Abstract
Combination treatment with pioglitazone and metformin are utilized clinically in the treatment of type II diabetes. Treatment with this drug combination reduced the development of aerodigestive cancers in this patient population. Our goal is to expand this treatment into clinical lung cancer chemoprevention. We hypothesized that dietary delivery of metformin/pioglitazone would prevent lung adenoma formation in A/J mice in a B[a]P-induced carcinogenesis model while modulating chemoprevention and anti-inflammatory biomarkers in residual adenomas. We found metformin (500 & 850 mg/kg/day) and pioglitazone (15 mg/kg/day) produced statistically significant decreases in lung adenoma formation both as single agent treatments and in combination, compared to untreated controls, after 15 weeks. Treatment with metformin alone and in combination with pioglitazone resulted in statistically significant decreases in lung adenoma formation at both early and late stage interventions. Pioglitazone alone resulted in significant decreases in adenoma formation only at early treatment intervention. We conclude oral metformin is a viable chemopreventive treatment at doses ranging from 500 to 1000 mg/kg/day. Pioglitazone at 15 mg/kg/day is a viable chemopreventive agent at early stage interventions. Combination metformin and pioglitazone performed equal to metformin alone and better than pioglitazone at 15 mg/kg/day. Since the drugs are already FDA approved, rapid movement to human clinical studies is possible.
Keywords: pioglitazone, metformin, lung cancer, chemoprevention, mouse model
Introduction
Aerodigestive malignancies (lung; head and neck) affect millions worldwide and approximately 90% of these malignancies are attributed to tobacco use. Those treated for an initial malignancy are at risk for second primary malignancies attributable to “field cancerization” (1). “Field cancerization” occurs as a result of prolonged exposure to toxicants found in cigarette smoke, resulting in the development of a field of initiated but morphologically normal appearing cells in the damaged lung epithelium that contain a mutation in an oncogene or tumor suppressor gene (2–4). Continued exposure of the lung parenchyma to environmental toxicants, as occurs in smokers, results in further genetic or epigenetic damage to the initiated cells from genotoxic carcinogens and lung tumor promoters. Minimal change in survival for either malignancy for over a generation mandates novel approaches for those affected with tobacco-associated field cancerization in primary and secondary prevention settings.
Both pioglitazone and metformin are type II diabetes therapies which may have off-target use as aerodigestive cancer chemoprevention agents (5–14). Pioglitazone is a thiazolidinedione (TZD) peroxisome proliferator-activated receptor gamma (PPARγ) activator with chemoprevention capacity preclinically and clinically in both head and neck and lung carcinoma (7,12–19). This drug class has been demonstrated to have anti-inflammatory effects targeting NFκB in both diabetes and cancer. Observational studies derived from the Veterans Administration Veterans Integrated Service Network (VA VISN) database have shown greater than 35% reductions in both head and neck and lung cancer incidence in diabetics treated with TZD drugs (20,21).
Initial interest in metformin as an anticancer agent has come from clinical and epidemiologic research which has shown, for type II diabetes patients prescribed metformin, reduced cancer incidence and/or mortality (22–31). This has provided an empiric basis for its evaluation in the clinical setting. One of the effects of metformin is reducing cell growth and proliferation via attenuation of the insulin/IGF-1R pathway, which inhibits PI3K/Akt/mTOR signaling. In one recent study by Dennis et al., dietary metformin reduced lung cancer burden in the NNK model of mouse carcinogenesis by over 70%, with a potential mechanism being downregulation of IGF-1 receptor phosphorylation (5). Additionally, several recent studies using metformin point to NFκB attenuation by this agent with respect to both angiogenic and matrix metalloproteinase activity in both cancer and atherogenesis (28,32–37). To summarize, both pioglitazone and metformin have been demonstrated to prevent tobacco smoke-induced lung tumor development in preclinical models; however, the pharmacological effectiveness of each agent individually in preventing lung tumor development has been deemed modest. Therefore, it is imperative to elucidate potential additive effects on lung cancer of these agents in combination in a prevention setting.
We presently explore this in an A/J mouse carcinogenesis model used for our chemoprevention work for several decades using a fixed dose combination of pioglitazone plus metformin. The FDA approved two fixed-dose combinations of pioglitazone plus metformin (15 mg/500 mg ACTOPLUS MET® and 15 mg/850 mg ACTOPLUS MET®) for human use, and generic brands of these medicines have been available in the U.S. since December 2012. Therefore, if successful, there could be direct translation to humans with this single drug which combines both agents.
Materials and Methods
Pulmonary tumor formation
Seven-week-old female A/J mice were fed pellet diet NIH-07 7022 (Harlan Teklad Diets, Madison WI) and acclimated to the facility for two weeks. Mice were weighed one day after arrival and then weekly. Mice were then switched to D62 semi-purified diet (Research Diets Inc., New Brunswick, NJ) consisting of 27% vitamin-free casein, 59% corn starch, 10% corn oil, 4% salt mix (USP XIV), and a complete mixture of vitamins. We employ the D62 diet for chemoprevention studies as other diet preparations, providing a more complete complement of nutrients and vitamins, have been found to be chemopreventive in their own right (e.g. soy inositols) (38,39). Animal diet was replenished twice weekly. At 11 weeks of age, the mice were given the first of three administrations of 3 mg benzo[a]pyrene (B[a]P) (TCI America)/kg of body weight in 0.2 mL cottonseed oil by oral gavage (Days 1, 4 and 8). Mice were randomized into treatment groups by weight the day prior to the first administration of test agents and reweighed once per week.
Experimental diet administration
Experimental diets were started one week after last dose of B[a]P. Pioglitazone and metformin were received from the NCI DCP chemical repository. Pioglitazone (15 mg/kg/day) and metformin (500, 850 and 1000 mg/kg/day) alone and in combination were prepared in the D62 diet. The addition of 1000 mg/kg/day metformin as a dietary additive was included in the second study. A third experiment tested lower doses of metformin only at 235 and 470 mg/kg/day.
