver resection (Supplemental Fig. 1A).
TABLE 1.
Blood pressure, acid base balance, and hemoglobin at various time points
| Characteristic | Group | ||
|---|---|---|---|
| PHx | PHx and bleeding | HAES | |
| Mean arterial blood pressure (mmHg) | |||
| Baseline | 98 ± 5 | 99 ± 6 | 104 ± 5 |
| PHx | 94 ± 6 | 74 ± 14* | 72 ± 7* |
| Resuscitation | NA | NA | 132 ± 5* |
| pH | |||
| Baseline | 7.33 ± 0.02 | 7.27 ± 0.01 | 7.30 ± 0.01 |
| PHx | 7.33 ± 0.01 | 7.27 ± 0.01 | 7.30 ± 0.01 |
| Resuscitation | NA | NA | 7.14 ± 0.02* |
| HCO3 (mEq/L) | |||
| Baseline | 21.3 ± 0.7 | 20.0 ± 1.0 | 20.9 ± 0.9 |
| PHx | 23.0 ± 0.4 | 16.8 ± 1.0* | 17.0 ± 1.0* |
| Resuscitation | NA | NA | 12.9 ± 1.0* |
| Hemoglobin (Gr%) | |||
| Baseline | 13.7 ± 1.0 | 12.8 ± 1.0 | 12.7 ± 0.9 |
| PHx | 14.0 ± 0.5 | 8.3 ± 1.0* | 9.0 ± 1.0* |
| Resuscitation | NA | NA | 8.1 ± 1.0* |
Values shown are means ± sd; n = 6 rats per group. NA, not applicable. *P < 0.05 vs. baseline.
Bleeding attenuates and delays liver regeneration
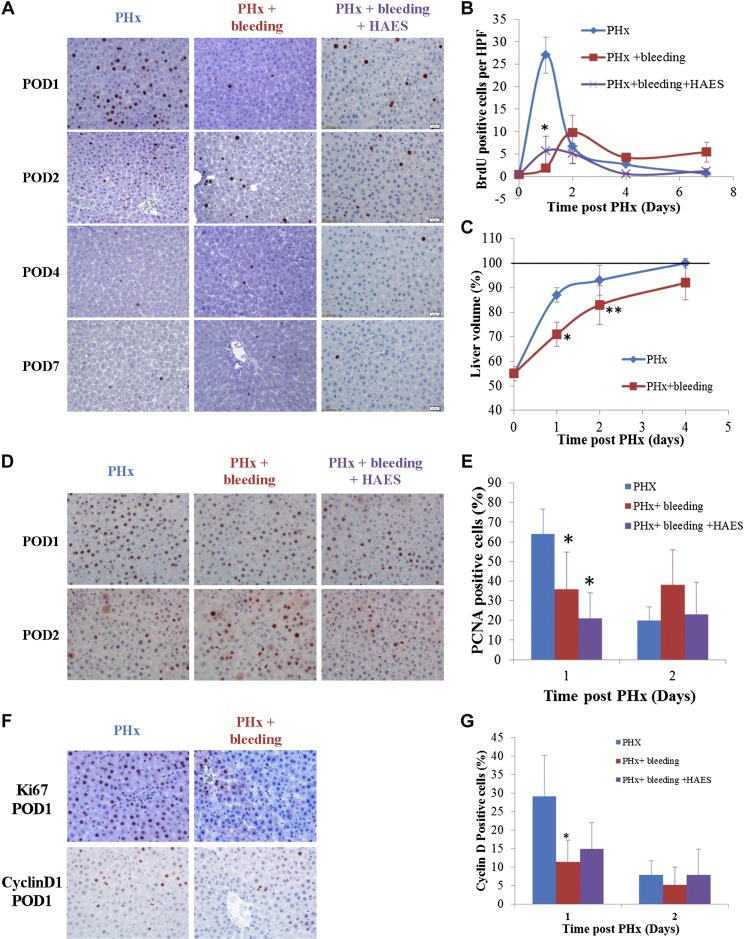
Fifty percent PHx induced a burst of hepatocyte proliferation, which peaked at 24 h after PHx (Fig. 1A, B). Acute bleeding markedly attenuated and delayed the regenerative process. As shown in Fig. 1A, B, hepatocyte proliferation on POD 1 and the following days after PHx and bleeding was significantly reduced (P < 0.0001), with only a few BrdU-labeled hepatocytes detected in liver samples compared to rats that underwent PHx without additional bleeding. Moreover, peak hepatocyte proliferation in the PHx and bleeding group appeared only 48 h after PHx (Fig. 1B). We further measured liver volume on POD 1, 2, and 4 after PHx in both the PHx and the PHx and bleeding groups (n = 4–6 rats/group/time point). Liver volume restoration was significantly attenuated on the first 2 d after PHx and bleeding (Fig. 1C). Nevertheless, the livers of rats subjected to PHx and bleeding reached almost 100% of their initial volume by POD 4 after PHx (Fig. 1C).
Figure 1.
Acute bleeding with or without volume resuscitation (HAES) attenuates regenerative process after 50% PHx. Hepatocyte proliferation was assessed at POD 1, 2, 4, and 7 for rats subjected to PHx (blue), PHx and bleeding (red), or PHx bleeding and HAES resuscitation (purple). Completion of cell proliferation was determined by BrdU incorporation assay (A, B), while entry into cell cycle was assessed by PCNA (D, E). Verification of BrdU results was carried out using Ki-67 staining on slides of livers from POD 1 to eliminate the possibility of inaccessibility due to hypovolemia (F). Cyclin D1 was analyzed to assess progression through G1 phase of cell cycle (F, G). Post-PHx liver volume (C) was evaluated as percentage (means ± sd) of pre-PHx volume in rats subjected to PHx (blue) or PHx and bleeding (red). Representative liver sections of rats humanely killed on indicated days after PHx were immunostained for BrdU (A), PCNA (D), Ki-67 (F), and cyclin D1 (F). Quantification of BrdU-labeled hepatocytes (B) or cyclin D1 (G) and percentage of PCNA-positive nuclei (E) in each slide was conducted by counting in 10 high-power fields per slide. Data represent means ± sd (n = 6 rats/time point); *P < 0.01, **P < 0.05 vs. PHx alone.
