Abstract
Though efficacious and affordable treatments for gout are widely available, gout is still not well controlled in many countries of the world including China.
To investigate patient adherence to gout management recommendations and potential barriers in Chinese male gout patients, a survey was carried out by telephone interview in male patients registered in the gout clinic at Peking Union Medical College Hospital. Adherence to dietary and medication recommendations was measured by a food frequency questionnaire and proportion of cumulative time adherent to chemical urate-lowering therapy (ULT), respectively. Dietary adherence was defined as consumption of alcohol, seafood and animal organs less than once per month, and reduced red meat after dietary counseling. Medication adherence was defined as ULT ≥80% of time in the past 12 months for patients with indications. Logistic regression models were used to identify patient characteristics associated with management adherence. Reasons for nonadherence were also sought by open-end questions.
Dietary and medication adherence were 44.2% and 21.9%, respectively. Older age (odds ratio [OR] 7.90, 95% confidence interval [CI] 2.49–25.04 for age ≥60), higher serum uric acid (sUA) levels (OR 3.53, 95% CI 1.42–8.75 for the highest quartile), and tophi (OR 2.31, 95% CI 1.12–4.77) were associated with dietary adherence independently, while tophi (OR 14.05, 95% CI 2.67–74.08) and chronic kidney disease (OR 16.66, 95% CI 2.63–105.37) were associated with medication adherence independently. Reasons that patients reported for nonadherence to medication included remission after treatment (35.3%), concerns for potential side effects (22.7%), insufficient patient education (8.7%), and adverse events (8.2%).
Patient adherence to gout management recommendations is poor in China. Older age, increased disease burden, and specific comorbidities were associated with management adherence.
Keywords: dietary, gout, management adherence, urate-lowering therapy
1. Introduction
The prevalence of gout and hyperuricemia appears to be increasing in China over the past 3 decades coinciding with the rapidly developing economy. A systemic review demonstrated that the prevalence of hyperuricemia in the China mainland is 21.6% (95%CI: 18.9%–24.6%) in the male population and 8.6% (95%CI: 8.2%–10.2%) in the female population.[1] There is no study assessing the national prevalence of gout in China, but 3 large cross-section studies conducted in coastal areas reported the prevalence to be 0.15% to 1.14%, the ratio of male to female was 5–21:1.[2–4]
Gout is probably the best understood and most manageable among all common rheumatic diseases due to its well understood pathophysiology.[5] Since gout is induced by the deposition of monosodium urate crystals in synovial fluid and other tissues, lowering serum uric acid (sUA) is the fundamental target of the treatment. The European League Against Rheumatism (EULAR) and American College of Rheumatology (ACR) guidelines emphasize that the treatment of gout requires both nonpharmacological and pharmacological modalities. All patients should receive counseling on appropriate lifestyle changes including weight loss, dietary modifications, and reduction of alcohol consumption. Urate-lowing therapy (ULT) is indicated in patients with an overload of uric acid such as tophi, recurrent acute attacks, or arthropathy,[6,7] and the goal of ULT is set to achieve a sUA level target at a minimum of 360 μmol/L or lower.[7]
Though efficacious and affordable treatments for gout are widely available, gout is still not well controlled in many countries of the world including China. Nonadherence to management recommendations is one major factor that prohibits optimal medical management.[8] A recent national study from the United Kingdom showed that the ULT adherence was very poor, the percentage of adherent patients is only 39.66%.[9] A systemic review by De Vera demonstrated ULT adherence to be 10% to 46% in developed countries.[10] The data are still unknown in China. There are big differences between China and developed countries when comparing culture, economy, and health systems. It is possible that the treatment adherence of gout and its associated factors may be different in China than these developed countries. Additionally, even though lifestyle intervention is widely recommended as a core treatment of gout, no data about the adherence to lifestyle recommendation are available. This retrospective study was initiated to further investigate the adherence to medication and dietary recommendations in gout patients being treated in a Chinese academic clinical setting.