Dose finding chemoprevention study
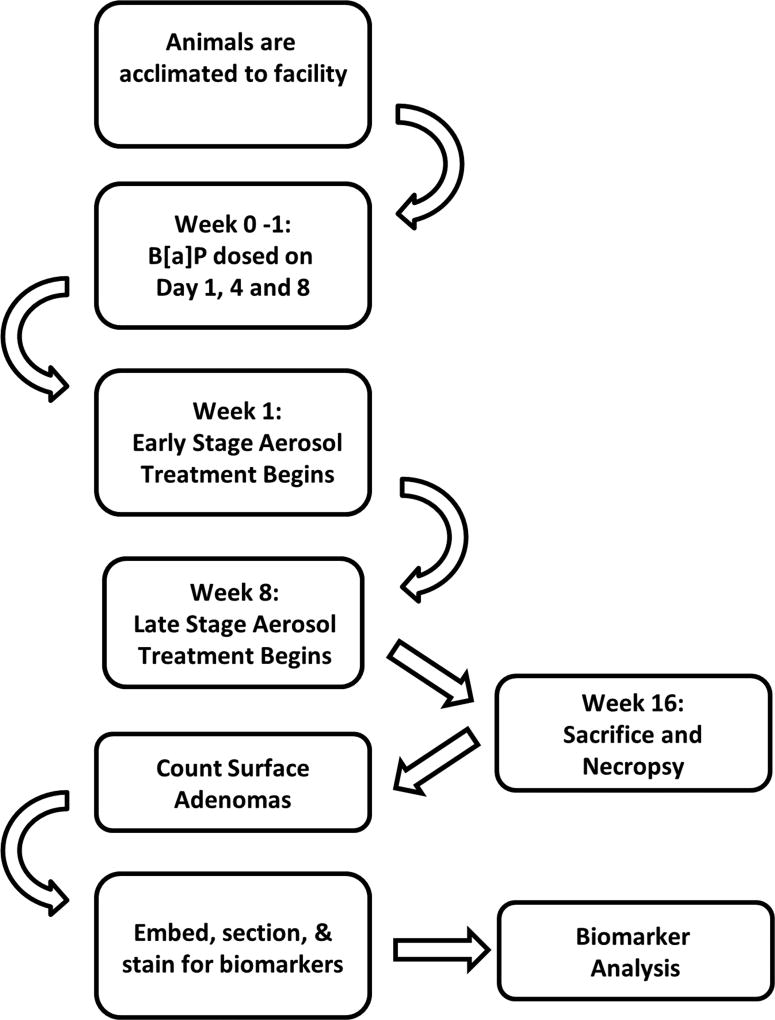
In the dose finding chemoprevention study, 192 seven-week-old female A/J mice were acclimated to the facility for two weeks, weighed, and received carcinogen (B[a]P by oral gavage) on days 1, 4, and 8. One week after the last dose of B[a]P, animals were randomized into six groups of 32 mice per group based on weight and placed on experimental diets as shown in Fig. 1. Animals were continued on the feeding schedule, weighed weekly, and monitored for weight loss, lethargy, rough hair coat, or other signs of ill health. For this experiment, all treatment groups were fed experimental diet for a total of 15 weeks.
Figure 1.
Infographic depiction of experimental timeline.
Intervention stage chemoprevention study
In the intervention stage chemoprevention study, 224 seven-week-old female A/J mice were acclimated to the facility and all procedures performed as in the dose finding study. Metformin (12 mg/g and 10.2 mg/g) and/or pioglitazone (0.18 mg/g) (w/w) were administered in the diet. Early stage agent intervention began seven days after the last dose of B[a]P and continued until termination of the animals. Late stage agent intervention began eight weeks after the last dose of B[a]P (Supplementary Table 1). Experiment termination occurred 16 weeks post carcinogen, resulting in 15 weeks of treatment for the “early” stage group and eight weeks of treatment for the “late” stage group (Figure 1). All lung lobes were preserved in 10% formalin and surface lung adenoma counts were performed. Six animals were euthanized or found dead during the treatment period which is less than the typical attrition of 12–24 animals usually expected for carcinogenesis experiments. Any data from these animals were censored from analysis.
Compliance
All experimental procedures are carried out according to University of Minnesota Department of Environmental Health and Safety requirements which abide by regulatory requirements set at the local, state, and federal level. All studies were conducted with the approval of the Institutional Animal Care and Use Committee at The University of Minnesota, under NIH Animal Welfare Assurance number A3456.
Immunohistochemistry (IHC)
Immunohistochemistry evaluations were performed after surface tumor counts were completed. After counting surface tumors, lungs were fixed in 10% neutral buffered formalin were processed into paraffin blocks. Lungs were sectioned at three levels, 75 microns apart, with eight to sixteen 4 µm unstained sections saved at each level. H&E-stained slides were examined by light microscopy to monitor the presence of tumors and to select slides for subsequent IHC.
For IHC, 4 µm formalin-fixed, paraffin-embedded sections were deparaffinized and rehydrated, followed by antigen retrieval using Tris EDTA buffer, pH 9.0, in a steamer. After blocking endogenous peroxidase and application of a protein block (Dako), immunohistochemistry for cyclin D1 and Ki-67 was performed on a Dako Autostainer using rabbit monoclonal antibodies obtained from Biocare Medical (#CRM 307 and CRM 325, respectively). A rabbit EnVision™+ HRP-polymer kit (Dako, #K4010) was used for detection with diaminobenzidine as the chromogen. Mayer’s Hematoxylin (Dako) was used as the counterstain. Primary antibodies were substituted with negative control rabbit IgG (Biocare Medical, #NC495H) for negative control slides. Positive control tissues included murine small intestine, spleen, and colon adenomas, and human tonsil; negative control tissues included murine skeletal and cardiac muscle. Digital images were collected via a Spot Insight 4 MP CCD Scientific Color Digital camera (Diagnostic Instruments) mounted on a Nikon E-800 microscope (Nikon Plan Apo 20×/0.95 lens).
IHC evaluations were done using light microscopy with tumor immunoreactivity subjectively graded on a 1 to 4 scale based on the extent and intensity of immunolabeling for the marker of interest in any given tumor. The pathologist was blinded from knowing experimental versus control groups for this analysis. Based on their relative abundance in any given animal, from one to 11 tumors were evaluated per animal, and mean scores noted for each animal. Data was obtained from three animals per group.
Cell culture experiments
Beas-2B, SV40 immortalized bronchial epithelial cells were a kind of gift from Reuben Lotan (MD Andersen Cancer Center). These cells were grown in keratinocyte serum free medium (KSFM) (Life Technologies, Carlsbad, CA) supplemented with L-glutamine (2mM), human recombinant epidermal growth factor (5ng/mL), and bovine pituitary extract (50µg/mL) at 37°C in 5% CO2. Cell line was authenticated by short tandem repeat genotyping performed by the Genetic Resources Core Facility at Johns Hopkins University followed by analysis of allele values in the AACR STR, CLIMA, and DSMZ databases. Cell proliferation was determined via MTT assay. Cells were plated at 5×103 cells/well in 96-well tissue culture plates and drugs added at day zero. MTT was added to the culture media at 0.5 mg/mL and incubated at 37°C for four hours, solubilized in isopropyl alcohol/DMSO, and absorbance read at 560 nm. Six replicates per data point were analyzed and experiments repeated thrice.
Statistical Methods
Data were analyzed in a group wise fashion for differences in tumor counts and changes in animal body weights between control and individual experimental groups by ANOVA testing (one-way) for each experiment, unless otherwise noted. Dunnett's post testing was additionally routinely employed to determine which groups were statistically different from the control groups or if the combination treatments were statistically different from the single agent treatments. One-way ANOVA results are presented as F ratio with degrees of freedom and the p value. Data in the charts were presented as a Mean +/− SEM for each group. P<0.05 was used as a cutoff for statistical significance on testing.
