Abstract
Nearly 75% of breast tumors express estrogen receptor (ER), and will be treated with endocrine therapy, such as selective estrogen receptor modulator (SERM), tamoxifen, or aromatase inhibitors. Despite their proven success, as many as 40–50% of ER+ tumors fail to respond to endocrine therapy and eventually recur as aggressive, metastatic cancers. Therefore, preventing and/or overcoming endocrine resistance in ER+ tumors remains a major clinical challenge. Deregulation or activation of the nuclear factor κB (NFκB) pathway has been implicated in endocrine resistance and poor patient outcome in ER+ tumors. As a consequence, one option to improve on existing anti-cancer treatment regimens may be to introduce additional anti-NFκB activity to endocrine therapy drugs. Our approach was to design and test SERM-fumarate co-targeting hybrid drugs capable of simultaneously inhibiting both ER, via the SERM, raloxifene, and the NFκB pathway, via fumarate, in breast cancer cells. We find that the hybrid drugs display improved anti-NFκB pathway inhibition compared to either raloxifene or fumarate. Despite some loss in potency against the ER pathway, these hybrid drugs maintain anti-proliferative activity in ER+ breast cancer cells. Furthermore, these drugs prevent clonogenic growth and mammosphere formation of ER+ breast cancer cells. As a proof-of-principle, the simultaneous inhibition of ER and NFκB via a single bifunctional hybrid drug may represent a viable approach to improve the anti-inflammatory activity and prevent therapy resistance of ER-targeted anti-cancer drugs.
Electronic supplementary material
The online version of this article (doi:10.1007/s12672-017-0294-5) contains supplementary material, which is available to authorized users.
Keywords: Breast cancer, Estrogen receptor, NFκB pathway, Co-targeting drugs
Introduction
Breast cancer is the most commonly diagnosed cancer among American women and claims over 40,000 lives each year. About 75% of breast tumors express estrogen receptor α (ERα), and ER remains the single most important driver and prognostic factor in breast cancer. Patients with ER+ tumors are typically treated with endocrine therapy to prevent activation of ER, such as aromatase inhibitors, or direct ER antagonism using the selective ER modulator (SERM) tamoxifen and the selective ER down-regulator (SERD) fulvestrant (ICI182780). Although the 5-year survival rates are generally good, it is estimated that up to 50% of ER+ tumors fail on endocrine therapy and recur as aggressive, therapy resistant, or metastatic tumors. As a result, more women die each year of ER+ breast cancer than ER-negative breast cancers [1, 2].
Although multiple pathways and factors contribute to endocrine resistance, increasing evidence points to the inflammatory nuclear factor κB (NFκB) pathway as a key player. In ER+ tumors, numerous studies have shown that a deregulated, or constitutively active NFκB pathway is associated with hormone-independence, both chemo and endocrine therapy failure, and an elevated risk of early relapse [3–7]. Furthermore, chemical inhibition of NFκB activity can restore sensitivity to ER antagonists in cell-based models of resistance [8, 9]. Together, these findings suggest that simultaneous inhibition of both ER and the inflammatory NFκB pathway would be beneficial against aggressive ER+ breast cancer disease (Fig. 2a). While multiple advancements have been made towards therapeutic targeting of the NFκB pathway (e.g., proteasome inhibitors and upstream kinase inhibitors), most have failed in the clinic due to inhibition of other non-NFκB targets and adverse toxic side effects [10]. Thus, safe and effective targeting of the NFκB pathway, especially in solid tumors, remains a major challenge. We have previously established that the anti-inflammatory drug, dimethyl fumarate (DMF), effectively inhibits the NFκB pathway in breast cancer cells and exhibits promising anti-cancer activities [11]. Furthermore, the fumarate functional group is required and sufficient to confer anti-NFκB activity when conjugated to other drugs as we have previously demonstrated with aspirin [12]. Therefore, we hypothesized that adding a fumarate moiety to current ER antagonists may confer similar anti-NFκB properties.
Fig. 2.
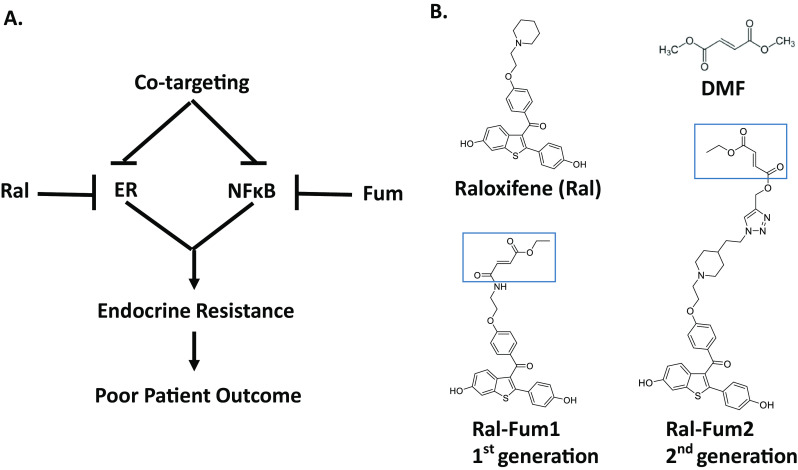
Hybrid drug co-targeting strategy and chemical structures are indicated. a Schematic representation of a hybrid drug capable of targeting simultaneously both ER and NFκB and its potential benefit in breast cancer. b Chemical structures of the parent drugs, raloxifene and dimethyl fumarate (DMF), along with the co-targeting drugs are shown. The fumarate moiety of Ral-Fum hybrids is highlighted
Simultaneous targeting of ER and NFκB can be achieved by combination therapies that independently inhibit those pathways. Alternatively, a hybrid drug strategy can be utilized. By definition, a hybrid drug is a chemical entity capable of interacting simultaneously with multiple targets. We reasoned that a single-agent hybrid may be superior based on purported advantages such as: (i) enhanced affinity and efficacy over parent drugs, (ii) improved pharmacokinetic profile, (iii) reduced side effects, and (v) improved patient compliance [13]. In this paper, several SERMs and SERDs were screened for their anti-NFκB activity, which was found to be either absent or modest. The potent, clinically relevant SERM, raloxifene, was selected as the scaffold for development of hybrid drugs containing a fumarate functionality. Unsurprisingly, after incorporating the fumarate functionality, potency on ER was lowered. However, both fumarate hybrids studied inhibited NFκB signaling equally. Importantly, the raloxifene-fumarate hybrid drugs remained capable of blocking estrogen-induced proliferation, clonogenic growth, and mammosphere formation of ER+ breast cancer cells. This anti-proliferative activity in breast cancer combined with inhibition of NFκB signaling indicates a viable, novel strategy to treat aggressive ER+ breast cancers.
