Abstract
To explore the relationship between neutrophil-to-lymphocyte ratio (NLR) and diabetic peripheral neuropathy (DPN) in type 2 diabetes mellitus.
A total of 557 newly diagnosed Type 2 Diabetes Mellitus (T2DM) patients were recruited, including 397 T2DM patients without complication (DM group) as well as 160 T2DM patients complicated with DPN (DPN group). Student t test, Mann–Whitney U test, or χ2 test was applied to the data of the 2 groups, including the levels of neutrophils and lymphocytes as well as the NLR values of peripheral blood and other biochemistry indexes; Pearson correlation analysis was used to calculate the correlation of NLR and detected factors; risk factors of DPN were estimated via logistic regression analysis and multivariate analysis.
The values of triglyceride (TG), neutrophils, fasting insulin, urinary albumin, and 2 hour postglucose in DPN group were significantly higher than those of the DM group, whereas the number of lymphocytes of DPN group was considerably lower than that of the DM group (P < .05 respectively); NLR values were remarkably higher in DPN group compared with those of DM group (2.58 ± 0.50 vs 2.18 ± 0.61, P < .001); logistic regression analysis showed that NLR (P = .002, OR = 4.960, 95% CI = 1.843–13.349) was a risk factor of DPN. Multivariate logistic regression analysis showed that DPN was independently related to NLR (P = .002, OR = 4.960, 95% CI = 1.843–13.349). The ROC curve analysis confirmed that the optimal cut-off point, specificity, and sensitivity in diagnosing DPN by NLR were 2.13%, 48.1%, and 81.3% respectively.
Our results showed that NLR is significantly correlated with DPN, which suggested that NLR may be an independent risk factor of DPN.
Keywords: diabetic peripheral neuropathy, neutrophil-to-lymphocyte ratio, Type 2 diabetes mellitus
1. Introduction
Diabetic peripheral neuropathy (DPN) is one of the most common chronic complications of diabetes mellitus (DM). Studies of American Diabetes Association (ADA) showed that 26.4% of the Type 2 Diabetes Mellitus (T2DM) patients are complicated by painful DPN,[1] while up to 50% of the DPN patients may be asymptomatic.[2] Further development of DPN can lead to diabetic foot, ulcers, infection, and amputation, seriously affecting the quality of patients’ life.[2] Diagnosing DPN depends on clinical examination and nerve conduction study (NCS). But only large myelinated nerve fibers injury can be detected by traditional electrophysiological methods. However, the development of DPN involves different diameters of peripheral sensory and motor nerve fibers,[3,4] which creates difficulties for early detection and diagnosis of DPN. Therefore, searching for a reliable indicator for the precautions and early diagnosis of DPN are of most significance.
The pathogenesis of DPN is so complex that it remains to be explored. Research has shown that early microvascular disorders[5–7] and inflammatory processes[8,9] play important roles in the occurrence and development of DPN. As an indicator of inflammation, which receives many concerns recently, neutrophil-to-lymphocyte ratio (NLR) has a close relationship with cancer,[10,11] cardiovascular diseases,[12,13] and diabetic microvascular concurrency diseases.[14–16] It is also an independent risk factor for the cerebral hemorrhage in T2DM patients[17] and a prognostic factor in patients with various operable tumors.[18]
To our knowledge, there is no study providing consistent evidence on the link between NLR and DPN. Under this circumstance, we aimed to investigate the relationship between NLR levels and DPN by comparing the NLR levels in T2DM patients with and without DPN.
2. Materials and methods
2.1. Subjects
All participants signed an informed consent before the procedure. The research program is in line with the “Declaration of Helsinki” and approved by the Ethics Committee of Zhujiang Hospital and the Chinese People's Liberation Army General Hospital.
This study was conducted between May 2014 and May 2015 in Zhujiang Hospital and Chinese People's Liberation Army General Hospital. One thousand five hundred twenty-nine T2DM patients were selected. After screening, 557 newly diagnosed T2DM patients were eligible and recruited in this study (Fig. 1). After further search for DPN, there were 397 T2DM patients (DM group) (111 males, 286 females) with an average age of 62.34 ± 9.61 years; and 160 T2DM patients complicated with DPN (DPN group) (50 males, 110 females) with an average age of 62.38 ± 10.05 years. The diagnosis of T2DM was according to the 1998 World Health Organization consulting criteria[15] and DPN was based on the 2006 American Diabetes Association criteria.[2] Patients were excluded from the study if they had: coronary artery disease; myocardial infarction, heart failure; active infection; severe tissue damage; acute massive hemorrhage; acute poisoning; cancer; blood diseases that affect neutrophils and lymphocytes (e.g., myeloproliferative disease and leukemia). Patients on medication that may affect neutrophils and lymphocytes were excluded as well. All the patients had signed a written informed consent.
Figure 1.
