Abstract
Rationale:
Pulmonary epithelioid hemangioendothelioma (P-EHE) is a rare tumor, with no established standard treatment. Overexpression of vascular endothelial growth factor receptor 2 (VEGFR-2) has been reported in some P-EHE patients. Apatinib, a new small molecule tyrosine kinase inhibitor that specifically targets VEGFR-2, has therapeutic benefits in some advanced tumors. However, its efficacy in P-EHE cases has not been reported.
Patient concerns:
Herein, we presented a 44-year-old man with recurrent hemoptysis for approximately 9 years.
Diagnoses:
After hospitalization, relevant examinations were conducted. The disease was subsequently diagnosed as P-EHE.
Interventions:
The patient underwent pulmonary lobectomy, but subsequently developed multiple metastases. Within the tumor, CD31, CK, and Vimentin were found to be positive, while CD34 was negative. Apatinib was initially administered 250 mg daily doses and after 1 month was increased to 500 mg daily.
Outcomes:
He showed noticeable symptomatic improvements and positive imaging changes in the first month of treatment. However, the disease progressed in the following month, despite the increased apatinib dose.
Lessons:
Apatinib is possibly a new treatment for P-EHE. However, further clinical trials are necessary to confirm an effective dose and the efficacy and safety of apatinib in P-EHE treatment.
Keywords: apatinib, chemotherapy, lung cancer, pulmonary epithelioid hemangioendothelioma, VEGFR inhibitors
1. Introduction
Pulmonary epithelioid hemangioendothelioma (P-EHE) is a rare tumor. It was originally described as an intravascular, bronchiolar, and alveolar tumor (IVBAT) of the lung in 1975 by Dail and Liebow.[1] The term “epithelioid hemangioendothelioma” (EHE) was introduced in 1982 by Weiss and Enzinger, which shows its biological features between both hemangioma and angiosarcoma.[2] EHE was classified as a low- to intermediate-grade malignant vascular tumor, with metastatic potential, in the recent World Health Organization (WHO 2015) classification.[3] The tumor has a low prevalence and preferentially occurs in females in an approximately 3:1 gender.[4] The clinical behavior of EHE tumors is unpredictable, with the lungs and liver being the most frequently affected organs. The clinical manifestations of P-EHE are heterogeneous, with majority of patients being symptomatic with weight loss, cough, hemoptysis, chest pain, pleural effusion, or dyspnea.[5,6] P-EHE typically manifests with bilateral lung and multiple pleura nodules that are usually discovered incidentally by imaging. Biopsy, histology, and immunohistochemistry are essential for diagnosis. The typical macroscopic appearance of EHE is rubbery or having a cartilage-like consistency, with a gray-white to yellow-brown cut surface. The typical microscopic appearance, usually showing low-grade atypia, includes hypercellular periphery of the nodules, hyalinization, hypocellular, necrosis, or calcification of the nodule centers. The nuclei are round or oval with abundant cytoplasm. Lumens formed by the epithelioid tumor cells that contain red blood cells may be observed. Vascular antigens, such as CD31, CD34, Fli-1, or Ulex-1, are expressed in most P-EHE, while CD31 is relatively specific and sensitive. Other antigens, such as vimentin, CK, and EMA, are also partially expressed in P-EHE.[7–9] However, considering its rarity and unpredictable clinical behavior, a standard treatment for this malignancy has not been established, without a large clinical trial to guide therapy having been conducted. Surgical resection, radiotherapy, and chemotherapy have been reported to treat P-EHE, but these modalities have shown varying effectiveness. The significant risk factors for P-EHE include: male gender, cough, hemoptysis, chest pain, multiple unilateral nodules, pleural effusion, and metastases to multiple sites.[6] The clinical outcome of P-EHE is variable, which ranges from spontaneous regression without treatment to rapid disease progression and death, even with aggressive intervention and management. Kitaichi et al analyzed 21 P-EHE patients throughout Asia using questionnaires. Survival ranged from 0.5 to 12.0 years during the follow-up period, with 3 cases being classified as partial spontaneous regression.[5] Bagna et al[10] reported a 5-year survival probability of 60% in 75 P-EHE patients, with those having poor prognosis factors showing a median survival of less than 1 year. Therefore, it is prudent to develop novel therapies for EHE. Given the vascular endothelial origin of EHE, inhibitors of vascular endothelial growth factors (VEGF) can be considered promising treatment options for multifocal EHE that does not qualify for surgical intervention.[11] Moreover, vascular endothelial growth factors receptor-2 (VEGFR-2) was reported to be overexpressed in some cases of P-EHE.[12] Apatinib, a tyrosine kinase inhibitor (TKI) that selectively binds to VEGFR-2, exerts broad anti-tumor effects,[13] which is a potential treatment for this refractory tumor. To the best of our knowledge, this is the first case of metastatic P-EHE treated with apatinib. We also reviewed the literature in the current report by summarizing treatments and outcomes for P-EHE, with a discussion on the effect of VEGFR inhibitors in P-EHE cases.
2. Case report
A 44-year-old man was admitted to our hospital on May 26, 2016 due to recurrent hemoptysis for approximately 9 years. The patient had been in good health until 2007, when coughing with small amounts of bright red blood, without obvious sputum and fever, presented. He was suspected of pulmonary tuberculosis at that time and treated with antituberculosis pharmacotherapy. However, the patient had poor compliance and used the prescribed medicine for 1 month. Hemoptysis repeated with small amounts of bright red blood until October 2015, when the hemoptysis presented with approximately 100 mL of blood on one occasion. He was admitted to another hospital, where a thoracic computed tomography (CT) scan showed a round 5.1 × 4.9 cm nodule in the right middle lobe of the lung, with several small nodules surrounding the lager nodule, as well as presenting with a right encapsulated pleural effusion with pleural calcification, without hilar and mediastinal lymphadenopathy. After a period of administering medication for the nodules, the patient felt no improvement and was subsequently transferred to another hospital for surgery. Lobectomy of the right lung middle lobe, with an empyema evacuation and pleural decortication, was conducted in January 2016. Postoperative histopathological examination showed chronic, inflammatory changes with cavity formation, scattered multinucleated giant cells, mass fibrinoid necrosis, and moderate heterocyst of the excisional pulmonary tissue. Immunohistochemical examination revealed that CD31, CK, and Vimentin were positive and CD34 was negative. Antiacid stain, PSM stain, and PAM stain were all found to be negative. The patient was subsequently diagnosed with P-EHE. Two months after surgery, the hemoptysis relapsed and gradually worsened. The patient sought treatment at several other hospitals, but did not obtain a favorable therapeutic effect. On May 26, 2016, the patient coughed 3 times with bright red blood and sputum, had chest pain and dyspnea, and was therefore admitted to our hospital.