2. Methods
2.1. Study population and design
Peking Union Medical College Hospital (PUMCH) Gout Clinics has been built since 2008. In our clinics, all doctors are senior attending from general medicine division. We supplied lifestyle advices and dietary education with all patients diagnosed with gout. For patients with ULT indications such as ≥2 gout attacks per year and/or tophi, we also provided with ULT medication recommendations according to the ACR and EULAR guidelines. Once the diagnosis was made, we registered their demographic data and clinical characteristics in our Gout Clinics Database. Due to July 31, 2013, we had recruited 539 subjects (528 men and 11 women) in this database and all subjects satisfied the ACR classification criteria for gout.[11] To investigate patient adherence to gout management recommendations and barriers, all subjects were selected from the database. Since women only accounted for very small proportion in our database, we removed all 11 women from our study to exclude the potential influence of sex. Three hundred fourty one male patients successfully completed a survey administered by telephone in 2014. The study was approved by the medical ethical review board of PUMCH, and informed consent was obtained from all participating patients before administration of the survey.
2.2. Adherence measures
Patients filled out a food frequency questionnaire to document their adherence to dietary recommendations for treatment of gout. Dietary adherence was defined as consumption of alcohol (beer, wine, liquor, and yellow rice wine), seafood, or internal organs less than once a month, and decreased intake of red meat after dietary counseling. Patients also completed a survey in which they document how often they were taking their ULT medications. For patients with indications for ULT (gout flare ≥2 attacks per year and/or tophi), adherence was defined at ULT ≥80% of days in past 12 months. Patients were also asked to document reasons for their inability to adhere to their medication regimen. Patient explanations for medication nonadherence were also sought by open-end questions. The last sUA in past 12 months was also documented to determine if sUA target had achieved after ULT.
2.3. Covariates
Patient characteristics were assessed to identify covariates of adherence. These included demographic factors (sex, age, ethnicity, education level, resident area), clinical characteristics at baseline (course of disease, gout flare frequency, cumulative involved joints, tophi, body mass index [BMI], sUA), previous ULT treatment, specific comorbidities (hypertension, diabetes, dyslipidemia, chronic kidney disease [CKD], nephrolithiasis, coronary artery disease [CAD], and cerebrovascular disease [CVD]), and time interval between baseline and the survey. Hypertension, diabetes, and dyslipidemia were defined by history of physician diagnosis. CKD was defined as glomerular filtration rate (GFR) <60 mL/min/1.73 m2, which was estimated by using the simplified modification of diet in renal disease study equation. Nephrolithiasis was established by medical history and ultrasound results. CAD was defined by history of previous myocardial infarction or radiological diagnosis. CVD was defined by previous stroke history.
2.4. Statistics
Descriptive statistics were used to determine the treatment adherence. We used univariable logistic regression to estimate the independent association between each patient characteristics and adherence. Multivariable logistic regression models were set up to estimate the strength of the association between patient characteristics and adherence. A forward conditional method was used to determine the variables to include in the multivariable models, including all variables considered to be potentially important and with P values <.05 in univariable logistic regression. Variables with P values <.10 remained in the final models. PASW 18.0, chicago, US for Windows was used for data collection, data validation, data selection, and statistical analysis.
3. Results
3.1. Baseline characteristics
A total of 341 eligible male patients were successfully interviewed via telephone. The mean age was 45 (±12). The median course of disease was 5 years. Eighty seven (25.5%) developed tophi. The mean sUA was 572 (±123) μmol/L and only eight (3.7%) patients had sUA <360 μmol/L. Comorbidities were common, including dyslipidemia (51.6%), hypertension (44.3%), nephrolithiasis (25.4%), and CKD (11.3%). Based on the ACR and EULAR guidelines, 265 (77.7%) had an indication for ULT. Hundred (41.5%) patients were once prescribed ULT at some point in their disease course. The study population baseline characteristics were shown in Tables 1 and 2.
Table 1.
Dietary adherence according to the baseline characteristics.