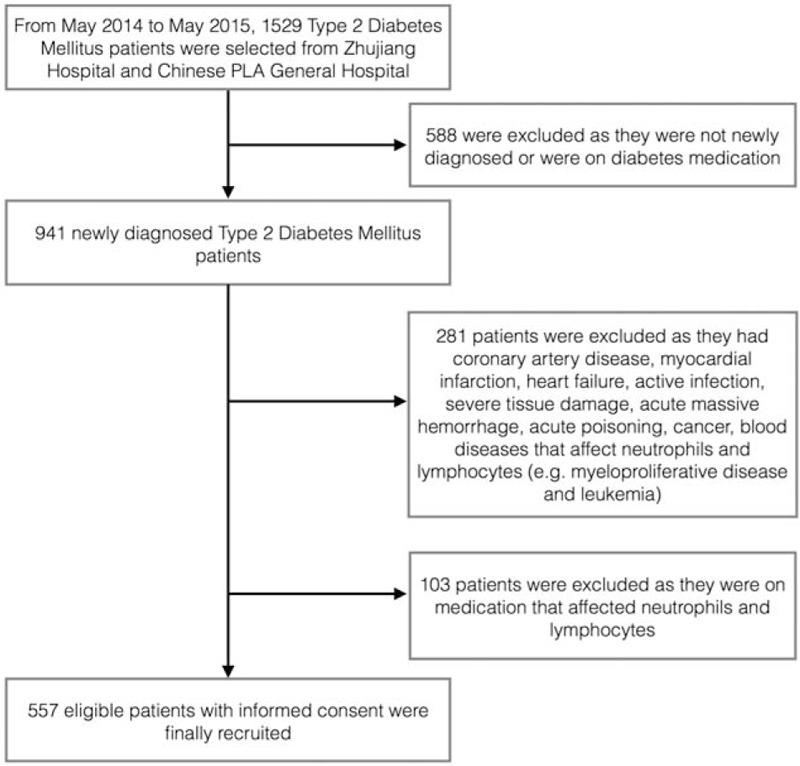
Flow chart showing the patients included in the study.
2.2. Research methods
All study subjects received a uniform questionnaire survey and physical examination, including measurement of height, weight, body mass index (BMI), systolic blood pressure (SBP) and diastolic blood pressure (DBP), fasting peripheral venous blood extraction for fasting plasma glucose (FPG), fasting insulin (Fins), triglycerides (TG), total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), hemoglobin A1c (HbA1c), creatinine (Cr), uric acid (UA), white blood cell (WBC), urinary albumin, high-sensitivity C-reactive protein (hsCRP), tumor necrosis factor-a (TNF-a), interleukin-6 (IL-6), whole blood count, and 2 hour postglucose (2hPG). Status of smoking and alcohol consumption were also included. HOMA-IR evaluates insulin resistance (IR) through HOMA-IR = (FPG × Fins)/22.5.[19]
2.3. Definition
Diabetes was diagnosed based on the World Health Organization consulting criteria (i.e., fasting plasma glucose [FPG] ≥7.0 mmol/L [126 mg/dL] and/or a 2-h postglucose value ≥11.1 mmol/L [200 mg/dL]).[20]
Diabetic peripheral neuropathy was defined as the presence of symptoms and/or signs of peripheral nerve dysfunction in people with diabetes after the exclusion of other causes (Combinations of more than 1 following test have > 87% sensitivity in detecting DPN: pinprick, temperature, and vibration perception (using a 128-Hz tuning fork), 10-g monofilament pressure sensation at the distal halluces, and ankle reflexes).[2]
Neutrophil-to-lymphocyte ratio, a novel potential indicator of inflammation, is the ratio of neutrophil to lymphocyte.
Smoking status was classified into current smoker and nonsmoker. Smokers were those who had smoked at least 100 cigarettes during their lifetimes and, at the time of the interview, reported smoking every day or some days.[21]
Alcohol consumption was determined by the DHQ (nondrinker, occasional drinker, consumption < 20 g/d, consumption ≥20 g/d). The average amount of alcohol consumption for each individual was calculated using the data on the frequency (number of days per week) and the amount of consumption of common alcoholic beverages.[22]
2.4. Statistical methods
The statistical analyses were performed by using software (SPSS 19.0). Continuous variables were presented as mean ± SD values, and categorical variables were expressed as percentages. Student t test (independent sample t test) was used for continuous variables, those with normal distribution and Mann–Whitney U test were used for continuous variables those without normal distribution. The χ2 test was used for categorical variables. Pearson correlation analysis was used to assess the relationship of DPN and the other data. Those factors showed significance in univariate logistic regression analysis and were pooled in multivariate analysis to identify independent risk factors of DPN. Receiver operator characteristic (ROC) curve analysis was performed to identify the optimal cut-off point of NLR (at which sensitivity and specificity would be maximal) for the prediction of DPN. Two-tailed P values of less than .05 were considered to indicate statistical significance.
