Abstract
Rationale:
Acinar cell carcinomas (ACCs) and mixed acinar-endocrine carcinomas (MAECs) of the pancreas are rare, accounting for only 1% of pancreatic tumors. Although both typically present at an advanced stage, chemotherapeutic regimes have not yet been standardized.
Patient concerns:
A 65-year-old man presented with a large mass in the pancreatic tail with multiple liver metastases.
Diagnosis, Interventions, Outcomes:
He was initially treated with gemcitabine for suspected ductal carcinoma of the pancreas, but no response was observed. S-1, administered as second-line chemotherapy, showed an approximately 38% reduction in the size of the primary tumor and metastatic deposits with therapeutic effects being maintained for 12 months. When the tumor progressed again, he underwent a percutaneous liver biopsy, which led to the diagnosis of MAEC. Combination therapy with cisplatin and etoposide targeting the endocrine component was administered, and this was based on the endocrine component potentially being less sensitive to S-1 than the ACC element. However, therapy was stopped due to the development of neutropenia, and the patient is currently receiving best supportive care.
Lessons:
Given the previous studies suggested that S-1 is more effective for ACCs than gemcitabine, MAECs may also respond to S-1 chemotherapy, similar to ACCs. Another potential interpretation is that S-1 was effective when the condition was ACC, and eventually showed decreased effectiveness when the condition shifted to MAEC. Future studies are needed to conclude whether S-1 chemotherapy truly works against MAECs or induces endocrine differentiation in ACCs as a part of the drug-resistance process.
Keywords: acinar cell carcinoma, chemotherapy, mixed acinar-endocrine carcinoma of the pancreas, neuroendocrine carcinoma, S-1
1. Introduction
Acinar cell carcinomas (ACCs) of the pancreas are rare, and account for only 1% of pancreatic exocrine tumors.[1] ACCs may express endocrine markers such as chromogranin and synaptophysin in a limited number of cells. ACCs with the expression of endocrine markers in >30% of cancer cells are referred to as mixed acinar-endocrine carcinomas (MAECs).[2,3] Due to their rarity, a treatment protocol for unresectable MAECs has not yet been standardized.
We herein present a 65-year-old man with MAEC, who showed a partial response (PR) for 12 months with S-1 chemotherapy. These results suggest that S-1 chemotherapy may be effective for MAEC of the pancreas.
2. Case report
A 65-year-old man with no particular previous medical history presented with persistent epigastric pain for 1 month. He did not have a family history of pancreatic cancer. His physical examination was unremarkable. Laboratory tests revealed elevations in hepatobiliary enzymes and slight renal dysfunction: gamma-glutamyl transpeptidase (116.0 U/L; normal range: 12–70 U/L), alkaline phosphatase (762.0 U/L; normal range: 110–370 U/L), creatinine (107.8 μmol/L; normal range: 54–92 μmol/L), and the estimated glomerular filtration rate (47.1 mL/min/1.73 m2; normal range: 60 < mL/min/1.73 m2). He also had elevated levels of carbohydrate antigen 19-9 (56.6 U/mL; normal range: <37.0 U/mL), carcinoembryonic antigen (17.2 μg/L; normal range: <5.0 μg/L), and Span-1 (73.0 U/mL; normal range: <30.0 U/mL), while DUPAN-2 was within the normal range (44 U/mL; normal range: <150 U/mL). He underwent abdominal computer tomography (CT), which revealed an 8.0-cm mass in the tail of the pancreas and multiple liver metastases (Fig. 1A). On endoscopic ultrasound, the lesion appeared to be a hypovascular tumor surrounded by the pancreatic parenchyma with increased blood flow. Endoscopic ultrasound-guided fine needle aspiration biopsy was attempted, but was not diagnostic because of its small size.
Figure 1.