Similar to the tumor animal counts, immunohistochemistry data were analyzed in a group wise fashion for changes in expression of any of the markers versus the control. Scores were assigned to each sample based on intensity of stain and relative number of nuclei stained. Kruskal-Wallis nonparametric testing was used to determine if staining intensity of treated groups differed from the control group. All results indicating p<0.05 were considered statistically significant.
Results
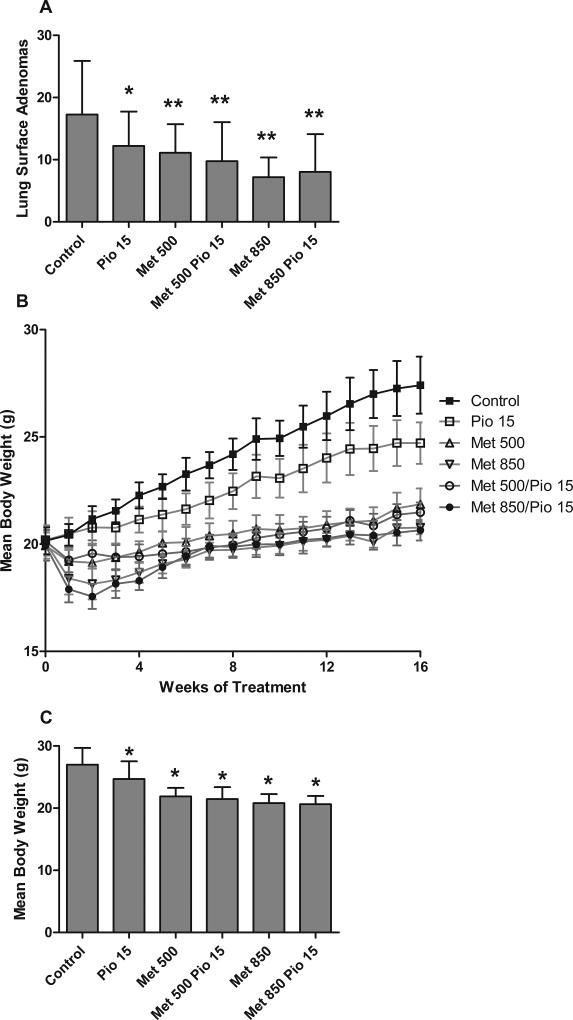
We first tested dietary pioglitazone (15 mg/kg/day) and metformin (500 and 850 mg/kg/day) alone and in combination in a B[a]P mouse carcinogenesis model to evaluate efficacy. Adenoma formation was reduced in all treatment groups (single and combination dosing) versus the control group (Figure 2A) (p < 0.0001, one-way ANOVA). Both metformin doses resulted in statistically significant adenoma reductions from control (t-test: 500: p = 0.001; 850: p ≤ 0.0001). There was a greater reduction in adenoma counts in the 850 mg/kg metformin group (58.3% reduction from control) versus the 500 mg/kg metformin group (35.7% reduction from control) (t-test: p = 0.0003). Pioglitazone (15 mg/kg/day) alone also demonstrated a significant 29.3% tumor reduction compared to the control group (t-test: p = 0.0098).
Figure 2.
Determination of dosing for pioglitazone and metformin. (A) By one-way ANOVA analysis, a statistically significant difference in adenoma formation in all treatment groups versus the control group was observed (F(5,171)=11.06,p<0.0001). (t-test versus control: * = p <0.01, ** = p <0.001) (B) Body weights over the duration of the dose finding study. (C) Endpoint body weights were significantly different by one-way ANOVA analysis (F(5,171=46.35,p<0.0001) and lower in treated groups versus the control group by Dunnett’s post testing (* = p <0.05).
When the two drugs were used in combination, 15 mg/kg/day pioglitazone and 500 mg/kg/day metformin reduced tumor formation by 43.5% from control (t-test: p = 0.004) and pioglitazone with 850 mg/kg/day metformin reduced tumor formation by 53.5% from control (t-test: p < 0.0001). Pioglitazone and 500 mg/kg/day metformin reduced tumor formation by 12.2% from 500 mg/kg/day metformin alone and by 20.1% from pioglitazone alone, which was not significant (Figure 2A). However, although the use of 850 mg/kg/day metformin and 15 mg/kg/day pioglitazone did not significantly decrease tumor formation from 850 mg/kg/day metformin alone, it did significantly decrease tumor formation from pioglitazone alone (Dunnett’s post test: p <0.05 and t-test: p = 0.0078).
Interestingly, throughout the experiment there was no overt toxicity with regard to animal physical appearance, behavior, tolerance of diet, rough coat, etc. However, there were weight differences which reflected an initial weight loss, followed by a partial recovery and a slower rate of weight gain primarily in the metformin treated animals. We observed a 20–30% weight difference in the animals treated with 500 and 850 mg/kg/day metformin compared to other groups over the course of the experiment (Figure 2B). By one-way ANOVA testing, the endpoint weight differences between the control group and the metformin treated groups were significant at p < 0.001 (Figure 2C). Therefore, either agent was associated with lack of weight gain; however, lack of weight gain was greater in groups treated with metformin compared to pioglitazone alone. As described in greater detail in the Discussion section, we believe treatment with metformin may have resulted in metabolic changes in the mice, which may have prevented full energy utilization of dietary intake, and resulted in weight gain differences in control versus experimental animals.
