Abstract
Background:
Parkinson disease (PD) is a neurodegenerative disease characterized by chronic and progressive loss of dopaminergic neurons in substansia nigra pars compacta. Oxidative stress is proposed to play a critical role in the pathogenesis of PD. Uric acid (UA), as an important physiological antioxidant, is identified a molecular predictor associated with a decreased risk and a slower disease progression for PD and potential neuroprotectant of PD by increasing epidemiological and clinical evidences. Within this review, we will present a comprehensive overview of the data linking UA to PD in recent years.
Methods:
We searched PubMed, EMBASE, Web of Science databases for relevant studies. Any observational or experimental studies that evaluated UA and PD were our goal of searching the electric databases.
Results:
Twelve studies that evaluated UA and PD were identified in this review. We reviewed the roles of UA in the pathogenesis of PD, the association of UA with morbidity, severity/progression, nonmotor symptoms, motor complications of PD, with an attempt to provide new ideas for diagnosis and treatment in PD.
Conclusion:
Our findings supported that lots of clinical and epidemiological data observed lower UA levels in PD patients. Manipulation of UA or its precursors’ concentration could be effective to treat or prevent PD. However, it is still suspectable that higher UA levels are better enough to PD patients. Furthermore, for the complex nature of PD and its heterogeneous genetic and environmental influences, it is inadequate for just manipulating UA in treating the disease.
Keywords: Parkinson disease, systemic review, uric acid
1. Introduction
Parkinson disease (PD), which can affect over 1% of the population above 65 years of age,[1] is a neurodegenerative disease characterized by chronic and progressive loss of dopaminergic neurons in substansia nigra pars compacta. PD symptoms include rigidity, postural instability, tremor at rest, and slowness or absence of voluntary movement, and even neuropsychiatric symptoms.[2] The pathological hallmarks of PD include progressive degeneration of dopamine neurons, as well as accumulation of α-synuclein positive Lewy bodies in afflicted brain regions.[3,4] The diagnosis of PD in living patients is mainly based on the clinical presence of bradykinesia and one other motor feature (rest tremor or cogwheel rigidity), which have been outlined in the United Kingdom Brain Bank criteria.[5] However, diagnosis of all PD patients accurately is difficult only using these strict criteria. What is worsen, making a diagnosis may be too late until the motor symptoms developed, especially if we want to intervene early in the course of the disease.[6] Hence, developing reliable diagnostic markers and therapeutic goals of early PD is necessary.
Among currently hypotheses, oxidative stress has been proposed to play a key role leading to the degeneration of nigrostriatal dopaminergic pathway, and may represent a central common pathway in the complex convergence of genetic and environmental etiologic factors.[7] Oxidative stress takes parts in the pathogenesis of PD by enhanced enzymatic and nonenzymatic oxidation of dopamine, calcium influx through L-type calcium channels, and PD-linked genes, etc.[7,8] What is more, oxidative stress intertwines with almost all other mechanisms that have been implicated in PD, including protein misfolding and aggregation, mitochondrial dysfunction, cell cycle reactivation, apoptosis, and excito-toxicity.[9,10] Although several biological candidates of oxidative damage, including selegiline,[11] vitamin E,[12] coenzyme 10 (CoQ10),[13,14] and mostly recently creatine,[15,16] are elevated for early diagnosis to identify at-risk groups and disease modification, the results have disappointingly failed to show clear benefits.[17] As an important physiological antioxidant, uric acid (UA), mainly as the urate in human body, can scavenge free oxygen radicals[18] and interact other antioxidant systems.[19,20] Currently, increasing epidemiological and clinical evidences have supported the view higher UA levels were associated with a decreased risk and a slower disease progression for PD.[21,22]
Within this review, we will first present a comprehensive overview of the data linking UA to PD in recent years. And then, we review the role of UA in the pathogenesis of PD, the relationship between UA and PD, with an attempt to provide new ideas for diagnosis and treatment in PD.
2. Materials and methods
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)[23] reporting guidelines for the conduct of systematic review and meta-analysis of intervention trials.
2.1. Search strategy
Three electronic databases (PubMed, EMBASE, and Web of Science) were searched and we used terms and Boolean operators as follows: “(uric acid OR urate OR UA) AND (Parkinson's disease OR Parkinson disease OR parkinsonian OR parkinsonism OR PD).” We did not limit the year of publication, publication status, or language. And there was also no limitation on any particular study design: randomized or nonrandomized clinical trials, cohort, and case–control studies. In addition, we also checked the references of the articles manually to identify other potentially relevant publications. We did not seek unpublished articles. The study selection was then independently performed by 2 of the authors, and any different opinions were resolved through discussion.
