Abstract
Rare genetic variants in the core endocannabinoid system genes CNR1, CNR2, DAGLA, MGLL and FAAH were identified in molecular testing data from 6,032 patients with a broad spectrum of neurological disorders. The variants were evaluated for association with phenotypes similar to those observed in the orthologous gene knockouts in mice. Heterozygous rare coding variants in CNR1, which encodes the type 1 cannabinoid receptor (CB1), were found to be significantly associated with pain sensitivity (especially migraine), sleep and memory disorders—alone or in combination with anxiety—compared to a set of controls without such CNR1 variants. Similarly, heterozygous rare variants in DAGLA, which encodes diacylglycerol lipase alpha, were found to be significantly associated with seizures and neurodevelopmental disorders, including autism and abnormalities of brain morphology, compared to controls. Rare variants in MGLL, FAAH and CNR2 were not associated with any neurological phenotypes in the patients tested. Diacylglycerol lipase alpha synthesizes the endocannabinoid 2-AG in the brain, which interacts with CB1 receptors. The phenotypes associated with rare CNR1 variants are reminiscent of those implicated in the theory of clinical endocannabinoid deficiency syndrome. The severe phenotypes associated with rare DAGLA variants underscore the critical role of rapid 2-AG synthesis and the endocannabinoid system in regulating neurological function and development. Mapping of the variants to the 3D structure of the type 1 cannabinoid receptor, or primary structure of diacylglycerol lipase alpha, reveals clustering of variants in certain structural regions and is consistent with impacts to function.
Introduction
The endocannabinoid system (ECS) plays an important role in the regulation of neurological activity throughout the central and peripheral nervous system [1–3], as well as in the regulation of cell division, metabolic, and immune processes in a variety of other tissues [4–6]. The type 1 cannabinoid receptor (CB1), encoded by the CNR1 gene, is a key component of the ECS. CB1 is the most abundant G-protein coupled receptor in the brain, and is present at high levels in the neocortex, hippocampus and cerebellum [7]. CB1 is activated by the natural endocannabinoid agonists N-arachidonoylethanolamine (AEA), 2-arachidonoyl glycerol (2-AG) and a variety of related compounds [8]. It also binds the phytocannabinoid Δ9-tetrahydrocannabinol (THC), and a wide variety of synthetic agonists and antagonists [9]. At the cellular level, CB1 is located primarily on presynaptic termini of GABAergic and glutamatergic neurons in the brain [10, 11], where it binds 2-AG released by postsynaptic termini to down-regulate neurotransmitter release [12].
The type II cannabinoid receptor (CB2), encoded by the CNR2 gene, is expressed in cells of the immune system (recently reviewed by Turcotte et al [13]) and is strongly induced in activated microglia in the brain [14]. CB2 is activated by the endocannabinoids AEA and 2-AG, and by the phytocannabinoids THC and β‒caryophyllene [13, 15].
2-AG is the most abundant endocannabinoid in the brain, and is generated through the action of diacylglycerol lipase α (encoded by the DAGLA gene) by hydrolysis of diacylglycerol [16]. 2-AG is a mediator of retrograde signaling to presynaptic CB1 receptors to regulate neurotransmitter release [12]. 2-AG acts over longer distances during early development due to low monoacylglycerol lipase levels to regulate neuronal development at axonal growth cones [17, 18]. 2-AG is degraded by the action of monoacylglycerol lipase (encoded by the MGLL gene). AEA is generated mainly through the activity of N-acyl phosphatidylethanolamine phospholipase D, encoded by the NAPEPLD gene, and is degraded mainly by fatty acid amide hydrolase, encoded by the FAAH gene [19, 20].
Common single nucleotide polymorphisms in or near CNR1, CNR2, FAAH and MGLL have been reported to be associated with a variety of clinical phenotypes in candidate gene association studies (substance abuse disorders, cardiovascular disease risk factors, irritable bowel syndrome, migraine, chronic pain and mood disorders) [21–30]. The effect sizes are generally small, however, and replication studies in larger independent cohorts have been met with mixed results [31–35]. In contrast, the impact of rare genetic variation in genes associated with the endocannabinoid system has not been studied systematically. There are several reports of pathogenic deletions and duplications involving the ECS genes CNR1, CNR2, DAGLA, MGLL and FAAH with associated developmental phenotypes in the Decipher [36] and Clinvar [37] databases. There is also a reported association of DAGLA duplications with spinocerebellar ataxia-20 in OMIM [38] (entry 608687). However, the size of those structural variants is generally large (24 Mb on average) and they all impact multiple genes.
In this study, we investigate the phenotypic impact of rare missense variants in the core ECS genes: CNR1, CNR2, DAGLA, MGLL and FAAH, which encode CB1, the type 2 cannabinoid receptor (CB2), diacylglycerol lipase alpha, monoglyceride lipase, and fatty acid amide hydrolase, respectively. Phenotypes for were selected for evaluation based on their presence in mouse knockout strains for each gene.
Methods
Two diagnostic gene panels were used in this study (S1 Table). These panels were designed to identify chromosomal alterations associated mitochondrial disorders (NucSEEK Comprehensive– 1207 genes including CNR1, MGLL and FAAH) and epilepsy (EpiSEEK Comprehensive– 471 genes including CNR1, CNR2, DAGLA, MGLL and FAAH). Genetic sequencing data was generated from over 6,000 individuals using one or both of those gene panels in our CLIA/CAP certified laboratory, and clinical diagnostic reports were created, as follows.
DNA was extracted from saliva or blood samples using Genfind v2 (Beckman Coulter). Sequence-ready libraries were prepared using HaloPlex® target capture kits (Agilent Technologies) in conjunction with DREAM PCR amplification [39, 40] to avoid sample contamination. Sequencing was performed on the Illumina MiSeq platform to generate paired 250 base reads at average coverage levels of 465 for NucSEEK and 565 for EpiSEEK. Sequence reads were trimmed to remove adapters and low quality bases using Cutadapt and FastQC before alignment to the hg19 (GRCh37) reference sequence using BWA-MEM [41]. Qualimap [42] and Picard were used to generate alignment QC metrics such as insert size distribution, mismatch rates, and GC bias. Variant calls were generated by an ensemble [43] approach using FreeBayes, Platypus [44] and the GATK [45] UnifiedGenotyper [46]. For annotation and clinical review, a custom platform integrating data from multiple tools and databases was used (Ensembl variant effect predictor [47], SnpEff [48], Human Phenotype Ontology (HPO) [49], OMIM [38], ClinVar [50], 1000 genomes [51], ExAc [52], and dbNSFP [53]). SmartPCA [54] was used to stratify patients into subgroups based on analysis of the variants with respect to 1000 Genomes data. These data were stored in a database and reviewed in the context of clinical phenotype data provided by the patient’s physician by Variant Scientists, Genetic Counselors, and Laboratory Directors. A comprehensive, user-friendly report was then generated for the patient’s physician including pathogenicity scores generated according to ACMG guidelines [55].