3. Results
3.1. Patient characteristics
The differences in age, sex, BMI, SBP, DBP, Fins, IR, TC, HDL-C, LDL-C, HbA1c, Cr, UA, WBC, hsCRP, TNF-a, IL-6, and alcohol consumption between the DPN group and the DM group were not statistically significant (P > .05). All of the characteristics of the groups and the laboratory data are outlined in Table 1. Mean NLR values of the patients with DPN were significantly higher than the DM group (2.58 ± 0.50 vs 2.18 ± 0.61, P < .001) (Fig. 2). The mean neutrophil, FPG, TG, urinary albumin, 2hPG values of the DPN group and its proportion of smokers were significantly higher, while the mean lymphocyte values of the DPN group were significantly lower than those of the DM group (P < .05).
Table 1.
Characteristics and laboratory data of the groups.
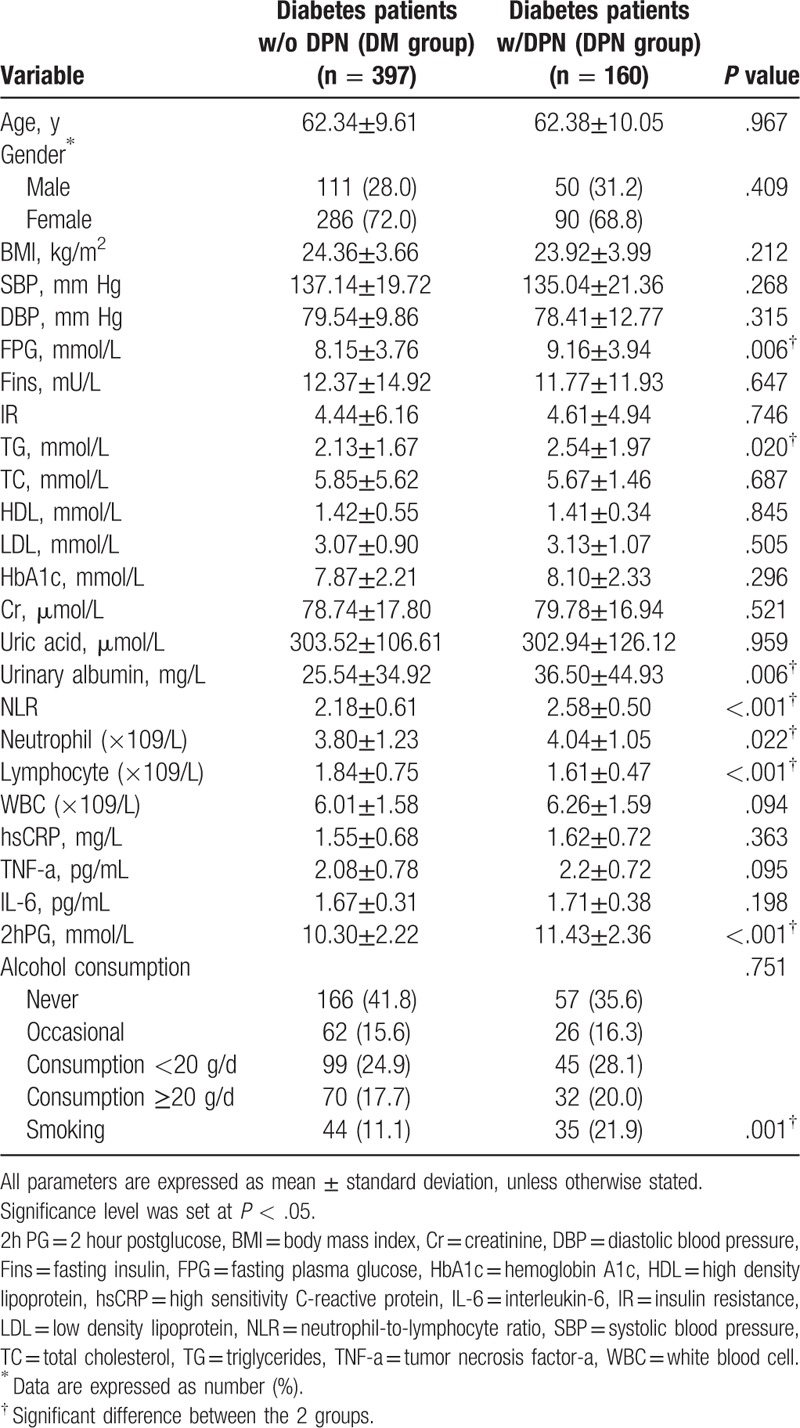
Figure 2.