2.2. Eligibility criteria and quality assessment
Studies were considered eligible if they met the following criteria: the study enrolled patients with PD; the studies evaluated UA and PD. Letters, comments, editorials, and practice guidelines were excluded. Two authors independently reviewed the titles and abstracts of potentially relevant studies. Any discrepancy was resolved by consensus with a third author.
2.3. Statistical analysis
We will first present a comprehensive overview of the data linking UA to PD in recent years. We reviewed the roles of UA in the pathogenesis of PD, the association of UA with morbidity, severity/progression, nonmotor symptoms, motor complications of PD, with an attempt to provide new ideas for diagnosis and treatment in PD.
3. Results
A total of 267 articles were initially searched from the PubMed, EMBASE, and Web of Science databases. After duplicating, title screening, and abstract or full text screening, 12 studies that evaluated UA and PD were identified in this review. We reviewed the roles of UA in the pathogenesis of PD, the association of UA with morbidity, severity/progression, nonmotor symptoms, motor complications of PD, with an attempt to provide new ideas for diagnosis and treatment in PD.
3.1. UA and morbidity of PD
Massive clinical and epidemiological studies found that UA levels are inversely correlated with development and progression of PD. A study in south Spain revealed that patients with PD had lower serum UA concentrations than controls and UA concentration might have a protective effect against PD and could influence its clinical progression.[24] Gao et al[25] conducted a nested case–control study based on 90,214 participants of 3 ongoing US cohorts and observed that men, but not women, with higher urate concentrations had a lower future risk of developing PD, suggesting that UA could be protective against PD risk or could slow disease progression during the preclinical stage of the disease. A Mendelian randomization analysis adds to the evidence of a causal protective effect of high UA levels to PD.[26] McFarland research supported the inverse association between UA levels and PD in a postmortem study as well.[27]
Nevertheless, there are researchers who held the slightly different view. Jain et al[28] assessed association of urate and incident PD over 14 years in the Cardiovascular Health Study in an elderly community-based cohort of adults, showing a U-shaped effect between urate and PD risk in men. The risk of developing PD was significantly increased for low urate range but not for the high in men.[28] Similar research in China declared that UA in a certain threshold of PD has a protective effect within limits.[29] Which is the optimum concentration range for PD patients? More researches are needed to confirm these preliminary findings and to fully understand UA's physiological effects.
3.2. UA and the severity /progression of PD
Schwarzschild et al[22] selected progression to clinical disability sufficient to warrant dopaminergic therapy as primary study endpoint, and observed inverse association between uricemia and PD progression in man in the “Parkinson Research Examination of CEP-1347 Trial” (PRECEPT) study.[23] Another study including 774 patients with early PD revealed that higher concentrations of urate in serum and cerebrospinal fluid could delay PD progression and the relationship was robust in men, but weak and nonsignificant among women.[22] Andreadou et al[30] reported that the level of serum UA decreased with the increase of Hoehn and Yahr staging. Clinical studies above confirmed UA as a severity indicator of PD especially in men.
3.3. Sex difference in the UA-PD relationship
Sex difference has been observed in studies regarding UA and PD severity/progression among patients with PD.[28,31–33] Between 1990 and 2004, O’Reilly et al[34] conducted a nested case–control study among participants of the female-only Nurses’ Health Study, suggesting that UA was not strongly associated with lower rates of PD among women. Schwarzschild et al[22] reported that PD risk declined with increasing serum urate and the association was stronger in men relative to women in a prospective study including 804 US patients with PD during 2002 to 2004.[23] In the case–control study including 161 patients with PD and 178 controls from southern Spain, Jesus et al[24] found that serum UA concentration was inversely associated with disease severity in men but not in women by assessing the modified the Hoehn and Yahr scale. Similarly, in a aforementioned study, UA levels in cortical and striatal tissue were lower in PD than in controls in men only.[27] The biological mechanisms underlying such sex specificity remain not unequivocal. Reasons for this discrepancy may include First, sex-specific hormones, especially estrogen, might promote more efficient renal clearance of UA and play a key role in dopamine modulation and neuroprotection of dopamine neurons.[35] Second, female PD patients weigh less than male PD patients, resulting in a relative increase in the concentrations of levodopa in the plasma of female PD patients.[36] Third, several cardiovascular risk factors, such as hypercholesterolemia[37] and hypertension,[38] have been reported to be associated with an increased risk of PD in women but not in men.