The patient worked at a rubber company and did not smoke, but did consume 150 to 200 mL of wine on a daily basis for almost 30 years; he had no other peculiar medical history. The patient's father did die of lung cancer.
The patient's temperature was 36.1°C, the pulse was 82 bpm, respirations were 21 breaths per minute, blood pressure was 128/75 mm Hg, and his pain score was 6 on a 10 scale.
On physical examination, the patient appeared fatigued and pale. An old oblique scar, about 20 cm in length, was observed on his right chest. Neither lymphadenopathy nor rash was detected. Breath was slightly short and rough sounding, but this was absent in the right middle and lower lung.
Hematologic laboratory and blood chemical tests were unremarkable. The urine test showed microalbuminuria and a stool test showed the presence of occult blood (2+). A thoracic CT scan was performed on May 26, 2016 (Fig. 1), showing increased bilateral lung markings and diffuse lesions, including multiple ill-defined nodules, with the largest (2.1 × 2.4 cm) surrounded by ground-glass opacities and multiple bilateral chest wall and pleural thickening. A cephalic CT scan and a liver ultrasound showed multiple metastases. After a multidisciplinary consultation, considering the patient had no chemotherapeutic and radiotherapeutic indications, and since other VEGFR inhibitors had been reported for the treatment of EHE, he was prescribed apatinib monotherapy with an initial daily dose of 250 mg. The patient began to take apatinib on May 31, 2016. Dramatically, hemoptysis, chest pain, and dyspnea markedly decreased after the 1-month of apatinb administration, with no side effects being observed. Another thoracic CT scan was performed on June 28, 2016 (Fig. 2), showing bilateral lung wild markings that were more clear than before, and the multiple nodules had decreased in size with less pleural thickening. As the patient seemed to improve, the apatinib dose was increased to 500 mg daily on July 1, 2016. Unfortunately, the patient suddenly coughed about 200 mL of bright red blood on July 3, 2016 with a blood pressure of 150/105 mm Hg. Hypertension was controllable. Selective embolization of the bronchial artery was operated on July 8, 2016, but the patient coughed bright red blood again on July 10, 2016. The general condition of the patient precipitously declined with progressive asthenia, dyspnea, and chest pain. A third thoracic CT scan was performed on July 17, 2016 (Fig. 3), showing once again diffuse lesions of the bilateral lungs, which were more obvious than previously, with multiple ill-defined nodules surrounded by ground-glass opacities and an additional mass in the right lower chest wall invading the liver with several new small mediastinal lymphadenopathies. Soon after, rash, chest tightness, ecchymosis, headache, inability to use the right limbs, and a deflection of the left angle of the mouth developed one after the other. His disease progressed and the treatment was terminated. Six months after surgery, the patient died of respiratory failure (Table 1).
Figure 1.
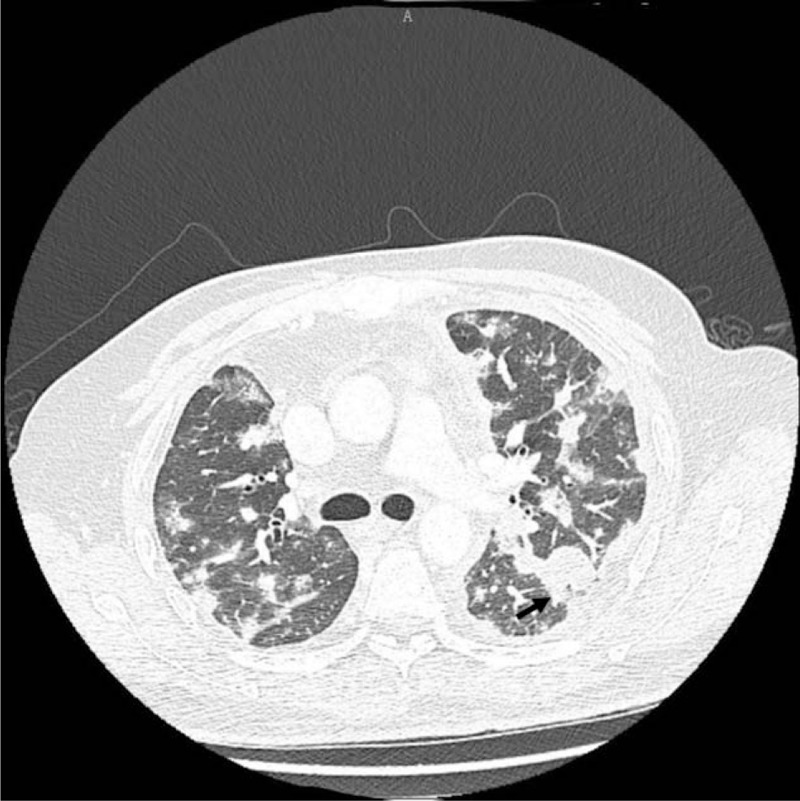
Thoracic CT reveals increased bilateral lung markings and diffuse lesions, including multiple ill-defined nodules, with the largest one (the arrow, 2.1 × 2.4 cm) surrounded by ground-glass opacities and multiple bilateral chest wall and pleural thickening. CT = computed tomography.
Figure 2.