Materials and Methods
Reagents and Drug Synthesis
ARN-810 was purchased from MedChem Express. Estrogen (17β-estradiol, E2), 4-hydroxy-tamoxifen, raloxifene, ICI182780, and DMF were purchased from Sigma. TNFα was purchased from R&D Systems. Raloxifene-fumarate hybrids, Ral-Fum 1 and Ral-Fum 2, and raloxifene-succinate (Ral-Succ) were synthesized according to the schemes shown in Supplemental Fig. 1. Ral-Fum 1 (5) and Ral-Succ (3) were obtained by the acylation of (4-(2-aminoethoxy)phenyl) (6-methoxy-2-(4-methoxyphenyl)benzo[b]thiophen-3yl) methanone (1) with ethyl fumaroyl or succinoyl chloride, followed by O-demethylation with BF3 * SMe2 in CHCl3 at low temperatures. The use of boron tribromide for O-deprotection led to the complete loss of the ester group. Non-aqueous click chemistry was chosen for the generation of Ral-Fum 2, an analog retaining the piperidine ring of raloxifene. This was accomplished by starting from raloxifene substituted with an azidoethyl group on the piperidine ring (6) and attaching the fumarate using click chemistry, with CuI * PPh3 as the catalyst. The reaction could be carried out in THF to avoid ester hydrolysis. The structures and purity were confirmed by LC-MS/MS and NMR spectroscopy.
Cell Lines, Culture Conditions, and Drug Treatments
The human estrogen receptor (ER) positive breast cancer cell lines, MCF-7, and T47D, were obtained from Dr. Debra Tonetti (University of Illinois at Chicago, USA) and authenticated. These cells were routinely maintained in RPMI 1640 media (Invitrogen Life Technologies) with phenol red supplemented with 10% FBS, 1% non-essential amino acids, 2 mmol/L L-glutamine, 1% antibiotics penicillin-streptomycin, and 6 ng/mL insulin. The ER− breast cancer cell line, MDA-MB-231, was obtained from Dr. Clodia Osipo (Loyola University Chicago, USA) and routinely maintained in IMEM media (Corning) supplemented with 5% FBS, 1% non-essential amino acids, 2 mM L-glutamine, and 1% antibiotics penicillin-streptomycin. Prior to measuring ER activity, cells were seeded for 72 h in phenol red-free media with reduced 5% charcoal-dextran stripped FBS. For dual luciferase assays, cells were pretreated with drugs for 1 h, followed by induction with E2 (10 nM) or TNFα (10 ng/mL) for 4 h. For gene expression studies, cells were pretreated with drugs for 1 h, followed by induction with E2 (10 nM) or TNFα (10 ng/mL) for 2 h.
Luciferase Reporter Assay
MCF-7 cells were transiently co-transfected with an NFκB-RE or ERE luciferase construct (Clontech) along with the renilla luciferase construct, pGL4.70 (Promega), and dual luciferase assays were carried out as previously described [14].
RT-Quantitative PCR (QPCR)
Total RNA was isolated using the Trizol method, then reverse transcribed (RT), and analyzed by QPCR performed as previously described [15]. Fold change was calculated using the ΔΔCt method with 36B4 serving as the internal control. QPCR primer sequences are available upon request.
Proliferation Assay
MCF-7 cells were seeded in phenol red-free media at 5000 cells per well in 24-well plates. After 48 h, media was replaced and varying concentrations of drugs along with E2 (10 nM) were added. After 72 h of treatment, cells were washed twice with PBS, followed by fixing and staining in 1% crystal violet in methanol and water (1:4). Stained cells are solubilized in 1% SDS and colorimetric density was measured at 570 nm via a plate reader.
Mammosphere (MS) Assay
Breast cancer cells were seeded at single-cell density on low attachment plates in media described by Dontu et al., supplemented with 1% methyl cellulose to prevent cellular aggregation [16]. Inhibitors were added the next day. After 7 days in culture, the diameter of MS was measured and the number of MS ≥75 μm in diameter was counted.
Clonogenic Assay
MCF-7 cells were seeded at clonogenic density of 1000 cells per well in 6-well plates in estrogenized phenol red media. After overnight attachment, cells were treated as indicated. Media was changed every 3–4 days and cells were re-treated for 2 weeks. After 2 weeks, colonies were stained with 1% crystal violet in methanol and water (1:4) and imaged via ImageJ software. Colony area was estimated automatically via the ColonyArea ImageJ plugin [17].
Statistical Analysis
Data are presented as mean ± SEM from at least three independent determinations. Statistical analysis consisted of one- or two-way ANOVA followed by Tukey posttest, or t test, as appropriate. IC50s are calculated with GraphPad Prism software.
