Abstract
The majority of microorganisms present a community lifestyle, establishing biofilm ecosystems. However, little is known about its formation in emergent Candida species involved in catheter-related infections. Thus, various techniques may be used in the biofilm detection to elucidate structure and clinical impact. In this context, we report the ability of emergent Candida species (Candida haemulonii, C. lusitaniae, C. pelliculosa, C.guilliermondii, C. famata and C. ciferrii) on developing well structured biofilms with cell viability and architecture, using optical coherence tomography (OCT). This new approach was compared with XTT analyses and Scanning Electron Microscopy (SEM). A positive correlation between oxidative activity (XTT) and OCT results (r = 0.8752, p < 0.0001) was observed. SEM images demonstrated cells attachment, multilayer and morphologic characteristics of the biofilm structure. C. lusitaniae was the emergent species which revealed the highest scattering extension length and oxidative metabolism when evaluated by OCT and XTT methods, respectively. Herein, information on C. ciferri biofilm structure were presented for the first time. The OCT results are independently among Candida strains and no species-specific pattern was observed. Our findings strongly contribute for clinical management based on the knowledge of pathogenicity mechanisms involving emergent yeasts.
Introduction
Several biological mechanisms involved in fungal infections are not yet fully understood. Therefore, a major concern involves the understanding and characterization of emergent Candida species [1]. The sudden emergence of previously uncommon and apparently harmless yeast species as agents of invasive candidiasis may be attributed to several factors such as the use of medical devices including catheter and biofilm formation [2,3]. In particular, systemic infections by emergent yeasts as Candida ciferrii, C. famata, C. guilliermondii, C. haemulonii, C. lusitaniae, and C. pelliculosa, had increased the mortality rate, especially when associated with severe underlying pathologies and the use of medical devices [4].
Interestingly, the majority of microorganisms survive in nature because of their community style of life as biofilm ecosystems [5]. Biofilms are structured microbial communities attached to either biotic or abiotic surface embedded in an exopolymeric matrix constituted mainly by carbohydrates, hexosamines, uronic acids, proteins and nucleic acids [6]. The biofilm architecture maintenance may be ensured by the formation of canals and columns, which allow the passage of nutrients and oxygen for the entire microbial community.
The biofilm formation is essential for yeasts protection against host defense mechanisms and commonly used antifungal drugs. Biofilm formation in implant devices is also an important medical concern, leading to clinical failure [7,8]. During the last decades, unusual yeast species have emerged as agents of invasive candidiasis and strong cause of death [9].
Classically, C. albicans develop highly structured biofilms with multiple cell types as budding yeast-form cells, pseudohyphae and true hyphae encased in an extracellular matrix. Commonly, Candida non-albicans biofilms form extracellular matrix but do not produce true hyphae [10]. Thus, its formation is an important feature for yeast virulence, and studies regarding this complex structure by emergent Candida species are still incipient [11, 12]. Various techniques may be used in the biofilm detection commonly SEM and metabolic activity evaluation by XTT (2,3-Bis-(2-Methoxy-4-Nitro-5-Sulfophenyl)-2H-Tetrazolium-5-Carboxanilide inner salt) [12,13]. Although rarely applied, imaging techniques may be an option in the detection of fungal biofilm. Thus, optical coherence tomography (OCT) is a well-established, low-coherence interferometric technique that performs high-resolution, ultrafast, noninvasive, and cross-sectional tomographic imaging, This optical technique evaluates interference patterns of backscattering light to build images, in depth, of biological structures as Candida albicans biofilm [14, 15]. In this context, the purpose of our study was to evaluate the potential use of OCT on analyzing the ability of emergent Candida species (Candida haemulonii, C. lusitaniae, C. pelliculosa, C. guilliermondii, C. famata and C. ciferrii) to develop well structured biofilms with cell viability and architecture.
Results
Emergent Candida strains
The emergent Candida species included in the present study were as follows: Candida haemulonii (3 strains), C. lusitaniae (3), C. pelliculosa (3), C. guilliermondii (4), C. famata (1) and C. ciferri (1). All yeast cultures evaluated in this study were previously isolated from critically ill patients, identified by MALDI TOF-MS and then were kept in the URM Culture Collection, Pernambuco, Brazil.