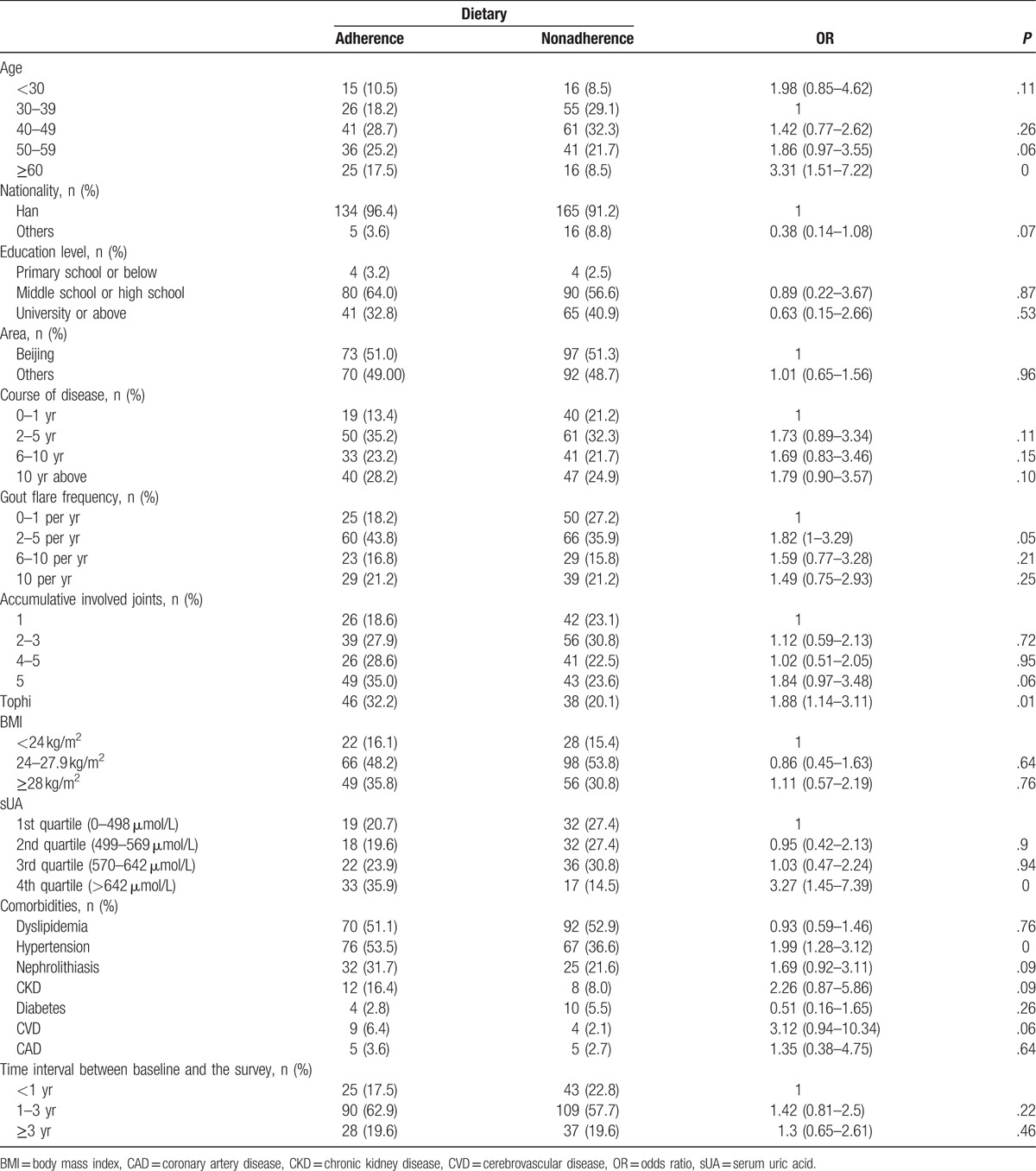
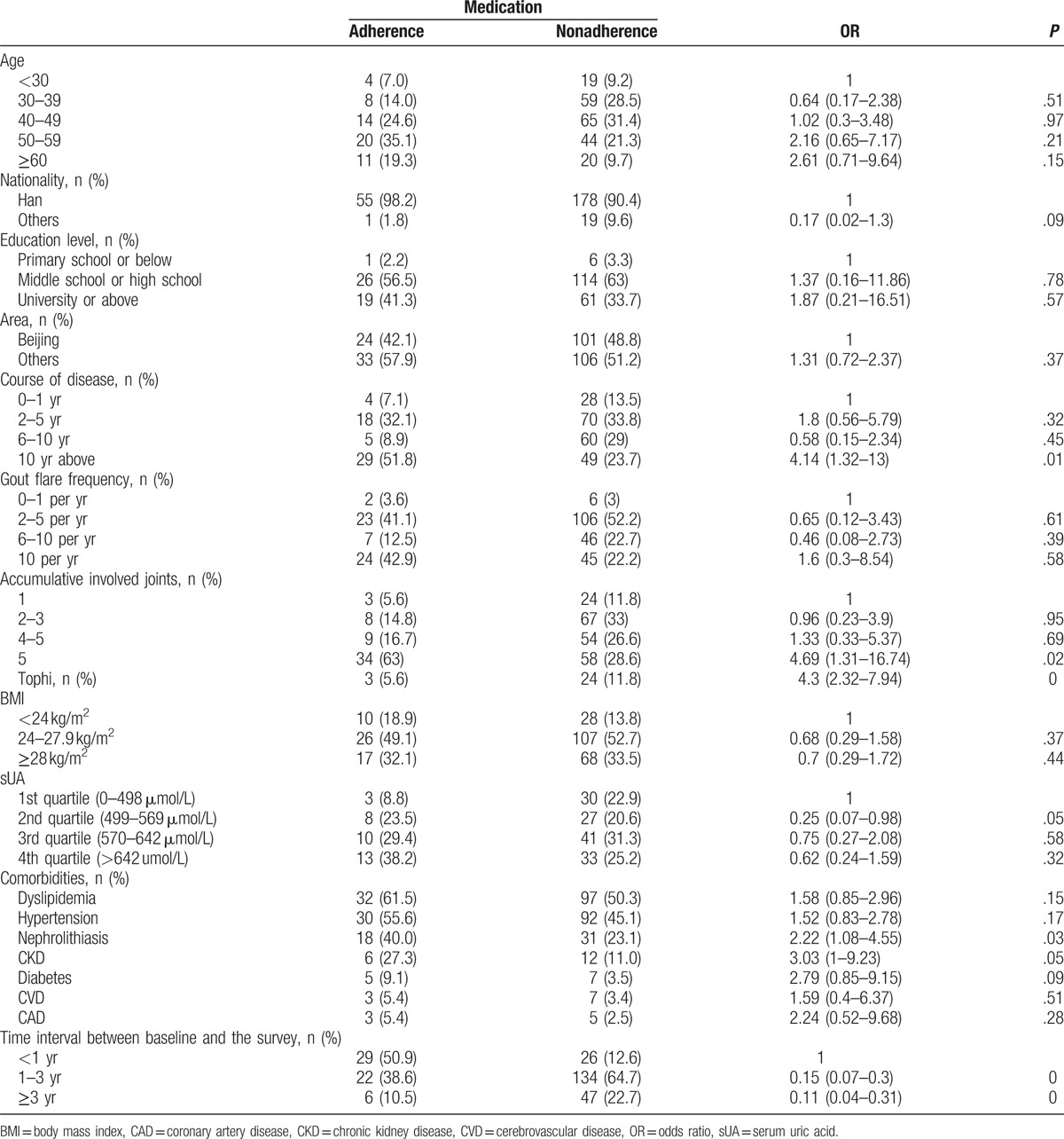
Table 2.
Medication adherence according to the baseline characteristics.
3.2. Dietary adherence
Based on data collected from a food frequency questionnaire, dietary adherence was 43.1%. 91.9% of patients consumed seafood less than once a month, 94.0% consumed internal organs less than once a month, and 63.2% consumed alcohol less than once a month. Among patients who consumed alcohol at least once a month, 94.3% chose liquor. The percentage of patients choosing beer, wine, and yellow rice wine was much lower corresponding to values of 9.8%, 4.1%, and 0.8%, respectively. 62.8% of patients admitted to decreased intake of red meat after dietary counseling.
In the adjusted analyses, older age (OR 7.90, 95% CI 2.49–25.04 for age ≥60 compare with age between 30 and 39), higher sUA levels (OR 3.53, 95% CI 1.42–8.75 for the highest quartile), and tophi (OR 2.31, 95% CI 1.12–4.77) were associated with dietary adherence independently (Table 3).
Table 3.
Characteristics associated with treatment adherence of gout.

3.3. Medication adherence
Figure 1 describes the distribution of the percentage of days covered with ULT. 40.9% patients with ULT indication did not took any ULT in past 12 months. Medication adherence was 21.6%.
Figure 1.

The percentage of days ULT was taken in past 12 months. ULT = urate-lowering therapy.
In the adjusted analyses, tophi (OR 14.05, 95% CI 2.67–74.08) and chronic kidney disease (OR 16.66, 95% CI 2.63–105.37) were associated with medication adherence independently (Table 3).