Results
Profiling SERMs and SERDs for Anti-NFκB Activity in ER+ Breast Cancer Cells
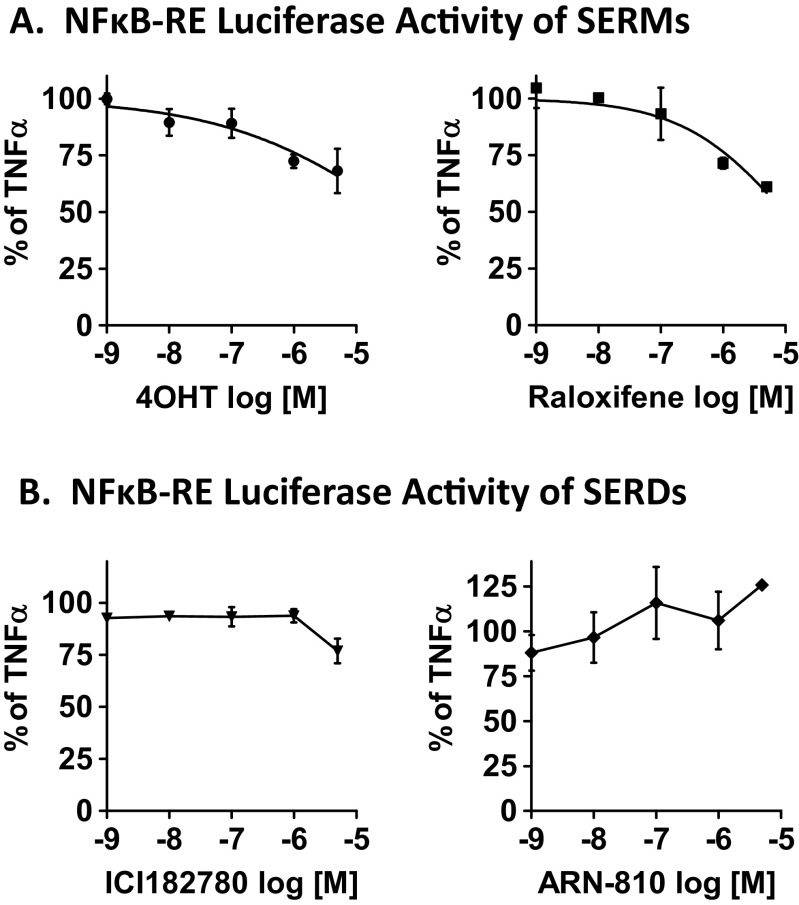
Fumarate esters provide clinical anti-inflammatory and immunomodulatory activity through multiple pathways [18]. We view fumarate as the chemical moiety of choice to antagonize the NFκB pathway, based on its proven anti-NFκB activity in breast cancer cells [11] and its acceptable clinical safety profile [19]. To block ER activity, several FDA approved drugs are available. Interestingly, extensive crosstalk has been reported between ER and the NFκB pathway that ranges from a classical mutual-transrepression mechanism to a synergistic cooperativity between the two [15, 20]. As a consequence, ER ligands from agonists such as estrogen, to tissue selective SERMs, to SERDs display a wide spectrum of activity on the NFκB pathway. To prioritize among ER antagonists for breast cancer therapy, we first investigated whether clinically relevant SERMs and SERDs are capable of inhibiting NFκB signaling in ER+ MCF-7 human breast cancer cells. NFκB activity was measured via a dual luciferase reporter assay of the NFκB-response element (NFκB-RE) as indicated in Fig. 1. The pro-inflammatory cytokine, tumor necrosis factor (TNFα), was used to activate the NFκB pathway. Two SERMs, the classical endocrine therapy drug, tamoxifen (active metabolite 4-hydroxy-tamoxifen, 4OHT) and raloxifene, an approved drug for post-menopausal osteoporosis and for breast cancer prevention in high risk patients, were tested. Two SERDs, ICI182780 (ICI), and the orally bioavailable SERD, ARN-810, were also tested. We find these drugs to be rather ineffective at doses relevant to ER modulation used in breast cancer cells. However, there are notable differences among these two classes of ER antagonists. The SERMs (Fig. 1a) are more potent inhibitors of NFκB signaling. The SERDs (Fig. 1b) showed no inhibitory activity, except for the modest inhibition by ICI at 5 μM (<25% inhibition). Our survey indicates that the benzothiophene SERM, raloxifene, is slightly more potent at inhibiting NFκB activity even compared to 4OHT (39 vs. 32% at 5 μM, respectively). Raloxifene’s anti-NFκB activity was also corroborated on a classical NFκB-target gene, intercellular adhesion molecule 1 (ICAM1) (Supplemental Fig. 2a), and in a second ER+ breast cancer cell line, T47D (Supplemental Fig. 2b). The calculated inhibitory concentration at 50% (IC50) is ~40 μM. Therefore, raloxifene was used as the anti-ER drug of choice in our hybrid drug design.
Fig. 1.
The effect of ER antagonists, SERMs (a) and SERDs (b), on NFκB activity in ER+ breast cancer cells. MCF-7 cells were transfected with NFκB-RE and renilla reporter plasmids. Cells were then pretreated with various concentrations of drugs for 1 h, followed by TNFα (10 ng/mL) for 4 h to activate the NFκB pathway. Each drug’s inhibitory activity was calculated as percentage of TNFα alone, which is set to 100%. Data are presented as mean ± SEM
Raloxifene-Fumarates Have Anti-NFκB and Anti-ER Activity
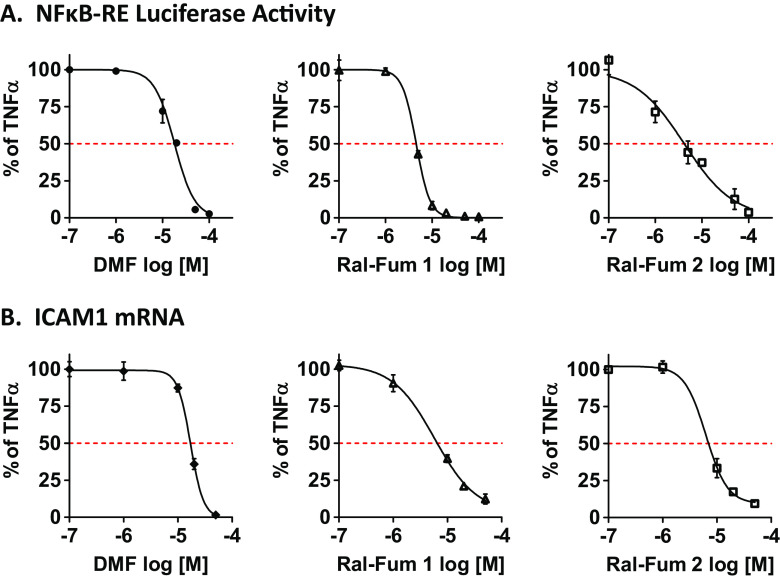
One approach to enhance the inhibitory activity of raloxifene on the NFκB pathway is by designing hybrid agents consisting of the parent SERM linked to additional functional groups or moieties with known anti-NFκB activity (Fig. 2). One such functionality is the fumarate, which we have previously shown to inhibit the NFκB pathway [11]. In Fig. 2, the co-targeting strategy and the chemical structures of Ral-Fum hybrids are shown. The first generation of raloxifene-fumarate hybrid (Ral-Fum 1) consisted of a benzothiophene-based synthetic intermediate conjugated to fumarate via an amide bond (Fig. 2b). The second generation, Ral-Fum 2, consisted of the full raloxifene structure conjugated to fumarate via an amine linker. The anti-NFκB activity was profiled in ER+ human breast cancer cells, MCF-7, by a dual luciferase reporter assay of the NFκB-RE shown in Fig. 3a, and by the expression of a bona-fide NFκB-target gene, intercellular adhesion molecule 1 (ICAM1), shown in Fig. 3b. We find that both co-targeting agents Ral-Fum 1 and Ral-Fum 2 inhibit the NFκB pathway on both assays with similar potency IC50 =4–5 μM. Ral-Fum 2 displays again similar potency against the NFκB pathway in a second ER+ breast cancer cell line, T47D, as well as in an ER− cell line, MDA-MB-231 (Supplemental Fig. 3). This shows an improvement over the parent drug, dimethyl fumarate (DMF), which has an IC50 =20 μM (Fig. 3). Overall, we conclude that Ral-Fum hybrids have improved inhibitory potency on NFκB, and it is likely independent of ER given their equal activity in either ER+ or ER− breast cancer cell lines.