Quantitative analyses of biofilms: Oxidative activity and optical coherence tomography (OCT)
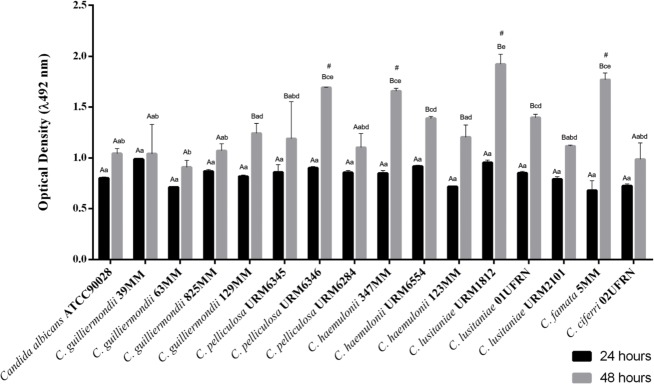
During the oxidative activity with the colorimetric assays based on XTT reduction, we observed that the emergent Candida strains used in the study were able to form an active biofilm. Quantitative XTT analyses revealed that mature stages with highest metabolic activities occurred at 48 hours of incubation (Fig 1).
Fig 1. Oxidative activity biofilm for emergent Candida strains developed at 24 and 48 hours.
Data represent the mean and standard deviation (SD) of the XTT absorbance during biofilm production in two independent experiments with at least three replicates (n≥ 6). For the analysis, Tukey's multiple comparisons test was performed for all averages obtained at the 5% level of significance. Different capital letters indicate significant difference in biofilm production in relation to time (24 and 48 hours) for a single Candida isolate. Different lowercase letters indicate significant difference in biofilm production among Candida isolates. The "#" symbol represents the isolates that have excelled in biofilm production in relation to the others, but they do not differ each other.
Fig 1. shows the mean OD 492 nm for each strain, for biofilms formed after 24 and 48 hours of incubation. C. guilliermondii strains did not present a significant variation for biofilm formation detected by XTT activity, exhibiting a homogenous quantitative pattern. This characteristic was not verified among the other emergent strains, such as C. pelliculosa, C. haemulonii and C. lusitaniae, which presented considerable differences in optical densities caused by XTT activity values for the strains within the same species.
Samples were analyzed using OCT, which exhibited the extension of changes in the catheters discs. In the OCT images, red shades represent higher scattering areas. Pixel intensity distributions (A-scan) of selected regions are also presented, indicating that the amplitude of the scattered light decrease at deeper areas under the biofilm surface (Fig 2). The OCT results are independently among Candida strains and no species-specific pattern.
Fig 2.
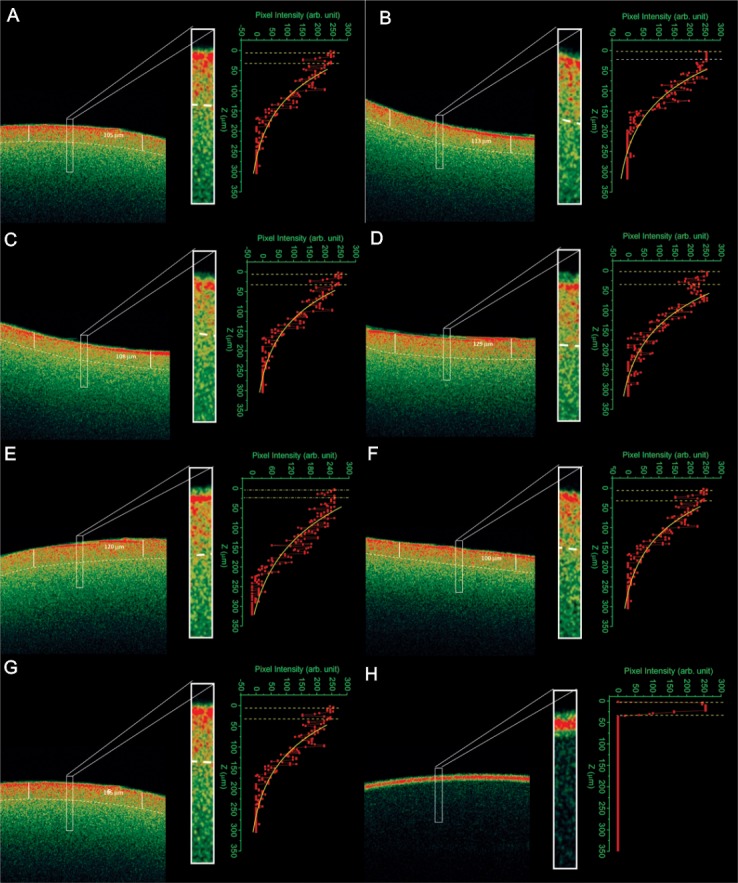
Optical coherence tomography indicating the the extension of changes in the sample structure due to the presence of emergent yeast in catheter discs: (A) Candida guilliermondii, (B) C. pelliculosa, (C) C. haemulonii, (D) C. lusitaniae, (E) C. famata, (F) C. ciferri and (G) C. albicans ATCC 90028. The control (H) is disc free of biofilm.