Molecular basis for bleeding-induced attenuation of liver regeneration
Cell cycle arrest
Further studies were undertaken to assess the molecular mechanisms that contribute to the observed attenuation of the regenerative process with bleeding. For the first step, in order to eliminate the possibility that bleeding by itself interfered with BrdU absorption, we stained parallel liver samples from the PHx and bleeding group for Ki-67. As can be seen in Fig. 1F, there were only sparse nuclei positive for Ki-67 in rats that underwent PHx and bleeding, thus indicating that proliferation is inhibited. Furthermore, in order to elucidate whether bleeding caused complete inhibition of the entire hepatocyte proliferation process or instead induced cell cycle arrest after initiation of the proliferation process, we stained liver samples for PCNA, a marker of entry into cell cycle. As shown in Fig. 1D, E, the percentage of hepatocyte nuclei expressing PCNA was significantly reduced in the PHx and bleeding group compared to the PHx group on POD 1 (36% compared to 65%, respectively, P < 0.01). Nevertheless, it was not completely blocked, thus confirming that bleeding interfered with hepatocyte proliferation by two mechanisms: first, by inhibiting cells from starting proliferation (about 45% reduction), and second, by causing hindrance in G1/S progression, as BrdU labeling was almost diminished, as shown in Fig. 1B. We further immunostained for cyclin D1, a protein that is required for the progression through the G1 phase of the cell cycle, driving cells into S phase. In samples from the PHx and bleeding group, the percentage of hepatocyte nuclei expressing cyclin D1 was significantly reduced compared to the PHx group on POD 1 (3-fold; Fig. 1F, G). These results strengthen the hindrance in G1/S progression.
Hepatocyte hypertrophy
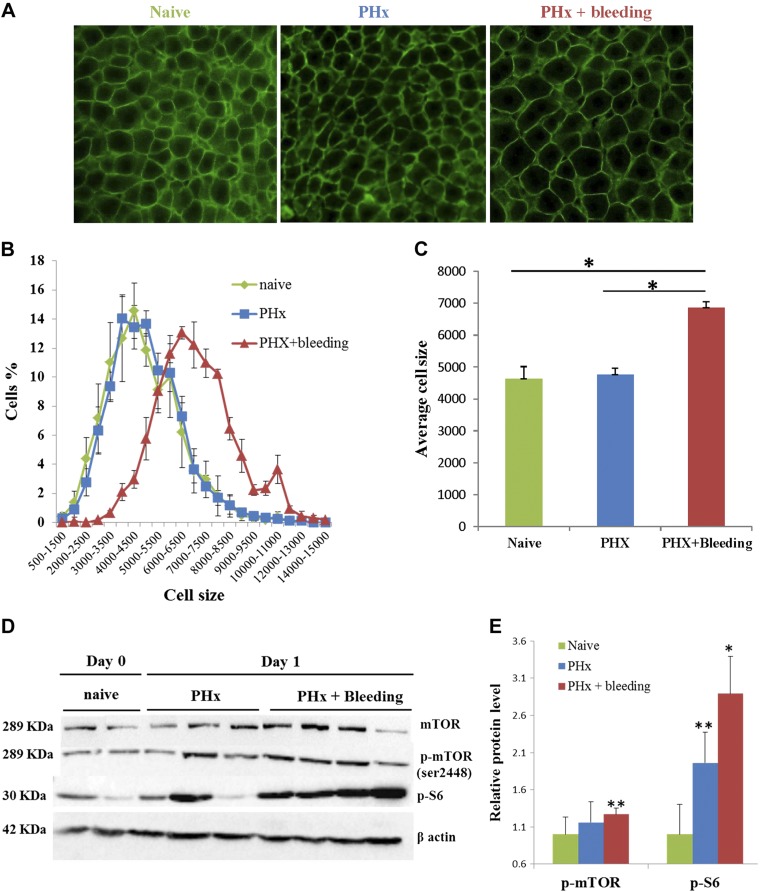
Because the livers restored their initial volume by POD 4 in the PHx and bleeding group (Fig. 1C), with hardly any detectable proliferation (Fig. 1B, F), we hypothesized that liver regeneration is a function of cell hypertrophy rather than cell proliferation. Assessment of hepatocyte size 1 d after PHx demonstrated that in rats that underwent only PHx, hepatocytes were not different compared to hepatocytes in age-matched naive rats. In rats that underwent PHx and bleeding, however, hepatocytes were 50% bigger (P = 0.03, 2-tailed Mann-Whitney test; Fig. 2A–C). Hepatocyte hypertrophy was maintained in rats subjected to PHx and bleeding at least until POD 4 after hepatectomy (Supplemental Fig. 2). The Akt/mTORC1 pathway is a key mediator of cell growth in many cellular systems, including certain settings of liver regeneration (13, 14). We therefore examined whether bleeding influences components of this pathway. Western blot analysis of liver extracts revealed that on POD 1 after hepatectomy, phosphorylation of mTOR and S6 were markedly increased in rats subjected to PHx and bleeding compared to PHx only (Fig. 2D, E). To assess the functional significance of mTORC1 signaling for liver regeneration in rats subjected to PHx and bleeding, we treated additional rats with the mTORC1 inhibitor rapamycin. This treatment resulted in a significant elimination of bleeding-induced hepatocyte hypertrophy along with inhibition of cell proliferation (Supplemental Fig. 2).
Figure 2.
Bleeding generates hyperplastic to hypertrophic switch in regenerative response after PHx. A) Representative microphotographs of β-catenin (green) immunofluorescence staining of liver sections from rats before (left) and on POD 1 after PHx for PHx only (middle) or PHx and bleeding (right). B) Cell size distribution before (naive, green) and on POD 1 after PHx in livers of PHx only (blue) or PHx and bleeding (red) rats (n = 5 rats/group). C) Mean cell size of hepatocytes in the distribution shown in B. D) Western blot analysis of mTOR, phosphorylated mTOR, and phosphorylated S6 in livers from naive rats and rats humanely killed on POD 1 after PHx. E) Western blot quantification. Data are mean ± sem fold change vs. naive for same blot (n = 5). **P < 0.05 vs. naive.