Retrospective, aggregate analysis of variants in the core ECS genes for this study utilized only the pre-existing clinical information provided at the time of testing. Individuals were excluded if they did not sign a consent form for research use of their clinical testing data, or if the available clinical information was absent or inadequate. Adequate clinical information included an informative clinical summary, a comprehensive evaluation, or a completed checklist of standardized terms by a treating physician. Clinical summaries were translated to HPO terms by genetic counselors using the PhenoTips [56] software tool. A limited data set, containing only the above-described information and no personal identifiers, was used for the present study. Families were not contacted in regards to the current study. Thus, per the Office of Human Research Protection of the U.S. National Institutes of Health, this study does not qualify as human subjects research, and informed consent is unnecessary. However, the study was approved by the Courtagen Life Sciences ethics committee.
Rare variants in CNR1, CNR2, DAGLA, MGLL and FAAH having an allele frequency of approximately 0.003 or less in the ExAC database were identified. A Pubmed search of the literature relating to mouse knockouts of each of the five genes was carried out, and a list of the phenotypes described in those studies was compiled (S2 Table). The mouse phenotypes were translated into general terms that could be related to human phenotypes in our test database (S2 Table). Comprehensive lists of HPO terms were generated from a query of all scored tests in our database in which the five ECS genes were present (S3 and S4 Tables). The terms were evaluated to generate subsets of HPO and text terms corresponding to each general phenotype for use as database queries (S3 and S4 Tables). Counts of subjects with individual or multiple neurological phenotypes were generated through text-based queries of summary clinical phenotype data associated with de-identified reports and stored in a database. The phenotypes were scored positive if any of the included terms matched one or more of the clinical terms provided by the patient’s physician. Non-neurological phenotypes were not evaluated because they were poorly represented in the clinical database, as almost all of the patients were being tested for neurological disorders.
A set of non-carrier controls were selected for each gene. The total number of non-carriers was 3,777 for DAGLA and CNR2, and 5,979 for CNR1, FAAH and MGLL. To simplify the analysis, 950 controls were selected at random for CNR1 and DAGLA, and ~500 controls were selected at random for CNR2, FAAH and MGLL. If the rare variant data for a particular gene was derived from two gene panels (this was the case for CNR1, FAAH, and MGLL), a proportional number of controls were selected from subjects sequenced using each of those panels. Counts of subjects with individual or multiple phenotypes were generated through text-based queries of summary clinical phenotype data as described above. Two-tailed Fisher exact tests were performed to evaluate the possible association of phenotypes present in carriers with rare variants, compared to controls, using a tool available at vassarstats.net (clinical research calculator number 3). The resulting P-values were Bonferroni corrected by multiplying by the number of general phenotypes tested (corrected P-values >1 were scored as 1). No correction was made for the number of genes tested, since they are all from a single pathway.
Results
The numbers of subjects harboring rare variants were 22 for CNR1, 11 for CNR2, 35 for DAGLA, 34 for MGLL and 53 for FAAH. Data on the rare variants detected, including genomic location, coding consequences, allele frequencies in the ExAC, GnomAD and CLS databases, Polyphen2 and SIFT scores, PhyloP conservation and ExAC gene constraint values are summarized in S5 Table. The human clinical phenotypes and HPO terms associated with each of the mouse knockout phenotypic categories detected are also presented in S5 Table.
The results of Fisher exact tests for phenotypes putatively associated rare variants in the genes CNR2, MGLL and FAAH did not reveal any significant association between rare variants and any phenotype or combination tested (S6 Table). The results for CNR1 and DAGLA, however, revealed several significant associations between the presence of heterozygous rare coding variants and neurological phenotypes as indicated in Table 1.
Table 1. Association of mouse knockout phenotypes with rare variants in CNR1 and DAGLA.
| Gene* | Phenotype | Cases | Controls | OR | P-value |
|---|---|---|---|---|---|
| CNR1 | Pain sensitivity | 9/22 | 156/950 | 3.5 | 0.036 |
| CNR1 | Anxiety | 7/22 | 122/950 | 3.2 | 0.131 |
| CNR1 | Memory disorder | 3/22 | 1/950 | 150 | 0.0003 |
| CNR1 | Sleep disorder | 6/22 | 33/950 | 10.4 | 0.001 |
| CNR1 | Urinary frequency | 2/22 | 19/950 | 4.9 | 0.301 |
| CNR1 | Seizures | 11/22 | 402/950 | 1.4 | 1.000 |
| CNR1 | Hearing loss | 1/22 | 31/950 | 1.4 | 1.000 |
| CNR1 | Pain sensitivity and anxiety | 5/22 | 47/950 | 5.7 | 0.030 |
| CNR1 | Sleep disorder and anxiety | 4/22 | 8/950 | 26.2 | 0.001 |
| CNR1 | Pain sensitivity and memory disorder | 3/22 | 1/950 | 150 | 0.000 |
| CNR1 | Pain sensitivity, memory and sleep disorder | 3/22 | 1/950 | 150 | 0.000 |
| CNR1 | Pain sensitivity, anxiety and memory disorder | 2/22 | 0/950 | N/A | 0.003 |
| CNR1 | Pain sensitivity, anxiety, memory and sleep disorder | 2/22 | 0/950 | N/A | 0.003 |
| CNR1 | Pain sensitivity and sleep disorder | 3/22 | 7/950 | 21.3 | 0.005 |
| DAGLA | Anxiety | 2/35 | 177/950 | 0.26 | 0.211 |
| DAGLA | Neurodevelopmental disorder | 27/35 | 448/950 | 3.78 | 0.001 |
| DAGLA | Anxiety and neurodevelopmental disorder | 2/35 | 98/950 | 0.53 | 1.000 |
| DAGLA | Seizures | 30/35 | 595/950 | 3.84 | 0.032 |
| DAGLA | Seizures and neurodevelopmental disorder | 22/35 | 332/950 | 3.15 | 0.003 |
*For each gene, the phenotypes that were evaluated are listed along with the number of cases or controls in which that phenotype was present out of the total number. Also listed are the odds ratio (OR), and P-values corrected for multiple testing. Confidence intervals for the odds ratios are provided in an extended version of the table in the supporting information (S7 Table).