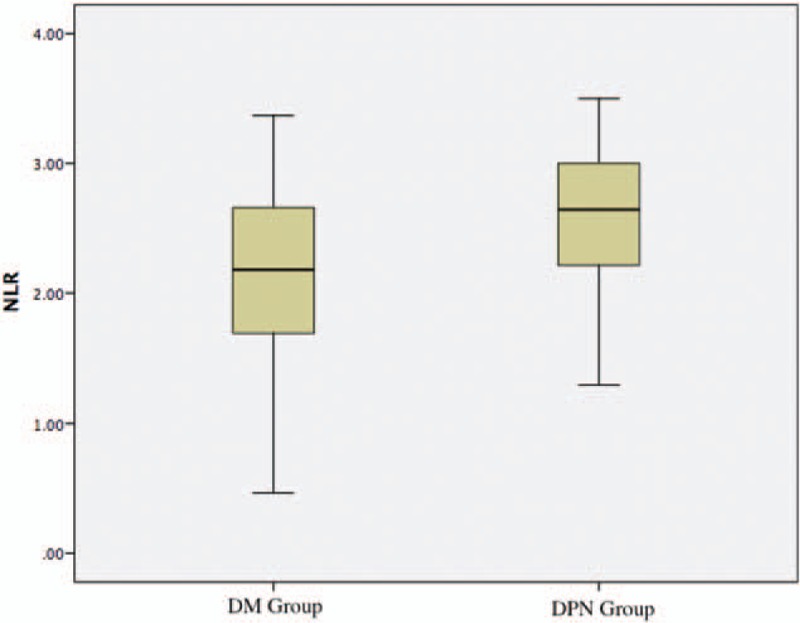
Mean NLR values of the patients in DPN group and DM group. DM = diabetes mellitus, DPN = Diabetic Peripheral Neuropathy, NLR = neutrophil-to-lymphocyte ratio.
3.2. Relationship between DPN and other indexes
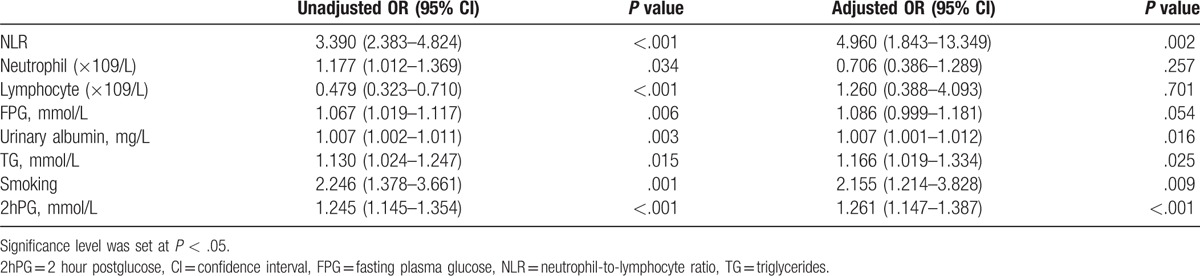
In the DPN group, there was a positive correlation between DPN and neutrophil (r = 0.091, P = .032), lymphocyte (r = −0.150, P < .001), TG (r = 0.106, P = .013) according to Pearson correlation analysis. The multivariate logistic regression models included age, sex, BMI, SBP, DBP, the levels of FPG, Fins, IR, TG, TC, HDL-C, LDL-C, HbA1c, Cr, UA, NLR, neutrophil, lymphocyte, WBC, 2hPG, alcohol consumption, and current smoking status among the DPN group and DM group. Results in multivariate logistic regression analysis showed that DPN was independently related to NLR (P = .002, OR = 4.960, 95% CI = 1.843–13.349), urinary albumin (P = .016, OR = 1.007, 95% CI = 1.001–1.012), TG (P = .025, OR = 1.166, 95% CI = 1.019–1.334), and smoking (P = .009, OR = 2.155, 95% CI = 1.214–3.828) (Table 2).
Table 2.
Univariate and multivariate regression analyses of independent variables for DPN.
3.3. Sensitivity, specificity analysis, and ROC
ROC curve analysis was applied to determine the cut-off point of CRP and NLR (Fig. 2). The cut-off point of NLR was 2.13, sensitivity and specificity were 81.3% and 48.1% respectively. And the area under the curve (AUC) was 0.691 (95% CI, 0.645–0.737; P < .001) (Fig. 3).
Figure 3.