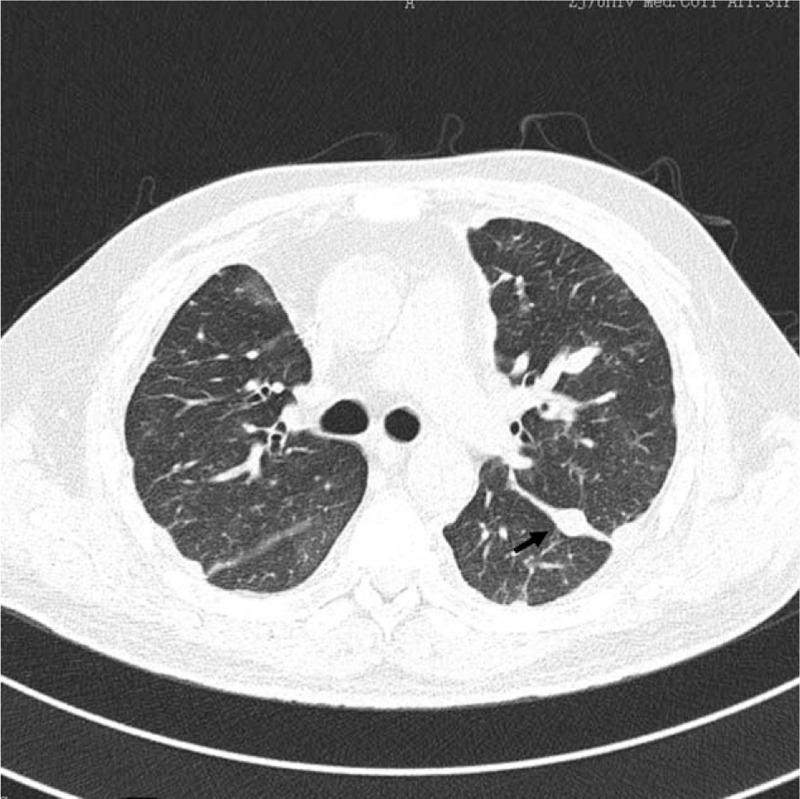
Thoracic CT reveals bilateral lung wild markings that were more clear than before, and the multiple nodules had decreased in size, the largest one (the arrow, 0.8 × 1.2 cm), with unnoticeable surrounding ground-glass opacities and less pleural thickening.
Figure 3.
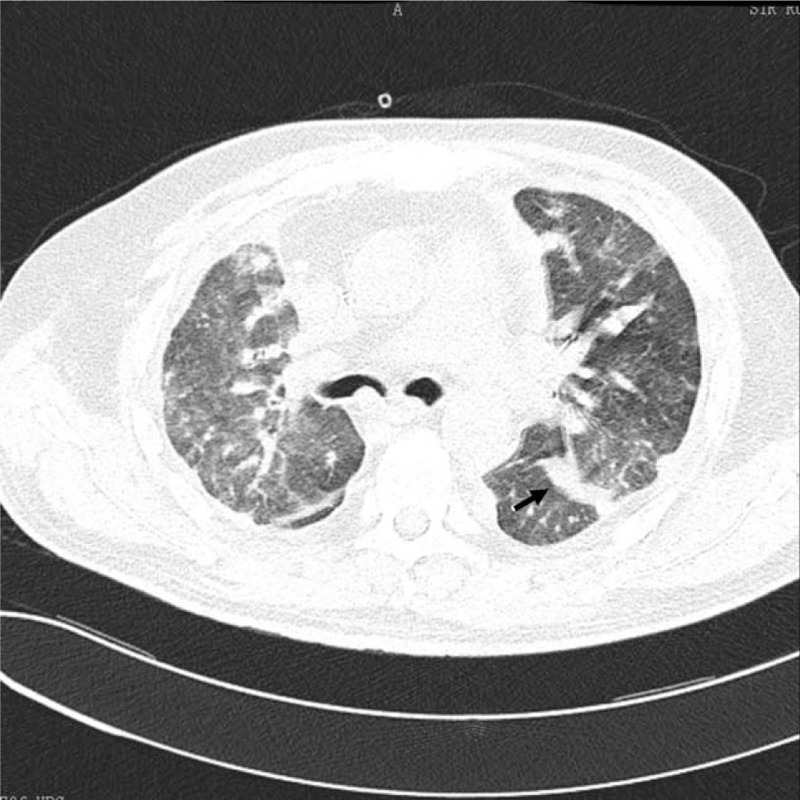
Thoracic CT reveals once again diffuse lesions of bilateral lungs, which were more obvious than previously, with multiple ill-defined nodules, the largest one (the arrow, 1.4 × 2.1 cm), surrounded by ground-glass opacities.
Table 1.
Timeline of patient's management.