The allele frequencies of the ECS gene variants in our database of genetic testing results (CLS database) were compared with those in the public reference databases ExAC and gnomAD [57]. Across all five genes, the rare variants had an average allele frequency of 0.0004 in ExAC and gnomAD. The rare variants were enriched in the CLS database compared to gnomAD by 4.5-fold for CNR1 and 6.7-fold for DAGLA (not including variants with zero frequency gnomAD). Eight of the 24 CNR1 variants, and three of the 36 DAGLA variants had zero frequency in gnomAD. The fraction of variants with zero frequency in gnomAD for the other genes was 1/10 for CNR2, 8/53 for FAAH and 3/40 for MGLL.
We looked for shared phenotypes amongst subjects carrying the same rare variant. Two of the CNR1 variants were observed in multiple samples (p.Glu93Lys in 2 samples, and p.Ala419Glu in 5 samples)—S5 Table. All subjects with p.Glu93Lys had seizures and developmental delay. Two of the subjects with p.Ala419Glu had the shared phenotypes: anxiety, sleep disorder, abnormality of the autonomic nervous system and a third had a sleep disorder combined with seizures. Those phenotypes were absent in the other subjects with p.Ala419Glu.
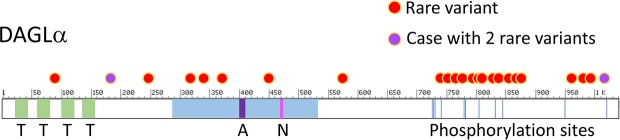
Four of the DAGLA variants were observed in multiple samples (p.His810Gln in 7 samples, p.Arg815His in 5 samples, p.Ala858Val in 2 samples, and p.Leu988Val in 3 samples)—S5 Table. Three of the subjects with p.His810Gln had infantile spasms and two had focal seizures. 5/6 of the subjects with p.Arg815His had seizures and developmental disorders and two had a brain abnormality. The subjects with p.Ala858Val did not have shared phenotypes. All three subjects with p.Leu988Val had seizures; two had developmental delay and the third had a brain abnormality in addition. One subject (ECS158) had two DAGLA variants, p.Arg197Trp and p.Ter1043GlnextTer29; the chromosomal phasing of those variants is unknown.
Additional genes with variants that could contribute to the observed phenotypes in the subjects, based on clinical reports, are noted in S5 Table.
Several 3D x-ray crystal structures of CB1 were published recently, by two groups [58–60]. We mapped the position of the rare coding variants onto one of those structures with bound agonist, PDB ID: 5XRA, as well as onto the primary structure and domain organization of the enzyme (Fig 1). Of the variants that could be mapped, 6/7 were in the 7-transmembrane cannabinoid binding domain (Fig 1). The other CNR1 variants, located near the N- or C-terminus, could not be mapped since those regions were deleted from the crystalized proteins. A similar exercise was performed for DAGLA using the primary structure and domain organization of the enzyme as described by Reisenberg, et al. [61] and NCBI Reference Sequence: NP_006124.1, since a crystal structure is not available. Most of the DAGLA variants mapped to the C-terminal end of the protein, a region that contains many regulatory phosphorylation sites (Fig 2).
Fig 1.
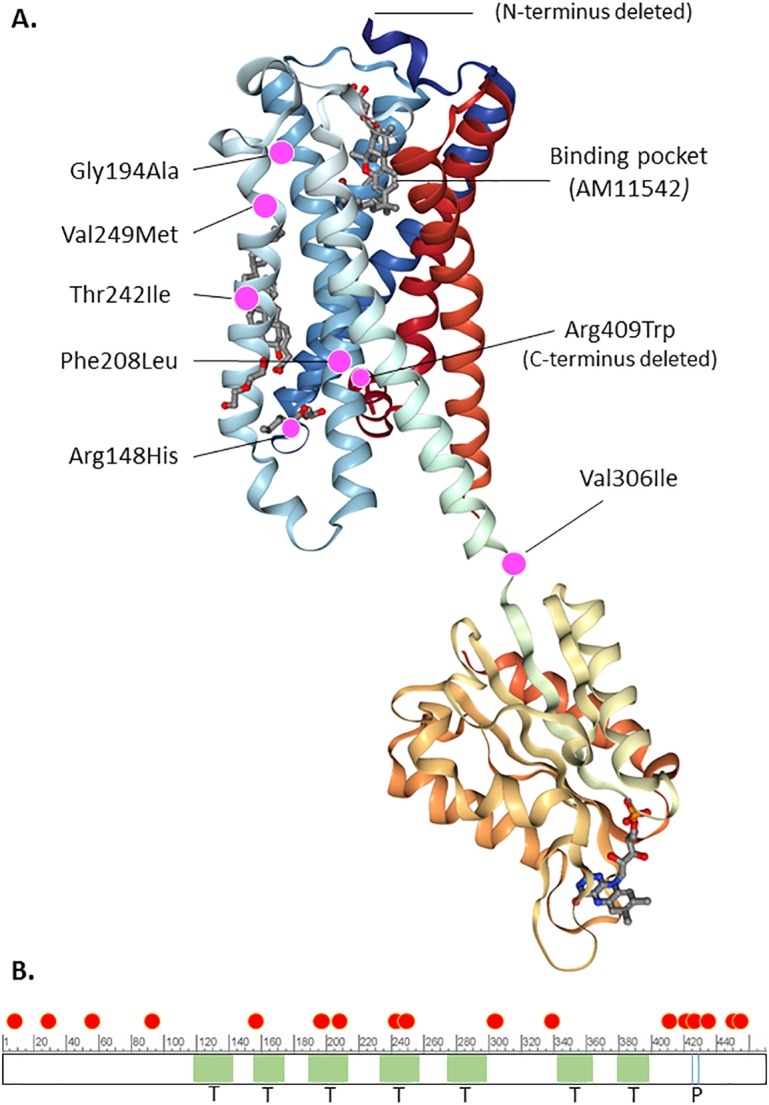
A. Depiction of the x-ray crystal structure of the type I cannabinoid receptor with bound agonist AM11542 from PDB ID 5XRA. The location of rare coding variants that could be mapped to the structure are shown by magenta dots, with annotations indicating the amino acid substitutions and the cannabinoid binding site. B. The location of rare coding variants relative to domains of the enzyme and regulatory phosphorylation sites are indicated, based on NCBI Reference Sequence: NP_001153731.1. Red dot: rare variant; T (light green shading): transmembrane domain; P (blue vertical lines): phosphorylation sites.
Fig 2. Mapping of rare variants to diacylglycerol lipase alpha.
The location of rare coding variants relative to domains of the enzyme and regulatory phosphorylation sites are indicated, based on NCBI Reference Sequence: NP_006124.1. Red dot: rare variant; purple dots: two variants present in one subject; T (light green shading): transmembrane domain; light blue shading: lipase domain; A (purple shading): Active site; N (magenta shading): Nucleophilic elbow; blue vertical lines: phosphorylation sites.