Changes with bleeding in serum IL-6 levels
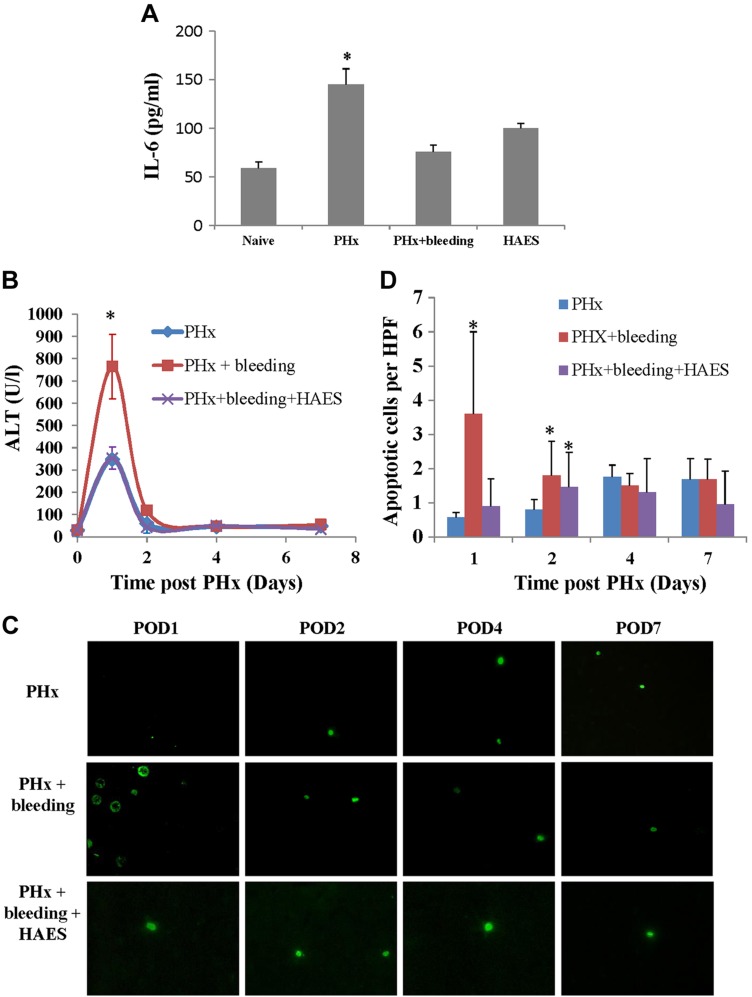
IL-6 is known to play a critical role in the initiation phase of liver regeneration (1, 2). Twenty-four hours after PHx, IL-6 levels were 2.5-fold higher than baseline, whereas after PHx and bleeding, no increases in IL-6 levels were observed (Fig. 3A).
Figure 3.
A) Serum IL-6 levels after PHx. IL-6 concentrations were measured from blood samples collected 24 h after PHx (n = 6 rats/group; *P < 0.01 vs. naive rat serum levels). B–D) Liver injury and apoptosis after PHx. B) Rat liver enzyme levels were measured on POD 1, 2, 4, and 7 after PHx in different groups (n = 6 rats per group). *P < 0.01 vs. PHx. C) Representative images of apoptotic cells in different groups (green fluorescence; original magnification, ×400). D) Diagrams of TUNEL-positive cells counted in 10 high-power fields per slide. Data represent means ± sd (n = 6 rats/time point). *P < 0.01 vs. PHx alone.
Bleeding causes mild liver injury without major effect on liver function
Liver function
Serum bilirubin levels were not elevated in both the PHx and the PHx and bleeding groups at any time point after hepatectomy (data not shown) compared to naive rats. We further checked the prothrombin time for naive rats and for rats on POD 1 and 2 after PHx. In both groups, measurements were within normal limits on POD 1 and 2 after PHx and were not significantly different between the groups (Supplemental Fig. 1B).
Liver injury and apoptosis
The delay in liver regeneration due to bleeding was associated with increased liver injury, as reflected by the significantly elevated ALT levels (2.5-fold, P < 0.001; Fig. 3B) 1 d after PHx and bleeding compared to PHx only. Liver apoptosis was also significantly increased with bleeding (compared to PHx only) (Fig. 3C, D). Nevertheless, the overall number of apoptotic cells was low, suggesting this mechanism is of less importance.
Bleeding after PHx was associated with prolonged liver hypoxia
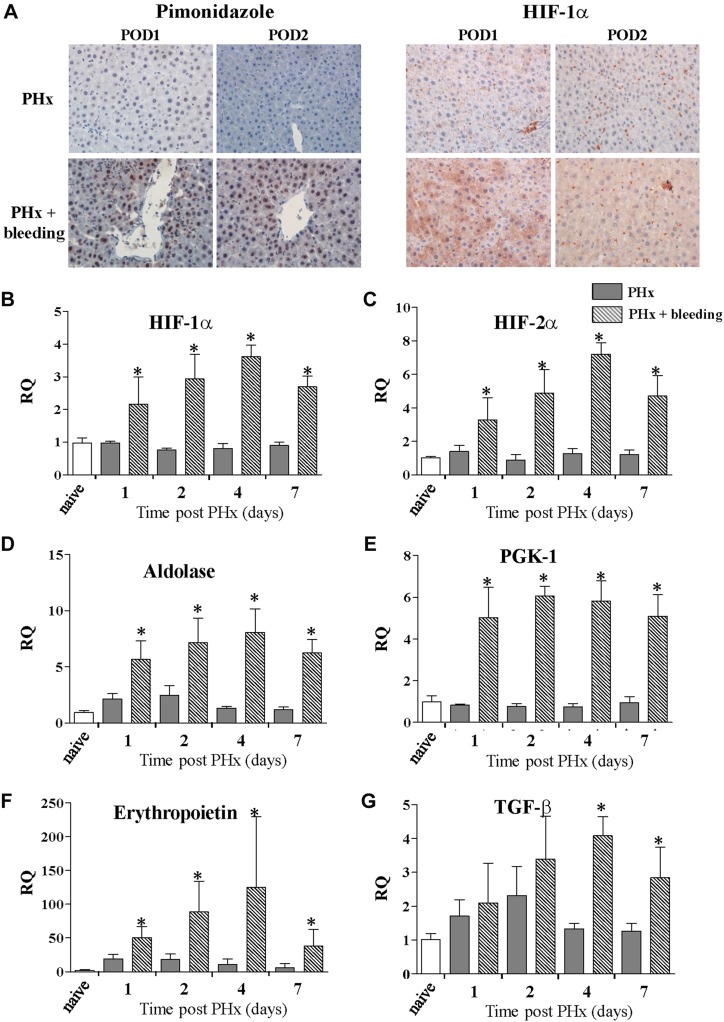
We further studied whether the combined effect of PHx and bleeding was associated with liver hypoxia. Pimonidazole histochemical staining detected liver hypoxia that was most prominent on the first 2 d after PHx in rats that underwent PHx and bleeding in the zone around the central vein. The stain was both cytoplasmic and nuclear. In contrast, only minimal staining was observed in the liver of rats that had undergone PHx only (Fig. 4A). Real-time PCR demonstrated significant up-regulation in the RNA level of the α subunits of HIF-1α and HIF-2α up to 1 wk, with a peak at POD 4 (Fig. 4B, C), only in the PHx and bleeding group. Because previous publications showed that HIF-1α is not readily detectable in hepatocytes and seems to be mainly localized in peroxisomes rather than nuclei (15), we stained the corresponding liver samples for HIF-1α. On POD 1, we found elevated HIF-1α via both cytoplasmic and nuclear staining (Fig. 4A). On POD 2, mainly cytoplasmic staining was detected (Fig. 4A).