3. Discussion
A standard treatment and consensual management for P-EHE are not presently available because of its low incidence and heterogeneous clinical manifestations. Therefore, we reviewed the case reports of P-EHE published in English, focused therapy and survival years and excluded studies did not state detailed patient information (Table 2).[4,6,7,14–62] In total, 63 patients were included, comprised of 22 males and 41 females. Age ranged from 13 to 76-years-old, with a mean age of 43.6 years. Nineteen patients (30%) had unilateral pulmonary nodules, and 44 (70%) had bilateral pulmonary nodules, and 26 (41%) had extrapulmonary metastases. In asymptomatic patients, watchful follow-up without intervention is 1 clinical option. Conservative therapy without treatment on some asymptomatic patients has been reported, with one of these patients maintaining a complete response and another was still alive after 10 years without treatment.[4,44] Jinghong and Lirong[40] reported an interesting P-EHE case with a unilateral dominant mass. The patient had been asymptomatic without treatment for approximately 23 years, before she began to cough and then underwent a lobectomy. Surgical resection alone is an available option, being proposed on the condition that the lesions are unilateral or limited, which typically results in a positive outcome since no extrapulmonary metastases are present. Notably, extensive lung resection offers the same survival as a wedge resection.[10] Since only 9% of patients present with lymph node metastasis, the prognostic value of lymph node invasion and resection remains unclear.[63] Baba et al[45] reported the longest follow-up case, which had no recurrence of 8 years after surgery, while Adamane et al[16] reported a 20-years-old boy who died on the 5th postoperative day. However, in some patients with bilateral multiple nodules that cannot be completely resected, surgery remains a treatment option, but is usually followed by chemotherapy or radiotherapy, according to the condition of the patient. Radiation therapy is considered ineffective for P-EHE, because of the tumor's slow growth and radiobiological characteristics, but it can be used as a symptomatic palliative treatment to control bone metastasis presentation or relieve pain associated with bone involvement.[10,64] Various chemotherapies, which also show variable effectiveness, have been reported for unresectable or metastatic P-EHE cases. Paclitaxel or platinum-based chemotherapy tends to be the most utilized chemotherapy regents. Paclitaxel alone did not stop progression in 2 cases.[14,23] A combination chemotherapy of carboplatin and paclitaxel resulted in short-term stable disease with no change in the nodule size for 1 patient, but this effect was not observed in another patient.[33,46] Platinum combined with etoposide was used in a P-EHE patient after 20 asymptomatic years.[58] The same combination chemotherapy resulted in progressive disease in 3 other patients,[30,54,56] but showed partial regression in another patient.[34] Although ifosfamide and anthracyclines are commonly used in the treatment of patients with advanced soft tissue sarcomas, their effect on P-EHE remains unclear. van Kasteren et al[61] reported an advanced P-EHE patient who poorly tolerated doxorubicin, which did not result in stopping tumor progression. Schattenberg et al and Sardaro et al each reported a patient who showed no response to chemotherapy consisting of adriamycin or epirubicin with ifosfamide, respectively.[31,51] Although Geramizadeh et al[28] reported a patient treated with postoperative chemotherapy including doxorubicin, ifosfamide, mesna, and dacarbazine was stable during 6 months of follow-up, this may due be to unilateral nodules and no metastasis. Since P-EHE has a vascular origin, bevacizumab, interferon-2α, sorafenib, and sunitinib have been administered to patients with advanced P-EHE. Belmont et al[56] reported the first case of a P-EHE treated with carboplatin, paclitaxel, and bevacizumab, as a third choice postoperative chemotherapy, which resulted in stable disease for at least 13 months. The same combination chemotherapy improved patients’ systems and obtained disease stabilization in 2 cases,[33,39] but failed to stop disease progression in 1 case.[46] Considering its antiangiogenic activity, interferon-2α also has been used in several cases, with disease stabilization observed, but the adverse response of interferon therapy should be paid attention.[52] Both sorafenib and sunitinib are VEGFR inhibitors that have antiangiogenic ability, but P-EHE patients did not respond to them in a few case studies.[29,30,32] However, another VEGFR inhibitor, pazopanib, was given to a P-EHE patient for more than 2 years, which resulted in stable disease.[19] Combination chemotherapy, including antiangiogenic drugs, appears to benefit P-EHE patients. However, further large studies are needed to determine the efficacy of these treatments for P-EHE patients.