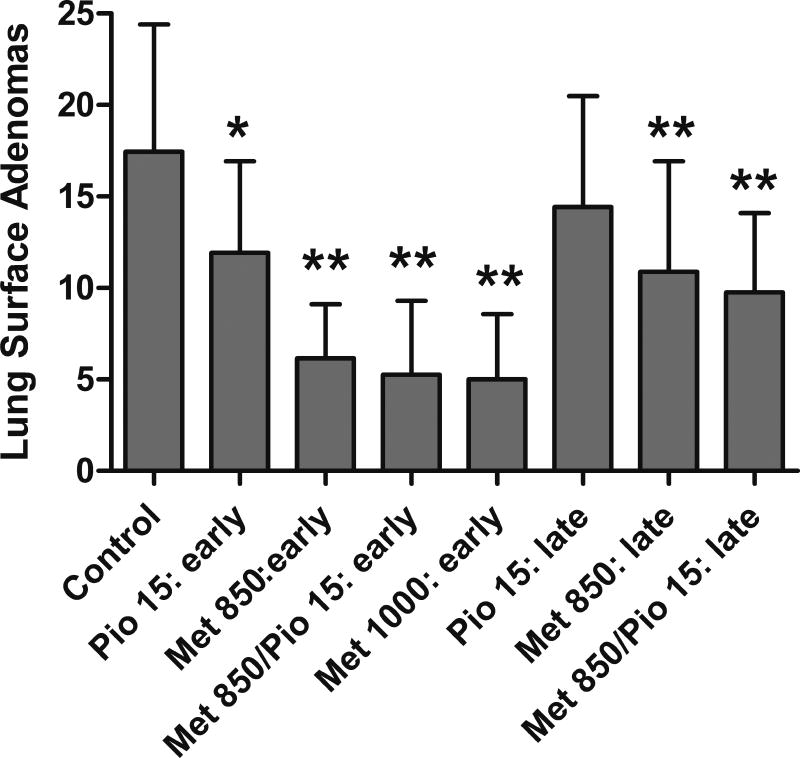
Next, we examined the effect of combination therapy pioglitazone and metformin on early versus late stage carcinogenesis (Figure 3). This experiment allowed us to add an additional dose of 1000 mg/kg/day metformin, and to confirm previous combination treatment results. Further, we tested the intervention at a much later post-initiation time point. At the early stage, treatment with pioglitazone, metformin, or both agents resulted in reductions in adenoma counts from 32–71%. Analysis via one-way ANOVA showed significant differences between treatment groups (F(4, 126)=34.57,p<0.0001) and all groups were significantly lower in surface adenoma count than the control by Dunnett’s post test (p < 0.05). Treatment with pioglitazone alone at 15 mg/kg/day resulted in 32% reduction in adenoma formation (t-test: p = 0.0023). Treatment with metformin alone at 850 mg/kg/day or 1000 mg/kg/day resulted in adenoma reductions of 65% and 71% respectively (t-test: p < 0.0001). Combination pioglitazone/metformin treatment resulted in 70% adenoma reductions from control (t-test: p < 0.0001) and 56% from pioglitazone alone (t-test: p < 0.0001). Combination treatment did not significantly lower adenoma formation over metformin alone.
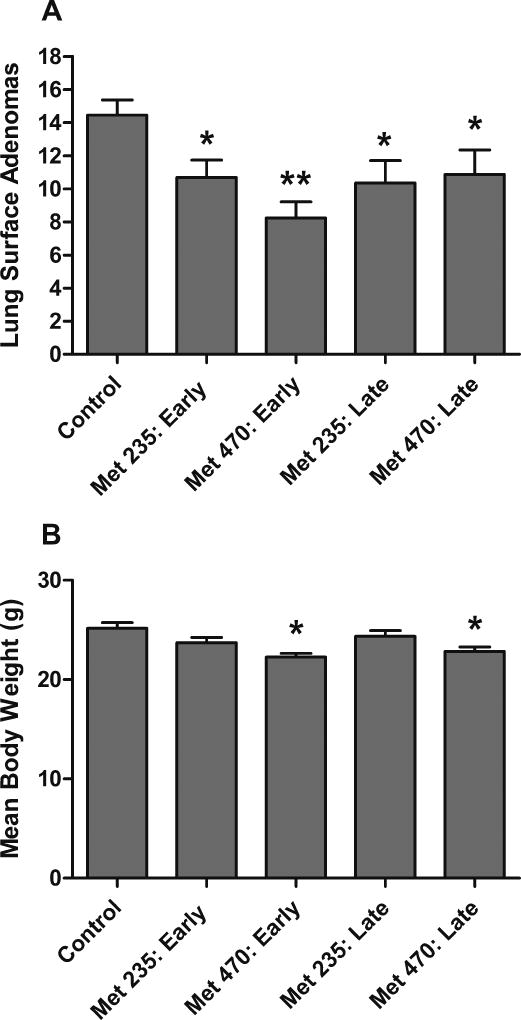
Figure 3.
Effect of pioglitazone and metformin in an early versus late stage intervention model. Treatment with metformin alone and in combination with pioglitazone resulted in statistically significant decreases in lung adenoma formation at both early and late stage interventions versus control. Pioglitazone alone resulted in significant decrease in adenoma formation only at the early treatment intervention. (* = p <0.01, ** = p <0.001)
Late stage treatment with either pioglitazone, metformin, or both agents resulted in reductions adenoma counts from 17–41% (Figure 3). Treatment with 15 mg/kg/day pioglitazone alone resulted in 17% reduction in adenoma formation which trended towards, however did not reach, statistical significance (t-test: p = 0.1126). Treatment with metformin alone at 850 mg/kg/day resulted in adenoma reductions of 38% (t-test: p = 0.0007). Combination pioglitazone and metformin treatment resulted in 44% adenoma reductions versus control (t-test: p < 0.0001) and 32% versus pioglitazone alone (t-test: p = 0.0029). One-way ANOVA analysis found significant differences between the treatment groups (F(3,98)=8.894,p<0.0001) and by Dunnett’s post test, metformin alone and with pioglitazone was significantly lower in surface adenoma count than the control (p < 0.05). In summary, treatment with metformin alone and in combination with pioglitazone resulted in statistically significant decreases in lung adenoma formation at both early and late stage interventions versus control and versus pioglitazone alone (Figure 3). Treatment with pioglitazone alone resulted in a significant decrease in adenoma formation only at the early treatment intervention.
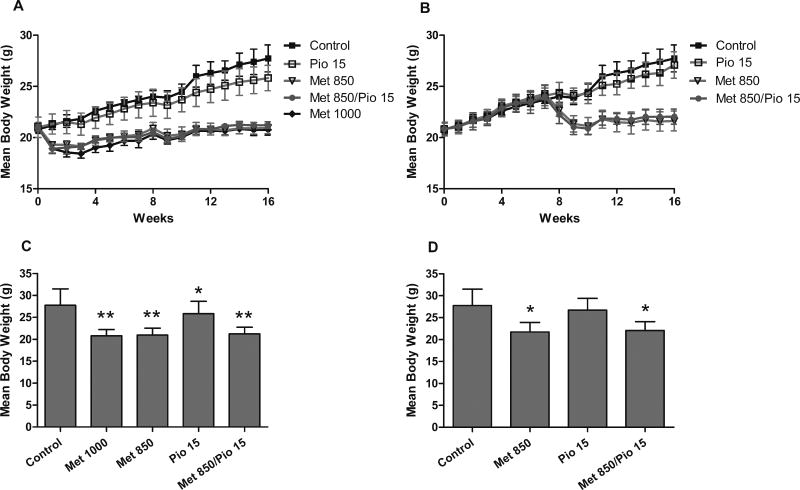
As in the first experiment, animals in the metformin groups experienced a 15% decrease in weight upon treatment onset, in both the early and late stage administration, followed by a partial recovery and an overall slower rate of weight gain over the duration of the experiment (Figure 4A & B). This resulted in final body weights significantly different among treatment groups in both the early stage administration (F(4,125)=48.32,p<0.0001) (Figure 4C) and the late stage administration (F(3,98)=33.58,p<0.0001) (Figure 4D) by one-way ANOVA analysis. By Dunnett’s post test, the metformin and the pioglitazone treated animals are significantly lower in body weight than the control at the early intervention and in the late intervention study, only the metformin treated animals (not the pioglitazone alone) are significantly lower in body weight than the controls (p <0.05).