However, some scholars found that, after adjusting for age factors, PD patients’ UA concentration was lower than the control group, but no gender differences.[28] Probably due to neuroprotective effect of UA in PD increases in a dose-dependent manner, the average UA concentration was generally higher in male PD patients than that in female PD patients. For accurate biological mechanisms underlying such sex specificity remain unclear, more studies are warranted to understand sex differences in the UA–PD relationship.
3.4. Relationship between UA and nonmotor symptoms (NMS) in PD
Despite characterized by motor issues such as tremor at rest, rigidity, bradykinesia, PD also carries various types of NMS,[39,40] which are a supplementary burden and are often very difficult to manage satisfactorily.[41] Reports have examined the relations between UA levels and cognition in PD patients. Euser et al[42] observed that higher serum UA levels were associated with a better cognitive function and a decreased risk of dementia later in life. In a study carried on 40 PD patients, researchers have discussed the relation between plasma UA levels and cognition, suggesting that urine UA levels were associated with worse cognitive performance in PD.[43] The subsequent study supported the association remained stable under 3-year follow-up period and cognitive changes observed were subtle.[44] Moccia et al[45] evaluated the usefulness of baseline serum UA as a marker of NMS progression in newly diagnosed PD and observed that UA might be related not only to NMS presence but also to NMS progression, at least with regard to the attention/memory, depression/anxiety, and cardiovascular domains.
Although a lot of studies reflecting a benefit of higher serum UA levels on cognition, others’ reports showed a contrary result. Gonzalez-Aramburu et al[46] observed a significant correlation between serum UA levels and the presence of hypertension, diabetes, and hypercholesterolemia, and after adjusting for these variables, serum UA levels did not have a significant impact on the risk of cognitive impairment in PD patients. Another study also reported that higher levels of serum UA were associated with a decreased risk of dementia and better cognitive function later in life, but only after adjusting for cardiovascular risk factors.[42]
This nondeterminacy may be explained as: First, researches above were different from the outcomes, sample size, and duration, which would generate deviation. Second, UA may independently affect cognitive function. As UA reflects to some extent the nutritional status, lower UA could reflect a reduction in intakes as may be observed in subjects with progressive cognitive impairment. On the contrary, UA reacts with peroxynitrite, and it will also generate free radicals to accelerate the process. It might have both deleterious and beneficial effects on neural function.
3.5. Relationship between UA and motor complications in PD
When PD progresses to the advanced stage, after treatment especially with levodopa, serious motor complications such as dyskinesias and motor fluctuations develop. Wearing-off fluctuation (WOF) is one of the motor fluctuations. Jiro et al examined the association between serum UA concentration and the occurrence of WOF in 123 Japanese PD patients, observing when the concentration above 6 mg/dL, serum UA was inversely correlated with development of WOF. This inverse association is significant in men but not in women.[47]
3.6. Hyperuricemia and PD
For a lower risk of PD among individuals with the highest levels of serum UA, hypothesis would be expected to find a lower risk of PD among individuals with hyperuricemia or gout, which is the major complication of long-standing hyperuricemia.[48] Alonso et al[49] used data including 1052 PD cases and 6634 controls matched by age, sex, medical practice, and year of enrollment, from a large computerized database in the United Kingdom. After adjusting for matching factors and smoking, individuals with a history of gout had a 31% lower risk of developing PD than those without PD. Researchers in Canada observed similar associations by a cohort design, comparing the incidence of PD in a group of 11,258 gout patients and 56,199 controls matched on age, sex, date of gout diagnosis, and length of medical record.[50]
Although 2 above studies evaluated the association between gout and PD risk and both reported an inverse association, researchers in Denmark found no associations between the use of antigout medications and risk of PD, preserving the possibility that there may not be an important effect of hyperuricaemia on PD in humans.[51] A recent systematic review and meta-analysis either did not provide support for an inverse relationship between gout and risk of PD.[52] To date, the association between hyperuricemia and risk of PD has been uncertain, so further long-term follow-up studies would be required to clarify the relationship.