Table 2.
Summary of the pulmonary epithelioid hemangioendothelioma case reports in the current literatures.
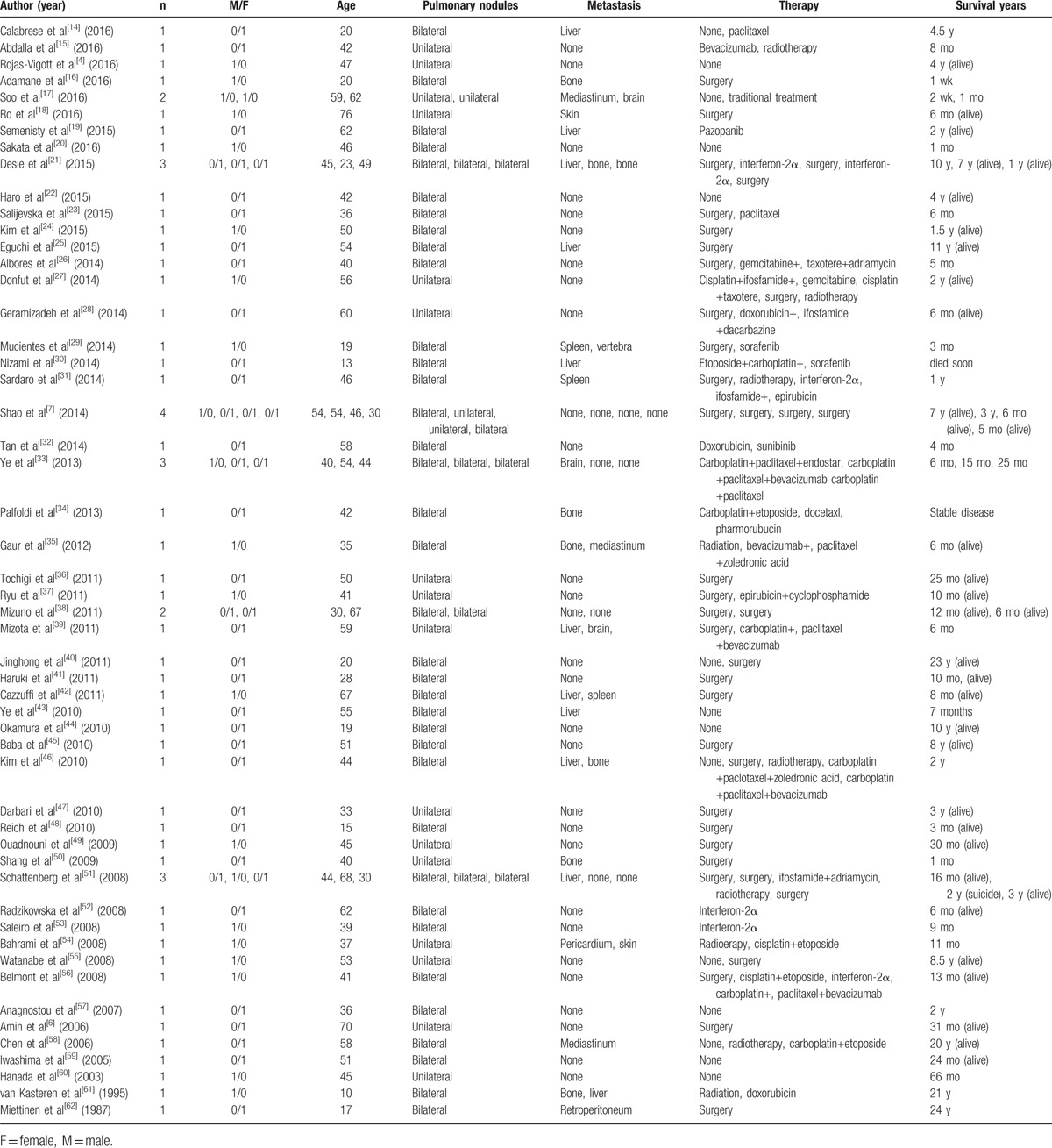
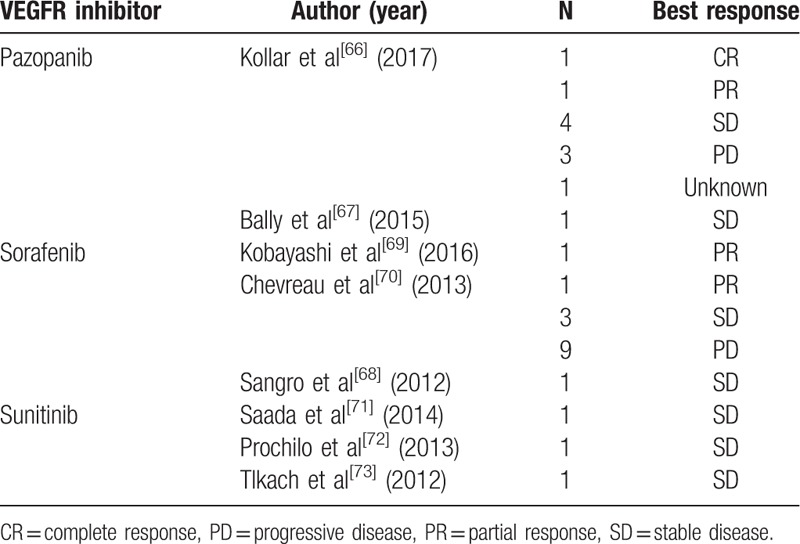
In addition, these 3 VEGFR inhibitors, sorafenib, pazopanib, and sunitinib, were previously reported in the treatment of other EHE cases (Table 3). All of these agents are multitargeted tyrosine kinase inhibitors acting not only on the VEGFR-1/2/3 tyrosine kinases but also other targets.[13,65] Kollar et al[66] retrospectively studied 10 EHE patients who were treated with pazopanib, and found a clinical benefit (response or stable disease) rate of 60% and a median progression-free survival of 26.3 months. While Bally et al[67] reported that pazopanib resulted in a longer period of disease stabilization (over 8 years) for 1 patient. Durable stabilization was also observed in sorafenib therapy, with partial response for 5 years and stable disease in almost 2 years being observed in previous reports.[68,69] In a phase 2 study of progressive EHE patients treated with sorafenib, the 2-, 4-, 6-, and 9-month progression-free survival was 84.6% (11 of 13), 46.4% (6 of 13), 38.4% (5 of 13), and 30.7% (4 of 13), respectively.[70] In contrast, only 3 EHE patients who were treated with sunitinib have been previously reported, with stabilization lasting about 10 months, 2 years, and 6 years, respectively.[71–73] In total, the clinical benefit rate of VEGFR inhibitors was 55.1% (16 of 29), with several observed adverse events, not including bleeding, but was manageable in the limited literature of EHE. However, the VEGFR expression level of these EHE patients who benefited from VEFGR inhibitors is unknown.
Table 3.
Vascular endothelial growth factor inhibitors and their best response to epithelioid hemangioendothelioma in the literatures.
In our clinical case, we used apatinib monotherapy since the patient had multiple organ metastases and could not tolerate conventional chemotherapy. Apatinib, a small molecule acting as a TKI of vascular endothelial growth factor receptor that selectively binds to VEGFR-2, is one of the latest developed oral administered, antiangiogenic agents that can potentially treat a variety of advanced solid tumors.[13] Moreover, Stacher et al[12] reported VEGFR-2 overexpression in 5 of 8 P-EHE cases. In addition, we have reviewed the biology of VEGFR-1, -2, and -3. The precise function of VEGFR-1 is still under debate. One proposition is that VEGFR-1, both the membrane-bound form and the soluble form, is a negative regulator of VEGFR action, serving as a decoy receptor preventing VEGF binding to VEGFR-2.[74] VEGFR-2 plays an important role in the development of angiogenesis and hematopoiesis,[74] and appears to mediate the vast majority of the known cellular responses to VEGF.[75] Furthermore, heterodimerization with VEGFR-2 is necessary for VEGFR-3 to exert its function[76]. Considering the key role of the VEGF-VEGFR system, especially VEGFR-2 in angiogenesis and metastasis of tumor, the application of targeted therapy may be feasible in P-EHE patients. To the best of our knowledge, this is the first report of apatinib monotherapy to treat P-EHE. After receiving apatinib for about a month, the patient showed dramatic improvement in the clinical status, as well as on CT imaging (Fig. 2). However, the patient's disease progressed and his general status gradually deteriorated after dose escalation. Previously, a phase III trial of apatinib in advanced gastric cancer patients reported an adverse event rate of bleeding of 19.9% for any grade and 3.4% for grade 3 or 4 bleeding with a daily dose of 850 mg.[77] Therefore, this may be the cause of the hemoptysis observed in our case. However, EHE tumors have a wide spectrum of behavior from indolent to aggressive, so there is uncertainty as to whether the increased dose or tumor characteristics contributed to the patient's deterioration. Therefore, it is important to balance chemotherapy dose and tumor control. When the vascular origin of P-EHE is considered, targeted antiangiogenic agents, such as apatinib and others, appear to be beneficial for treatment. However, more studies are needed to identify the clinicopathological features of patients who benefit from VEGFR inhibitors, as well as whether 1 particular VEGFR inhibitor is optimal for a specific patient with EHE.
In conclusion, antiangiogenic therapy may be a promising treatment for advanced P-EHE due to the expression of VEGFR-2 in this malignancy. Apatinib as a targeted therapy may have a more promising role in treating selected advanced P-EHE patients than multitargeted agents acting on various tyrosine kinases. However, further studies using large sample sizes are required to determine the safety and efficacy of apatinib for treating P-EHE.