Figure 4.
Effect of treatment on animal weight gain. Animals in the metformin groups experienced a 15% decrease in weight upon treatment onset in both the early stage administration and the late stage administration followed by a weight plateau and a slower rate of weight gain (A & B), resulting in final body weights lower than non-metformin treated animals (C & D). (t-test: * = p <0.05, ** = p <0.0001).
We performed one additional experiment with lower doses of metformin alone. Metformin was added to the diet at 235 and 470 mg/kg/day at early and late stage post-initiation time points. The most efficacious group was the high dose administered at the early stage, having an average of 8.3 tumors per animal compared with 14.5 in the control group, an inhibition of 43%. The early stage low dose and both late stage doses showed inhibition in the range of 25%–28%. The groups were found to be significantly different by one-way ANOVA analysis (F(4,105)=3.533,p=0.0095) and the early stage 470 mg/kg/day metformin treatment was found to be significantly lower than the control by Dunnett’s post test (p < 0.05) (Figure 5A). At these lower doses, the 470 mg/kg/day metformin treatment groups showed a 10% decrease in weight gain (Figure 5B). The endpoint body weights were found to be significantly different via one-way ANOVA analysis (F(4,105)=5.153,p=0.0008) and only the 470 mg/kg/day metformin treatment groups were significantly different from the control by Dunnett’s post test (p < 0.05) (Figure 5B). Other than the change in rate of weight gain, no other indicators of overt toxicity were observed. This data supports the concept that even five-fold lower doses of metformin from our maximum dietary dose (1000 mg/kg/day) still result in significant reduction in adenoma formation.
Figure 5.
Effect of low dose 235 and 470 mg/kg/day dietary metformin on lung adenoma formation. (A) Treatment with metformin alone resulted in statistically significant decreases in lung adenoma formation at the early stage intervention and a strong trend toward decreases by one-way ANOVA. (B) At the conclusion of the experiment, the animals treated with 470 mg/kg/day metformin experienced a small lack of weight gain of approximately 10%. (t-test: * = p <0.05, ** = p <0.0001)
Immunohistochemistry in residual adenomas
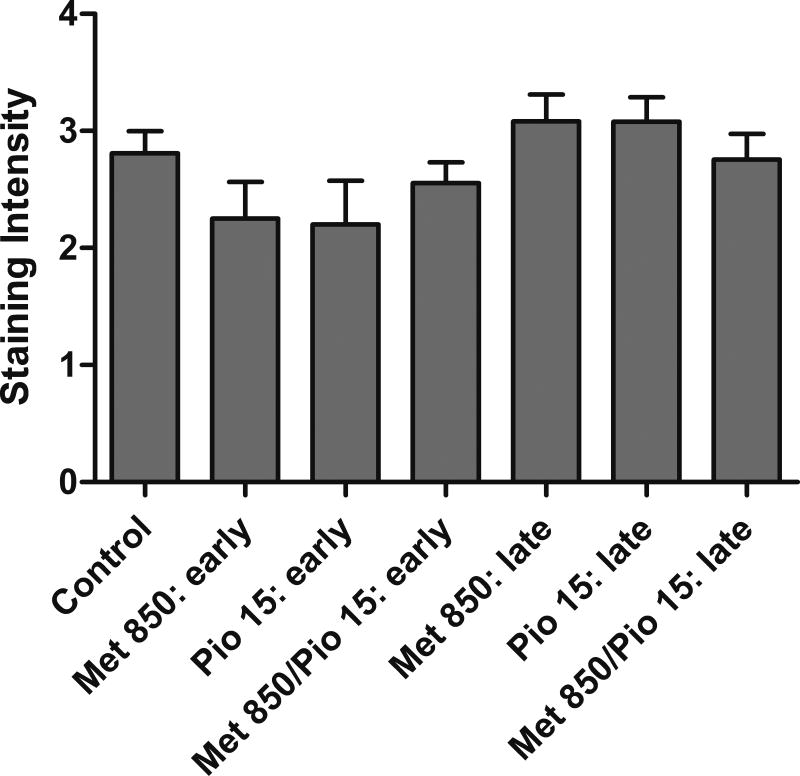
Adenomas from both early and late stage treatment groups (excluding the 1000 mg/kg/day metformin alone group) were analyzed via IHC for cell cycle markers. Positive and negative control stains for each marker are presented in supplementary data (Figure S1). Immunohistochemical analysis of the cell proliferation marker, cyclin D1, showed no statistically significant differences in staining intensity or observed stained nuclei (Figure 6). Immunohistochemical analysis of Ki-67 did not show any epitope staining in the lung adenoma tissues. In the final experiment with 235 mg/kg/day and 470 mg/kg/day dietary metformin, cyclin D1 staining was also not reduced (data not shown).
Figure 6.
Immunohistochemical analysis of cyclin D1 in lung adenoma tissue. Adenomas from each treatment group (excluding the 1000 mg/kg/day metformin alone) from early and late stage interventions were analyzed via IHC for cyclin D1. No statistically significant differences in staining intensity or observed stained nuclei were detected after Kruskal Wallis testing.
In vitro analysis of metformin and pioglitazone
We concluded the project with exploratory MTT experiments utilizing combination metformin/pioglitazone treatment of human immortalized bronchial epithelial Beas-2B cells. We treated cells with 10 µM pioglitazone in serum free media with or without 5–20 µM metformin for 1–3 days. Doses in this range are achievable serum concentrations in vivo. We found significant decreases in Beas-2B proliferation with 10 µM pioglitazone in several experiments with no further decreases in proliferation noted with the addition of 5–20 µM metformin (Supplementary Figure 2). This is consistent with published data on metformin effects on in vitro cell growth. Often, metformin doses need to be in the mM range in order for changes in proliferation to be observed (40).
Discussion
Considerable preclinical and clinical epidemiologic data indicates both biguanides, such as metformin, and thiazolidinediones, such as pioglitazone, demonstrate lung cancer prevention effects (5,22,23,31). Thus far, no unifying mechanism of action ties together the chemoprevention effects of either of these agents in aerodigestive cancer. In experimental lung carcinogenesis, data suggest AKT associated pathways can be targeted by metformin as a potential lead strategy for their use (5).
In our current study, we hypothesized metformin and pioglitazone dual therapy might be more useful in a prevention setting than either agent alone. We chose doses of metformin and pioglitazone at the exact ratio they are available at as an FDA-approved combination agent for type II diabetes treatment (15 mg pioglitazone with 500 or 850 mg metformin). We found metformin (500 and 850 mg/kg/day) and pioglitazone (15 mg/kg/day) produced statistically significant decreases in lung adenoma formation both as single agent treatments and in combination compared to untreated control. Adenoma reduction ranged between 30–60% with either metformin dose alone or with the addition of pioglitazone. Pioglitazone alone was effective in producing 30% reductions in adenoma when applied in early stage testing.