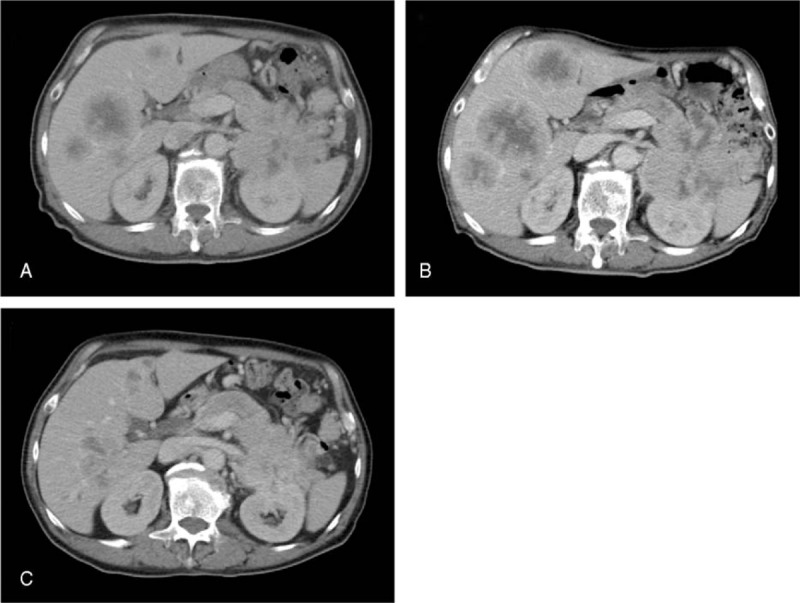
Findings of contrast-enhanced computed tomography (CT). (A) CT before the treatment showing an 8 × 8-cm mass in the pancreatic tail and liver metastasis. (B) On CT taken 6 months after the initiation of chemotherapy with gemcitabine, the primary pancreatic tumor and liver metastases both appeared to be progressive. (C) On CT taken 12 months after the initiation of chemotherapy with S-1, size reductions were observed in the primary tumor and liver metastases.
Based on imaging features and elevations in tumor markers, chemotherapy was initiated for suspected ductal carcinoma of the pancreas, with gemcitabine being administered at a dose of 700 mg/m2 on days 1, 8, and 15, of every 28-day cycle. After 7 courses, the primary pancreatic tumor and liver metastases both appeared to be progressive on CT (Fig. 1B). S-1 was administered orally at a daily dose of 80 mg/m2 from days 1 to 28 of each 42-day cycle. Due to the development of side effects such as nausea and a poor appetite, the protocol was changed to daily administration for 2 weeks with a 2-week interval as a cycle. The first follow-up CT after starting S-1 showed an approximately 38% reduction in the size of the primary tumor and liver metastases, according to the Response Evaluation Criteria In Solid Tumors (RECIST) protocol.[4] After PR had been sustained for 12 months after the initiation of S-1 chemotherapy (Fig. 1C), tumors started increasing in size.
Percutaneous liver biopsy was performed in order to evaluate chemotherapeutic effects on tissue. The liver biopsy specimen consisted predominantly of viable cancer cells arranged in an acinar or trabecular pattern. The cells had round, enlarged nuclei, and occasional mitotic figures. On immunostaining, cells were diffusely positive for BCL10 (a marker for acinar cells), chromogranin A, and synaptophysin, leading to the diagnosis of MAEC (Fig. 2).
Figure 2.