3.7. Probable mechanisms of UA influencing PD
As the end product of purine metabolism, UA in humans circulates at high concentrations near the limits of its solubility and accounts for approximately 60% of the free radical scavenging activity in human serum.[53] Results from in vitro and in vivo experimental studies suggested that UA could be a potential neuroprotective agent.[54]
UA, as a potent antioxidant, may counter oxidative stress in PD. Elisabeth et al investigated serum UA levels in PD patients compared with age-matched healthy controls and results suggested that there may be increased consumption of UA as a scavenger in PD.[30] Similar studies had lent support to this finding.[55] However, Radhika et al held the point that serum UA was homeostatically regulated at a lower level in PD patients by evaluating 24-hour urinary excretion of UA and its oxidized metabolite, allantoin. They speculated that the chronically lower levels of UA may contribute to relatively lower protection against damage from oxidative stress in PD.[56] Although several studies assumed an association between UA and oxidative stress in PD, they do not prove directly and sample size was relatively small. Moreover, results may be interfered by variety of inflammatory and neurodegenerative diseases. Therefore, its role of resisting oxidative stress in PD needs further elucidation. Further prospective studies with larger sample sizes should be conducted to confirm such a relationship.
UA has been shown to have iron-chelating property. Iron is an essential element in the metabolism of all cells. Neuronally, iron is involved in myelination and neurotransmission. Iron accumulation, particularly in the substantia nigra, plays significant roles in the death of dopaminergic neurons, which is one of pathogenesis of PD.[57,58] Also, toxic iron can promote α-synuclein misfolding and aggregation contributing to the pathogenesis of PD.[59,60] UA has been shown to have iron-chelating property by forming stable complexes with Fe3+, and diminishing the oxidizing potential of Fe3+. Level of Fe3+ in PD patients was higher than normal individuals, and the low UA concentration probably weakens the iron-chelating capacity and may expose the dopaminergic neurons to iron toxicity in PD.[59,61]
Although the neurodegenerative molecular mechanism of PD remains unclear, a large number of studies show that genetic factors contribute to the pathogenesis of PD. Approximately 5% of patients with clinical features of PD have clear familial etiology, which show a classical recessive or dominant mendelian mode of inheritance.[62] A recent study revealed that PD-associated genes played important roles in nigral degeneration,[63] and serum UA levels might modify susceptibility to PD together with genetic variability. Besides, SLC2A9 has been indicated as the most effective of all UA transporters, and SLC2A9 variants have been shown to influence circulating UA levels. Facheris et al[64] observed an association between the rs1014290 polymorphism within SLC2A9 and an earlier age at onset of PD in 664 PD individuals from 3 European centers, suggesting that SLC2A9 genetic variants influenced age of onset of PD probably through influencing on circulating UA levels.
The specific pathology of PD is characterized by the degeneration of dopaminergic neurons in the substantia nigra of the midbrain. In an animal model of PD developed using 6-OHDA, the nigrostriatal neurons displayed a typical morphology of cells undergoing apoptosis.[65,66] Cell apoptosis is programmed mainly through 2 pathways: the mitochondrial pathway and the endoplasmic reticulum pathway, which interact with each other to regulate the apoptosis of cells. Caspase-3 activation is an important pathway of mitochondrial apoptosis.[67] Researchers in China revealed that UA could inhibit 6-Hydroxydopamine, which induces caspase-3-dependent cleavage in catecholaminergic cell line (PC12 cells) and counter the apoptosis of dopaminergic neurons in the substantia nigra.[68] The probable mechanisms of UA in the diagnosis and treatment of PD can be shown in Fig. 1.
Figure 1.
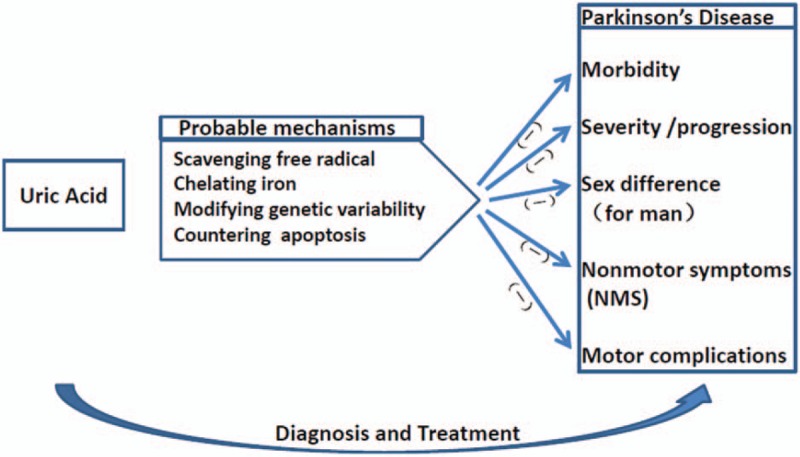
The probable mechanisms of uric acid in the diagnosis and treatment of Parkinson disease.