Figure 4.
Acute bleeding causes transient liver hypoxia with elevation of HIF-targeted gene expression during regenerative process. A) Liver sections were analyzed by immunohistochemical detection of pimonidazole, a marker of cellular hypoxia (left) and HIF-1α (right). There was no hepatic hypoxia in livers of rats exposed to PHx alone (top), while increased hypoxia was detected as regeneration proceeded (bottom) in rats subjected to PHx and bleeding. Elevated hepatic hypoxia caused elevated levels of HIF-1α staining in rats subjected to PHx and bleeding. B, C) HIF-1α (B) and HIF-2α (C) expression kinetics in liver after PHx with (stripes) or without (gray) bleeding. D–G) Expression kinetics of HIF target genes known to be involved in short-term adaptation to hypoxic conditions was analyzed in liver samples after PHx alone (gray) and PHx and bleeding (stripes). Results shown for glycolytic enzymes aldolase (D) and PGK-1 (E); and glycoprotein hormone erythropoietin (F) and TGF-β (G). Normalized data are provided as relative expression of target gene (n = 6 rats/time point) to naive animals (n = 3; ∆∆Ct method; means ± sd). *P < 0.01.
Furthermore, we analyzed the expression of HIF-targeted genes known to be involved in short-term adaptation to hypoxic conditions. The glycolytic enzymes aldolase and PGK-1 were both up-regulated in liver samples of rats that underwent PHx and bleeding, whereas no induction was found in rats that underwent PHx alone (Fig. 4D, E). The glycoprotein hormone erythropoietin is one of the most studied hypoxia-inducible genes. PHx alone induced a short and transient up-regulation of erythropoietin expression in liver samples, with a peak on POD 1 and 2. In the PHx and bleeding group, a significantly more pronounced and sustained increase in erythropoietin expression was observed, peaking at POD 4 after PHx (Fig. 4F). TGF-β is known as one of the key mediators of the liver regeneration process. TGF-β is a profibrogenic and antiproliferative protein with pleiotropic functions depending on the cellular context. In parallel to the results observed for erythropoietin expression, we detected a moderate transient increase in TGF-β expression in liver samples from rats that underwent PHx alone. In contrast, a marked and sustained increase in TGF-β expression was observed in rats that underwent PHx and bleeding, with peak expression at POD 4 after PHx (Fig. 4G).
Moderate bleeding does not cause liver injury and hypoxia
In an additional experimental group, we checked whether bleeding of 3 ml alone (i.e., without PHx) can induce liver hypoxia and injury. ALT levels remained within the normal range on POD 1 and 2 after bleeding. No BrdU staining was noted in these livers. Further analysis for HIF-1α, HIF-2α, and HIF-targeted genes by real-time PCR indicated that bleeding alone did not cause hypoxic effects (Supplemental Fig. 3).
Effect of bleeding on gene expression profiling
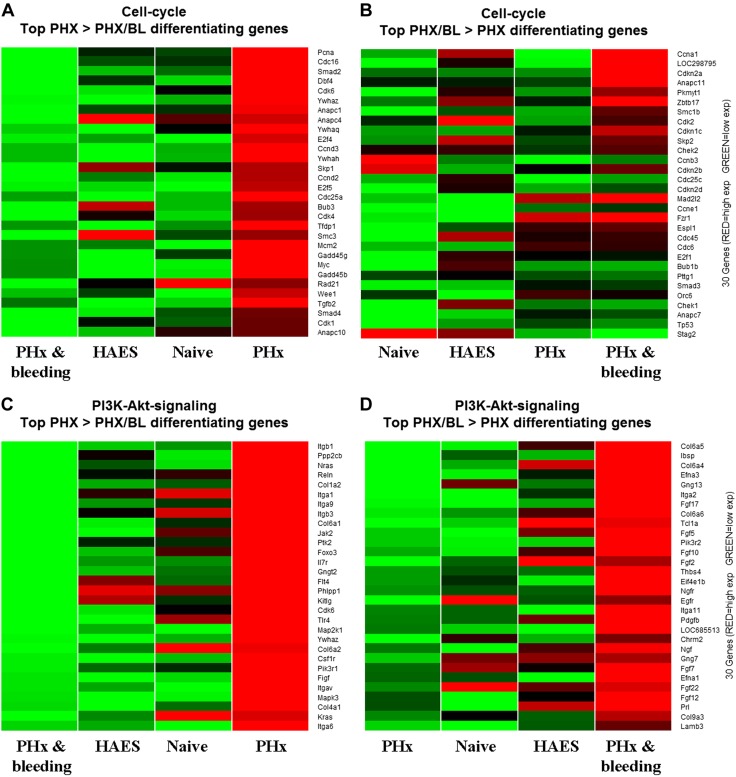
In an additional experiment that aimed to further explore the molecular basis for the attenuated liver regeneration due to bleeding, we performed genome-scale gene expression profiling. We assessed the DNA expression profile in the liver 24 h after intervention using the Affymetrix array because DNA replication after PHx reaches a maximum at this time point (16). Bioinformatic statistical analysis revealed numerous genes that were significantly altered at 24 h after PHx and bleeding vs. PHx alone. Subsequently, we reviewed the annotation groups and enriched pathways of the altered genes. We identified 29 statistically significant pathways containing the differentially expressed genes (Table 2). Among these pathways, we identified the cell cycle, DNA replication, PI3K-Akt, purine, and pyrimidine metabolism pathways. From these we depicted 2 pathways: cell cycle and PI3K-Akt. We present in Fig. 5 the top 30 altered genes for each pathway as heat maps demonstrating a substantial difference in gene expression profiles between these groups.
TABLE 2.