Discussion
We report a set of 155 rare missense or short deletion variants in the core ECS genes CNR1, CNR2, DAGLA, FAAH and MGLL. A subset of those variants, in CNR1 and DAGLA, were found to be statistically associated with neurological phenotypes by case control analysis at the gene level. It is perhaps not surprising that CNR2, FAAH and MGLL were not associated with any phenotypes. Those genes are significantly more tolerant to missense and loss of function (LOF) variation than CNR1 and DAGLA, based on ExAC gene constraint values [57, 62]. Furthermore, our testing focuses on neurological phenotypes, so metabolic or immunological phenotypes associated with those genes may not have been reported.
The CNR1 variants were found to be associated with headache (including migraine), sleep and memory disorders, alone or in combination with anxiety, compared to a control set of 950 randomly selected patients without such CNR1 variants. The Fisher odds ratios for the associated variants ranged from 4.5 up to 150, with the highest ratios being associated with sleep disorder plus headache (18.9), sleep disorder plus anxiety (26.2), memory disorder with or without headache (150) and the combined phenotypes of headache, memory disorder and anxiety and/or sleep disorder, which did not occur in any of the controls (Table 1).
Based on ExAC gene constraint values [57, 62], the CNR1 gene is predicted to be somewhat tolerant of single missense and loss of function variants (missense z score = 2.7 and pLI = 0.15), but intolerant of dual heterozygous LOF variants (pRec = 0.78). All but one of the variants that could be mapped to the 3D structure of CB1 were in the 7-transmembrane domain. One additional variant, Ile339Val, would have mapped to the cytoplasmic domain if the region containing it had not been substituted in the 5XRA sequence. While we have no direct evidence that the variants we discovered impair function of the receptor, we note that three of the variants, p.Gly194Ala, p.Val249Met and p.Thr242Ile, map near the cannabinoid binding pocket in the 3-dimensional structure (Fig 1). In addition, two other variants, p.Arg148His and p.Val306Ile, map near the boundary between the 7-transmembrane and cytoplasmic signaling domains. Such variants could affect signal transduction of the receptor. There were four variants (at residues 7, 27,57 and 93) that could not be mapped since the N-terminus was deleted from the structure. The N-terminal region is not required for cannabinoid binding, but may play a role in regulating the stability and surface expression of CB1[63]. We also note that the PolyPhen 2 deleteriousness scores of the CNR1 variants are generally high (mean PP2 score = 0.85 with 11/24 ≥ 0.95).
Mice that are experimentally manipulated to harbor a non-functional CNR1 gene (CB1 knockout), or that are treated with CB1 antagonists, display behaviors that suggest impacts related to pain sensitivity, anxiety, memory and sleep, amongst others (S2 Table). The behaviors include elevated avoidance, freezing and risk-assessment behaviors [64], accelerated early learning and memory decline [65], food-seeking, and non-REM sleep alterations [66, 67]. There is a strong correlation between those phenotypes and those we observed in the present study (anxiety, sleep and memory disorders). Three of the eight subjects with rare CNR1 variants and headaches in our study also have memory disorders (mainly short term memory), and two of those have comorbid anxiety and sleep disorders.
Two of the subjects with migraine headaches harbor variants in other genes that have previously been associated with migraine (TRAP1 and CACNA1A) [68, 69]. However, the clinical correlation of the variants present in those subjects (TRAP1 p.Ile253Val in subject ECS031 and CACNA1A p.Ser2423_Gly2424del in subject ECS100) with migraine and anxiety is uncertain. Interestingly, 2/5 subjects with a CNR1 p.Ala419Glu variant (ExAC MAF = 0.00038) had the shared phenotypes: anxiety, sleep disorder, and abnormality of the autonomic nervous system. Only one of the other three subjects with that variant, however, had any of those phenotypes. These observations suggest that additional factors (genetic or environmental) may contribute to expression of the CNR1-associated phenotypes. Additional work will be required to resolve this.
The CB1 receptor encoded by CNR1 binds the natural endocannabinoid agonists AEA and 2-AG. A theory of Clinical Endocannabinoid Deficiency (CED), first described by Russo, posits that migraine, fibromyalgia, irritable bowel syndrome and comorbid anxiety may be related manifestations of reduced endocannabinoid tone, specifically AEA and 2-AG levels [70, 71]. These conditions frequently occur together, and a growing body of evidence suggests that they respond well to cannabinoid therapy, particularly THC [71]. Our results suggest that impaired CB1 signaling may be associated with increased susceptibility to a related set of clinical phenotypes: migraine, sleep and memory disorders with co-morbid anxiety. It would be interesting to explore whether migraine, anxiety and sleep disorders in patients with rare deleterious variants in CNR1 could be alleviated by treatment with CB1 agonists that could effectively stimulate the impaired and/or remaining functional CB1 receptors. Compounds that increase endocannabinoid levels such as CBD [72], or inhibitors of fatty acid amyl hydrolase, monoacylglycerol lipase, or fatty acid binding proteins [73, 74], might also be effective. We note that a recent observational study of 121 adults with migraine reported a decrease in mean headache frequency from 10.4 to 4.6 headaches per month (p<0.0001) for patients receiving medicinal cannabis [75]. However, the genetic basis (if any) of migraine in those patients was not investigated, and the therapeutic effect of THC could be a result of other mechanisms [75]. It is not clear whether high levels of THC could effectively remediate defective CB1 receptors because of its partial agonist nature and potential antagonistic effects on AEA and 2-AG signaling. Furthermore, the effects of THC may be different from AEA and 2-AG due to biased agonism [76, 77].
DAGLA encodes diacylglycerol lipase alpha, which is the major enzyme involved in 2-AG biosynthesis in the central nervous system. 2-AG levels have been reported to drop by ~80% in DAGLA knockout mice, resulting in pronounced anxiety and depression-like behaviors [78], and increased severity of kainate-induced seizures[79]. Developmental alterations observed in DAGLA knockout mice include altered cholinergic innervation of CA1 pyramidal cells of the hippocampus [18], and compromised adult neurogenesis in the hippocampus and subventricular zone [80]. In addition, altered axon formation in the midbrain-hindbrain region (associated with vision and locomotion) has been observed in response to transient morpholino DAGLA knockdown in zebrafish [81].