4. Conclusion
Lots of clinical and epidemiological data observed lower UA levels in PD patients. This change has an influence on morbidity, severity/progression, NMS, etc, of PD. Current diagnosis of PD mainly depends on clinical manifestation and lack of objective biochemical markers, which is inimical to early diagnosis. For the close association of UA and PD, UA concentration (of serum, cerebrospinal fluid, urine, etc.) may be an eligible biomarker in diagnosis and prognosis of early PD. But as a biomarker of PD, UA is not specific enough on its own to distinguish PD and non-PD diagnoses. Nevertheless, it may be combined with other markers, such as imaging,[69] hyposmia,[70] rapid eye movement (REM) sleep behavior disorder (RBD),[71] and α-synuclein,[72] to form a more useful composite indicator of PD diagnoses in early parkinsonism.
What is more, manipulation of UA or its precursors’ concentration could be effective to treat or prevent PD. A recent trial provides evidence that long-term administration of oral inosine, a urate precursor, could increase UA and probably benefit PD in a dose-dependent fashion.[73] However, it is still suspectable that higher UA levels are better enough to PD patients. Previous epidemiologic research has shown that hyperuricemia is a predisposing factor for the incidence of hypertension and cardiovascular disease,[74] and increased serum UA could also lead to the development of nephrolithiasis.[75] Which threshold of UA concentrations is optimum for PD, not only benefits PD treatment but also does not give rise to aforementioned side effects, needs intensive studies in next step. Furthermore, for the complex nature of PD and its heterogeneous genetic and environmental influences, it is inadequate for just manipulating UA in treating the disease.
Inevitably, current studies of UA and PD still show many defects, whereas detection and manipulation of UA will be promising in the diagnosis and treatment of PD in later researches.
Footnotes
Abbreviations: CoQ10 = coenzyme 10, NMS = nonmotor symptoms, PD = Parkinson disease, RBD = rapid eye movement sleep behavior disorder, REM = rapid eye movement, UA = uric acid, WOF = wearing-off fluctuation.
ZY, SZ, DW, and MF are joint first authors.
Funding/support: The authors declare that no funding was received for this meta-analysis.
Elegy: Zhange Yu, MD, had done a major work for this study. Unfortunately, he has left us forever. He passed away peacefully on May 17, 2017, and he was only early 30s. We will embalm him forever. We believe he will still keep shined in the Heaven. God bless him!
All datasets on which the conclusions are based are presented in the main paper.
The authors declare that they have no competing interests.
References
- [1].de Lau LM, Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol 2006;5:525–35. [DOI] [PubMed] [Google Scholar]
- [2].Fahn S, Sulzer D. Neurodegeneration and neuroprotection in Parkinson disease. NeuroRx 2004;1:139–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sulzer D, Surmeier DJ. Neuronal vulnerability, pathogenesis, and Parkinson's disease. Mov Disord 2013;28:715–24. [DOI] [PubMed] [Google Scholar]
- [4].Lees AJ, Hardy J, Revesz T. Parkinson's disease. Lancet (London, England) 2009;373:2055–66. [DOI] [PubMed] [Google Scholar]
- [5].Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mehta SH, Adler CH. Advances in biomarker research in Parkinson's disease. Curr Neurol Neurosci Rep 2016;16:7. [DOI] [PubMed] [Google Scholar]
- [7].Jiang T, Sun Q, Chen S. Oxidative stress: a major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson's disease and Alzheimer's disease. Progr Neurobiol 2016;147:1–9. [DOI] [PubMed] [Google Scholar]
- [8].Surmeier DJ, Guzman JN, Sanchez-Padilla J, et al. The origins of oxidant stress in Parkinson's disease and therapeutic strategies. Antioxid Redox Signal 2011;14:1289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mosharov EV, Larsen KE, Kanter E, et al. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron 2009;62:218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cubukcu HC, Yurtdas M, Durak ZE, et al. Oxidative and nitrosative stress in serum of patients with Parkinson's disease. Neur Sci 2016;37:1793–8. [DOI] [PubMed] [Google Scholar]