We observed weight differences between groups at the end of the experiment which reflected both an initial weight loss at the initiation of 500 and 850 mg/kg metformin treatment as well as reduced weight gain while under metformin treatment. It is likely that metformin at 500 and 850 mg/kg/day resulted in metabolic changes preventing full energy utilization in the diet at a time in murine life where weight gain is a feature of normal growth. Both metformin and pioglitazone have been categorized, particularly in studies of longevity and aging, as calorie restriction mimetics (CRM). CRMs are agents which mimic calorie restriction in the diet while not causing a decrease in food intake. Other than the differences in weight gain, no other signs of overt toxicity were observed in any of the groups. During replenishment of diet throughout the experiment, food consumption of all treatment groups was observed to be similar. On further analysis of effects of metformin in humans, weight loss is a common observance and up to 5% weight loss is experienced by patients given prescription doses of metformin for three months or more (41,42). Molecular mechanisms contributing to weight loss have been increases in muscle, adipose, and liver AMPK levels, leading to decreases in carbohydrate uptake, leptin, liver gluconeogenesis, and fat and cholesterol synthesis (42).
When we tested combination pioglitazone/metformin treatment for early and late stage post-carcinogen initiation effects, the treatments again resulted in a statistically significant decrease in adenoma formation. Treatment with metformin alone and in combination with pioglitazone resulted in statistically significant decreases in lung adenoma formation at both early and late stage interventions. The addition of pioglitazone did not augment the reductive effect observed with metformin at either stage. Treatment with pioglitazone alone resulted in a significant decrease in adenoma formation only at the early treatment intervention. Similar to our previous observations, animals in the metformin groups experienced a 15% decrease in weight upon treatment onset in both the early stage administration and the late stage administration, followed by slower rates of weight gain resulting in final body weights 15% lower than non-metformin treated animals. No other evidence of overt toxicity was observed in any treatment group, including the higher 1000 mg/kg/day metformin dose. Therefore, it is not trivial to rectify the complete etiology of some of the tumorigenesis effects we observed, since a CRM such as metformin would be expected to have multiple metabolic effects which are anti-tumorigenic. There is established literature showing intentional calorie restriction, animal starvation, and overt drug toxicities causing decreased food intake can be individually associated with reduced tumorigenesis (43–48).
We made it a project goal to handle the adenoma specimens in the manner they would be handled in a human clinical trial, with preservation and short term storage in formalin before blocking and cutting. Also, the efficacy of the prevention effects made biomarker determination difficult. This was attributable to the decrease in both number and size of the lung adenomas in the treated groups. We thus limited our analyses to assessment of cyclin D1 and Ki-67 expression levels due to their key roles in cell growth and proliferation as well as the decreased amount of tissue available from the animals. Immunohistochemistry analysis of the cell proliferation marker, cyclin D1, indicated no obvious effect of treatment on cyclin D1 expression, with similar scores observed across the groups. Although there were slightly lower scores in treatment groups, this was not considered to be significant due to the relatively small magnitude of the effect. Immunohistochemistry analysis of Ki-67 did not provide any epitope staining in the lung adenoma tissues. This was attributed to prolonged fixation and storage in formalin. It has been well documented that prolonged formalin fixation results in deterioration or absence of immunolabelling for many markers (49,50). In future studies, tissues will be formalin-fixed for 24 hours and stored in 70% ethanol prior to processing into paraffin blocks, conditions which work well for routine detection of Ki-67 and other markers in our hands.
We conclude oral metformin is a viable chemopreventive treatment at doses ranging from 235 to 1000 mg/kg/day at both early and late stage interventions. Pioglitazone at 15 mg/kg/day is a viable chemopreventive agent at early stage interventions. Combination metformin and pioglitazone perform equal to metformin alone and better than 15 mg/kg/day pioglitazone alone. These are promising preclinical findings which could be advanced to clinical prevention studies with pioglitazone, metformin, or ACTOplus Met®. If this drug combination were to make it to a clinical trial, the likely agent, ACTOplus Met®XR, contains a 30:1000 ratio of pioglitazone:metformin, quite close to the ratio we used in our combination study of 15:500 pioglitazone:metformin.
Supplementary Material
Acknowledgments
Financial support: This work was supported by the NCI (Subcontract for Preclinical Contract N01-CN-25002-78, Primary Contract Number: Contract #HHSN-261201200015I, F.G. Ondrey) and the Lions Multiple District 5M Hearing Foundation of Minnesota, (Translational Biomarker Initiatives for Medical Students, F.G. Ondrey).
We wish to express sincere and deep gratitude to our mentor and colleague, Dr. Lee Wattenberg, for his help with this project. His intellectual founding contributions to the field of cancer prevention have allowed for us to proceed in a logical stepwise fashion on these experiments. Dr. Wattenberg passed away in December 2014, during the funding period of this project and we wish to acknowledge his input during the early planning and design phases of this work.
Footnotes
Conflict of interest statement: The authors report no conflict of interest.