Fig. 3.
Co-targeting Ral-Fum hybrids have anti-NFκB activity in breast cancer cells. a MCF-7 cells were transfected with NFκB-RE and renilla reporter plasmids. Cells were then pretreated with various concentrations of drugs for 1 h, followed by TNFα (10 ng/mL) for 4 h to activate the NFκB pathway. Each drug’s inhibitory activity was calculated as percentage of TNFα alone, which is set to 100%. b MCF-7 cells were pretreated with various concentrations of drugs for 1 h, followed by TNFα (10 ng/mL) for 2 h to activate the NFκB pathway. mRNA expression of ICAM1 was measured by RT-QPCR. Each drug’s inhibitory activity was calculated as percentage of TNFα alone, which is set to 100%. IC50s are calculated with GraphPad Prism software
We have previously shown that DMF’s anti-NFκB activity is attributed to its electrophilic nature, and its mechanism of action is via covalent protein modification [11]. To determine whether this chemical reactivity is required in the co-targeting hybrids, we tested raloxifene-succinate (Ral-Succ), the saturated analog of Ral-Fum devoid of the fumarate’s double bond, hence unable to form covalent protein adducts. We find that Ral-Succ is unable to inhibit the NFκB pathway illustrated in Supplemental Fig. 4. This suggests that Ral-Fum hybrids, similar to DMF, require fumarate’s chemical reactivity to inhibit the NFκB pathway in breast cancer cells.
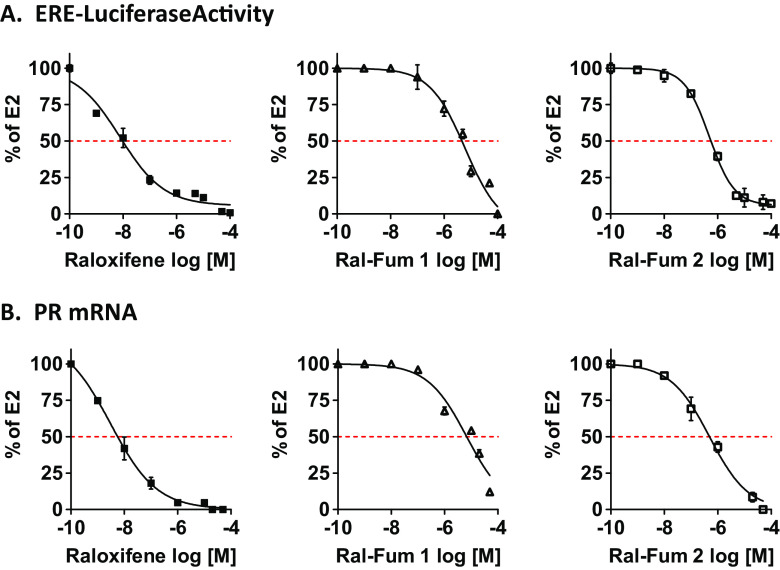
Next, we profiled the anti-ER activity of Ral-Fum co-targeting agents. As mentioned above, first generation versus second generation of raloxifene-fumarate hybrids differ in their SERM portion and linkage to fumarate (Fig. 2b). While Ral-Fum 1 consists of a benzothiophene-based synthetic intermediate conjugated to fumarate via an amide bond, Ral-Fum 2 consists of the full raloxifene structure, including its piperidine arm. The fumarate was then conjugated by click chemistry while the piperidine nitrogen remained intact as a tertiary amine, hence an amine linker (Fig. 2b). ER antagonism was measured in ER+ human breast cancer cells, MCF-7, by a dual luciferase reporter assay of the ER-response element (ERE) shown in Fig. 4a, and by the expression of a bona-fide ER-target gene, progesterone receptor, PR, shown in Fig. 4b, in the presence of estrogen (E2). We find that both Ral-Fum hybrids show significant loss of potency compared to the parent drug raloxifene, which exhibits IC50s of 8 and 3 nM on ERE luciferase and PR gene expression, respectively. Ral-Fum 1 is the weakest ER antagonist IC50 =6 μM, while Ral-Fum 2 is an order of magnitude more potent: IC50 =500 nM. Since the benzothiophene core is identical in both hybrids, the difference in potency is attributed to the piperidine ring of the side arm present in Ral-Fum 2 that is critical for helix 12 placement in an antagonist ER conformation [21]. Instead, the fumarate moiety is predicted to have no effect on ER. Indeed, DMF by itself has no inhibitory activity against ER, as illustrated in Supplemental Fig. 5 on both ER-induced target genes, trefoil factor 1 (TFF1) and early growth response 3 (EGR3), or on ERE luciferase activity.