Statistically significant reduced pathways due to bleeding based on robust multichip average preprocess
| Pathway | Name | P | q | Set size | Path size |
|---|---|---|---|---|---|
| rno04110 | Cell cycle | 2.38E−10 | 6.46E−08 | 121 | 127 |
| rno04510 | Focal adhesion | 2.14E−06 | 2.90E−04 | 185 | 206 |
| rno04144 | Endocytosis | 5.50E−06 | 4.97E−04 | 262 | 290 |
| rno00230 | Purine metabolism | 1.10E−05 | 5.17E−04 | 166 | 182 |
| rno03030 | DNA replication | 1.18E−05 | 5.17E−04 | 32 | 36 |
| rno04142 | Lysosome | 1.31E−05 | 5.17E−04 | 119 | 129 |
| rno05206 | MicroRNAs in cancer | 1.33E−05 | 5.17E−04 | 133 | 143 |
| rno03040 | Spliceosome | 1.56E−05 | 5.27E−04 | 112 | 138 |
| rno00240 | Pyrimidine metabolism | 4.77E−05 | 1.34E−03 | 95 | 107 |
| rno04151 | PI3K-Akt signaling pathway | 4.95E−05 | 1.34E−03 | 308 | 336 |
| rno05200 | Pathways in cancer | 7.00E−05 | 1.71E−03 | 371 | 400 |
| rno05205 | Proteoglycans in cancer | 7.58E−05 | 1.71E−03 | 192 | 205 |
| rno04068 | FoxO signaling pathway | 1.16E−04 | 2.43E−03 | 125 | 136 |
| rno05166 | HTLV-I infection | 1.81E−04 | 3.50E−03 | 256 | 295 |
| rno04115 | p53 signaling pathway | 2.26E−04 | 4.09E−03 | 64 | 71 |
| rno05203 | Viral carcinogenesis | 2.72E−04 | 4.45E−03 | 194 | 239 |
| rno04611 | Platelet activation | 2.88E−04 | 4.45E−03 | 112 | 127 |
| rno03008 | Ribosome biogenesis in eukaryotes | 2.96E−04 | 4.45E−03 | 79 | 88 |
| rno05169 | Epstein-Barr virus infection | 3.54E−04 | 5.00E−03 | 195 | 231 |
| rno04120 | Ubiquitin-mediated proteolysis | 3.69E−04 | 5.00E−03 | 128 | 141 |
| rno05220 | Chronic myeloid leukemia | 4.05E−04 | 5.16E−03 | 68 | 76 |
| rno03013 | RNA transport | 4.19E−04 | 5.16E−03 | 144 | 165 |
| rno04810 | Regulation of actin cytoskeleton | 4.79E−04 | 5.57E−03 | 202 | 221 |
| rno04670 | Leukocyte transendothelial migration | 5.12E−04 | 5.57E−03 | 111 | 120 |
| rno04512 | ECM-receptor interaction | 5.14E−04 | 5.57E−03 | 75 | 84 |
| rno05322 | Systemic lupus erythematosus | 5.37E−04 | 5.60E−03 | 87 | 136 |
| rno04210 | Apoptosis | 6.77E−04 | 6.79E−03 | 119 | 141 |
| rno05100 | Bacterial invasion of epithelial cells | 1.05E−03 | 1.00E−02 | 71 | 81 |
| rno05161 | Hepatitis B | 1.07E−03 | 1.00E−02 | 122 | 139 |
ECM, extracellular matrix; HTLV-I, human T-lymphotropic virus I.
Figure 5.
Effect of bleeding on cell cycle A, B) and PI3K-Akt pathway (C, D) genes. Liver samples from naive rats and rats subjected to PHx, to PHx and bleeding, or to PHx and bleeding and HAES resuscitation (n = 3 rats/group) were analyzed by Affymetrix arrays. Functional analysis of differentially expressed genes on POD 1 after PHx revealed numerous genes that were altered by more than 2-fold. Top 30 altered genes for PHx > PHx and bleeding (A, C) and for PHx and bleeding > PHx (B, D) are visualized as heat maps of gene expression, with red indicating up-regulated and green down-regulated genes.
Colloid resuscitation after bleeding does not restore liver regenerative capacity
Several studies previously suggested that the immediate increased portal blood flow after PHx is important in triggering some of the early changes essential for the regenerative process (2). In order to explore whether the hypovolemic effect of bleeding contributes to its observed inhibitory effect on liver regeneration kinetics, an additional group of animals that received colloid resuscitation after PHx and bleeding was assessed. HAES resuscitation did not improve liver regeneration after bleeding. In these rats, the regeneration progress was similar to that observed in rats subjected to PHx and bleeding (Fig. 1). As with bleeding, regeneration was diminished and BrdU-labeled cells in liver samples were scarce (Fig. 1). Further, IL-6 levels did not increase (Fig. 3). With colloid resuscitation, however, no injury to the liver was observed (Fig. 3).
DISCUSSION
Nowadays, improved survival rates of resections compared to nonsurgical strategies in the treatment of patients with liver lesions have been reported (17–20). Accordingly, radical surgical approaches have been implemented, resulting in smaller remnant liver volumes. Intraoperative variables greatly influence patient outcomes and therefore must be considered (21, 22). Operative blood loss, which is not a rare event during extended liver operations, is a critical predictor of posthepatectomy liver failure, perioperative morbidity, and mortality (22). The present study was therefore undertaken to explore the effect of bleeding on the liver regenerative process. Preservation of the regenerative process is critical to avoid postresection liver failure, which has been shown to have a major impact on patient outcome (23). Data from the present study demonstrate that bleeding during PHx attenuates and delays the regenerative process. Moreover, it causes a switch from hepatocyte proliferation to hypertrophy.
One of the remarkable features of the liver is its endogenous capacity to proliferate and regenerate after PHx (2). Hepatocyte proliferation during liver regeneration is characterized primarily by 2 critical phases: the priming phase, which consists of a reversible passage of hepatocytes from the G0 to the G1 state, and the progression phase, where the hepatocytes are committed for the G1-to-S transition (1, 24). For the priming phase to occur, it is critical that TNF-α and IL-6 are secreted (1, 2). Data from the current study provide evidence that the occurrence of bleeding during PHx affects all early phases of liver regeneration. First, with bleeding, the initial trigger did not take place (i.e., the anticipated increase in IL-6 levels was sparse). Additionally, in the array analysis, we identified reduced levels of genes involved in the cell cycle G1-S checkpoint (cyclin A, MCM6, and PCNA), which was later confirmed by immunostaining with PCNA, a marker of cell cycle entry. Moreover, bleeding also hindered G1/S progression, as only few hepatocytes stained positive for BrdU, Ki-67, and cyclin D1. Furthermore, we identified reduced levels of genes involved in G2-M cell cycle (Cdc25 and Cdk1) in the PHx and bleeding group. We also observed elevation in genes that are known to constrain cell proliferation (Cdkn1a, Cdkn1b, and Cdkn1c) in the PHx and bleeding group.
Inhibition of cell cycle progression during liver regeneration has been previously shown to result in enlarged hepatocytes (i.e., in Stat3- or Cdk1-deficient mice) (25, 26). Moreover, hepatocyte hypertrophy was demonstrated to be the first response in normal liver regeneration (14) and in pregnant mice (13). Our findings indicate that hypertrophy, rather than proliferation, is the main mechanism by which the liver regains its volume when bleeding occurs during liver resection. The Akt/mTORC1 signaling axis was shown to be an important pathway for hypertrophy in liver regeneration (13, 14). Indeed, the Affymetrix and Western blot results confirmed that the Akt/mTOR pathway was activated after bleeding and PHx compared to PHx alone. Moreover, by treating rats with rapamycin hepatocytes, hypertrophy was inhibited. Since mTOR inhibitors (sirolimus and everolimus) became a safe alternative to standard immunosuppression therapy after organ transplantation, including liver transplantation (27, 28), according to our current results, it is important to further consider the optimal timing and contraindications when transplanting small-for-size livers because it requires hepatocyte proliferation (29).