Heterozygous rare variants in DAGLA were found to be significantly associated with seizures, developmental disorders and abnormalities of brain morphology compared to controls. Based on ExAC gene constraint values, the DAGLA gene is predicted to be intolerant of missense and LOF variants (missense z score = 5.3 and pLI = 0.95 [57, 62]. We observed that 15/23 of the unique variants observed in our study map to the C-terminal third of the DAGLA coding sequence, a region that is predicted to contain numerous regulatory phosphorylation sites based on aggregate data from several biochemical studies [61]. This region appears to be more tolerant of variation than the catalytic domain, in which variation was sparse, despite its similar size. In contrast to mouse knockout models, anxiety phenotypes appear to be depleted in the clinical subjects—but the result was not significant. This raises the possibility that some of the variants might augment diacylglycerol lipase function. Further studies will be required to evaluate that possibility.
The frequency of seizures and developmental disorders in subjects with rare DAGLA variants were each elevated by a factor of approximately 4 compared to controls. The developmental disorders observed included several types of brain abnormalities: abnormalities of the cerebral white matter, brain malformations, posterior delayed myelination, heterotopia, and porencephaly with encephalomalacia. The prevalence of developmental disorders and brain abnormalities in these subjects aligns with the observations in animal models. Only two of the 35 samples with rare DAGLA variants harbored a likely pathogenic variant in another gene known to be associated with seizures and developmental delay (GRIN2A in subject 061 and MEF2C in subject 044). This represents about half the number of positive tests that would be expected based on the overall positive rate of the epiSEEK panel in our patient population (13%). Further work will be necessary to define whether rare variants in DAGLA are causatively associated with seizures and developmental disorders.
While the variants described in this study occur at extremely low frequencies in the general population, we suspect that patient populations with disorders of the nature studied here will be enriched for them. The allele frequencies observed in our test database for the rare CNR1 and DAGLA variants were approximately 4.5-fold higher, on average, than those observed in the ExAC database. It will be interesting to evaluate what fraction of patients with migraine, with or without anxiety, sleep, or memory disorders, harbor low frequency or rare variants in CNR1. Similarly, the prevalence of rare DAGLA variants in patients with developmental disorders should be investigated.
Supporting information
Column 2 lists the genes contained in the NucSEEK gene panel.
(XLSX)
Column 2 gives the general phenotype that was used to select human phenotpes and HPO terms. Column 3 indicates whether the phenotypes are neurological. Column 4 provides PMIDs for corresponding mouse knockout publications.
(XLSX)
Column 2 provides the corresponding HPO descriptions. Columns 3–13 indicate the HPO terms corresponding to each of the general phenotypes derived from mouse knockouts according to the headers in row 3 (a "1" indicates that the term is included). Row 1 provides the strings of query terms used to evaluate the presence of general phenotypes in clinical reports of carrier and control subjects. Row 2 provides the frequencies of patients with the corresponding phenotypes amongst the 5464 patients.
(XLSX)
Column 2 provides the corresponding HPO descriptions. Columns 3–8 indicate the HPO terms corresponding to each of the general phenotypes derived from mouse knockouts according to the headers in row 3 (a "1" indicates that the term is included). Row 1 provides the strings of query terms used to evaluate the presence of general phenotypes in clinical reports of carrier and control subjects. Row 2 provides the frequencies of patients with the corresponding phenotypes amongst the 5464 patients.
(XLSX)
Headers in row 1 are self explanatory.
(XLSX)
(XLSX)
Columns identical to Table 1: Gene, Phenotypes that were evaluated, Number of cases or controls in which that phenotype was present out of the total number, odds ratio (OR), and P-value corrected for multiple testing.
(XLSX)
Acknowledgments
This study was supported by Courtagen Life Sciences, Inc. We thank Josee Dupuis for advice on the statistical analysis, and Ethan Russo and Anne Conyers-Hom for helpful comments on the manuscript.
Data Availability
The raw sequencing data used for this study was submitted to the short read archive at NCBI and available under BioProject accession number PRJNA413705. The Biosample accession numbers are SAMN07763820 through SAMN07763970.
Funding Statement
The author(s) received no specific funding for this work. However they were employees of a commercial company (Courtagen Life Sciences). The company provided support in the form of salaries for authors DS, CS, TF, RB and KM, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the "author contributions" section.
References
- 1.Iannotti FA, Di Marzo V, Petrosino S. Endocannabinoids and endocannabinoid-related mediators: Targets, metabolism and role in neurological disorders. Prog Lipid Res. 2016;62:107–28. doi: 10.1016/j.plipres.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 2.Lowin T, Straub RH. Cannabinoid-based drugs targeting CB1 and TRPV1, the sympathetic nervous system, and arthritis. Arthritis Res Ther. 2015;17:226 doi: 10.1186/s13075-015-0743-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maldonado R, Banos JE, Cabanero D. The endocannabinoid system and neuropathic pain. Pain. 2016;157 Suppl 1:S23–32. [DOI] [PubMed] [Google Scholar]
- 4.Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013;17(4):475–90. doi: 10.1016/j.cmet.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 5.Cabral GA, Ferreira GA, Jamerson MJ. Endocannabinoids and the Immune System in Health and Disease. Handb Exp Pharmacol. 2015;231:185–211. doi: 10.1007/978-3-319-20825-1_6 [DOI] [PubMed] [Google Scholar]
- 6.Pyszniak M, Tabarkiewicz J, Luszczki JJ. Endocannabinoid system as a regulator of tumor cell malignancy—biological pathways and clinical significance. Onco Targets Ther. 2016;9:4323–36. doi: 10.2147/OTT.S106944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu SS, Mackie K. Distribution of the Endocannabinoid System in the Central Nervous System. Handb Exp Pharmacol. 2015;231:59–93. doi: 10.1007/978-3-319-20825-1_3 [DOI] [PubMed] [Google Scholar]
- 8.Pertwee RG. Endocannabinoids and Their Pharmacological Actions. Handb Exp Pharmacol. 2015;231:1–37. doi: 10.1007/978-3-319-20825-1_1 [DOI] [PubMed] [Google Scholar]