- [11].Yoritaka A, Mori H, Hattori N. Treatment of mild camptocormia with selegiline in patients with Parkinson's disease. Eur Neurol 2016;76:35–9. [DOI] [PubMed] [Google Scholar]
- [12].Zhang SM, Hernan MA, Chen H, et al. Intakes of vitamins E and C, carotenoids, vitamin supplements, and PD risk. Neurology 2002;59:1161–9. [DOI] [PubMed] [Google Scholar]
- [13].Beal MF, Oakes D, Shoulson I, et al. A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: no evidence of benefit. JAMA Neurol 2014;71:543–52. [DOI] [PubMed] [Google Scholar]
- [14].Horstink MW, van Engelen BG. The effect of coenzyme Q10 therapy in Parkinson disease could be symptomatic. Arch Neurol 2003;60:1170–2. author reply 1172–1173. [DOI] [PubMed] [Google Scholar]
- [15].Kieburtz K, Tilley BC, Elm JJ, et al. Effect of creatine monohydrate on clinical progression in patients with Parkinson disease: a randomized clinical trial. JAMA 2015;313:584–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bender A, Koch W, Elstner M, et al. Creatine supplementation in Parkinson disease: a placebo-controlled randomized pilot trial. Neurology 2006;67:1262–4. [DOI] [PubMed] [Google Scholar]
- [17].Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron 2003;39:889–909. [DOI] [PubMed] [Google Scholar]
- [18].Ames BN, Cathcart R, Schwiers E, et al. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A 1981;78:6858–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kuzkaya N, Weissmann N, Harrison DG, et al. Interactions of peroxynitrite with uric acid in the presence of ascorbate and thiols: implications for uncoupling endothelial nitric oxide synthase. Biochem Pharmacol 2005;70:343–54. [DOI] [PubMed] [Google Scholar]
- [20].Hink HU, Santanam N, Dikalov S, et al. Peroxidase properties of extracellular superoxide dismutase: role of uric acid in modulating in vivo activity. Arterioscler Thromb Vasc Biol 2002;22:1402–8. [DOI] [PubMed] [Google Scholar]
- [21].Ascherio A, LeWitt PA, Xu K, et al. Urate as a predictor of the rate of clinical decline in Parkinson disease. Arch Neurol 2009;66:1460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Schwarzschild MA, Schwid SR, Marek K, et al. Serum urate as a predictor of clinical and radiographic progression in Parkinson disease. Arch Neurol 2008;65:716–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Chinese Integr Med 2009;7:889–96. [PMC free article] [PubMed] [Google Scholar]
- [24].Jesus S, Perez I, Caceres-Redondo MT, et al. Low serum uric acid concentration in Parkinson's disease in southern Spain. Eur J Neurol 2013;20:208–10. [DOI] [PubMed] [Google Scholar]
- [25].Gao X, O’Reilly EJ, Schwarzschild MA, et al. Prospective study of plasma urate and risk of Parkinson disease in men and women. Neurology 2016;86:520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Simon KC, Eberly S, Gao X, et al. Mendelian randomization of serum urate and Parkinson disease progression. Ann Neurol 2014;76:862–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].McFarland NR, Burdett T, Desjardins CA, et al. Postmortem brain levels of urate and precursors in Parkinson's disease and related disorders. Neurodegener Dis 2013;12:189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jain S, Ton TG, Boudreau RM, et al. The risk of Parkinson disease associated with urate in a community-based cohort of older adults. Neuroepidemiology 2011;36:223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhou Q, Cai C, Qiu J, et al. Clinical study of the protective effect of uric acid on Parkinson's disease. J Med Theory Pract 2016;29:1401–2. 1405. [Google Scholar]
- [30].Andreadou E, Nikolaou C, Gournaras F, et al. Serum uric acid levels in patients with Parkinson's disease: their relationship to treatment and disease duration. Clin Neurol Neurosurg 2009;111:724–8. [DOI] [PubMed] [Google Scholar]