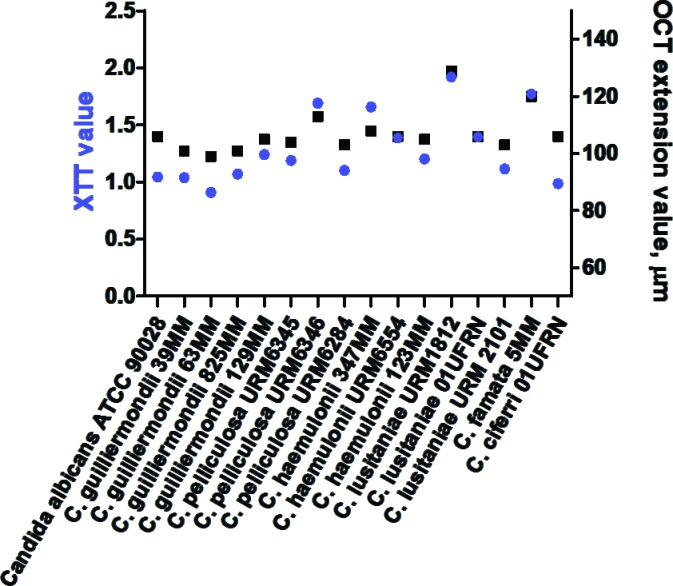
The correspondence between the XTT and the measured OCT values are shown in Fig 3. There was a significant positive correlation between oxidative activity and optical coherence tomography in biofilm development (Pearson correlation test, r = 0.8752, p < 0.0001). Furthermore, correspondence in results were visually demonstrated by SEM through observation of cells attachment, multilayer and morphologic characteristics (Fig 4).
Fig 3. Ample structure changes and metabolism of emergent Candida biofilms observed by optical coherence tomography (OCT) and oxidative activity (XTT).
The results correlation shows positive distribution (Pearson correlation test, r = 0.8752, p < 0.0001).
Fig 4.
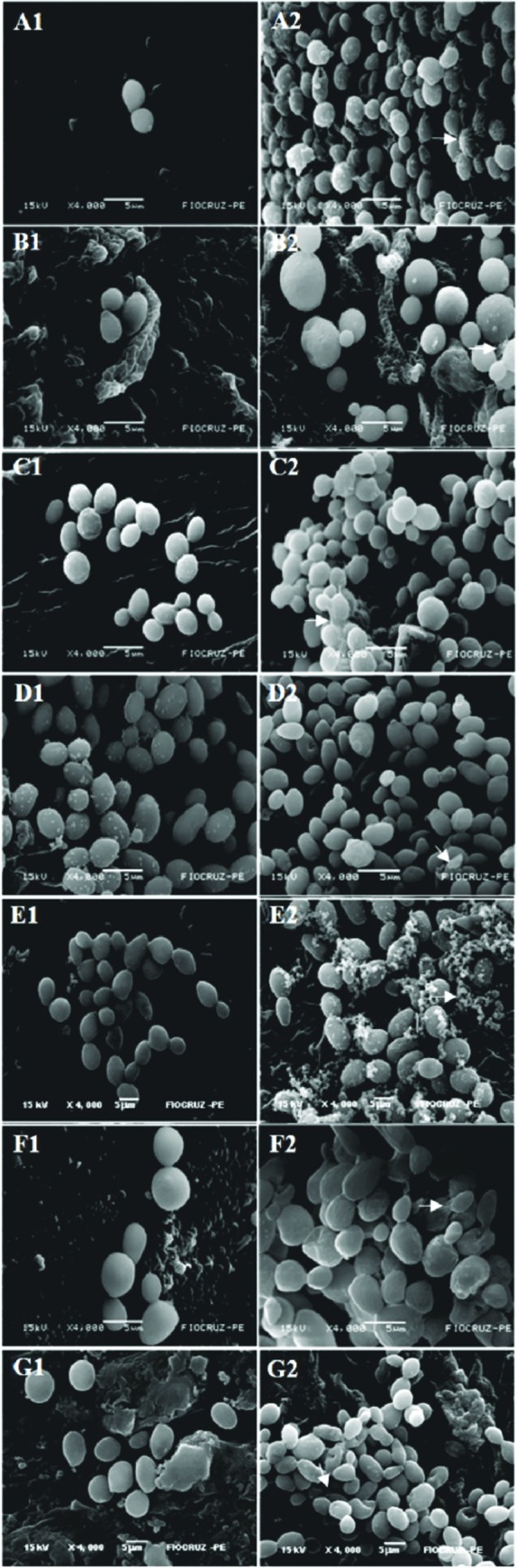
Scanning electron microscopy of 24h (A1-G1) and 48h (A2-G2) biofilms formed on catheter discs for emergent Candida strains. Candida guilliermondii (A1, A2), C. pelliculosa (B1, B2), C. haemulonii (C1, C2), C. lusitaniae (D1, D2), C. famata, (E1, E2) and C. ciferri (F1, F2). The control (G1, G2) is C. albicans ATCC90028. Arrow indicates the presence of the extracellular matrix within the biofilm. Magnification x4.000.