- 9.Bow EW, Rimoldi JM. The Structure-Function Relationships of Classical Cannabinoids: CB1/CB2 Modulation. Perspect Medicin Chem. 2016;8:17–39. doi: 10.4137/PMC.S32171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramirez-Franco J, Bartolome-Martin D, Alonso B, Torres M, Sanchez-Prieto J. Cannabinoid type 1 receptors transiently silence glutamatergic nerve terminals of cultured cerebellar granule cells. PLoS One. 2014;9(2):e88594 doi: 10.1371/journal.pone.0088594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SH, Ledri M, Toth B, Marchionni I, Henstridge CM, Dudok B, et al. Multiple Forms of Endocannabinoid and Endovanilloid Signaling Regulate the Tonic Control of GABA Release. J Neurosci. 2015;35(27):10039–57. doi: 10.1523/JNEUROSCI.4112-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanimura A, Yamazaki M, Hashimotodani Y, Uchigashima M, Kawata S, Abe M, et al. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron. 2010;65(3):320–7. doi: 10.1016/j.neuron.2010.01.021 [DOI] [PubMed] [Google Scholar]
- 13.Turcotte C, Blanchet MR, Laviolette M, Flamand N. The CB2 receptor and its role as a regulator of inflammation. Cell Mol Life Sci. 2016;73(23):4449–70. doi: 10.1007/s00018-016-2300-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, Dittel BN. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem. 2005;95(2):437–45. doi: 10.1111/j.1471-4159.2005.03380.x [DOI] [PubMed] [Google Scholar]
- 15.Gertsch J, Leonti M, Raduner S, Racz I, Chen JZ, Xie XQ, et al. Beta-caryophyllene is a dietary cannabinoid. Proc Natl Acad Sci U S A. 2008;105(26):9099–104. doi: 10.1073/pnas.0803601105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163(3):463–8. doi: 10.1083/jcb.200305129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long LE, Lind J, Webster M, Weickert CS. Developmental trajectory of the endocannabinoid system in human dorsolateral prefrontal cortex. BMC Neurosci. 2012;13:87 doi: 10.1186/1471-2202-13-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keimpema E, Alpar A, Howell F, Malenczyk K, Hobbs C, Hurd YL, et al. Diacylglycerol lipase alpha manipulation reveals developmental roles for intercellular endocannabinoid signaling. Sci Rep. 2013;3:2093 doi: 10.1038/srep02093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fowler CJ, Jonsson KO, Tiger G. Fatty acid amide hydrolase: biochemistry, pharmacology, and therapeutic possibilities for an enzyme hydrolyzing anandamide, 2-arachidonoylglycerol, palmitoylethanolamide, and oleamide. Biochem Pharmacol. 2001;62(5):517–26. [DOI] [PubMed] [Google Scholar]
- 20.Basavarajappa BS. Critical enzymes involved in endocannabinoid metabolism. Protein Pept Lett. 2007;14(3):237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herman AI, Kranzler HR, Cubells JF, Gelernter J, Covault J. Association study of the CNR1 gene exon 3 alternative promoter region polymorphisms and substance dependence. Am J Med Genet B Neuropsychiatr Genet. 2006;141B(5):499–503. doi: 10.1002/ajmg.b.30325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russo P, Strazzullo P, Cappuccio FP, Tregouet DA, Lauria F, Loguercio M, et al. Genetic variations at the endocannabinoid type 1 receptor gene (CNR1) are associated with obesity phenotypes in men. J Clin Endocrinol Metab. 2007;92(6):2382–6. doi: 10.1210/jc.2006-2523 [DOI] [PubMed] [Google Scholar]
- 23.Lu AT, Ogdie MN, Jarvelin MR, Moilanen IK, Loo SK, McCracken JT, et al. Association of the cannabinoid receptor gene (CNR1) with ADHD and post-traumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(8):1488–94. doi: 10.1002/ajmg.b.30693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agrawal A, Wetherill L, Dick DM, Xuei X, Hinrichs A, Hesselbrock V, et al. Evidence for association between polymorphisms in the cannabinoid receptor 1 (CNR1) gene and cannabis dependence. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(5):736–40. doi: 10.1002/ajmg.b.30881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Proudnikov D, Kroslak T, Sipe JC, Randesi M, Li D, Hamon S, et al. Association of polymorphisms of the cannabinoid receptor (CNR1) and fatty acid amide hydrolase (FAAH) genes with heroin addiction: impact of long repeats of CNR1. Pharmacogenomics J. 2010;10(3):232–42. doi: 10.1038/tpj.2009.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carey CE, Agrawal A, Zhang B, Conley ED, Degenhardt L, Heath AC, et al. Monoacylglycerol lipase (MGLL) polymorphism rs604300 interacts with childhood adversity to predict cannabis dependence symptoms and amygdala habituation: Evidence from an endocannabinoid system-level analysis. J Abnorm Psychol. 2015;124(4):860–77. doi: 10.1037/abn0000079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadhasivam S, Zhang X, Chidambaran V, Mavi J, Pilipenko V, Mersha TB, et al. Novel associations between FAAH genetic variants and postoperative central opioid-related adverse effects. Pharmacogenomics J. 2015;15(5):436–42. doi: 10.1038/tpj.2014.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong BS, Camilleri M, Eckert D, Carlson P, Ryks M, Burton D, et al. Randomized pharmacodynamic and pharmacogenetic trial of dronabinol effects on colon transit in irritable bowel syndrome-diarrhea. Neurogastroenterol Motil. 2012;24(4):358–e169. doi: 10.1111/j.1365-2982.2011.01874.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Camilleri M, Kolar GJ, Vazquez-Roque MI, Carlson P, Burton DD, Zinsmeister AR. Cannabinoid receptor 1 gene and irritable bowel syndrome: phenotype and quantitative traits. Am J Physiol Gastrointest Liver Physiol. 2013;304(5):G553–60. doi: 10.1152/ajpgi.00376.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juhasz G, Lazary J, Chase D, Pegg E, Downey D, Toth ZG, et al. Variations in the cannabinoid receptor 1 gene predispose to migraine. Neurosci Lett. 2009;461(2):116–20. doi: 10.1016/j.neulet.2009.06.021 [DOI] [PubMed] [Google Scholar]