Fig. 4.
Co-targeting Ral-Fum hybrids have anti-ER activity in breast cancer cells. a MCF-7 cells were transfected with ERE and renilla reporter plasmids. Cells were then treated with various concentrations of drugs for 1 h, followed by E2 (10 nM) for 4 h to activate ER. Each drug’s inhibitory activity was calculated as percentage of E2 alone, which is set to 100%. b MCF-7 cells were treated with various concentrations of drugs for 1 h, followed by E2 (10 nM) for 2 h. mRNA expression of PR was measured by RT-QPCR. Each drug’s inhibitory activity was calculated as percentage of E2 alone, which is set to 100%
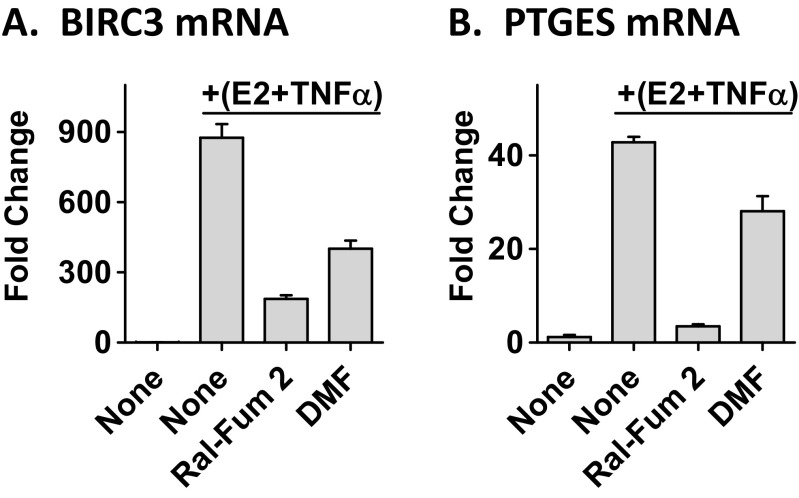
More recently, the classical paradigm of antagonistic crosstalk between ER and the NFκB pathway in breast cancer has been challenged. We have shown that ER and NFκB can also act together in a positive manner to synergistically increase transcription of a subset of genes that correlates with poor patient outcome following endocrine treatment [15]. The Ral-Fum 2 hybrid showed enhanced potency at inhibiting ER and NFκB crosstalk genes such as, baculoviral IAP repeat containing 3 (BIRC3) and prostaglandin E synthase (PTGES) (Fig. 5), further supporting its purported benefit of the dual-targeting nature.
Fig. 5.
Ral-Fum 2 inhibits ER and NFκB crosstalk genes. MCF-7 cells were treated with 10 μM Ral-Fum 2 or 10 μM DMF for 1 h, followed by E2+TNFα for 2 h. mRNA expression of crosstalk genes, BIRC3 and PTGES, was measured by RT-QPCR
Raloxifene-Fumarates Block Proliferation, Clonogenic Growth, and Mammosphere Formation of ER+ Breast Cancer Cells
To determine the therapeutic value of the Ral-Fum hybrids in breast cancer, we measured the anti-proliferative (Fig. 6a), anti-clonogenic (Fig. 6b), and anti-mammosphere formation (Fig. 6c) activity of these drugs. Ral-Fum 2 (IC50 =500 nM) is more potent than Ral-Fum 1 (IC50 =3 μM) at blocking estrogen-induced proliferation of ER+ breast cancer cells. Given that ER fueled by estrogen is the main driver of proliferation in ER+ breast cancer cells, we find as expected that the anti-proliferative activity of Ral-Fum hybrids mimics the ER antagonism observed in Fig. 4. We find that anti-clonogenic activity of Ral-Fum 1 (IC50 =2 μM) versus Ral-Fum 2 (IC50 =16 nM) also follows the same trend given that this assay relies on both cell proliferation in estrogenized growth media and cell survival under clonogenic cell density. In both of these assays, raloxifene alone shows an efficacy consistent with its ER IC50 in the low nanomolar range. Other important breast cancer phenotypes are anchorage-independent growth and self-renewal, which can be measured in the mammosphere (MS) assay. MS culture also enriches for breast cancer stem cells (CSCs); these are a subset of cells within the tumor endowed with tumorigenic potential, are refractory to therapy, and contribute to recurrence and metastasis [22–27]. We have shown that DMF inhibits MS formation of breast cancer cells by inhibiting the NFκB pathway [11]. Ral-Fum 2 inhibits MS formation more potently compared to either raloxifene or DMF at their respective ER or NFκB pathway IC50s (Fig. 6c). Together, these data support the feasibility of a dual-targeting approach to improve on existing endocrine therapy drugs.
Fig. 6.