Acknowledgments
The authors thank Editage (www.editage.com) for English language editing.
Footnotes
Abbreviations: CT = computed tomography, EHE = epithelioid hemangioendothelioma, P-EHE = pulmonary epithelioid hemangioendothelioma, TKI = tyrosine kinase inhibitor, VEGFR = vascular endothelial growth factor receptor.
Ethical approval is not necessary because that it is a case report and literature review, and we do not use any special methods.
Informed consent is not given because that the patient is died, and the use of entirely anonymized CT images does not contain any identifying marks and is not accompanied by text that might identify the individual concerned.
The authors have no conflicts of interest to disclose.
References
- [1].Corrin B, Manners B, Millard M, et al. Histogenesis of the so-called “intravascular bronchioloalveolar tumour”. J Pathol 1979;128:163–7. [DOI] [PubMed] [Google Scholar]
- [2].Weiss SW, Enzinger FM. Epithelioid hemangioendothelioma: a vascular tumor often mistaken for a carcinoma. Cancer 1982;50:970–81. [DOI] [PubMed] [Google Scholar]
- [3].Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015;10:1243–60. [DOI] [PubMed] [Google Scholar]
- [4].Rojas-Vigott R, Castro CM, Mendez SR. Pulmonary-epithelioid hemangioendothelioma: a case report of spontaneous regression: track: supportive care and others. J Thorac Oncol 2016;11:S231–2.27676560 [Google Scholar]
- [5].Kitaichi M, Nagai S, Nishimura K, et al. Pulmonary epithelioid haemangioendothelioma in 21 patients, including three with partial spontaneous regression. Eur Respir J 1998;12:89–96. [DOI] [PubMed] [Google Scholar]
- [6].Amin RM, Hiroshima K, Kokubo T, et al. Risk factors and independent predictors of survival in patients with pulmonary epithelioid haemangioendothelioma. Review of the literature and a case report. Respirology 2006;11:818–25. [DOI] [PubMed] [Google Scholar]
- [7].Shao J, Zhang J. Clinicopathological characteristics of pulmonary epithelioid hemangioendothelioma: a report of four cases and review of the literature. Oncol Lett 2014;8:2517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sardaro A, Bardoscia L, Petruzzelli MF, et al. Epithelioid hemangioendothelioma: an overview and update on a rare vascular tumor. Oncol Rev 2014;8:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Murali R, Zarka MA, Ocal IT, et al. Cytologic features of epithelioid hemangioendothelioma. Am J Clin Pathol 2011;136:739–46. [DOI] [PubMed] [Google Scholar]
- [10].Bagan P, Hassan M, Le Pimpec Barthes F, et al. Prognostic factors and surgical indications of pulmonary epithelioid hemangioendothelioma: a review of the literature. Ann Thorac Surg 2006;82:2010–3. [DOI] [PubMed] [Google Scholar]
- [11].Bialas M, Papla B, Bulanda A. Immunohistochemical investigation of selected endothelial markers in pulmonary epithelioid haemangioendothelioma. Pol J Pathol 2011;62:236–40. [PubMed] [Google Scholar]
- [12].Stacher E, Gruber-Mosenbacher U, Halbwedl I, et al. The VEGF-system in primary pulmonary angiosarcomas and haemangioendotheliomas: new potential therapeutic targets? Lung Cancer 2009;65:49–55. [DOI] [PubMed] [Google Scholar]
- [13].Zhang H. Apatinib for molecular targeted therapy in tumor. Drug Des Devel Ther 2015;9:6075–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Calabrese C, Gilli M, De Rosa N, et al. Role of FDG-PET scan in staging of pulmonary epithelioid hemangioendothelioma. Open Med (Wars) 2016;11:158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Abdalla A, Seedahmed E, Bachuwa G, et al. Lung lobar collapse as the first manifestation of pulmonary epithelioid haemangioendothelioma diagnosed with fibreoptic bronchoscopy. BMJ Case Rep 2016;2016.pii: bcr2016216411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Adamane SA, Deodhar KK, Gupta AM, et al. Pulmonary hemangioendothelioma with osteoclast-like giant cells: a rare observation. Indian J Pathol Microbiol 2016;59:398–400. [DOI] [PubMed] [Google Scholar]
- [17].Soo CI, Ng BH, Tan EL, et al. Ambiguous presentations of pulmonary epithelioid hemangioendothelioma: two case reports of a rare pulmonary malignancy. SAGE Open Med Case Rep 2016;4: 2050313X16650323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ro HS, Shin JY, Roh SG, et al. A rare case of pulmonary epithelioid hemangioendothelioma presenting with skin metastasis. Arch Plast Surg 2016;43:284–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Semenisty V, Naroditsky I, Keidar Z, et al. Pazopanib for metastatic pulmonary epithelioid hemangioendothelioma-a suitable treatment option: case report and review of anti-angiogenic treatment options. BMC Cancer 2015;15:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sakata KK, Gotway MB, Smith ML, et al. Pulmonary epithelioid hemangioendothelioma diagnosed with endobronchial biopsies: a case report and literature review. J Bronchology Interv Pulmonol 2016;23:168–73. [DOI] [PubMed] [Google Scholar]
- [21].Desie N, Van Raemdonck DE, Ceulemans LJ, et al. Combined or serial liver and lung transplantation for epithelioid hemangioendothelioma: a case series. Am J Transplant 2015;15:3247–54. [DOI] [PubMed] [Google Scholar]