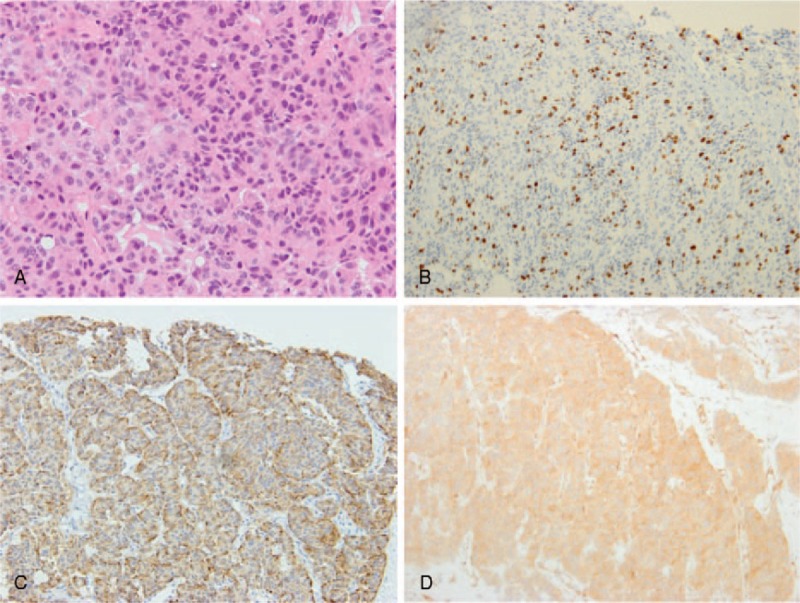
Histopathological findings of MAEC. (A) The tumor consisted of tumor cells arranged in an acinar or trabecular architecture (hematoxylin and eosin (H&E) staining; magnification, ×100). (B) The Ki-67 index was approximately 55%. (C and D) Tumor cells were diffusely immunoreactive to chromogranin (C) and BCL10 (D).
Chemotherapy with cisplatin (65 mg/m2 on day 1) and etoposide (80 mg/m2 on days 1–3) was administered as a third-line treatment. However, leukopenia (1300 leukocytes/mm3) with neutropenia (520 neutrophils/mm3) developed on day 16. Therefore, chemotherapy was discontinued and the treatment plan was switched to best supportive care.
3. Discussion
The pancreas consists of exocrine and endocrine components. Although acinar tissue accounts for >90% of the entire pancreas, ACCs account for only 1% of pancreatic tumors. Furthermore, MAECs are markedly rarer with only several dozen cases being described in the literature. According to a recent literature review by Liu et al,[5] MAECs are often diagnosed in middle-aged individuals with a male predominance, and may develop in any part of the pancreas, with ∼50% being located in the pancreatic head.
Histologically, MAECs are positive for both acinar (e.g., trypsin, chymotrypsin, lipase, and BCL10) and endocrine markers (e.g., chromogranin A and synaptophysin). MAECs may be divided into 2 groups based on histological appearance: One is a collision tumor, in which acinar and endocrine components are relatively separated, while the other is intermingled tumors consisting of cancer cells with both acinar and endocrine phenotypes.[6] The presented case corresponded to the latter group because tumor cells were diffusely positive for chromogranin A, synaptophysin, and BCL10.
A standardized management protocol for pancreatic MAECs has not yet been established. It is generally agreed that surgery is the sole curative therapy for resectable tumors.[1] In a literature review by Holen et al,[7] patients who underwent surgery (n = 16) as first-line therapy had longer survival (36 months) than those who had nonsurgical treatments (n = 21, 14 months). Tumor recurrence was observed in approximately 50% of R0, 75% of R1, and ∼100% of R2 patients, indicating that micrometastases were already present even in clinically resectable MAECs.
The rarity of MAECs has also restricted the discussion of appropriate chemotherapy for unresectable cases. Since MAECs have 2 cellular components, chemotherapy regimens that are effective for both elements are ideal. Previous case studies indicated that fluorouracil-based regimens are effective against ACCs.[7,8] Holen et al[7] also analyzed 22 different chemotherapy regimens used in 18 patients. None of the patients examined showed a complete response (CR), while PR was observed in 2 patients and stable disease (SD) in 7. Of the 2 patients with PR, 1 was administered a combination of irinotecan, fluorouracil, and leucovorin, while the other received an experimental regimen of cytarabine, cisplatin, and caffeine. The most common chemotherapy associated with SD was fluorouracil.[7] In another study by Seki et al,[8] 1 out of 3 patients receiving S-1 (an oral prodrug of fluorouracil) achieved PR, while 4 patients who received gemcitabine had limited effects.