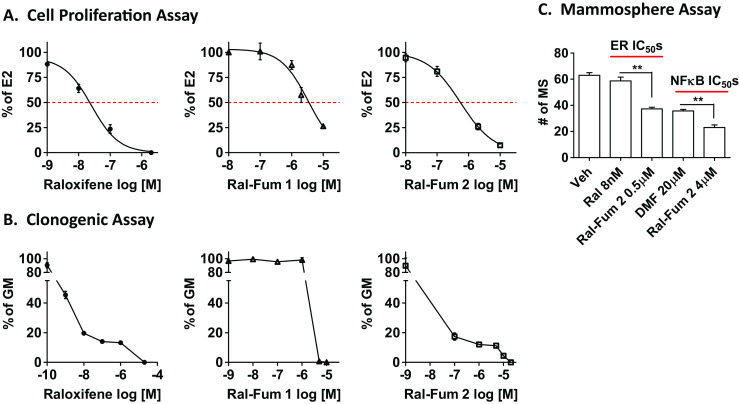
Co-targeting Ral-Fum hybrids block proliferation, clonogenic growth, and mammosphere formation of breast cancer cells. a MCF-7 cells were treated with various drug concentrations in the presence of E2 (10 nM) for 72 h. Each drug’s inhibitory activity was calculated as percentage of E2 alone, which is set to 100%. b MCF-7 cells are seeded as single cells in estrogenized growth media (GM), and then treated with the indicated concentration every 3–4 days for 2 weeks. Colonies were stained and quantified with ImageJ. Each drug’s inhibitory activity was calculated as percentage of GM alone, which is set to 100%. c MCF-7 cells were seeded as single cells on low attachment plates in MS media. DMSO (Veh) or drugs were added next day, and 7 days later the number of MS (MS >75 μm in diameter) was quantified. Double asterisks indicate (**) P < 0.01
Discussion
In breast cancer, development of resistance in ER+ tumors remains a major clinical problem that limits the usefulness of endocrine therapy. Therefore, a substantial number of breast cancer patients would benefit from an alternative approach to address endocrine resistance. Increasing evidence supports an important role for aberrant NFκB activity in endocrine resistance (Reviewed in [20, 28]), thus addition of an agent that can inhibit NFκB activity may improve patient outcome. We find that bifunctional hybrid drugs consisting of clinically relevant moieties aimed at inhibiting both ER and the NFκB pathway, as demonstrated herein, represent a new and viable strategy for treating ER+ breast cancers.
The classical paradigm for ER and the NFκB pathway interaction is described as mutually antagonistic. This was first noted by the clinical observation that pregnancy, which is characterized by excess estrogens, ameliorates inflammatory diseases such as rheumatoid arthritis, inflammatory bowel disease (Crohn’s disease and ulcerative colitis), and multiple sclerosis [29]. Additional molecular approaches further supported this antagonistic crosstalk by demonstrating the ability of estrogen-activated ER to quell NFκΒ signaling [30]. This led to the quest for ER ligands with agonist or non-classical activity at ER as a new class of anti-inflammatory agents [31–34]. However, for therapeutic applications in ER+ breast cancer therapy, a ligand is preferred with antagonist activity at ER. The effects of the clinically relevant SERMs and SERDs on NFκB activity in breast cancer cells have yet to be fully characterized. We showed that a SERM like raloxifene is a weak NFκB pathway inhibitor, yet more potent than SERDs, such as fulvestrant (ICI) and ARN-810.
To enhance raloxifene’s anti-NFκB activity, we synthesized raloxifene-fumarate hybrid drugs. These bifunctional hybrids show similar potency against the NFκB pathway, but differences in ER antagonism. We find that maintaining an intact tertiary amine in raloxifene’s piperidine arm is important for ER antagonistic activity. The cationic tertiary amine is a component of the side chains of most SERMs including tamoxifen, baxedoxifene, and raloxifene, and is implicated in displacement of helix 12 to yield an antagonist ER conformation [21]. Regarding NFκB inhibition, similar to our prior findings [12], we conclude that the fumarate moiety is sufficient and required to bestow anti-NFκB activity in breast cancer cells, and is independent of ER affinity. This conclusion is based on similar NFκB IC50s for Ral-Fum 2 on either ER+ or ER– breast cancer cells. Furthermore, fumarate (DMF) on its own has no activity on ER. We do observe an improved potency for raloxifene-fumarate hybrids compared to DMF, which we speculate results from increased cell permeability conferred by the raloxifene moiety.
DMF is a clinical anti-inflammatory drug functioning via activation of nuclear factor (erythroid-derived 2)-like 2 (Nrf2) and other immunomodulatory pathways [18]. We have previously shown that DMF, as an electrophilic small molecule, inhibits NFκB signaling in breast cancer cells by covalent modification of a key NFκB transcription factor p65 (RelA) [11]. Similarly, Ral-Fum hybrids rely on fumarate’s chemical reactivity to inhibit the NFκB pathway. Covalent inhibitors, by reacting irreversibly with protein targets, can produce more complete and sustained pharmacological effects [35]. More recently, Cravatt and colleagues demonstrated that a hybrid fumarate ester of Bruton’s tyrosine kinase (BTK) inhibitor, ibrutinib, undergoes enzymatic cleavage on a time scale that preserves rapid and sustained BTK inhibition, while thwarting more off-target reactivity in cell and animal models [36]. We still have to determine the hybrid’s cleavage rate within the cells due to the labile nature of that conjugation, but we envision that fumarate may endow similar kinetic selectivity against the NFκB pathway specifically in ER+ breast cancer cells. Overall, the SERM-fumarate strategy shown here provides the first attempt and proof-of-principle that this is a viable approach to improve the anti-inflammatory activity of ER-targeted anti-cancer drugs for breast cancer therapy.
Electronic supplementary material
Synthetic scheme for synthesizing Ral-Fum hybrids. (PPTX 106 kb)
Raloxifene inhibits ICAM1 gene expression and NFκB-RE activity. (A) MCF-7 cells were treated with various concentrations of drugs for 1 h, followed by TNFα (10 ng/mL) for 2 h to activate the NFκB pathway. mRNA expression of ICAM1 was measured by RT-QPCR. Drug’s inhibitory activity was calculated as % of TNFα alone, which is set to 100%. (B) T47D cells were transfected and treated with raloxifene as described in Fig. 1. (PPTX 126 kb)
Ral-Fum 2 inhibits NFκB-RE activity in breast cancer cells. (A) T47D and (B) MDA-MB-231 cells were transfected and treated as described in Fig. 1. (PPTX 108 kb)
Raloxifene-Succinate (Ral-Succ) fails to inhibit the NFκB pathway. MCF-7 cells were treated with 10 μM Ral-Succ for 1 h, followed by TNFα (10 ng/mL) for 2 h to activate the NFκB pathway. mRNA expression of ICAM1 was measured by RT-QPCR. (PPTX 46 kb)
DMF fails to inhibit ER in breast cancer cells. MCF-7 cells were treated with 10 μM DMF for 1 h, followed by E2. (A) mRNA expression of ER-target genes, pS2 and EGR3, was measured by RT-QPCR. (B) ERE luciferase activity was measured as described in Fig. 4. (PPTX 70 kb)
Acknowledgments
Authors’ Contributions
IK conceived and coordinated the study, performed and analyzed most of the experiments, and wrote the paper. MIS synthesized the co-targeting agents. SDB performed the luciferase assays. GRJT and JF contributed to the conceptualizing and drafting of the paper. All authors reviewed the results and approved the final version of the manuscript.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Financial Support
This work was supported by grants provided by the National Institute of Health (NIH), R01 CA200669 to JF, and CA121107 to GRJT, and by a postdoctoral fellowship grant from Susan G. Komen for the Cure® to IK (PDF12229484).