References
- 1.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6(5):963–8. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 2.Sozzi G, Miozzo M, Pastorino U, Pilotti S, Donghi R, Giarola M, et al. Genetic evidence for an independent origin of multiple preneoplastic and neoplastic lung lesions. Cancer Res. 1995;55(1):135–40. [PubMed] [Google Scholar]
- 3.Park IW, Wistuba II, Maitra A, Milchgrub S, Virmani AK, Minna JD, et al. Multiple clonal abnormalities in the bronchial epithelium of patients with lung cancer. J Natl Cancer Inst. 1999;91(21):1863–8. doi: 10.1093/jnci/91.21.1863. [DOI] [PubMed] [Google Scholar]
- 4.Wistuba II, Mao L, Gazdar AF. Smoking molecular damage in bronchial epithelium. Oncogene. 2002;21(48):7298–306. doi: 10.1038/sj.onc.1205806. [DOI] [PubMed] [Google Scholar]
- 5.Memmott RM, Mercado JR, Maier CR, Kawabata S, Fox SD, Dennis PA. Metformin prevents tobacco carcinogen--induced lung tumorigenesis. Cancer Prev Res (Phila) 2010;3(9):1066–76. doi: 10.1158/1940-6207.CAPR-10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu H, Zhang J, Pan J, Zhang Q, Lu Y, Wen W, et al. Chemoprevention of lung carcinogenesis by the combination of aerosolized budesonide and oral pioglitazone in A/J mice. Mol Carcinog. 2011;50(12):913–21. doi: 10.1002/mc.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ihle NT, Lemos R, Schwartz D, Oh J, Halter RJ, Wipf P, et al. Peroxisome proliferator-activated receptor gamma agonist pioglitazone prevents the hyperglycemia caused by phosphatidylinositol 3-kinase pathway inhibition by PX-866 without affecting antitumor activity. Mol Cancer Ther. 2009;8(1):94–100. doi: 10.1158/1535-7163.MCT-08-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nemenoff RA, Weiser-Evans M, Winn RA. Activation and Molecular Targets of Peroxisome Proliferator-Activated Receptor-gamma Ligands in Lung Cancer. PPAR Res. 2008;2008:156875. doi: 10.1155/2008/156875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, James M, Wen W, Lu Y, Szabo E, Lubet RA, et al. Chemopreventive effects of pioglitazone on chemically induced lung carcinogenesis in mice. Mol Cancer Ther. 2010;9(11):3074–82. doi: 10.1158/1535-7163.MCT-10-0510. [DOI] [PubMed] [Google Scholar]
- 10.Ondrey F. Peroxisome proliferator-activated receptor gamma pathway targeting in carcinogenesis: implications for chemoprevention. Clin Cancer Res. 2009;15(1):2–8. doi: 10.1158/1078-0432.CCR-08-0326. [DOI] [PubMed] [Google Scholar]
- 11.Harris G, Ghazallah RA, Nascene D, Wuertz B, Ondrey FG. PPAR activation and decreased proliferation in oral carcinoma cells with 4-HPR. Otolaryngol Head Neck Surg. 2005;133(5):695–701. doi: 10.1016/j.otohns.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 12.Chang TH, Szabo E. Induction of differentiation and apoptosis by ligands of peroxisome proliferator-activated receptor gamma in non-small cell lung cancer. Cancer Res. 2000;60(4):1129–38. [PubMed] [Google Scholar]
- 13.Chang TH, Szabo E. Enhanced growth inhibition by combination differentiation therapy with ligands of peroxisome proliferator-activated receptor-gamma and inhibitors of histone deacetylase in adenocarcinoma of the lung. Clin Cancer Res. 2002;8(4):1206–12. [PubMed] [Google Scholar]
- 14.Avis I, Martínez A, Tauler J, Zudaire E, Mayburd A, Abu-Ghazaleh R, et al. Inhibitors of the arachidonic acid pathway and peroxisome proliferator-activated receptor ligands have superadditive effects on lung cancer growth inhibition. Cancer Res. 2005;65(10):4181–90. doi: 10.1158/0008-5472.CAN-04-3441. [DOI] [PubMed] [Google Scholar]
- 15.Wright SK, Wuertz BR, Harris G, Abu Ghazallah R, Miller WA, Gaffney PM, et al. Functional activation of PPARγ in human upper aerodigestive cancer cell lines. Mol Carcinog. 2016 doi: 10.1002/mc.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han EJ, Im CN, Park SH, Moon EY, Hong SH. Combined treatment with peroxisome proliferator-activated receptor (PPAR) gamma ligands and gamma radiation induces apoptosis by PPARγ-independent up-regulation of reactive oxygen species-induced deoxyribonucleic acid damage signals in non-small cell lung cancer cells. Int J Radiat Oncol Biol Phys. 2013;85(5):e239–48. doi: 10.1016/j.ijrobp.2012.11.040. [DOI] [PubMed] [Google Scholar]
- 17.Bren-Mattison Y, Meyer AM, Van Putten V, Li H, Kuhn K, Stearman R, et al. Antitumorigenic effects of peroxisome proliferator-activated receptor-gamma in non-small-cell lung cancer cells are mediated by suppression of cyclooxygenase-2 via inhibition of nuclear factor-kappaB. Mol Pharmacol. 2008;73(3):709–17. doi: 10.1124/mol.107.042002. [DOI] [PubMed] [Google Scholar]
- 18.Hazra S, Batra RK, Tai HH, Sharma S, Cui X, Dubinett SM. Pioglitazone and rosiglitazone decrease prostaglandin E2 in non-small-cell lung cancer cells by up-regulating 15-hydroxyprostaglandin dehydrogenase. Mol Pharmacol. 2007;71(6):1715–20. doi: 10.1124/mol.106.033357. [DOI] [PubMed] [Google Scholar]
- 19.Han S, Sidell N, Fisher PB, Roman J. Up-regulation of p21 gene expression by peroxisome proliferator-activated receptor gamma in human lung carcinoma cells. Clin Cancer Res. 2004;10(6):1911–9. doi: 10.1158/1078-0432.ccr-03-0985. [DOI] [PubMed] [Google Scholar]
- 20.Govindarajan R, Ratnasinghe L, Simmons DL, Siegel ER, Midathada MV, Kim L, et al. Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J Clin Oncol. 2007;25(12):1476–81. doi: 10.1200/JCO.2006.07.2777. [DOI] [PubMed] [Google Scholar]
- 21.Govindarajan R, Siegel ER, Simmons DL, Lang NP. Thiazolidinedione (TZD) exposure and risk of squamous cell carcinoma of head and neck (SCCHN) Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings Part I. 2007 Jun 20;25(18S)(Supplement):1511–2007. [Google Scholar]
- 22.Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res. 2010;3(11):1451–61. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- 23.Franciosi M, Lucisano G, Lapice E, Strippoli GF, Pellegrini F, Nicolucci A. Metformin therapy and risk of cancer in patients with type 2 diabetes: systematic review. PLoS One. 2013;8(8):e71583. doi: 10.1371/journal.pone.0071583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodwin PJ, Stambolic V. Obesity and insulin resistance in breast cancer--chemoprevention strategies with a focus on metformin. Breast. 2011;20(Suppl 3):S31–5. doi: 10.1016/S0960-9776(11)70291-0. [DOI] [PubMed] [Google Scholar]