Patient-reported reasons for medication nonadherence included remission after treatment (35.3%), concerns for potential side effects (22.7%), insufficient patient education (8.7%), and adverse events (8.2%) (Table 4). Other reasons include preferring Traditional Chines Medicine, having gout flare after ULT, treatment failure, family plan, inconvenient to get medicine, and forgetting to take pills. Among patients who underwent remission after treatment, 91.1% has <2 gout flares per year, however, only 4.3% (N = 2) patients had sUA levels <360 μmol/L.
Table 4.
Major reason leading to medication nonadherence in gout patients (n = 207).

4. Discussion
The study was a retrospective cohort study which evaluated patient adherence to gout management recommendations in China. Older age, increased disease burden defined by increased sUA and presence of tophi, and specific comorbidities such as CKD were associated with increased treatment adherence.
Consumption of specific dietary foods like alcohol, seafood, and internal organ have been proven to be risk factors for gout.[12,13] Surveys based on the Chinese population also demonstrated a relationship between certain dietary foods (seafood, meat, and alcohol) and sUA.[4,14] Unfortunately, there is no available studies to date which focus on dietary adherence among gout patients. While conducting our study, we found the adherence to dietary recommendation is poor among Chinese gout patients. Harrold's survey on 240 gout patients showed that only a minority of patients were aware of common foods known to trigger acute gout attacks (e.g., seafood [23%], beef [22%], pork [7%], and beer [43%]).[15] A small cross-sectional study by Shulten reported that gout patients were often unaware of which foods and beverages to avoid (alcohol, n = 14 [48%]; beer, n = 18 [62%]; seafood, n = 29 [100%]; meat, n = 7 [24%]; beef/pork/lamb, n = 24 [83%]).[16] In our study, most patients avoided beer, seafood, and internal organs which are well-established risk factors for gout, however, consumption of liquor and red meat were high. This result suggests that besides those well-known risk food for gout, doctors may need spend more time on educating gout patients to avoid liquor and red meat during dietary counseling. We also found that patients with higher sUA level and tophi showed a better adherence to dietary recommendation, which indicated that disease burden might urge patients to change their eating habit.
Like many chronic diseases, medication adherence needs to be improved in patients with gout. Briesacher compared medication adherence rates among patients with different chronic diseases based on their health care claims data from the United States. 36.8% of individuals with gout successfully achieved daily medication adherence rates of 80% or greater compared with 72.3% for hypertension, 65.4% for type II diabetes, 60.8% for seizure disorders, 54.6% for hypercholesterolemia, and 51.2% for osteoporosis. Our study also showed poor medication adherence among our gout patients in China. In De Vera's systemic review, he summarizes the factors associated with poor medication adherence in those suffering from gout. Younger age, more gout flares, no tophi, African–American race, lower socioeconomic status, current smoker, and no provider visits for gout prior to initiating ULT were associated with a higher rate of nonadherence. Among medical comorbities, hypertension, diabetes, and CVD were associated with higher medication adherence.[10] In our study, patients with tophi or CKD demonstrated superior medication adherence. Both conditions are markers for worse disease severity which may urge patients to comply with treatment plans.
In our survey, the most common reason for medication nonadherence was remission of the disease after treatment. The data also showed that even if symptoms remitted after treatment, sUA became or remained elevated once ULT was discontinued. It appears that patients with gout often take medications only if they have clinical symptoms. Even though there are few studies focusing on optimal dosing for ULT, the goal of ULT is to achieve a sUA level target of 6 mg/dL or less in order to prevent acute attacks and to facilitate tophus regression.[17] The results indicate that it is important to emphasize the significance of the sUA target when educating patients on their disease. Patient concerns regarding potential side effects also led to an increased rate of medication nonadherence. In China, there is a common cultural saying that every medicine brings side effects. Potential adverse effects of ULT range from mild abdominal discomfort to severe hypersensitive reaction. Patients need to be well educated on the benefits versus risks of ULT before starting the treatment and should be monitored for potential adverse reactions during clinical follow up.