Qualitative analyses of biofilms: Evaluation of architecture in catheter discs
During the first 24 hours of incubation, C. lusitaniae strains exhibited intense biofilm formation, followed by C. famata, C. haemulonii and C. albicans reference strain. However, the other species (C. ciferri, C. pelliculosa and C. guilliermondii) showed sparse yeast cells adhered to catheter discs’ surface, typical of initial stage of the biofilm formation. Nevertheless, in 48 hours of exposure, all yeast strains showed a mature biofilm on the surface of the discs.
Discussion
The Candida species evaluated in this study are rarely described in clinical cases of invasive fungal infections. However, when this condition occurs, these yeasts are commonly isolated from the bloodstream of patients with poor prognostic [16–17].
The fact that these emergent yeast species are able to form biofilms may be clinically relevant. In fact, Tumbarello et al. [18] found that patients with candidemia caused by non-biofilm-forming Candida spp. strains had better outcomes. In contrast, the biofilm-forming strains showed a significant association with poor prognoses.
In addition, Iturrieta-Gonzalez et al. [19] described that Trichosporon, another emergent yeast genus, showed levels of biofilm formation similar or greater than those described for Candida spp. and that biofilm-forming cells were at least 1,000 times more resistant to antifungals than their planktonic counterparts. These data reinforce the likely importance of biofilm formation by the rare Candida species used in the present study.
The evaluation using the XTT reduction and the biofilms images demonstrated the metabolic viability inside matrix in all tested yeasts, with significant differences between species and within different strains of the same species. In contrast, C. guilliermondii isolates showed no significant intra-specific variations, demonstrating a homogenous pattern of viability for sessile cells present in the biofilms. Interestingly, the high XTT OD 492nm obtained for C. lusitaniae (URM 1812) biofilm is in agreement with the literature because emergent yeasts may form viable multicellular structures in inert surfaces as catheters and invasive devices or colonize valves in humans [12].
Based on light scattering, OCT images showed the extension of changes inside the catheter structure, indicating the pathogenicity of these emerging yeast species. A clear image difference can be observed among the samples with and without (control) biofilm (Fig 2). The presence of the biofilm enhances light scattering of the sample, increasing the brightness of undersurface areas of the OCT biofilm images. Scattering can be attributed to the biofilm microstructure on the catheter. Thus, it was observed that the OCT values are higher for those isolates with the high XTT activity. Few studies have been developed using these techniques to clarify structure and metabolism of Candida biofilms [12, 15]. This is the first study to explore OCT technique to evaluate emergent clinical yeasts. The OCT technique offers advantages once it clearly proved to be non-invasive, real-time, privileging in-situ analysis of biofilm layers. Moreover this biofilm keep its original structure without the performance of a destructive process enabling the assessment of biofilm roughness and surface area [20].
Through of the scanning electron microscopy, it was visually verified that all biofilm formation stages occurred in the yeasts tested within 48 hours. Thus, with a significant increase of cell proliferation with amorphous material, representing the extracellular matrix and featuring a mature biofilm. Interestingly, rare yeast cells were adhered to the surface of the catheter disc during the first 24h in C. ciferri, C. pelliculosa and C. guilliermondii. C. lusitaniae (URM 1812) showed intense cell adhesion at this time of evaluation. This species showed a higher number of yeast cells adhered to the discs and intercellular adhesion. In addition, we observed in scanning electron microscopy that the biofilm of all species was composed of blastoconidia, but did not present filamentous forms. All emergent Candida biofilms appeared as discontinuous layers of blastoconidia anchored to the surface and rich in extracellular matrix but not hyphae, which was in accordance with the findings of Silva et al. [21] after evaluating C. glabrata, C. parapsilosis and C. tropicalis biofilm. As demonstrated by Chandra et al. [22] both RPMI as YNB media are used to form well-structured biofilms, however YNB enhances budding reproduction and RPMI stimulates filamentation. Their data indicate that biofilm growth was not morphology specific.