Contributor Information
Irida Kastrati, Phone: 312-355-2578, Email: ikastr2@uic.edu.
Jonna Frasor, Phone: 312-355-2583, Email: jfrasor@uic.edu.
References
- 1.Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res. 2007;9:R6. doi: 10.1186/bcr1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 3.Zhou Y, Eppenberger-Castori S, Marx C, Yau C, Scott GK, Eppenberger U, Benz CC. Activation of nuclear factor-kappaB (NFkappaB) identifies a high-risk subset of hormone-dependent breast cancers. Int J Biochem Cell Biol. 2005;37:1130–1144. doi: 10.1016/j.biocel.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Jones RL, Rojo F, A'Hern R, Villena N, Salter J, Corominas JM, Servitja S, Smith IE, Rovira A, Reis-Filho JS, Dowsett M, Albanell J. Nuclear NF-kappaB/p65 expression and response to neoadjuvant chemotherapy in breast cancer. J Clin Pathol. 2011;64:130–135. doi: 10.1136/jcp.2010.082966. [DOI] [PubMed] [Google Scholar]
- 5.Kubo M, Kanaya N, Petrossian K, Ye J, Warden C, Liu Z, Nishimura R, Osako T, Okido M, Shimada K, Takahashi M, Chu P, Yuan YC, Chen S. Inhibition of the proliferation of acquired aromatase inhibitor-resistant breast cancer cells by histone deacetylase inhibitor LBH589 (panobinostat) Breast Cancer Res Treat. 2013;137:93–107. doi: 10.1007/s10549-012-2332-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu H, Lee ES, Gajdos C, Pearce ST, Chen B, Osipo C, Loweth J, McKian K, De Los Reyes A, Wing L, Jordan VC. Apoptotic action of 17beta-estradiol in raloxifene-resistant MCF-7 cells in vitro and in vivo. J Natl Cancer Inst. 2003;95:1586–1597. doi: 10.1093/jnci/djg080. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Y, Eppenberger-Castori S, Eppenberger U, Benz CC. The NFkappaB pathway and endocrine-resistant breast cancer. Endocr Relat Cancer. 2005;12 Suppl 1:S37–S46. doi: 10.1677/erc.1.00977. [DOI] [PubMed] [Google Scholar]
- 8.Riggins RB, Zwart A, Nehra R, Clarke R. The nuclear factor kappa B inhibitor parthenolide restores ICI 182,780 (Faslodex; fulvestrant)-induced apoptosis in antiestrogen-resistant breast cancer cells. Mol Cancer Ther. 2005;4:33–41. doi: 10.1186/1476-4598-4-33. [DOI] [PubMed] [Google Scholar]
- 9.deGraffenried LA, Chandrasekar B, Friedrichs WE, Donzis E, Silva J, Hidalgo M, Freeman JW, Weiss GR. NF-kappa B inhibition markedly enhances sensitivity of resistant breast cancer tumor cells to tamoxifen. Ann Oncol. 2004;15:885–890. doi: 10.1093/annonc/mdh232. [DOI] [PubMed] [Google Scholar]
- 10.Garber K. The second wave in kinase cancer drugs. Nat Biotechnol. 2006;24:127–130. doi: 10.1038/nbt0206-127. [DOI] [PubMed] [Google Scholar]
- 11.Kastrati I, Siklos MI, Calderon-Gierszal EL, El-Shennawy L, Georgieva G, Thayer EN, Thatcher GR, Frasor J. Dimethyl fumarate inhibits the nuclear factor kappaB pathway in breast cancer cells by covalent modification of p65 protein. J Biol Chem. 2016;291:3639–3647. doi: 10.1074/jbc.M115.679704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kastrati I, Litosh VA, Zhao S, Alvarez M, Thatcher GR, Frasor J. A novel aspirin prodrug inhibits NFkappaB activity and breast cancer stem cell properties. BMC Cancer. 2015;15:845. doi: 10.1186/s12885-015-1868-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morphy R, Kay C, Rankovic Z. From magic bullets to designed multiple ligands. Drug Discov Today. 2004;9:641–651. doi: 10.1016/S1359-6446(04)03163-0. [DOI] [PubMed] [Google Scholar]
- 14.Pradhan M, Baumgarten SC, Bembinster LA, Frasor J. CBP mediates NF-kappaB-dependent histone acetylation and estrogen receptor recruitment to an estrogen response element in the BIRC3 promoter. Mol Cell Biol. 2012;32:569–575. doi: 10.1128/MCB.05869-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frasor J, Weaver A, Pradhan M, Dai Y, Miller LD, Lin CY, Stanculescu A. Positive cross-talk between estrogen receptor and NF-kappaB in breast cancer. Cancer Res. 2009;69:8918–8925. doi: 10.1158/0008-5472.CAN-09-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guzman C, Bagga M, Kaur A, Westermarck J, Abankwa D. ColonyArea: an ImageJ plugin to automatically quantify colony formation in clonogenic assays. PLoS One. 2014;9:e92444. doi: 10.1371/journal.pone.0092444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulze-Topphoff U, Varrin-Doyer M, Pekarek K, Spencer CM, Shetty A, Sagan SA, Cree BA, Sobel RA, Wipke BT, Steinman L, Scannevin RH, Zamvil SS. Dimethyl fumarate treatment induces adaptive and innate immune modulation independent of Nrf2. Proc Natl Acad Sci U S A. 2016;113:4777–4782. doi: 10.1073/pnas.1603907113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoefnagel JJ, Thio HB, Willemze R, Bouwes Bavinck JN. Long-term safety aspects of systemic therapy with fumaric acid esters in severe psoriasis. Br J Dermatol. 2003;149:363–369. doi: 10.1046/j.1365-2133.2003.05433.x. [DOI] [PubMed] [Google Scholar]
- 20.Frasor J, El-Shennawy L, Stender JD, Kastrati I (2014) NFkappaB affects estrogen receptor expression and activity in breast cancer through multiple mechanisms. Mol Cell Endocrinol [DOI] [PMC free article] [PubMed]