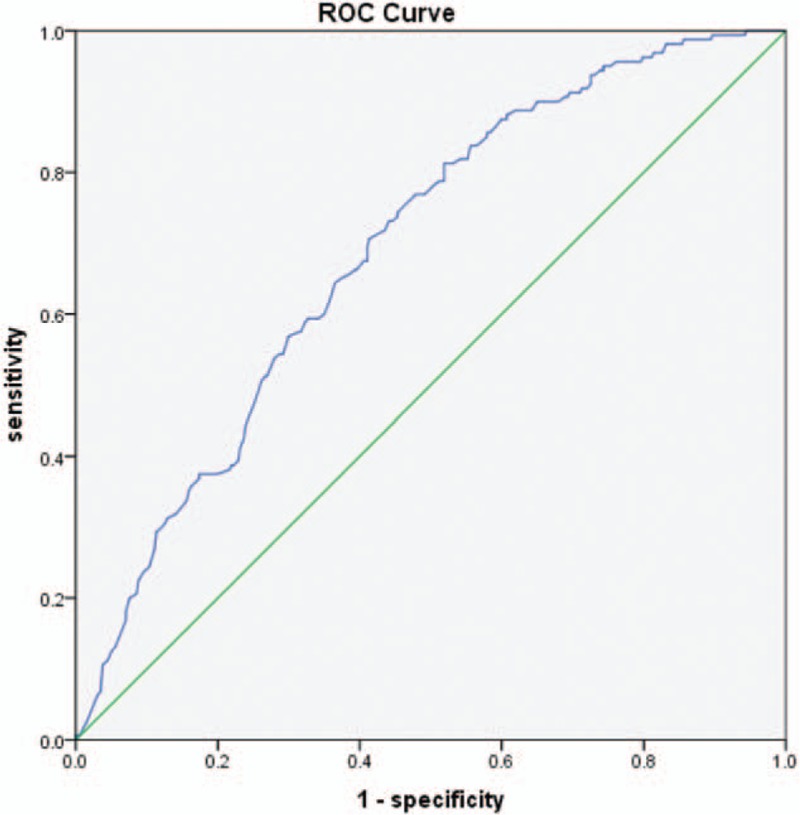
Receiver operating characteristic (ROC) analysis for neutrophil-to-lymphocyte ratio to predict DPN (area under curve is 0.691). DPN = Diabetic Peripheral Neuropathy.
4. Discussion
Diabetic peripheral neuropathy (DPN) is one of the most common complications for people with diabetes. The characteristics of DPN include insidious onset, slow development, and at an early stage there may be symmetry numbness, pain, paresthesia, but at advanced stages, there may be even ulcers and gangrene of the feet.[23] Therefore, the precaution and early diagnosis of DPN are of prime importance in improving the quality of life of diabetic patients.
T2DM and its complications have been proved to be an inflammatory disease and immune system dysfunction.[24,25] Research has shown that chronic inflammation contributes to the occurrence and development of DPN in diabetic patients.[8,9,26,27] Nuclear factor κB (NF-κB) can be induced by stimuli such as hyperglycemia and oxidative stress.[28] The activation of NF-κB will stimulate the inflammatory response by increasing the expression of ICAM-1, proinflammatory cytokines, and chemokines.[29] The overexpression of ICAM-1 results in the gathering of inflammatory cells that releases large quantities of cytokines to recruit more inflammatory cells.[30] Myeloperoxidase and reactive oxygen species are released during the activation of increased neutrophils, which may lead to enhanced oxidative stress and persistent inflammation. These cascades in inflammatory responses will eventually lead to an increase in neutrophils. On the other hand, T2DM and its complications may be associated with lymphopenia. Many clinical and experimental studies witnessed the similar lymphopenia in people with diabetes with microvascular, macrovascular, and other complications.[14–16,31,32] This may be attributed to the increased oxidative DNA damage and apoptosis in peripheral blood lymphocytes.[33]
NLR represents a combination of 2 major components of chronic inflammatory condition (high neutrophil and low lymphocyte). A high neutrophil value is a marker of the ongoing destructive nonspecific inflammatory process. A low lymphocyte value indicates relatively inadequate immune regulation as well as a quiescent immunity pathway.[34] Hence, elevated NLR can reveal the functional status of the immune system in the process of chronic inflammation.[35] In addition, compared with other leukocyte parameters (e.g., neutrophil, lymphocyte, and total leucocyte count), the stability of NLR is less influenced by physiological, pathological, and physical factors.[36,37]
On the other hand, it has been demonstrated that NLR is an independent risk factor for a DPN-related pathophysiological process named diabetic microangiopathy,[14–16] which affects the nutrition supply of neuronal and Schwann cells, causes nerve degeneration, and eventually leads to toperipheral neuropathy.
In our study, neutrophil is significantly higher in the DPN group than in the T2DM group while lymphocyte is significantly lower. Also, neutrophil is positively correlated with DPN while lymphocyte is negatively correlated with DPN. Those results indicated the different inflammatory degree between DPN and T2DM participants. NLR values were remarkably higher in the DPN group compared with those from the DM group (2.58 ± 0.50 vs 2.18 ± 0.61, P < .001). Logistic regression analysis showed that NLR (P = .002, OR = 4.960, 95% CI = 1.843–13.349) was an independent risk factor for DPN. Furthermore, multivariate logistic regression analysis in our study showed that NLR was an independent risk factor for DPN. Combining the results of multivariate logistic regression analysis and independent sample t test, we speculate that elevated NLR may predict a higher incidence of peripheral neuropathy in T2DM patients to some extent. The results suggest that NLR might not only contribute to the development of DPN but also serve as an auxiliary index in the early diagnosis of DPN. Since NLR is an inflammatory marker, it can be hypothesized that an appropriate control of chronic inflammation state in diabetic patients can preclude or alleviate DPN. Moreover, NLR may be an effective monitoring indicator for anti-inflammatory therapy.