Sparse investigations upon C. albicans biofilm architecture can be found in the literature [23]. However, currently, there are no studies characterizing the structures of some emergent yeasts. Moreover, we observed that C. ciferrii developed a classic biofilm, with cell viability, indicating the pathogenic potential of this species. Then, with microscopy, we confirmed that in addition to C. albicans, which was used in the trials as control species, emerging Candida species are also able to form biofilms in their entirety, showing the architecture in all stages. In addition, the chemical nature of the catheter, a material made up of polystyrene, polyurethane or polyvinyl chloride enable biofilm formation [24].
The knowledge of pathogenicity mechanisms of yeast biofilms is crucial for the development of new antifungal therapies and diagnostic strategies.
Materials and methods
Candida strains
Fifteen Candida clinical isolates obtained from the Micoteca URM Culture Collection, Medical Mycology Laboratory (MML) from Federal University of Pernambuco and Laboratory of Medical and Molecular Mycology (UFRN), Federal University of Rio Grande do Norte were analyzed in this study. In addition, C. albicans ATCC 90028 was used as reference strain. The strain descriptions are summarized in Table 1.
Table 1. Descriptions of the strains analyzed in the study obtained in the collection of culture URM, Laboratory of Medical Mycology (MML) of the federal university of pernambuco and laboratory of Medical and Molecular Mycology (UFRN), federal university of Rio Grande do Norte.
| Microorganism | Strain number | Substrate of origin |
|---|---|---|
| C. ciferri | 02UFRN | Blood |
| C. famata | 05MML | Blood |
| C. guilliermondii | 39MML | Blood |
| C. guilliermondii | 63MML | Blood |
| C. guilliermondii | 129MML | Blood |
| C. guilliermondii | 825MML | Blood |
| C. haemulonii | 123MML | Blood |
| C. haemulonii | 347MML | Blood |
| C. haemulonii | URM6554 | Nail |
| C. lusitaniae | 01UFRN | Blood |
| C. lusitaniae | URM1812 | - |
| C. lusitaniae | URM2101 | - |
| C. pelliculosa | URM6345 | Blood |
| C. pelliculosa | URM6346 | Blood |
| C. pelliculosa | URM6384 | Blood |
| C. albicans | ATCC 90028* | - |
-: Substrate of origin uninformed.
* Reference strain
Emergent Candida species identification by MALDI-TOF MS
Homogenous inoculum of yeast cells were grown and maintained on Yeast Extract Peptone Dextrose Agar medium (YEPD). Incubations were performed at 20h and strains were grown aerobically at 37°C according to Lima-Neto et al. [25]. In order to avoid changes in the protein expression pattern, the culture conditions and growth time were standardized as described above. One single colony was directly deposited onto a 196- position target plate (Bruker Daltonik GmbH), in duplicate for each strain.
Aliquots of 1μL of 70% formic acid were added and mixed gently with yeasts. When the liquid medium was almost evaporated, the preparation was overlaid with 1μL of saturated matrix solution {75mg/ml of α-cyano-4-hydroxycinnamic acid (CHCA) in ethanol/water/acetonitrile [1:1:1] with 0.03% trifluoroacetic acid (TFA)}. The isolates were deposited per plate in duplicate, and the matrix-sample was crystallized by air-drying at 25°C for 5 minutes.
The equipment used was MALDI TOF Autoflex III Mass Spectrometer (Bruker Daltonics Inc., USA/Germany) composed of a Nd:YAG (neodymium-doped yttrium aluminium garnet; Nd:Y3Al5O12) laser of 1064nm, set to a 66% power. The mass range from 2,000 to 20,000 Da was recorded using a linear mode with a delay of 104ns and an acceleration voltage of +20 kV. The resulting peak lists were exported to the software MALDI Biotyper™ 3.1 (Bruker Daltonics, Bremem, Germany) where the final identifications were achieved.
Quantitative analyses of biofilms
Inoculum and biofilm development for oxidative activity
Yeast strains were cultured aerobically at 37°C for 18h on Sabouraud Dextrose Agar (SDA) and then inoculated in Yeast Nitrogen Base (YNB) broth (Difco Laboratories, Detroit, MI, USA) supplemented with 50mM glucose. After 18h of incubation, cells were harvested, washed twice with PBS (pH 7.2) and resuspended in YNB supplemented with 100mM glucose. Candida strains suspensions were prepared to a concentration of 107 cells/mL evaluated using a spectrophotometer Genesis 10S UV-Vis (Thermo Scientific) at 530nm, corresponding to 80% transmittance.