- 21.Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engstrom O, Ohman L, Greene GL, Gustafsson JA, Carlquist M. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389:753–758. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- 22.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC, Wong H, Rosen J, Chang JC. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 24.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, Joshua B, Kaplan MJ, Wapnir I, Dirbas FM, Somlo G, Garberoglio C, Paz B, Shen J, Lau SK, Quake SR, Brown JM, Weissman IL, Clarke MF. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Croker AK, Allan AL. Cancer stem cells: implications for the progression and treatment of metastatic disease. J Cell Mol Med. 2008;12:374–390. doi: 10.1111/j.1582-4934.2007.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Velasco-Velazquez MA, Popov VM, Lisanti MP, Pestell RG. The role of breast cancer stem cells in metastasis and therapeutic implications. Am J Pathol. 2011;179:2–11. doi: 10.1016/j.ajpath.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hollier BG, Evans K, Mani SA. The epithelial-to-mesenchymal transition and cancer stem cells: a coalition against cancer therapies. J Mammary Gland Biol Neoplasia. 2009;14:29–43. doi: 10.1007/s10911-009-9110-3. [DOI] [PubMed] [Google Scholar]
- 28.Baumgarten SC, Frasor J. Inflammation: an instigator of more aggressive estrogen receptor (ER) positive breast cancers. Mol Endocrinol. 2012;26:360–371. doi: 10.1210/me.2011-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav. 2012;62:263–271. doi: 10.1016/j.yhbeh.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biswas DK, Singh S, Shi Q, Pardee AB, Iglehart JD. Crossroads of estrogen receptor and NF-kappaB signaling. Sci STKE. 2005;2005:pe27. doi: 10.1126/stke.2882005pe27. [DOI] [PubMed] [Google Scholar]
- 31.Chadwick CC, Chippari S, Matelan E, Borges-Marcucci L, Eckert AM, Keith JC, Jr, Albert LM, Leathurby Y, Harris HA, Bhat RA, Ashwell M, Trybulski E, Winneker RC, Adelman SJ, Steffan RJ, Harnish DC. Identification of pathway-selective estrogen receptor ligands that inhibit NF-kappaB transcriptional activity. Proc Natl Acad Sci U S A. 2005;102:2543–2548. doi: 10.1073/pnas.0405841102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nettles KW, Bruning JB, Gil G, Nowak J, Sharma SK, Hahm JB, Kulp K, Hochberg RB, Zhou H, Katzenellenbogen JA, Katzenellenbogen BS, Kim Y, Joachmiak A, Greene GL. NFkappaB selectivity of estrogen receptor ligands revealed by comparative crystallographic analyses. Nat Chem Biol. 2008;4:241–247. doi: 10.1038/nchembio.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nwachukwu JC, Srinivasan S, Bruno NE, Parent AA, Hughes TS, Pollock JA, Gjyshi O, Cavett V, Nowak J, Garcia-Ordonez RD, Houtman R, Griffin PR, Kojetin DJ, Katzenellenbogen JA, Conkright MD, Nettles KW. Resveratrol modulates the inflammatory response via an estrogen receptor-signal integration network. elife. 2014;3:e02057. doi: 10.7554/eLife.02057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Y, Gong P, Chen Y, Nwachukwu JC, Srinivasan S, Ko C, Bagchi MK, Taylor RN, Korach KS, Nettles KW, Katzenellenbogen JA, Katzenellenbogen BS. Dual suppression of estrogenic and inflammatory activities for targeting of endometriosis. Sci Transl Med. 2015;7:271ra279. doi: 10.1126/scitranslmed.3010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh J, Petter RC, Baillie TA, Whitty A. The resurgence of covalent drugs. Nat Rev Drug Discov. 2011;10:307–317. doi: 10.1038/nrd3410. [DOI] [PubMed] [Google Scholar]
- 36.Zaro BW, Whitby LR, Lum KM, Cravatt BF. Metabolically labile fumarate esters impart kinetic selectivity to irreversible inhibitors. J Am Chem Soc. 2016;138:15841–15844. doi: 10.1021/jacs.6b10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Synthetic scheme for synthesizing Ral-Fum hybrids. (PPTX 106 kb)
Raloxifene inhibits ICAM1 gene expression and NFκB-RE activity. (A) MCF-7 cells were treated with various concentrations of drugs for 1 h, followed by TNFα (10 ng/mL) for 2 h to activate the NFκB pathway. mRNA expression of ICAM1 was measured by RT-QPCR. Drug’s inhibitory activity was calculated as % of TNFα alone, which is set to 100%. (B) T47D cells were transfected and treated with raloxifene as described in Fig. 1. (PPTX 126 kb)
Ral-Fum 2 inhibits NFκB-RE activity in breast cancer cells. (A) T47D and (B) MDA-MB-231 cells were transfected and treated as described in Fig. 1. (PPTX 108 kb)
Raloxifene-Succinate (Ral-Succ) fails to inhibit the NFκB pathway. MCF-7 cells were treated with 10 μM Ral-Succ for 1 h, followed by TNFα (10 ng/mL) for 2 h to activate the NFκB pathway. mRNA expression of ICAM1 was measured by RT-QPCR. (PPTX 46 kb)
DMF fails to inhibit ER in breast cancer cells. MCF-7 cells were treated with 10 μM DMF for 1 h, followed by E2. (A) mRNA expression of ER-target genes, pS2 and EGR3, was measured by RT-QPCR. (B) ERE luciferase activity was measured as described in Fig. 4. (PPTX 70 kb)