Generally the microenvironment of an injured tissue is characterized by low levels of oxygen and glucose and high levels of inflammatory cytokines, which are necessary for the regenerative process (30, 31). Most of the hypoxic adaptations implicate gene expression driven by transcription factors such as HIFs (32, 33). Hypoxia and HIFs are crucial regulators of cell cycle progression and cell proliferation. Interestingly, however, HIF-1α and HIF-2α can prompt adverse effects on cell cycle progression, which is partly due to their molecular interactions with c-Myc and mTOR (34–36). Because of its physiologic oxygen gradient, the liver is a unique organ where maintenance oxygen homeostasis is critical for its specialized function. Yet in a previous study, Khan et al. (15) demonstrated that in hepatocytes that were subjected to hypoxia and reoxygenation, HIF-1α targets the peroxisome rather than the nucleus, where it colocalizes with von Hippel-Lindau and the HIF hydroxylases. Nevertheless, in a rat model of portal ligation combined with parenchymal transection, Schadde et al. (37) showed that different levels of hypoxia may play a critical role in liver regeneration through HIF-1α. In our study, rats subjected to PHx and bleeding demonstrated elevated HIF-1α both in the cytoplasm and the nuclei (Fig. 4A). Moreover, the elevation of HIF target genes also implies that nuclear translocation occurs. Previously Maeno et al. (38) demonstrated that the accumulation of HIF-1α protein in liver sinusoidal endothelial cells after 70% PHx in rats coincides with endothelial reconstruction. In these rats, HIF-1α was rapidly induced in the liver and remained up-regulated for up to 24 h (38, 39). In the current study, acute bleeding on top of PHx induced a marked and prolonged hypoxia along with enhanced expression of HIF and its target genes in the liver parenchyma for up to 4 d after PHx. This prolonged hypoxia after PHx and bleeding is possibly one of the critical contributors to mediate the delayed and attenuated regeneration process. HIF and its target genes are important for the initial phase of tissue regeneration (40). To our knowledge, however, ours is the first study to report that sustained up-regulation of HIF occurred simultaneously with an impaired regenerative process. Interestingly, TGF-β, which is a known suppressor of hepatocyte proliferation, remained up-regulated for a protracted period in rats that underwent bleeding and PHx vs. PHx alone. During regeneration, termination of this process is in part dependent on TGF-β and its balance with other growth factors (41). It is possible that the protracted high levels of TGF-β contributed to the attenuated regeneration. Nevertheless, we should note that our findings do not rule out the existence of other potential mechanisms that could also lead to the delayed regeneration we observed.
The liver plays a central role in maintaining overall energy balance by controlling carbohydrate and lipid metabolism. Oxygen is an important systemic signal that modulates metabolic activities in the liver. Previous studies suggested that HIF-1 and -2 have unique roles in the metabolic adaptation to hypoxia, while HIF-1 regulates the expression of glucose transporters and glycolytic enzymes; HIF-2 regulates β-oxidation and lipid droplet formation (42, 43). Recent data suggest that the metabolic response to hepatic insufficiency might play a critical role in this highly orchestrated process of liver regeneration (44). Usually PHx induces rapid accumulation of lipid droplets, which are assumed to be a source of energy and materials for regeneration. In the present rat model of 50% PHx there was, however, only slight evidence for lipid droplets, with no difference between the experimental groups (Supplemental Fig. 4).
It has been previously suggested that the immediate increase in portal blood flow after PHx is important in triggering some of the early events essential for the regenerative process (2). Because bleeding hindered the regenerative process, we further assessed whether the observed effect resulted from the hypovolemic and hypotensive effects. Considering that colloid resuscitation after PHx and bleeding did not recover any of the early regenerative processes, we speculate that the reduced red blood mass is the influencing factor leading to sustained hypoxic conditions in the remaining liver parenchyma.
In recent years, experimental studies have demonstrated that platelets play an important role in promoting liver regeneration after hepatectomy. One clinical study demonstrated that a decrease of the postoperative platelet percentage to below 60% after hepatectomy was associated with delayed liver function recovery and postoperative morbidity (45). Although platelet counts reached a minimum on POD 3 for all patients (45), when platelet counts returned to preoperative levels significantly earlier, it was indicative of better functional recovery. In the current study, a significantly prolonged reduction in platelet count was observed for the PHx and bleeding group compared to PHx alone, which probably contributed to the observed delayed regeneration.
In the process of liver regeneration, there is a remarkable redundancy between signals. Many of the signaling agents overlap in function, thus providing the missing contributions of the blocked or absent pathway, leading to complete regeneration. Yet liver surgery is frequently performed in elderly patients or in patients with a predamaged liver (e.g., in patients with liver fibrosis or cirrhosis). Further studies should thus characterize the effect of bleeding on liver regeneration under these conditions. Our results demonstrate for the first time the injurious effect of bleeding over PHx. It is thus logical to assess the effect of blood transfusion in the described rat model of PHx and bleeding. Indeed, this is the next step of our ongoing research, keeping in mind that previous studies from our lab showed blood transfusion to not always be beneficial to the liver (9, 10). Moreover, nowadays, restrictive blood-management protocols are endorsed, as reduced exposure to blood transfusions (with their inherent risks) results in improved patient safety and reduced costs (46). Thus, the results from the present study may hint at specific pathways to target in order to improve the regenerative process during PHx and bleeding without the need for transfusion.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank T. Asraf (Hadassah Hebrew University Medical Center) for performing histologic evaluation for liver samples of rats treated with rapamycin. This research was supported by the Israel Science Foundation (Grant 1157/12; to I.M.), a grant from the European Society of Anesthesiology (to R.A.), the Israeli Health Ministry (Grant 3-10999; to R.A.), and the German Israel Foundation (Grant I-1318-422.13/2015; to R.A., S.F. and I.M.). The authors declare no conflicts of interest.