Candida species biofilm formation was performed as described by Silva et al. [13]. Briefly, biofilms were grown in commercially available pre-sterilized, polystyrene, flat-bottomed 96-well microtiter plates (TPP; Trasadingen, Switzerland). Aliquots of 100μL of standard cell suspensions of yeasts (107 cells/mL) were transferred into each well and incubated for 1.5h (adhesion phase) at 37°C at 75rpm. After the adhesion phase, cell suspensions were gently aspirated and each well was washed twice with PBS to carefully remove any remaining planktonic cells. In order to allow the growth of biofilm (biofilm phase), 200μL of YNB supplemented with 100mM glucose was added to each well. The plates were incubated for 24 and 48h at 37°C at 75rpm in a shaking TE-424 (Tecnal). After 24h incubation, the medium was aspirated and, biofilms were washed twice with PBS followed by addition of 200μL of YNB medium. All assays were performed in triplicate.
Oxidative activity assay
The 2,3—Bis—(2—Methoxy—4—Nitro—5—Sulfophenyl) - 2H - Tetrazolium—5 Carboxanilide (XTT) (Sigma-Aldrich Corp.) was dissolved in PBS at a final concentration of 1mg/mL.
The solution was filter-sterilized and stored frozen at -70°C until use. Menadione solution (0.4mM; Sigma-Aldrich Corp.) was prepared immediately before each assay. For each assay, XTT solution was thawed on ice and mixed with menadione solution at a volume ratio of 20:1. Biofilms were washed twice with 200μL of PBS to remove no adherent cells.
Subsequently, 158μL of PBS with or without glucose at different concentrations, 40μL of XTT and 2μL of menadione were transferred to each well of 96-well plates. The plates were covered with aluminum foil and incubated in the dark at 37°C for 3h. Thereafter, 100μL of the solution was transferred to each well of new 96-well plates. The colorimetric changes were measured at 492nm wavelength using a microtiter plate reader (Spectra-MAX 340; Molecular Devices Ltd., Sunnyvale, CA, USA).
Inoculum and biofilm development for optical coherence tomography (OCT) assay
The evaluation of biofilm formation stages was followed using catheter discs. The tests for biofilm formation were developed according Vandenbosch et al. [26]. The strains were grown on Sabouraud dextrose at 37°C for 16h, and then were centrifuged and the supernatant removed for washing the cells with a 0.85% saline solution. Cells were resuspended in 1ml saline solution (Novolab, Geraardsbergen, Belgium) and inocula were subsequently diluted in YNB medium supplemented with 50mM glucose to obtain an optical density of 0.07 at 600nm.
After standardization of fungal inoculum, 1ml of a 1:100 dilution in YNB was added to each well containing catheter discs. The microtiter plates were incubated for 1h at 37°C. Subsequently, the discs were washed (three times) with 1mL saline solution to remove non-adherent cells aseptically transferred to a new well. Subsequently, 1mL of diluted YNB was added with a final glucose concentration of 0.2mM, and plates were incubated for 24h and 48h at 37°C for further analysis by OCT.
Optical coherence tomography imaging analysis
Analyzes were performed using OCT technique based on the evaluation of the light scattered by the object, and provides in-depth information of the structures. A 2D image was obtained by the combination of depth-resolved back-scatter light intensity profiles (A-scans) along the section of interest on the sample. In this work, a commercial spectral optical coherence tomography system (Ganymede from Thorlabs Inc.) was used. The image system exploits a super luminescent diode as light source, emitting infrared light, with wavelength centered at 930nm and spectral width of 100nm. With an A-scan rate of 29kHz, this system can produce 29 frames per second with 512 lines per frame and an axial resolution of 5 micrometers. A theoretical model based on an exponential decay fitting is used to describe light intensity distribution (A-scan) in the sample. The light scattering depth of the biofilm (OCT extension value) was determined, and quantified as the inverse of the exponential decay constant of the theoretical fitting.
Qualitative analyses of biofilms
Evaluation of architecture in catheter discs by scanning electron microscopy
The inoculum preparations and biofilm formation were performed as prior described in OCT assays according to Vandenbosch et al. [26]. The biofilms formed on the catheter discs were fixed in 2.5% glutaraldehyde in 0.1M Sodium Cacodylate buffer for two hours. After fixation, the disks were washed with 0.1M Cacodylate buffer three times for 10 minutes for removal of the entire fixative.
Subsequently, post-fixation was performed in a ratio of 1:1 osmium 2% + 0.1M Cacodylate buffer for 30min, allowing a higher contrast of the material.