The study has some limitations. We chose a self-designed questionnaire where patients self-reported dietary intake, prescription history, and medication adherence. Since this method largely relies on the patient to remember and provide the information, it may cause recall or lying bias. Because there is no widely accepted definition of dietary adherence in gout treatment, we evaluated the dietary adherence based on the evidence from nutritional epidemiological research and recommendation from ACR guidelines. The validity of this method needs to be tested in future studies. The population of this study came from an urban hospital, therefore, the results may not represent patients with gout in rural areas of China.
In conclusion, this retrospective cohort study has shown a poor adherence to management recommendations among gout patients in China. Future trials should be designed to test if any specific educational, lifestyle, or psychological therapy interventions lead to better treatment adherence in the gout patient population.
Footnotes
Abbreviations: ACR = American College of Rheumatology, BMI = body mass index, CAD = coronary artery disease, CKD = chronic kidney disease, CVD = cerebrovascular disease, EULAR = European League Against Rheumatism, GFR = glomerular filtration rate, PUMCH = Peking Union Medical College Hospital, sUA = serum uric acid, ULT = urate-lowering therapy.
Chinese Research Special Fund for Public Welfare Industry of Health (grant number 201502024 and 201302008).
Fund fromBeijing Municipal Bureau of Health (grant number 2011-4001-07).
The authors report no conflicts of interest.
References
- [1].Liu B, Wang T, Zhao HN, et al. The prevalence of hyperuricemia in China: a meta-analysis. BMC Public Health 2011;11:832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nan H, Qiao Q, Dong Y, et al. The prevalence of hyperuricemia in a population of the coastal city of Qingdao, China. J Rheumatol 2006;33:1346–50. [PubMed] [Google Scholar]
- [3].Zeng Q, Wang Q, Chen R, et al. Primary gout in Shantou: a clinical and epidemiological study. Chin Med J (Engl) 2003;116:66–9. [PubMed] [Google Scholar]
- [4].Miao Z, Li C, Chen Y, et al. Dietary and lifestyle changes associated with high prevalence of hyperuricemia and gout in the Shandong coastal cities of Eastern China. J Rheumatol 2008;35:1859–64. [PubMed] [Google Scholar]
- [5].Richette P, Bardin T. Gout. Lancet 2010;375:318–28. [DOI] [PubMed] [Google Scholar]
- [6].Zhang W, Doherty M, Bardin T, et al. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2006;65:1312–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken) 2012;64:1431–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dunbar-Jacob J, Mortimer-Stephens MK. Treatment adherence in chronic disease. J Clin Epidemiol 2001;54(Suppl):S57–60. [DOI] [PubMed] [Google Scholar]
- [9].Kuo CF, Grainge MJ, Mallen C, et al. Rising burden of gout in the UK but continuing suboptimal management: a nationwide population study. Ann Rheum Dis 2015;74:661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].De Vera MA, Marcotte G, Rai S, et al. Medication adherence in gout: a systematic review. Arthritis Care Res (Hoboken) 2014;66:1551–9. [DOI] [PubMed] [Google Scholar]
- [11].Wallace SL, Robinson H, Masi AT, et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum 1977;20:895–900. [DOI] [PubMed] [Google Scholar]
- [12].Choi HK, Atkinson K, Karlson EW, et al. Alcohol intake and risk of incident gout in men: a prospective study. Lancet 2004;363:1277–81. [DOI] [PubMed] [Google Scholar]
- [13].Choi HK, Atkinson K, Karlson EW, et al. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med 2004;350:1093–103. [DOI] [PubMed] [Google Scholar]
- [14].Xiong Z, Zhu C, Qian X, et al. Serum uric acid is associated with dietary and lifestyle factors in elderly women in suburban Guangzhou in Guangdong province of south China. J Nutr Health Aging 2013;17:30–4. [DOI] [PubMed] [Google Scholar]
- [15].Harrold LR, Mazor KM, Peterson D, et al. Patients’ knowledge and beliefs concerning gout and its treatment: a population based study. BMC Musculoskelet Disord 2012;13:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shulten P, Thomas J, Miller M, et al. The role of diet in the management of gout: a comparison of knowledge and attitudes to current evidence. J Hum Nutr Diet 2009;22:3–11. [DOI] [PubMed] [Google Scholar]
- [17].Sivera F, Andres M, Carmona L, et al. Multinational evidence-based recommendations for the diagnosis and management of gout: integrating systematic literature review and expert opinion of a broad panel of rheumatologists in the 3e initiative. Ann Rheum Dis 2014;73:328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]