- [22].Haro A, Saitoh G, Tamiya S, et al. Four-year natural clinical course of pulmonary epithelioid hemangioendothelioma without therapy. Thorac Cancer 2015;6:544–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Salijevska J, Watson R, Clifford A, et al. Pleural epithelioid hemangioendothelioma: literature summary and novel case report. J Clin Med Res 2015;7:566–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kim M, Chang J, Choi H, et al. Pulmonary epithelioid hemangioendothelioma misdiagnosed as a benign nodule. World J Surg Oncol 2015;13:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Eguchi K, Sawafuji M. Surgical management of a patient with bilateral multiple pulmonary epithelioid hemangioendothelioma: report of a case. Surg Today 2015;45:904–6. [DOI] [PubMed] [Google Scholar]
- [26].Albores J, Bando J, Barjaktarevic I. A 40-year-old woman with multilobar nodular densities and massive hemoptysis. Chest 2014;146:e134–7. [DOI] [PubMed] [Google Scholar]
- [27].Donfut AL, Lemaitre J, Van de Walle H, et al. Primitive pulmonary “malignant epithelioid hemangioendothelioma” versus epithelioid angiosarcoma. A case report and review of the literature. Acta Chir Belg 2014;114:143–5. [PubMed] [Google Scholar]
- [28].Geramizadeh B, Ziyaian B, Dehghani M, et al. Prolonged hemoptysis caused by primary pulmonary epithelioid hemangioendothelioma; a case report and review of the literature. Iran J Med Sci 2014;39(2 suppl):223–7. [PMC free article] [PubMed] [Google Scholar]
- [29].Mucientes P, Gomez-Arellano L, Rao N. Malignant pleuropulmonary epithelioid hemangioendothelioma—unusual presentation of an aggressive angiogenic neoplasm. Pathol Res Pract 2014;210:613–8. [DOI] [PubMed] [Google Scholar]
- [30].Nizami I, Mohammed S, Abouzied Mel D. Pulmonary epitheloid hemangioendothelioma PET CT findings and review of literature. Ann Saudi Med 2014;34:447–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sardaro A, Bardoscia L, Petruzzelli MF, et al. Pulmonary epithelioid hemangioendothelioma presenting with vertebral metastases: a case report. J Med Case Rep 2014;8:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tan GL, Takano A, Cheah FK, et al. A 58-year-old woman with dry cough and pulmonary nodules. Ann Acad Med Singapore 2014;43:59–61. [PubMed] [Google Scholar]
- [33].Ye B, Li W, Feng J, et al. Treatment of pulmonary epithelioid hemangioendothelioma with combination chemotherapy: report of three cases and review of the literature. Oncol Lett 2013;5:1491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Palfoldi R, Radacs M, Csada E, et al. Pulmonary epithelioid haemangioendothelioma studies in vitro and in vivo: new diagnostic and treatment methods. In Vivo 2013;27:221–5. [PubMed] [Google Scholar]
- [35].Gaur S, Torabi A, O’Neill TJ. Activity of angiogenesis inhibitors in metastatic epithelioid hemangioendothelioma: a case report. Cancer Biol Med 2012;9:133–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tochigi N, Tsuta K, Maeshima AM, et al. Malignant pulmonary epithelioid hemangioendothelioma with hilar lymph node metastasis. Ann Diagn Pathol 2011;15:207–12. [DOI] [PubMed] [Google Scholar]
- [37].Ryu HS, Lee SS, Choi HS, et al. A case of pulmonary malignant epithelioid hemangioendothelioma misdiagnosed as adenocarcinoma by fine needle aspiration cytology. Diagn Cytopathol 2011;39:801–7. [DOI] [PubMed] [Google Scholar]
- [38].Mizuno Y, Iwata H, Shirahashi K, et al. Pulmonary epithelioid hemangioendothelioma. Gen Thorac Cardiovasc Surg 2011;59:297–300. [DOI] [PubMed] [Google Scholar]
- [39].Mizota A, Shitara K, Fukui T. Bevacizumab chemotherapy for pulmonary epithelioid hemangioendothelioma with severe dyspnea. J Thorac Oncol 2011;6:651–2. [DOI] [PubMed] [Google Scholar]
- [40].Jinghong X, Lirong C. Pulmonary epithelioid hemangioendothelioma accompanied by bilateral multiple calcified nodules in lung. Diagn Pathol 2011;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Haruki T, Arai T, Nakamura H, et al. Pulmonary epithelioid hemangioendothelioma with PlGF expression: report of a case. Thorac Cardiovasc Surg 2011;59:128–30. [DOI] [PubMed] [Google Scholar]
- [42].Cazzuffi R, Calia N, Ravenna F, et al. Primary pulmonary epithelioid hemangioendothelioma: a rare cause of PET-negative pulmonary nodules. Case Rep Med 2011;2011:262674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ye B, Li W, Liu XY, et al. Multiple organ metastases of pulmonary epithelioid haemangioendothelioma and a review of the literature. Med Oncol 2010;27:49–54. [DOI] [PubMed] [Google Scholar]
- [44].Okamura K, Ohshima T, Nakano R, et al. A case of pulmonary epithelioid hemangioendothelioma surviving 10 years without treatment. Ann Thorac Cardiovasc Surg 2010;16:432–5. [PubMed] [Google Scholar]
- [45].Baba H, Tomiyasu M, Makino H, et al. Surgical resection of a primary pulmonary epithelioid hemangioendothelioma in bilateral lungs. Gen Thorac Cardiovasc Surg 2010;58:431–3. [DOI] [PubMed] [Google Scholar]
- [46].Kim YH, Mishima M, Miyagawa-Hayashino A. Treatment of pulmonary epithelioid hemangioendothelioma with bevacizumab. J Thorac Oncol 2010;5:1107–8. [DOI] [PubMed] [Google Scholar]
- [47].Darbari A, Singh D, Singh PK, et al. Pulmonary epithelioid hemangioendothelioma: a rare pulmonary tumor in differential diagnosis of bronchogenic carcinoma. Lung India 2010;27:37–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Reich S, Ringe H, Uhlenberg B, et al. Epithelioid hemangioendothelioma of the lung presenting with pneumonia and heart rhythm disturbances in a teenage girl. J Pediatr Hematol Oncol 2010;32:274–6. [DOI] [PubMed] [Google Scholar]