- [31].Chen H, Mosley TH, Alonso A, et al. Plasma urate and Parkinson's disease in the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol 2009;169:1064–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Weisskopf MG, O’Reilly E, Chen H, et al. Plasma urate and risk of Parkinson's disease. Am J Epidemiol 2007;166:561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].de Lau LM, Koudstaal PJ, Hofman A, et al. Serum uric acid levels and the risk of Parkinson disease. Ann Neurol 2005;58:797–800. [DOI] [PubMed] [Google Scholar]
- [34].O’Reilly EJ, Gao X, Weisskopf MG, et al. Plasma urate and Parkinson's disease in women. Am J Epidemiol 2010;172:666–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Behl C, Skutella T, Lezoualc’h F, et al. Neuroprotection against oxidative stress by estrogens: structure-activity relationship. Mol Pharmacol 1997;51:535–41. [PubMed] [Google Scholar]
- [36].Muller T, Woitalla D, Saft C, et al. Levodopa in plasma correlates with body weight of parkinsonian patients. Parkinsonism Relat Disord 2000;6:171–3. [DOI] [PubMed] [Google Scholar]
- [37].Simon KC, Chen H, Schwarzschild M, et al. Hypertension, hypercholesterolemia, diabetes, and risk of Parkinson disease. Neurology 2007;69:1688–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Qiu C, Hu G, Kivipelto M, et al. Association of blood pressure and hypertension with the risk of Parkinson disease: the National FINRISK Study. Hypertension 2011;57:1094–100. [DOI] [PubMed] [Google Scholar]
- [39].Borek LL, Amick MM, Friedman JH. Non-motor aspects of Parkinson's disease. CNS Spectr 2006;11:541–54. [DOI] [PubMed] [Google Scholar]
- [40].Georgescu D, Ancusa OE, Georgescu LA, et al. Nonmotor gastrointestinal disorders in older patients with Parkinson's disease: is there hope? Clin Interv Aging 2016;11:1601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pressley JC, Louis ED, Tang MX, et al. The impact of comorbid disease and injuries on resource use and expenditures in parkinsonism. Neurology 2003;60:87–93. [DOI] [PubMed] [Google Scholar]
- [42].Euser SM, Hofman A, Westendorp RG, et al. Serum uric acid and cognitive function and dementia. Brain 2009;132:377–82. [DOI] [PubMed] [Google Scholar]
- [43].Annanmaki T, Pessala-Driver A, Hokkanen L, et al. Uric acid associates with cognition in Parkinson's disease. Parkinsonism Relat Disord 2008;14:576–8. [DOI] [PubMed] [Google Scholar]
- [44].Annanmaki T, Pohja M, Parviainen T, et al. Uric acid and cognition in Parkinson's disease: a follow-up study. Parkinsonism Relat Disord 2011;17:333–7. [DOI] [PubMed] [Google Scholar]
- [45].Moccia M, Picillo M, Erro R, et al. Presence and progression of non-motor symptoms in relation to uric acid in de novo Parkinson's disease. Eur J Neurol 2015;22:93–8. [DOI] [PubMed] [Google Scholar]
- [46].Gonzalez-Aramburu I, Sanchez-Juan P, Sierra M, et al. Serum uric acid and risk of dementia in Parkinson's disease. Parkinsonism Relat Disord 2014;20:637–9. [DOI] [PubMed] [Google Scholar]
- [47].Fukae J, Ishikawa K, Hatano T, et al. Serum uric acid concentration is linked to wearing-off fluctuation in Japanese Parkinson's disease patients. J Parkinsons Dis 2014;4:499–505. [DOI] [PubMed] [Google Scholar]
- [48].Baker JF, Schumacher HR. Update on gout and hyperuricemia. Int J Clin Pract 2010;64:371–7. [DOI] [PubMed] [Google Scholar]
- [49].Alonso A, Rodriguez LA, Logroscino G, et al. Gout and risk of Parkinson disease: a prospective study. Neurology 2007;69:1696–700. [DOI] [PubMed] [Google Scholar]
- [50].De Vera M, Rahman MM, Rankin J, et al. Gout and the risk of Parkinson's disease: a cohort study. Arthritis Rheum 2008;59:1549–54. [DOI] [PubMed] [Google Scholar]
- [51].Schernhammer E, Qiu J, Wermuth L, et al. Gout and the risk of Parkinson's disease in Denmark. Eur J Epidemiol 2013;28:359–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ungprasert P, Srivali N, Thongprayoon C. Gout is not associated with a lower risk of Parkinson's disease: A systematic review and meta-analysis. Parkinsonism Relat Disord 2015;21:1238–42. [DOI] [PubMed] [Google Scholar]