Pearson correlation analysis showed that TG is positively correlated with DPN and multivariate logistic regression analysis showed that triglyceride, urine microalbumin, smoking, and 2hPG are risk factors for DPN as well. The study of Irina G Obrosova first proved the key roles of hypertriglyceridemia and increased fatty acid concentrations in contributing to prediabetic neuropathy through oxidative–nitrosative stress.[38] A high-fat diet will not only raise the production of superoxide and peroxynitrite in the vast nervorum but also lead to peripheral nerve dysfunction associated with prediabetes.[39]
Multivariate logistic regression analysis also showed that urine microalbumin, smoking, and 2hPG are risk factors for DPN. A meta-analysis by Carole Clai has provided evidence that smoking is closely linked with the incidence of DPN. Smoking has direct toxic effects, and it is associated with oxidative stress, systemic inflammation, and endothelial dysfunction, increasing the risk of nerve damage through these pathways.[40] Urine microalbumin is not only a significant sign of early renal impairment but also a sign of changes in the vascular system. Therefore, the presence of urine microalbumin can be an important predictor of microvascular and macrovascular complications of diabetes.[41] 2hPG has been proved to be an important risk factor for peripheral neuropathy in subjects with diabetes and prediabetes.[42,43] Hyperglycemia creates a proinflammatory microenvironment, accelerating inflammatory processes and the release of inflammatory biomarkers contributing to DPN.[44]
Previous studies have suggested that the Michigan Neurological Disease Screening Scale (MNSI), Diabetic Neuropathy Symptom (DNS) score, are good screening tools for diabetic neuropathy, and the Toronto Clinical Scoring System (CSS) can detect diabetic peripheral sensorimotor polyneuropathy (DSP) presence and severity.[45–47] However, those diagnosis methods of DPN are easily affected by subjective feelings of patients while NLR is an objective, economic indicator which can be more effective in predicting DPN. Furthermore, these methods ignore the screening of asymptomatic neuropathy.[48] In addition, high cost and time consuming also restrict the application of these traditional methods in extensive clinical screening for DPN. On the contrary, NLR can easily be calculated by the ratio of neutrophils to lymphocytes in peripheral blood. Moreover, the calculation of NLR is outstanding due to its high stability, high repeatability, and a relatively low cost.
There are limitations in our study. First, the relationship between the values of NLR and degrees of the severity of DPN was not explored. Further study in which participants are divided into different groups according to their severity of DPN is needed. Second, our sample is small and it is restricted to regions and race which may cause bias in those statistic results, so larger-scale research is demanded to further evaluate the application of NLR in DPN prediction.
Acknowledgments
The authors thank the Zhujiang Hospital of Southern Medical University, Guangdong, China and the Chinese People's Liberation Army General Hospital, Beijing, China for providing the data.
Footnotes
Abbreviations: 2hPG =2 hour postglucose, ADA = Studies of American Diabetes Association, BMI = body mass index, Cr = creatinine, CSS = Clinical Scoring System, DBP = diastolic blood pressure, DM = diabetes mellitus, DNS = Diabetic Neuropathy Symptom, DPN = Diabetic Peripheral Neuropathy, DSP= diabetic peripheral sensorimotor polyneuropathy, Fins = fasting insulin, FPG = fasting plasma glucose, HbAlc = hemoglobin A1c, HDL = high-density lipoprotein, hsCRP = high-sensitivity C-reactive protein, IL-6 = interleukin-6, IR = insulin resistance, LDL = low-density lipoprotein, MNSI = Michigan Neurological Disease Screening Scale, NCS = nerve conduction study, NF- κB = nuclear factor κB, NLR = neutrophil-to-lymphocyte ratio, ROC = receiver operator characteristic, SBP = body mass index, T2DM = Type 2 Diabetes Mellitus, TC = total cholesterol, TG = triglyceride, TNF-a = tumor necrosis factor-a, UA = uric acid, WBC = white blood cell.
TX, ZW, CP contributed equally to this work.
No current funding sources for this study.
Statement of Human and Animal Rights: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Its approval number is 2015-NFMK-025.
Statement of Informed Consent: Informed consent was obtained from all patients for being included in the study.
This work was supported by Nature Science Foundation of Guangdong Province, China (2017A030313473) and National Natural Science Foundation of China (81672267,81403215, 81774035).