- 31.Lieb W, Manning AK, Florez JC, Dupuis J, Cupples LA, McAteer JB, et al. Variants in the CNR1 and the FAAH genes and adiposity traits in the community. Obesity (Silver Spring). 2009;17(4):755–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hartman CA, Hopfer CJ, Haberstick B, Rhee SH, Crowley TJ, Corley RP, et al. The association between cannabinoid receptor 1 gene (CNR1) and cannabis dependence symptoms in adolescents and young adults. Drug Alcohol Depend. 2009;104(1–2):11–6. doi: 10.1016/j.drugalcdep.2009.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clarke TK, Bloch PJ, Ambrose-Lanci LM, Ferraro TN, Berrettini WH, Kampman KM, et al. Further evidence for association of polymorphisms in the CNR1 gene with cocaine addiction: confirmation in an independent sample and meta-analysis. Addict Biol. 2013;18(4):702–8. doi: 10.1111/j.1369-1600.2011.00346.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Miguel-Yanes JM, Manning AK, Shrader P, McAteer JB, Goel A, Hamsten A, et al. Variants at the endocannabinoid receptor CB1 gene (CNR1) and insulin sensitivity, type 2 diabetes, and coronary heart disease. Obesity (Silver Spring). 2011;19(10):2031–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melroy-Greif WE, Wilhelmsen KC, Ehlers CL. Genetic variation in FAAH is associated with cannabis use disorders in a young adult sample of Mexican Americans. Drug Alcohol Depend. 2016;166:249–53. doi: 10.1016/j.drugalcdep.2016.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swaminathan GJ, Bragin E, Chatzimichali EA, Corpas M, Bevan AP, Wright CF, et al. DECIPHER: web-based, community resource for clinical interpretation of rare variants in developmental disorders. Hum Mol Genet. 2012;21(R1):R37–44. doi: 10.1093/hmg/dds362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landrum MJ, Lee JM, Riley GR, Jang W, Rubinstein WS, Church DM, et al. ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 2014;42(Database issue):D980–5. doi: 10.1093/nar/gkt1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amberger JS, Bocchini CA, Schiettecatte F, Scott AF, Hamosh A. OMIM.org: Online Mendelian Inheritance in Man (OMIM(R)), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015;43(Database issue):D789–98. doi: 10.1093/nar/gku1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKernan KJ, Spangler J, Helbert Y, Zhang L, Tadigotla V. DREAMing of a patent-free human genome for clinical sequencing. Nat Biotechnol. 2013;31(10):884–7. doi: 10.1038/nbt.2703 [DOI] [PubMed] [Google Scholar]
- 40.McKernan KJ, Spangler J, Zhang L, Tadigotla V, McLaughlin S, Warner J, et al. Expanded genetic codes in next generation sequencing enable decontamination and mitochondrial enrichment. PLoS One. 2014;9(5):e96492 doi: 10.1371/journal.pone.0096492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H. Toward better understanding of artifacts in variant calling from high-coverage samples. Bioinformatics. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia-Alcalde F, Okonechnikov K, Carbonell J, Cruz LM, Gotz S, Tarazona S, et al. Qualimap: evaluating next-generation sequencing alignment data. Bioinformatics. 2012;28(20):2678–9. doi: 10.1093/bioinformatics/bts503 [DOI] [PubMed] [Google Scholar]
- 43.Trubetskoy V, Rodriguez A, Dave U, Campbell N, Crawford EL, Cook EH, et al. Consensus Genotyper for Exome Sequencing (CGES): improving the quality of exome variant genotypes. Bioinformatics. 2015;31(2):187–93. doi: 10.1093/bioinformatics/btu591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rimmer A, Phan H, Mathieson I, Iqbal Z, Twigg SR, Consortium WGS, et al. Integrating mapping-, assembly- and haplotype-based approaches for calling variants in clinical sequencing applications. Nat Genet. 2014;46(8):912–8. doi: 10.1038/ng.3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–303. doi: 10.1101/gr.107524.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43(5):491–8. doi: 10.1038/ng.806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yourshaw M, Taylor SP, Rao AR, Martin MG, Nelson SF. Rich annotation of DNA sequencing variants by leveraging the Ensembl Variant Effect Predictor with plugins. Brief Bioinform. 2015;16(2):255–64. doi: 10.1093/bib/bbu008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cingolani P, Platts A, Wang le L, Coon M, Nguyen T, Wang L, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin). 2012;6(2):80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kohler S, Doelken SC, Mungall CJ, Bauer S, Firth HV, Bailleul-Forestier I, et al. The Human Phenotype Ontology project: linking molecular biology and disease through phenotype data. Nucleic Acids Res. 2014;42(Database issue):D966–74. doi: 10.1093/nar/gkt1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Landrum MJ, Lee JM, Benson M, Brown G, Chao C, Chitipiralla S, et al. ClinVar: public archive of interpretations of clinically relevant variants. Nucleic Acids Res. 2016;44(D1):D862–8. doi: 10.1093/nar/gkv1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Genomes Project C, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. 2015;526(7571):68–74. doi: 10.1038/nature15393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song W, Gardner SA, Hovhannisyan H, Natalizio A, Weymouth KS, Chen W, et al. Exploring the landscape of pathogenic genetic variation in the ExAC population database: insights of relevance to variant classification. Genet Med. 2015. [DOI] [PubMed] [Google Scholar]
- 53.Liu X, Jian X, Boerwinkle E. dbNSFP v2.0: a database of human non-synonymous SNVs and their functional predictions and annotations. Hum Mutat. 2013;34(9):E2393–402. doi: 10.1002/humu.22376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904–9. doi: 10.1038/ng1847 [DOI] [PubMed] [Google Scholar]
- 55.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–24. doi: 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Girdea M, Dumitriu S, Fiume M, Bowdin S, Boycott KM, Chenier S, et al. PhenoTips: patient phenotyping software for clinical and research use. Hum Mutat. 2013;34(8):1057–65. doi: 10.1002/humu.22347 [DOI] [PubMed] [Google Scholar]
- 57.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536(7616):285–91. doi: 10.1038/nature19057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shao Z, Yin J, Chapman K, Grzemska M, Clark L, Wang J, et al. High-resolution crystal structure of the human CB1 cannabinoid receptor. Nature. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hua T, Vemuri K, Pu M, Qu L, Han GW, Wu Y, et al. Crystal Structure of the Human Cannabinoid Receptor CB1. Cell. 2016;167(3):750–62 e14. doi: 10.1016/j.cell.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hua T, Vemuri K, Nikas SP, Laprairie RB, Wu Y, Qu L, et al. Crystal structures of agonist-bound human cannabinoid receptor CB1. Nature. 2017;547(7664):468–71. doi: 10.1038/nature23272 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Reisenberg M, Singh PK, Williams G, Doherty P. The diacylglycerol lipases: structure, regulation and roles in and beyond endocannabinoid signalling. Philos Trans R Soc Lond B Biol Sci. 2012;367(1607):3264–75. doi: 10.1098/rstb.2011.0387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Samocha KE, Robinson EB, Sanders SJ, Stevens C, Sabo A, McGrath LM, et al. A framework for the interpretation of de novo mutation in human disease. Nat Genet. 2014;46(9):944–50. doi: 10.1038/ng.3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Andersson H D 'Antona AM, Kendall DA, Von Heijne G, Chin CN. Membrane assembly of the cannabinoid receptor 1: impact of a long N-terminal tail. Mol Pharmacol. 2003;64(3):570–7. doi: 10.1124/mol.64.3.570 [DOI] [PubMed] [Google Scholar]