- [53].Davies KJ, Sevanian A, Muakkassah-Kelly SF, et al. Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem J 1986;235:747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Waring WS. Uric acid: an important antioxidant in acute ischaemic stroke. QJM 2002;95:691–3. [DOI] [PubMed] [Google Scholar]
- [55].Zhu TG, Wang XX, Luo WF, et al. Protective effects of urate against 6-OHDA-induced cell injury in PC12 cells through antioxidant action. Neurosci Lett 2012;506:175–9. [DOI] [PubMed] [Google Scholar]
- [56].Sampat R, Young S, Rosen A, et al. Potential mechanisms for low uric acid in Parkinson disease. J Neural Transm (Vienna) 2016;123:365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wang N, Jin X, Guo D, et al. Iron chelation nanoparticles with delayed saturation as an effective therapy for Parkinson Disease. Biomacromolecules 2017;18:461–74. [DOI] [PubMed] [Google Scholar]
- [58].Dusek P, Schneider SA, Aaseth J. Iron chelation in the treatment of neurodegenerative diseases. J Trace Elem Med Biol 2016;38:81–92. [DOI] [PubMed] [Google Scholar]
- [59].Sian-Hulsmann J, Mandel S, Youdim MB, et al. The relevance of iron in the pathogenesis of Parkinson's disease. J Neurochem 2011;118:939–57. [DOI] [PubMed] [Google Scholar]
- [60].Berg D, Hochstrasser H. Iron metabolism in Parkinsonian syndromes. Mov Disord 2006;21:1299–310. [DOI] [PubMed] [Google Scholar]
- [61].Duan W, Ladenheim B, Cutler RG, et al. Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson's disease. J Neurochem 2002;80:101–10. [DOI] [PubMed] [Google Scholar]
- [62].Hatano T, Kubo S, Sato S, et al. Pathogenesis of familial Parkinson's disease: new insights based on monogenic forms of Parkinson's disease. J Neurochem 2009;111:1075–93. [DOI] [PubMed] [Google Scholar]
- [63].Gonzalez-Aramburu I, Sanchez-Juan P, Jesus S, et al. Genetic variability related to serum uric acid concentration and risk of Parkinson's disease. Mov Disord 2013;28:1737–40. [DOI] [PubMed] [Google Scholar]
- [64].Facheris MF, Hicks AA, Minelli C, et al. Variation in the uric acid transporter gene SLC2A9 and its association with AAO of Parkinson's disease. J Mol Neurosci 2011;43:246–50. [DOI] [PubMed] [Google Scholar]
- [65].In S, Hong CW, Choi B, et al. Inhibition of mitochondrial clearance and Cu/Zn-SOD activity enhance 6-hydroxydopamine-induced neuronal apoptosis. Mol Neurobiol 2016;53:777–91. [DOI] [PubMed] [Google Scholar]
- [66].Venderova K, Park DS. Programmed cell death in Parkinson's disease. Cold Spring Harb Perspect Med 2012;2: pii: a009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Hanrott K, Gudmunsen L, O’Neill MJ, et al. 6-hydroxydopamine-induced apoptosis is mediated via extracellular auto-oxidation and caspase 3-dependent activation of protein kinase Cdelta. J Biol Chem 2006;281:5373–82. [DOI] [PubMed] [Google Scholar]
- [68].Yan Y, Luo W, Zhu T, et al. Protective effect of uric acid on the cell damage of PC12 induced by 6-OHDA. Chinese J Clin Neuro Sci 2010;18:135–9. [Google Scholar]
- [69].Perlmutter JS, Norris SA. Neuroimaging biomarkers for Parkinson disease: facts and fantasy. Ann Neurol 2014;76:769–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Fullard ME, Tran B, Xie SX, et al. Olfactory impairment predicts cognitive decline in early Parkinson's disease. Parkinsonism Relat Disord 2016;25:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Postuma RB, Gagnon JF, Bertrand JA, et al. Parkinson risk in idiopathic REM sleep behavior disorder: preparing for neuroprotective trials. Neurology 2015;84:1104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Campbell MC, Koller JM, Snyder AZ, et al. CSF proteins and resting-state functional connectivity in Parkinson disease. Neurology 2015;84:2413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Schwarzschild MA, Ascherio A, Beal MF, et al. Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: a randomized clinical trial. JAMA Neurol 2014;71:141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med 2008;359:1811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Alonso A, Sovell KA. Gout, hyperuricemia, and Parkinson's disease: a protective effect? Curr Rheumatol Rep 2010;12:149–55. [DOI] [PubMed] [Google Scholar]