- 25.He X, Esteva FJ, Ensor J, Hortobagyi GN, Lee MH, Yeung SC. Metformin and thiazolidinediones are associated with improved breast cancer-specific survival of diabetic women with HER2+ breast cancer. Ann Oncol. 2012;23(7):1771–80. doi: 10.1093/annonc/mdr534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai SW, Liao KF, Chen PC, Tsai PY, Hsieh DP, Chen CC. Antidiabetes drugs correlate with decreased risk of lung cancer: a population-based observation in Taiwan. Clin Lung Cancer. 2012;13(2):143–8. doi: 10.1016/j.cllc.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Rozengurt E, Sinnett-Smith J, Kisfalvi K. Crosstalk between insulin/insulin-like growth factor-1 receptors and G protein-coupled receptor signaling systems: a novel target for the antidiabetic drug metformin in pancreatic cancer. Clin Cancer Res. 2010;16(9):2505–11. doi: 10.1158/1078-0432.CCR-09-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan BK, Adya R, Chen J, Lehnert H, Sant Cassia LJ, Randeva HS. Metformin treatment exerts antiinvasive and antimetastatic effects in human endometrial carcinoma cells. J Clin Endocrinol Metab. 2011;96(3):808–16. doi: 10.1210/jc.2010-1803. [DOI] [PubMed] [Google Scholar]
- 29.Vaccaro O, Masulli M, Bonora E, Del Prato S, Giorda CB, Maggioni AP, et al. Addition of either pioglitazone or a sulfonylurea in type 2 diabetic patients inadequately controlled with metformin alone: impact on cardiovascular events. A randomized controlled trial. Nutr Metab Cardiovasc Dis. 2012;22(11):997–1006. doi: 10.1016/j.numecd.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Xie Y, Wang YL, Yu L, Hu Q, Ji L, Zhang Y, et al. Metformin promotes progesterone receptor expression via inhibition of mammalian target of rapamycin (mTOR) in endometrial cancer cells. J Steroid Biochem Mol Biol. 2011;126(3–5):113–20. doi: 10.1016/j.jsbmb.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Zhang ZJ, Bi Y, Li S, Zhang Q, Zhao G, Guo Y, et al. Reduced risk of lung cancer with metformin therapy in diabetic patients: a systematic review and meta-analysis. Am J Epidemiol. 2014;180(1):11–4. doi: 10.1093/aje/kwu124. [DOI] [PubMed] [Google Scholar]
- 32.Cacicedo JM, Yagihashi N, Keaney JF, Jr, Ruderman NB, Ido Y. AMPK inhibits fatty acid-induced increases in NF-kappaB transactivation in cultured human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2004;324(4):1204–9. doi: 10.1016/j.bbrc.2004.09.177. [DOI] [PubMed] [Google Scholar]
- 33.Hattori Y, Suzuki K, Hattori S, Kasai K. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension. 2006;47(6):1183–8. doi: 10.1161/01.HYP.0000221429.94591.72. [DOI] [PubMed] [Google Scholar]
- 34.Huang NL, Chiang SH, Hsueh CH, Liang YJ, Chen YJ, Lai LP. Metformin inhibits TNF-alpha-induced IkappaB kinase phosphorylation, IkappaB-alpha degradation and IL-6 production in endothelial cells through PI3K-dependent AMPK phosphorylation. Int J Cardiol. 2009;134(2):169–75. doi: 10.1016/j.ijcard.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Isoda K, Young JL, Zirlik A, MacFarlane LA, Tsuboi N, Gerdes N, et al. Metformin inhibits proinflammatory responses and nuclear factor-kappaB in human vascular wall cells. Arterioscler Thromb Vasc Biol. 2006;26(3):611–7. doi: 10.1161/01.ATV.0000201938.78044.75. [DOI] [PubMed] [Google Scholar]
- 36.Li SN, Wang X, Zeng QT, Feng YB, Cheng X, Mao XB, et al. Metformin inhibits nuclear factor kappaB activation and decreases serum high-sensitivity C-reactive protein level in experimental atherogenesis of rabbits. Heart Vessels. 2009;24(6):446–53. doi: 10.1007/s00380-008-1137-7. [DOI] [PubMed] [Google Scholar]
- 37.Tan BK, Adya R, Chen J, Farhatullah S, Heutling D, Mitchell D, et al. Metformin decreases angiogenesis via NF-kappaB and Erk1/2/Erk5 pathways by increasing the antiangiogenic thrombospondin-1. Cardiovasc Res. 2009;83(3):566–74. doi: 10.1093/cvr/cvp131. [DOI] [PubMed] [Google Scholar]
- 38.Wattenberg LW. Studies of polycyclic hydrocarbon hydroxylases of the intestine possibly related to cancer. Effect of diet on benzpyrene hydroxylase activity. Cancer. 1971;28(1):99–102. doi: 10.1002/1097-0142(197107)28:1<99::aid-cncr2820280118>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 39.Wattenberg LW. Exogenous factors affecting polycyclic hydrocarbon hydroxylase activity. Adv Enzyme Regul. 1973;11:193–201. doi: 10.1016/0065-2571(73)90016-2. [DOI] [PubMed] [Google Scholar]
- 40.Griss T, Vincent EE, Egnatchik R, Chen J, Ma EH, Faubert B, et al. Metformin Antagonizes Cancer Cell Proliferation by Suppressing Mitochondrial-Dependent Biosynthesis. PLoS Biol. 2015;13(12):e1002309. doi: 10.1371/journal.pbio.1002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jarskog LF, Hamer RM, Catellier DJ, Stewart DD, Lavange L, Ray N, et al. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032–40. doi: 10.1176/appi.ajp.2013.12010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malin SK, Kashyap SR. Effects of metformin on weight loss: potential mechanisms. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):323–9. doi: 10.1097/MED.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 43.Harvey AE, Lashinger LM, Hays D, Harrison LM, Lewis K, Fischer SM, et al. Calorie restriction decreases murine and human pancreatic tumor cell growth, nuclear factor-κB activation, and inflammation-related gene expression in an insulin-like growth factor-1-dependent manner. PLoS One. 2014;9(5):e94151. doi: 10.1371/journal.pone.0094151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mizuno NK, Rogozina OP, Seppanen CM, Liao DJ, Cleary MP, Grossmann ME. Combination of intermittent calorie restriction and eicosapentaenoic acid for inhibition of mammary tumors. Cancer Prev Res (Phila) 2013;6(6):540–7. doi: 10.1158/1940-6207.CAPR-13-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Albanes D. Total calories, body weight, and tumor incidence in mice. Cancer Res. 1987;47(8):1987–92. [PubMed] [Google Scholar]
- 46.Kumar SP, Roy SJ, Tokumo K, Reddy BS. Effect of different levels of calorie restriction on azoxymethane-induced colon carcinogenesis in male F344 rats. Cancer Res. 1990;50(18):5761–6. [PubMed] [Google Scholar]
- 47.Reddy BS, Wang CX, Maruyama H. Effect of restricted caloric intake on azoxymethane-induced colon tumor incidence in male F344 rats. Cancer Res. 1987;47(5):1226–8. [PubMed] [Google Scholar]
- 48.Chen RF, Good RA, Engelman RW, Hamada N, Tanaka A, Nonoyama M, et al. Suppression of mouse mammary tumor proviral DNA and protooncogene expression: association with nutritional regulation of mammary tumor development. Proc Natl Acad Sci U S A. 1990;87(7):2385–9. doi: 10.1073/pnas.87.7.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oyama T, Ishikawa Y, Hayashi M, Arihiro K, Horiguchi J. The effects of fixation, processing and evaluation criteria on immunohistochemical detection of hormone receptors in breast cancer. Breast Cancer. 2007;14(2):182–8. doi: 10.2325/jbcs.976. [DOI] [PubMed] [Google Scholar]
- 50.Engel KB, Moore HM. Effects of preanalytical variables on the detection of proteins by immunohistochemistry in formalin-fixed, paraffin-embedded tissue. Arch Pathol Lab Med. 2011;135(5):537–43. doi: 10.5858/2010-0702-RAIR.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.