- [49].Ouadnouni Y, Bouchikh M, Achir A, et al. Pulmonary epithelioid hemangioendothelioma: a case report. Cases J 2009;2:8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Shang A, Wang X. Pulmonary epithelioid haemangioendothelioma mimicking central lung cancer. Respirology 2009;14:452–5. [DOI] [PubMed] [Google Scholar]
- [51].Schattenberg T, Kam R, Klopp M, et al. Pulmonary epithelioid hemangioendothelioma: report of three cases. Surg Today 2008;38:844–9. [DOI] [PubMed] [Google Scholar]
- [52].Radzikowska E, Szczepulska-Wojcik E, Chabowski M, et al. Pulmonary epithelioid haemangioendothelioma—interferon 2-alpha treatment—case report. Pneumonol Alergol Pol 2008;76:281–5. [PubMed] [Google Scholar]
- [53].Saleiro S, Barbosa M, Souto Moura C, et al. Epithelioid hemangioendothelioma—a rare pulmonary tumor. Rev Port Pneumol 2008;14:421–5. [PubMed] [Google Scholar]
- [54].Bahrami A, Allen TC, Cagle PT. Pulmonary epithelioid hemangioendothelioma mimicking mesothelioma. Pathol Int 2008;58:730–4. [DOI] [PubMed] [Google Scholar]
- [55].Watanabe S, Yano F, Kita T, et al. 18F-FDG-PET/CT as an indicator for resection of pulmonary epithelioid hemangioendothelioma. Ann Nucl Med 2008;22:521–4. [DOI] [PubMed] [Google Scholar]
- [56].Belmont L, Zemoura L, Couderc LJ. Pulmonary epithelioid haemangioendothelioma and bevacizumab. J Thorac Oncol 2008;3:557–8. [DOI] [PubMed] [Google Scholar]
- [57].Anagnostou V, Mossa E, Mihas S, et al. Epithelioid haemangioendothelioma of the lung presenting with pulmonary nocardiosis. In Vivo 2007;21:1123–6. [PubMed] [Google Scholar]
- [58].Chen TM, Donington J, Mak G, et al. Recurrence of pulmonary intravascular bronchoalveolar tumor with mediastinal metastasis 20 years later. Respir Med 2006;100:367–70. [DOI] [PubMed] [Google Scholar]
- [59].Iwashima D, Kobayashi J, Yagi T, et al. [A case of pulmonary epithelioid hemangioendothelioma detected on medical checkup]. Nihon Kokyuki Gakkai Zasshi 2005;43:595–9. [PubMed] [Google Scholar]
- [60].Hanada N, Namai S, Kobayashi C, et al. [Case report of pulmonary epithelioid hemangioendothelioma]. Nihon Kokyuki Gakkai Zasshi 2003;41:144–9. [PubMed] [Google Scholar]
- [61].van Kasteren ME, van der Wurff AA, Palmen FM, et al. Epithelioid haemangioendothelioma of the lung: clinical and pathological pitfalls. Eur Respir J 1995;8:1616–9. [PubMed] [Google Scholar]
- [62].Miettinen M, Collan Y, Halttunen P, et al. Intravascular bronchioloalveolar tumor. Cancer 1987;60:2471–5. [DOI] [PubMed] [Google Scholar]
- [63].Ross GJ, Violi L, Friedman AC, et al. Intravascular bronchioloalveolar tumor: CT and pathologic correlation. J Comput Assist Tomogr 1989;13:240–3. [DOI] [PubMed] [Google Scholar]
- [64].Aquilina K, Lim C, Kamel MH, et al. Epithelioid hemangioendothelioma of the spine. Report of two cases. J Neurosurg Spine 2005;3:393–9. [DOI] [PubMed] [Google Scholar]
- [65].Bhargava P, Robinson MO. Development of second-generation VEGFR tyrosine kinase inhibitors: current status. Curr Oncol Rep 2011;13:103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kollar A, Jones RL, Stacchiotti S, et al. Pazopanib in advanced vascular sarcomas: an EORTC Soft Tissue and Bone Sarcoma Group (STBSG) retrospective analysis. Acta Oncol 2017;56:88–92. [DOI] [PubMed] [Google Scholar]
- [67].Bally O, Tassy L, Richioud B, et al. Eight years tumor control with pazopanib for a metastatic resistant epithelioid hemangioendothelioma. Clin Sarcoma Res 2015;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sangro B, Inarrairaegui M, Fernandez-Ros N. Malignant epithelioid hemangioendothelioma of the liver successfully treated with Sorafenib. Rare Tumors 2012;4:e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kobayashi N, Shimamura T, Tokuhisa M, et al. Sorafenib monotherapy in a patient with unresectable hepatic epithelioid hemangioendothelioma. Case Rep Oncol 2016;9:134–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Chevreau C, Le Cesne A, Ray-Coquard I, et al. Sorafenib in patients with progressive epithelioid hemangioendothelioma: a phase 2 study by the French Sarcoma Group (GSF/GETO). Cancer 2013;119:2639–44. [DOI] [PubMed] [Google Scholar]
- [71].Saada E, Saint Paul MC, Gugenheim J, et al. Metastatic hepatic epithelioid hemangioendothelioma: long-term response to sunitinib malate. Oncol Res Treat 2014;37:124–6. [DOI] [PubMed] [Google Scholar]
- [72].Prochilo T, Savelli G, Bertocchi P, et al. Targeting VEGF-VEGFR pathway by sunitinib in peripheral primitive neuroectodermal tumor, paraganglioma and epithelioid hemangioendothelioma: three case reports. Case Rep Oncol 2013;6:90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Tolkach Y, Petrov S, Lerut E, et al. Epithelioid hemangioendothelioma of the kidney treated with sunitinib. Onkologie 2012;35:376–8. [DOI] [PubMed] [Google Scholar]
- [74].Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003;9:669–76. [DOI] [PubMed] [Google Scholar]
- [75].Holmes K, Roberts OL, Thomas AM, et al. Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal 2007;19:2003–12. [DOI] [PubMed] [Google Scholar]
- [76].Alam A, Herault JP, Barron P, et al. Heterodimerization with vascular endothelial growth factor receptor-2 (VEGFR-2) is necessary for VEGFR-3 activity. Biochem Biophys Res Commun 2004;324:909–15. [DOI] [PubMed] [Google Scholar]
- [77].Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol 2016;34:1448–54. [DOI] [PubMed] [Google Scholar]