Two washes were carried out for 10min in 0.1M Cacodylate buffer and distilled water. Subsequently, the samples were submitted to serial dehydration with 30%, 50%, 70%, 90% and 100% acetone for 5 minutes and dried at the critical point. The material was assembled in metal stubs containing carbon tape and silver ink (which serve as an electrons conductor) and then metallized by bombarding with gold, and analyzed using a FEI (Quanta 200 FEG). The processing of the samples followed the proposed protocol Duarte et al. [27].
Ethics statement
The Candida isolates were obtained after review board approval to use the samples from the Micoteca URM Culture Collection, Medical Mycology Laboratory (MML) from Federal University of Pernambuco and Laboratory of Medical and Molecular Mycology (UFRN), Federal University of Rio Grande do Norte. All Candida samples were anonymized in accordance to the ethics statement.
Statistical analysis
Assays were carried out in triplicate for each strain. Statistical analysis was calculated using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA) software. The results from the XTT colorimetric assay were statistically evaluated by analysis of variance with ANOVA and Tukey's test, with significance level p<0.05. The presented OCT average values and the corresponding standard deviations were obtained by the evaluation of 75 A-scan of each image. The relationship between quantitative analyses of biofilms was evaluated by the Pearson correlation test (p≤0.05).
Acknowledgments
The authors would like to thank the Department of Mycology from Federal University of Pernambuco, for their technical support and the Technological Development Program in Materials for Health (PDTIS) of FIOCRUZ, for the Electron Microscopy Service from Technology Platforms Nucleus/Aggeu Magalhães Research Center (FIOCRUZ-PE), CNPq and CAPES for financial support.
Data Availability
All relevant data are within the paper.
Funding Statement
The author Rejane Neves received financial support from FACEPE/Brazil to acquire reagents and the necessary materials and Melyna Leite-Andrade received a scholarship from CNPq/Brazil. This study was also funded by CAPES and the National Institute of Photonics (to REdA). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kullberg BJ, and Arendrup MC. Invasive Candidiasis. N. Engl. J. Med. 2015; 15: 373. [DOI] [PubMed] [Google Scholar]
- 2.Holland LM, Schröder SM, Turner SA, TaffH Andes D, Grózer Z, Gácser A, et al. Comparative Phenotypic Analysis of the Major Fungal Pathogens Candida parapsilosis and Candida albicans. PLoS Pathogens. 2014; 10:004365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silva FC, Viana VO, Araújo BP, Campos LANL, and Rosa LP. Prevalence of Candida yeasts in oral samples from children with AIDS and children exposed and not exposed to HIV served by SUS in the state of Bahia, Brazil. Rev. Gaúch. Odontol. 2015; 63: 7–12. [Google Scholar]
- 4.Li Y, Xu W, Jiang Z, Gao Y, Pang Y, Li L, et al. Neutropenia and invasive fungal infection in patients with hematological malignancies treated with chemotherapy: a multicenter, prospective, non-interventional study in China. Tumour Biol. 2014; 35: 5869–5876. doi: 10.1007/s13277-014-1777-4 [DOI] [PubMed] [Google Scholar]
- 5.Donlan RM. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 2002; 8: 881–890. doi: 10.3201/eid0809.020063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell KF, Zarnowski R, Andes DR. The Extracellular Matrix of Fungal Biofilms. Adv. Exp. Med. Biol. 2016; 931: 21–35. doi: 10.1007/5584_2016_6 [DOI] [PubMed] [Google Scholar]
- 7.Kojic EM, and Darouiche RO. Candida infections of medical devices. Clin. Microbiol. Rev. 2004; 17: 255–267. doi: 10.1128/CMR.17.2.255-267.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramage G, Saville SP, Thomas DP, and Lopez-Ribot JL. Candida biofilms: an update. Eukaryot. Cell. 2005; 4: 633–638. doi: 10.1128/EC.4.4.633-638.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arendrup MC, Dzajic E, Jensen RH, Johansen HK, Kjældgaard P, Knudsen JD, et al. Epidemiological changes with potential implication for antifungal prescription recommendations for fungaemia: data from a nationwide fungaemia surveillance programme. Clin. Microbiol. Infect. 2013; 19: 343–353. [DOI] [PubMed] [Google Scholar]