- 64.Litvin Y, Phan A, Hill MN, Pfaff DW, McEwen BS. CB1 receptor signaling regulates social anxiety and memory. Genes Brain Behav. 2013;12(5):479–89. doi: 10.1111/gbb.12045 [DOI] [PubMed] [Google Scholar]
- 65.Bilkei-Gorzo A, Drews E, Albayram O, Piyanova A, Gaffal E, Tueting T, et al. Early onset of aging-like changes is restricted to cognitive abilities and skin structure in Cnr1(-)/(-) mice. Neurobiol Aging. 2012;33(1):200 e11–22. [DOI] [PubMed] [Google Scholar]
- 66.Silvani A, Berteotti C, Bastianini S, Lo Martire V, Mazza R, Pagotto U, et al. Multiple sleep alterations in mice lacking cannabinoid type 1 receptors. PLoS One. 2014;9(2):e89432 doi: 10.1371/journal.pone.0089432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pava MJ, Makriyannis A, Lovinger DM. Endocannabinoid Signaling Regulates Sleep Stability. PLoS One. 2016;11(3):e0152473 doi: 10.1371/journal.pone.0152473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boles RG, Hornung HA, Moody AE, Ortiz TB, Wong SA, Eggington JM, et al. Hurt, tired and queasy: Specific variants in the ATPase domain of the TRAP1 mitochondrial chaperone are associated with common, chronic "functional" symptomatology including pain, fatigue and gastrointestinal dysmotility. Mitochondrion. 2015. [DOI] [PMC free article] [PubMed]
- 69.Riant F, Ducros A, Ploton C, Barbance C, Depienne C, Tournier-Lasserve E. De novo mutations in ATP1A2 and CACNA1A are frequent in early-onset sporadic hemiplegic migraine. Neurology. 2010;75(11):967–72. doi: 10.1212/WNL.0b013e3181f25e8f [DOI] [PubMed] [Google Scholar]
- 70.Russo EB. Clinical endocannabinoid deficiency (CECD): can this concept explain therapeutic benefits of cannabis in migraine, fibromyalgia, irritable bowel syndrome and other treatment-resistant conditions? Neuro Endocrinol Lett. 2008;29(2):192–200. [PubMed] [Google Scholar]
- 71.Russo EB. Clinical Endocannabinoid Deficiency Reconsidered: Current Research Supports the Theory in Migraine, Fibromyalgia, Irritable Bowel, and Other Treatment-Resistant Syndromes. Cannabis and Cannabinoid Research. 2016;1(1):154–65. doi: 10.1089/can.2016.0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rimmerman N, Juknat A, Kozela E, Levy R, Bradshaw HB, Vogel Z. The non-psychoactive plant cannabinoid, cannabidiol affects cholesterol metabolism-related genes in microglial cells. Cell Mol Neurobiol. 2011;31(6):921–30. doi: 10.1007/s10571-011-9692-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaczocha M, Rebecchi MJ, Ralph BP, Teng YH, Berger WT, Galbavy W, et al. Inhibition of fatty acid binding proteins elevates brain anandamide levels and produces analgesia. PLoS One. 2014;9(4):e94200 doi: 10.1371/journal.pone.0094200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pertwee RG. Elevating endocannabinoid levels: pharmacological strategies and potential therapeutic applications. Proc Nutr Soc. 2014;73(1):96–105. doi: 10.1017/S0029665113003649 [DOI] [PubMed] [Google Scholar]
- 75.Rhyne DN, Anderson SL, Gedde M, Borgelt LM. Effects of Medical Marijuana on Migraine Headache Frequency in an Adult Population. Pharmacotherapy. 2016;36(5):505–10. doi: 10.1002/phar.1673 [DOI] [PubMed] [Google Scholar]
- 76.Ibsen MS, Connor M, Glass M. Cannabinoid CB1 and CB2 Receptor Signaling and Bias. Cannabis Cannabinoid Res. 2017;2(1):48–60. doi: 10.1089/can.2016.0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khajehali E, Malone DT, Glass M, Sexton PM, Christopoulos A, Leach K. Biased Agonism and Biased Allosteric Modulation at the CB1 Cannabinoid Receptor. Mol Pharmacol. 2015;88(2):368–79. doi: 10.1124/mol.115.099192 [DOI] [PubMed] [Google Scholar]
- 78.Jenniches I, Ternes S, Albayram O, Otte DM, Bach K, Bindila L, et al. Anxiety, Stress, and Fear Response in Mice With Reduced Endocannabinoid Levels. Biol Psychiatry. 2016;79(10):858–68. doi: 10.1016/j.biopsych.2015.03.033 [DOI] [PubMed] [Google Scholar]
- 79.Sugaya Y, Yamazaki M, Uchigashima M, Kobayashi K, Watanabe M, Sakimura K, et al. Crucial Roles of the Endocannabinoid 2-Arachidonoylglycerol in the Suppression of Epileptic Seizures. Cell Rep. 2016;16(5):1405–15. doi: 10.1016/j.celrep.2016.06.083 [DOI] [PubMed] [Google Scholar]
- 80.Gao Y, Vasilyev DV, Goncalves MB, Howell FV, Hobbs C, Reisenberg M, et al. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J Neurosci. 2010;30(6):2017–24. doi: 10.1523/JNEUROSCI.5693-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martella A, Sepe RM, Silvestri C, Zang J, Fasano G, Carnevali O, et al. Important role of endocannabinoid signaling in the development of functional vision and locomotion in zebrafish. FASEB J. 2016;30(12):4275–88. doi: 10.1096/fj.201600602R [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Column 2 lists the genes contained in the NucSEEK gene panel.
(XLSX)
Column 2 gives the general phenotype that was used to select human phenotpes and HPO terms. Column 3 indicates whether the phenotypes are neurological. Column 4 provides PMIDs for corresponding mouse knockout publications.
(XLSX)
Column 2 provides the corresponding HPO descriptions. Columns 3–13 indicate the HPO terms corresponding to each of the general phenotypes derived from mouse knockouts according to the headers in row 3 (a "1" indicates that the term is included). Row 1 provides the strings of query terms used to evaluate the presence of general phenotypes in clinical reports of carrier and control subjects. Row 2 provides the frequencies of patients with the corresponding phenotypes amongst the 5464 patients.
(XLSX)
Column 2 provides the corresponding HPO descriptions. Columns 3–8 indicate the HPO terms corresponding to each of the general phenotypes derived from mouse knockouts according to the headers in row 3 (a "1" indicates that the term is included). Row 1 provides the strings of query terms used to evaluate the presence of general phenotypes in clinical reports of carrier and control subjects. Row 2 provides the frequencies of patients with the corresponding phenotypes amongst the 5464 patients.
(XLSX)
Headers in row 1 are self explanatory.
(XLSX)
(XLSX)
Columns identical to Table 1: Gene, Phenotypes that were evaluated, Number of cases or controls in which that phenotype was present out of the total number, odds ratio (OR), and P-value corrected for multiple testing.
(XLSX)
Data Availability Statement
The raw sequencing data used for this study was submitted to the short read archive at NCBI and available under BioProject accession number PRJNA413705. The Biosample accession numbers are SAMN07763820 through SAMN07763970.