References
- [1].Davies M, Brophy S, Williams R, et al. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care 2006;29:1518–22. [DOI] [PubMed] [Google Scholar]
- [2].Boulton AJ, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005;28:956–62. [DOI] [PubMed] [Google Scholar]
- [3].Chéliout-Héraut F, Zrek N, Khemliche H, et al. Exploration of small fibers for testing diabetic neuropathies. Joint Bone Spine 2005;72:412–5. [DOI] [PubMed] [Google Scholar]
- [4].Sumner CJ, Sheth S, Griffin JW, et al. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology 2003;60:108–11. [DOI] [PubMed] [Google Scholar]
- [5].Cameron NE, Eaton SE, Cotter MA, et al. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia 2001;44:1973–88. [DOI] [PubMed] [Google Scholar]
- [6].Malik RA, Tesfaye S, Thompson SD, et al. Endoneurial localisation of microvascular damage in human diabetic neuropathy. Diabetologia 1993;36:454–9. [DOI] [PubMed] [Google Scholar]
- [7].Tesfaye S, Chaturvedi N, Eaton SE, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352:341–350. [DOI] [PubMed] [Google Scholar]
- [8].Vincent AM, Callaghan BC, Smith AL, et al. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol 2011;7:573–83. [DOI] [PubMed] [Google Scholar]
- [9].Kampoli AM, Tousoulis D, Briasoulis A, et al. Potential pathogenic inflammatory mechanisms of endothelial dysfunction induced by type 2 diabetes mellitus. Curr Pharm Des 2011;17:4147–58. [DOI] [PubMed] [Google Scholar]
- [10].Cho H, Hur HW, Kim SW, et al. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother 2009;58:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Walsh SR, Cook EJ, Goulder F, et al. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol 2005;91:181–4. [DOI] [PubMed] [Google Scholar]
- [12].Uthamalingam S, Patvardhan EA, Subramanian S, et al. Utility of the neutrophil to lymphocyte ratio in predicting long-term outcomes in acute decompensated heart failure. Am J Cardiol 2011;107:433–8. [DOI] [PubMed] [Google Scholar]
- [13].Bhat T, Teli S, Rijal J, et al. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther 2013;11:55–9. [DOI] [PubMed] [Google Scholar]
- [14].Öztürk ZA, Kuyumcu ME, Yesil Y, et al. Is there a link between neutrophil-lymphocyte ratio and microvascular complications in geriatric diabetic patients? J Endocrinol Invest 2013;36:593–9. [DOI] [PubMed] [Google Scholar]
- [15].Huang W, Huang J, Liu Q, et al. Neutrophil-lymphocyte ratio is a reliable predictive marker for early-stage diabetic nephropathy. Clin Endocrinol (Oxf) 2015;82:229–33. [DOI] [PubMed] [Google Scholar]
- [16].Ulu SM, Dogan M, Ahsen A, et al. Neutrophil-to-lymphocyte ratio as a quick and reliable predictive marker to diagnose the severity of diabetic retinopathy. Diabetes Technol Ther 2013;15:942–7. [DOI] [PubMed] [Google Scholar]
- [17].Luo P, Li R, Yu S, et al. The relationship between neutrophil-to-lymphocyte ratio and intracerebral hemorrhage in type 2 diabetes mellitus. J Stroke Cerebrovasc Dis 2017;26:930–7. [DOI] [PubMed] [Google Scholar]
- [18].Yuan ZY, Gao SG, Mu JW, et al. Prognostic value of preoperative neutrophil-lymphocyte ratio is superior to platelet-lymphocyte ratio for survival in patients who underwent complete resection of thymic carcinoma. J Thorac Dis 2016;8:1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lou M, Luo P, Tang R, et al. Relationship between neutrophil-lymphocyte ratio and insulin resistance in newly diagnosed type 2 diabetes mellitus patients. BMC Endocr Disord 2015;15:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Alberti KG, Zimmet PZ. Definition, diagnosis, and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539–53. [DOI] [PubMed] [Google Scholar]
- [21].Thorne SL, Malarcher A, Maurice E, Caraballo R. Centers for Disease Control and Prevention (CDC). Cigarette smoking among adults—United States, 2007. MMWR Morb Mortal Wkly Rep 2008;57:1221–6. [PubMed] [Google Scholar]
- [22].Sasaki S, Yanagibori R, Amano K. Self-administered diet history questionnaire developed for health education: a relative validation of the test-version by comparison with 3-day diet record in women. J Epidemiol 1998;8:203–15. [DOI] [PubMed] [Google Scholar]
- [23].Callaghan BC, Cheng HT, Stables CL, et al. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol 2012;11:521–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia 1998;41:1241–8. [DOI] [PubMed] [Google Scholar]
- [25].Navarro JF, Mora C. Role of inflammation in diabetic complications. Nephrol Dial Transplant 2005;20:2601–4. [DOI] [PubMed] [Google Scholar]
- [26].Uceyler N, Rogausch JP, Toyka KV, et al. Differential expression of cytokines in painful and painless neuropathies. Neurology 2007;69:42–9. [DOI] [PubMed] [Google Scholar]