Glossary
- ALT
alanine aminotransferase
- BrdU
bromodeoxyuridine
- Gr%
granulocyte percentage
- HAES
hydroxyethyl starch
- HIF
hypoxia-inducible factors
- mTOR
mammalian target of rapamycin
- PCNA
proliferation cell nuclear antigen
- PGK-1
phosphoglycerate kinase 1
- PHx
partial hepatectomy
- POD
postoperative day
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
I. Matot, S. Frede, and R. Abramovitch designed research; S. Frede, N. Nachmansson, O. Duev, S. Schulz, K. Schroeder-Stein, and R. Abramovitch analyzed data; N. Nachmansson, O. Duev, S. Schulz, K. Schroeder-Stein, and R. Abramovitch performed research; I. Matot, S. Frede, and R. Abramovitch wrote the article; and all authors read a draft of the article, suggested improvements, and approved the final version.
REFERENCES
- 1.Gilgenkrantz H., Collin de l’Hortet A. (2011) New insights into liver regeneration. Clin. Res. Hepatol. Gastroenterol. 35, 623–629 [DOI] [PubMed] [Google Scholar]
- 2.Michalopoulos G. K. (2010) Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am. J. Pathol. 176, 2–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van den Broek M. A., Olde Damink S. W., Dejong C. H., Lang H., Malagó M., Jalan R., Saner F. H. (2008) Liver failure after partial hepatic resection: definition, pathophysiology, risk factors and treatment. Liver Int. 28, 767–780 [DOI] [PubMed] [Google Scholar]
- 4.Jaeschke H. (2003) Molecular mechanisms of hepatic ischemia–reperfusion injury and preconditioning. Am. J. Physiol. Gastrointest. Liver Physiol. 284, G15–G26 [DOI] [PubMed] [Google Scholar]
- 5.Kobelt F., Schreck U., Henrich H. A. (1994) Involvement of liver in the decompensation of hemorrhagic shock. Shock 2, 281–288 [DOI] [PubMed] [Google Scholar]
- 6.Wang P., Hauptman J. G., Chaudry I. H. (1990) Hepatocellular dysfunction occurs early after hemorrhage and persists despite fluid resuscitation. J. Surg. Res. 48, 464–470 [DOI] [PubMed] [Google Scholar]
- 7.Barash H., Gross E., Edrei Y., Pappo O., Spira G., Vlodavsky I., Galun E., Matot I., Abramovitch R. (2008) Functional magnetic resonance imaging monitoring of pathological changes in rodent livers during hyperoxia and hypercapnia. Hepatology 48, 1232–1241 [DOI] [PubMed] [Google Scholar]
- 8.Barash H., Gross E., Matot I., Edrei Y., Tsarfaty G., Spira G., Vlodavsky I., Galun E., Abramovitch R. (2007) Functional MR imaging during hypercapnia and hyperoxia: noninvasive tool for monitoring changes in liver perfusion and hemodynamics in a rat model. Radiology 243, 727–735 [DOI] [PubMed] [Google Scholar]
- 9.Matot I., Cohen K., Pappo O., Barash H., Abramovitch R. (2008) Liver response to hemorrhagic shock and subsequent resuscitation: MRI analysis. Shock 29, 16–24 [DOI] [PubMed] [Google Scholar]
- 10.Matot I., Katz M., Pappo O., Zelig O., Corchia N., Yedgar S., Barshtein G., Bennett-Guerrero E., Abramovitch R. (2013) Resuscitation with aged blood exacerbates liver injury in a hemorrhagic rat model. Crit. Care Med. 41, 842–849 Erratum in: Crit. Care Med. 2013;41:e41. [DOI] [PubMed] [Google Scholar]
- 11.Castro e Silva O., Kemp R., Sankarankutty A. K., Zucoloto S., Souza M. E., Evora P. R. (2007) The influence of hemorrhagic shock on rat liver regeneration after partial hepatectomy: serum aminotranspherases, mitochondrial function, and hepatocellular replication studies. Dig. Dis. Sci. 52, 2610–2615 [DOI] [PubMed] [Google Scholar]
- 12.Higgins G. M., Anderson R. M. (1931) Restoration of the liver of the white rat following partial surgical removal. Arch. Pathol. (Chic) 12, 186–202 [Google Scholar]
- 13.Gielchinsky Y., Laufer N., Weitman E., Abramovitch R., Granot Z., Bergman Y., Pikarsky E. (2010) Pregnancy restores the regenerative capacity of the aged liver via activation of an mTORC1-controlled hyperplasia/hypertrophy switch. Genes Dev. 24, 543–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyaoka Y., Miyajima A. (2013) To divide or not to divide: revisiting liver regeneration. Cell Div. 8, 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan Z., Michalopoulos G. K., Stolz D. B. (2006) Peroxisomal localization of hypoxia-inducible factors and hypoxia-inducible factor regulatory hydroxylases in primary rat hepatocytes exposed to hypoxia–reoxygenation. Am. J. Pathol. 169, 1251–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michalopoulos G. K., DeFrances M. C. (1997) Liver regeneration. Science 276, 60–66 [DOI] [PubMed] [Google Scholar]
- 17.Clavien P. A., Petrowsky H., DeOliveira M. L., Graf R. (2007) Strategies for safer liver surgery and partial liver transplantation. N. Engl. J. Med. 356, 1545–1559 [DOI] [PubMed] [Google Scholar]
- 18.Feng K., Yan J., Li X., Xia F., Ma K., Wang S., Bie P., Dong J. (2012) A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J. Hepatol. 57, 794–802 [DOI] [PubMed] [Google Scholar]
- 19.Huang J., Yan L., Cheng Z., Wu H., Du L., Wang J., Xu Y., Zeng Y. (2010) A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann. Surg. 252, 903–912 [DOI] [PubMed] [Google Scholar]
- 20.Kopetz S., Chang G. J., Overman M. J., Eng C., Sargent D. J., Larson D. W., Grothey A., Vauthey J. N., Nagorney D. M., McWilliams R. R. (2009) Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J. Clin. Oncol. 27, 3677–3683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cescon M., Vetrone G., Grazi G. L., Ramacciato G., Ercolani G., Ravaioli M., Del Gaudio M., Pinna A. D. (2009) Trends in perioperative outcome after hepatic resection: analysis of 1500 consecutive unselected cases over 20 years. Ann. Surg. 249, 995–1002 [DOI] [PubMed] [Google Scholar]
- 22. Jarnagin, W. R., Gonen, M., Fong, Y., DeMatteo, R. P., Ben-Porat, L., Little, S., Corvera, C., Weber, S., and Blumgart, L. H. (2002) Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann. Surg. 236, 397–406, discussion 406–397. [DOI] [PMC free article] [PubMed]