- 10.Silva S, Negri M, Henriques M, Oliveira R, Williams DW. Adherence and biofilm formation of non-Candida albicans Candida species. Trends Microbiol. 2011; 19: 241–247. doi: 10.1016/j.tim.2011.02.003 [DOI] [PubMed] [Google Scholar]
- 11.Botelho NS, Paula SB, Panagio LA, Pinge-Filho P, Yamauchi LM and Yamada-Ogatta SF. Candida Species Isolated from Urban Bats of Londrina-Parana, Brazil and their Potential Virulence. Zoonoses Public Health. 2012; 59: 16–22. doi: 10.1111/j.1863-2378.2011.01410.x [DOI] [PubMed] [Google Scholar]
- 12.Pannanusorn S, Fernandez V, and Romling U. Prevalence of biofilm formation in clinical isolates of Candida species causing bloodstream infection. Mycoses. 2013; 56:264–272. doi: 10.1111/myc.12014 [DOI] [PubMed] [Google Scholar]
- 13.Silva WJ, Seneviratne J, Parahitiyawa N, Rosa EAR, Samaranayake LP, and Del Bel Cury AA. Improvement of XTT Assay Performance for Studies Involving Candida albicans Biofilms. Braz. Dent. J. 2008; 19: 364–369. [DOI] [PubMed] [Google Scholar]
- 14.Fujimoto JG. Optical coherence tomography for ultrahigh resolution in vivo maging. Nature Biotechnology. 2003; 21: 1361–1367. doi: 10.1038/nbt892 [DOI] [PubMed] [Google Scholar]
- 15.Suzuki LC, Prates RA, Raele MP, Freitas AZ, and Ribeiro MS. Real time optical coherence tomography monitoring of Candida albicans biofilm in vitro during photodynamic treatment. Biophotonics: Photonic Solutions for Better Health Care II. 2010. [Google Scholar]
- 16.Agin H, Ayhan Y, Devrin I, Guilfidan G, Tulumoglu S, Kayserilli E. Fluconazole, Amphotericin-B, Caspofungin and Anidulafungin Resistent Candida ciferri: An Unknow Cause of Systemic Mycosis in a Child. Mycopathologia. 2011; 172: 237–239. doi: 10.1007/s11046-011-9418-6 [DOI] [PubMed] [Google Scholar]
- 17.Jung DS, Farmakiotis D, Jiang Y, Tarrand JJ, Kontoyiannis DP. Uncommon Candida Species Fungemia among Cancer Patients, Houston, Texas, USA. Emerging Infectious Diseases. 2015; 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tumbarello M, Fiori B, Trecarichi EM, Posteraro P, Losito AR, De Luca A, et al. Risk Factors and Outcomes of Candidemia Caused by Biofilm-Forming Isolates in a Tertiary Care Hospital. PLoS One. 2012; 7: e33705 doi: 10.1371/journal.pone.0033705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iturrieta-González IA, Padovan ACB, Bizerra FC, Hahn RC, Colombo AL. Multiple Species of Trichosporon Produce Biofilms Highly Resistant to Triazoles and Amphotericin B. Plos One. 2014; 9:10 doi: 10.1371/journal.pone.0109553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dreszer C, Wexler AD, Drusova S, Overdijk T, Zwijnenburg A, Flemming HC, et al. In-situ biofilm characterization in membrane systems using Optical Coherence Tomography: Formation, structure, detachment and impact of flux change. Water Research. 2014; 67: 243–254. doi: 10.1016/j.watres.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 21.Silva S, Henriques M, Martins A, Oliveira R, Williams D, Azeredo J. Biofilms of non-Candida albicans Candida species: quantifi cation, structure and matrix composition. Medical Mycology. 2009; 47: 681–689. doi: 10.3109/13693780802549594 [DOI] [PubMed] [Google Scholar]
- 22.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans: development architecture and drug resistance. Journal of Bacteriology. 2001; 138: 5385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fanning S, Mitchell AP. Fungal Biofilms. PLoS Pathog. 2012; 8: 1002585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wildgruber M, Lueg C, Borgmeyer S, Karimov I, Braun U, Kiechle M, et al. Polyurethane versus silicone catheters for central venous port devices implanted at the forearm. European Journal of Cancer. 2016; 59: 113e124. [DOI] [PubMed] [Google Scholar]
- 25.Lima-Neto R, Cledir Santos C, Lima N, Sampaio P, Pais C, Neves RP. Application of MALDI-TOF MS for requalification of a Candida clinical isolates culture collection. Brazilian Journal of Microbiology. 2014; 45: 515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vandenbosch D, Braeckmans K, Nelis HJ, Coenye TJ. Fungicidal activity of miconazole against Candida spp. Biofilms. Antimicrob Chemother. 2010; 65: 694–700. [DOI] [PubMed] [Google Scholar]
- 27.Duarte LC, Juchem PL, Pulz GM, Brum TMM, Chodur N, Liccardo A, et al. Aplicações de microscopia eletrônica de varredura (MEV) e sistema de energia dispersiva no estudo de gemas: exemplos brasileiros. Pesquisa em geociências.2003; 30: 3–15. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.