- [27].Doupis J, Lyons TE, Wu S, et al. Microvascular reactivity and inflammatory cytokines in painful and painless peripheral diabetic neuropathy. J Clin Endocrinol Metab 2009;94:2157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mohamed AK, Bierhaus A, Schiekofer S, et al. The role of oxidative stress and NF-kappaB activation in late diabetic complications. Biofactors 1999;10:157–67. [DOI] [PubMed] [Google Scholar]
- [29].Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 1997;336:1066–71. [DOI] [PubMed] [Google Scholar]
- [30].Kawamura N, Dyck PJ, Schmeichel AM, et al. Inflammatory mediators in diabetic and non-diabetic lumbosacral radiculoplexus neuropathy. Acta Neuropathol 2008;115:231–9. [DOI] [PubMed] [Google Scholar]
- [31].Wang R, Zhang J, Li Y, et al. Neutrophil–lymphocyte ratio is associated with arterial stiffness in diabetic retinopathy in type 2 diabetes. J Diabetes Complications 2015;29:245–9. [DOI] [PubMed] [Google Scholar]
- [32].Ulu S, Bucak A, Ulu MS, et al. Neutrophil–lymphocyte ratio as a new predictive and prognostic factor at the hearing loss of diabetic patients. Eur Arch Otorhinolaryngol 2014;271:2681–6. [DOI] [PubMed] [Google Scholar]
- [33].Shiny A, Bibin YS, Shanthirani CS, et al. Association of neutrophil-lymphocyte ratio with glucose intolerance: an indicator of systemic inflammation in patients with type 2 diabetes. Diabetes Technol Ther 2014;16:524–30. [DOI] [PubMed] [Google Scholar]
- [34].Azab B, Daoud J, Naeem FB, et al. Neutrophil-to-lymphocyte ratio as a predictor of worsening renal function in diabetic patients (3-year follow-up study). Ren Fail 2012;34:571–6. [DOI] [PubMed] [Google Scholar]
- [35].Luo P, Huang Y, Xu T, et al. Relationship between hyperuricemia and neutrophil-to-lymphocyte ratio in type 2 diabetes mellitus. Int J Clin Exp Pathol 2016;9:10833–8. [Google Scholar]
- [36].Núñez J, Núñez E, Bodí V, et al. Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. Am J Cardiol 2008;101:747–52. [DOI] [PubMed] [Google Scholar]
- [37].Gibson PH, Croal BL, Cuthbertson BH, et al. Preoperative neutrophil-lymphocyte ratio and outcome from coronary artery bypass grafting. Am Heart J 2007;154:995–1002. [DOI] [PubMed] [Google Scholar]
- [38].Lupachyk S, Watcho P, Hasanova N, et al. Triglyceride, nonesterified fatty acids, and prediabetic neuropathy: role for oxidative–nitrosative stress. Free Radic Biol Med 2012;52:1255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Vincent AM, Hayes JM, McLean LL, et al. Dyslipidemia-induced neuropathy in mice: the role of oxLDL/LOX-1. Diabetes 2009;58:2376–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Clair C, Cohen MJ, Eichler F, et al. The effect of cigarette smoking on diabetic peripheral neuropathy: a systematic review and meta-analysis. J Gen Intern Med 2015;30:1193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chuengsamarn S, Rattanamongkolgul S, Jirawatnotai S. Association between serum uric acid level and microalbuminuria to chronic vascular complications in Thai patients with type 2 diabetes. J Diabetes Complications 2014;28:124–9. [DOI] [PubMed] [Google Scholar]
- [42].Lu B, Hu J, Wen J, et al. Determination of peripheral neuropathy prevalence and associated factors in Chinese subjects with diabetes and pre-diabetes—ShangHai Diabetic neuRopathy Epidemiology and Molecular Genetics Study (SHDREAMS). PLoS One 2013;8:e61053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bongaerts BW, Rathmann W, Kowall B, et al. Postchallenge hyperglycemia is positively associated with diabetic polyneuropathy: the KORA F4 study. Diabetes Care 2012;35:1891–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Nguyen DV, Shaw LC, Grant MB. Inflammation in the pathogenesis of microvascular complications in diabetes. Front Endocrinol (Lausanne) 2012;3:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bril V, Perkins BA. Validation of the Toronto Clinical Scoring System for diabetic polyneuropathy. Diabetes Care 2002;25:2048–52. [DOI] [PubMed] [Google Scholar]
- [46].Feldman EL, Stevens MJ, Thomas PK, et al. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994;17:1281–9. [DOI] [PubMed] [Google Scholar]
- [47].Meijer JW, Smit AJ, Sonderen EV, et al. Symptom scoring systems to diagnose distal polyneuropathy in diabetes: the Diabetic Neuropathy Symptom score. Diabet Med 2002;19:962–5. [DOI] [PubMed] [Google Scholar]
- [48].Perkins BA, Olaleye D, Zinman B, et al. Simple screening tests for peripheral neuropathy in the diabetes clinic. Diabetes Care 2001;24:250–6. [DOI] [PubMed] [Google Scholar]