- 23.Hammond J. S., Guha I. N., Beckingham I. J., Lobo D. N. (2011) Prediction, prevention and management of postresection liver failure. Br. J. Surg. 98, 1188–1200 [DOI] [PubMed] [Google Scholar]
- 24.Fausto N., Campbell J. S., Riehle K. J. (2006) Liver regeneration. Hepatology 43(2 Suppl 1), S45–S53 [DOI] [PubMed] [Google Scholar]
- 25.Diril M. K., Ratnacaram C. K., Padmakumar V. C., Du T., Wasser M., Coppola V., Tessarollo L., Kaldis P. (2012) Cyclin-dependent kinase 1 (Cdk1) is essential for cell division and suppression of DNA re-replication but not for liver regeneration. Proc. Natl. Acad. Sci. USA 109, 3826–3831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haga S., Ogawa W., Inoue H., Terui K., Ogino T., Igarashi R., Takeda K., Akira S., Enosawa S., Furukawa H., Todo S., Ozaki M. (2005) Compensatory recovery of liver mass by Akt-mediated hepatocellular hypertrophy in liver-specific STAT3-deficient mice. J. Hepatol. 43, 799–807 [DOI] [PubMed] [Google Scholar]
- 27.Benzing C., Krezdorn N., Förster J., Hinz A., Atanasov G., Wiltberger G., Morgül M. H., Lange U. G., Schmelzle M., Hau H. M., Bartels M. (2015) Impact of different immunosuppressive regimens on the health-related quality of life following orthotopic liver transplantation. Clin. Transplant. 29, 1081–1089 [DOI] [PubMed] [Google Scholar]
- 28.Glover T. E., Watson C. J., Gibbs P., Bradley J. A., Ntzani E. E., Kosmoliaptsis V. (2016) Conversion from calcineurin to mammalian target of rapamycin inhibitors in liver transplantation: a meta-analysis of randomized controlled trials. Transplantation 100, 621–629 [DOI] [PubMed] [Google Scholar]
- 29.Lu H., Lu L., Zhang F., Zhai Y., Wang X. (2016) Living donor liver transplantation: where do we stand and where are we going? Hepatobiliary Surg. Nutr. 5, 141–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cramer T., Johnson R. S. (2003) A novel role for the hypoxia inducible transcription factor HIF-1alpha: critical regulation of inflammatory cell function. Cell Cycle 2, 192–193 [PubMed] [Google Scholar]
- 31.Karhausen J., Haase V. H., Colgan S. P. (2005) Inflammatory hypoxia: role of hypoxia-inducible factor. Cell Cycle 4, 256–258 [PubMed] [Google Scholar]
- 32.Schofield C. J., Ratcliffe P. J. (2004) Oxygen sensing by HIF hydroxylases. Nat. Rev. Mol. Cell Biol. 5, 343–354 [DOI] [PubMed] [Google Scholar]
- 33.Wang G. L., Jiang B. H., Rue E. A., Semenza G. L. (1995) Hypoxia-inducible factor 1 is a basic–helix–loop–helix–PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 92, 5510–5514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elorza A., Soro-Arnáiz I., Meléndez-Rodríguez F., Rodríguez-Vaello V., Marsboom G., de Cárcer G., Acosta-Iborra B., Albacete-Albacete L., Ordóñez A., Serrano-Oviedo L., Giménez-Bachs J. M., Vara-Vega A., Salinas A., Sánchez-Prieto R., Martín del Río R., Sánchez-Madrid F., Malumbres M., Landázuri M. O., Aragonés J. (2012) HIF2α acts as an mTORC1 activator through the amino acid carrier SLC7A5. Mol. Cell 48, 681–691 [DOI] [PubMed] [Google Scholar]
- 35.Mollenhauer M., Kiss J., Dudda J., Kirchberg J., Rahbari N., Radhakrishnan P., Niemietz T., Rausch V., Weitz J., Schneider M. (2012) Deficiency of the oxygen sensor PHD1 augments liver regeneration after partial hepatectomy. Langenbecks Arch. Surg. 397, 1313–1322 [DOI] [PubMed] [Google Scholar]
- 36.Wouters B. G., Koritzinsky M. (2008) Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat. Rev. Cancer 8, 851–864 [DOI] [PubMed] [Google Scholar]
- 37.Schadde E., Tsatsaris C., Swiderska-Syn M., Breitenstein S., Urner M., Schimmer R., Booy C., Z’graggen B. R., Wenger R. H., Spahn D. R., Hertl M., Knechtle S., Diehl A. M., Schläpfer M., Beck-Schimmer B. (2017) Hypoxia of the growing liver accelerates regeneration. Surgery 161, 666–679 [DOI] [PubMed] [Google Scholar]
- 38.Maeno H., Ono T., Dhar D. K., Sato T., Yamanoi A., Nagasue N. (2005) Expression of hypoxia inducible factor-1alpha during liver regeneration induced by partial hepatectomy in rats. Liver Int. 25, 1002–1009 [DOI] [PubMed] [Google Scholar]
- 39.Nath B., Szabo G. (2012) Hypoxia and hypoxia inducible factors: diverse roles in liver diseases. Hepatology 55, 622–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lokmic Z., Musyoka J., Hewitson T. D., Darby I. A. (2012) Hypoxia and hypoxia signaling in tissue repair and fibrosis. Int. Rev. Cell Mol. Biol. 296, 139–185 [DOI] [PubMed] [Google Scholar]
- 41.Karkampouna S., Ten Dijke P., Dooley S., Julio M. K. (2012) TGFβ signaling in liver regeneration. Curr. Pharm. Des. 18, 4103–4113 [DOI] [PubMed] [Google Scholar]
- 42.Liu Y., Ma Z., Zhao C., Wang Y., Wu G., Xiao J., McClain C. J., Li X., Feng W. (2014) HIF-1α and HIF-2α are critically involved in hypoxia-induced lipid accumulation in hepatocytes through reducing PGC-1α-mediated fatty acid β-oxidation. Toxicol. Lett. 226, 117–123 [DOI] [PubMed] [Google Scholar]
- 43.Rankin E. B., Rha J., Selak M. A., Unger T. L., Keith B., Liu Q., Haase V. H. (2009) Hypoxia-inducible factor 2 regulates hepatic lipid metabolism. Mol. Cell. Biol. 29, 4527–4538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang J., Rudnick D. A. (2014) Elucidating the metabolic regulation of liver regeneration. Am. J. Pathol. 184, 309–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takahashi K., Kurokawa T., Oshiro Y., Fukunaga K., Sakashita S., Ohkohchi N. (2016) Postoperative decrease in platelet counts is associated with delayed liver function recovery and complications after partial hepatectomy. Tohoku J. Exp. Med. 239, 47–55 [DOI] [PubMed] [Google Scholar]
- 46.Goodnough L. T., Maggio P., Hadhazy E., Shieh L., Hernandez-Boussard T., Khari P., Shah N. (2014) Restrictive blood transfusion practices are associated with improved patient outcomes. Transfusion 54, 2753–2759 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.