Based on these findings, fluorouracil may be more likely to exert therapeutic effects in ACCs than gemcitabine. Abraham et al[9] reported that the loss of Smad4 protein expression or p53 accumulation was not detected in ACCs, highlighting a significant difference in genetic features between ACCs and pancreatic ductal adenocarcinomas. Since ∼25% of ACCs are known to have abnormalities in the APC/beta-catenin pathway that cause colorectal cancer, fluorouracil-based chemotherapies are expected to have an effect on ACCs.
In terms of chemotherapy for high-grade endocrine neoplasms of the pancreas, combination chemotherapy using cisplatin and etoposide is currently the first choice.[10–12] However, several case reports also showed that S-1 was effective for patients with high-grade endocrine neoplasms of the pancreas.[13–17]
A single case of MAEC treated with S-1 is available in the literature. Kanemasa et al[18] treated a 63-year-old man with advanced pancreatic MAEC and multiple liver metastases using S-1. Liver biopsy from a metastatic deposit, which was performed before treatment was initiated, showed the features of ACC with only a small number of chromogranin-positive cells. Chemotherapy with S-1 (80 mg/m2 per day) led to a 10-month PR with the primary tumor and liver metastases being markedly reduced in size. After tumor progression was observed, gemcitabine was administered, but was not effective. He eventually died of liver failure 18 months after the initiation of S-1 chemotherapy. At autopsy, tumor morphology was nearly identical to that observed in pretreatment liver biopsy; however, the immunophenotype changed with endocrine features being positive in >30% tumor cells expressing chromogranin. The diagnosis was then revised to MAEC. The authors suspected that S-1 was less effective for the endocrine element than for the ACC component.
In our case, a histological diagnosis was not established before the initiation of chemotherapy. Since gemcitabine was less effective, S-1 was administered and led to a 12-month PR. At that point, we obtained tissue confirmation of the diagnosis of MAEC, and suspected that tumor regrowth was induced by the endocrine component, which was resistant to S-1. Therefore, chemotherapy with cisplatin and etoposide was initiated, but shortly stopped because of the development of side effects.
Based on the clinical courses of our case and the patient reported by Kanemasa et al,[18] we initially thought that MAECs may respond to S-1 chemotherapy, similar to ACCs. Another possibility is that S-1 therapy induced endocrine differentiation in ACCs of the pancreas during the drug-resistance process. In the case reported by Kanemasa et al the number of cancer cells expressing endocrine markers dramatically increased after S-1 chemotherapy. Although pretreatment biopsy was not available in our patient, posttreatment biopsy showed both acinar and endocrine differentiation. Therefore, a potential interesting interpretation is that S-1 chemotherapy showed PR when the condition was ACC, and eventually showed decreased effectiveness when the condition shifted to MAEC. Further studies using matched pre- and posttreatment biopsies are needed to conclude which possibility is more likely.
In conclusion, this report indicates that some cases of MAECs show a good response to S-1 chemotherapy, similar to ACCs. However, future studies are necessary to conclude whether S-1 chemotherapy truly works against MAECs or induces endocrine differentiation in ACCs as a part of the drug-resistance process.
Footnotes
Abbreviations: ACC = acinar cell carcinoma, CR = complete response, CT = computer tomography, MAEC = mixed acinar-endocrine carcinoma, PR = partial response, SD = stable disease.
Informed consent statement: Verbal consent for publication was obtained from the patient.
The authors have no conflicts of interest to disclose.
References
- [1].Kyriazi MA, Arkadopoulos N, Stafyla VK, et al. Mixed acinar-endocrine carcinoma of the pancreas: a case report and review of the literature. Cases J 2009;2:6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].La Rosa S, Adsay V, Albarello L, et al. Clinicopathologic study of 62 acinar cell carcinomas of the pancreas: insights into the morphology and immunophenotype and search for prognostic markers. Am J Surg Pathol 2012;36:1782–95. [DOI] [PubMed] [Google Scholar]
- [3].Klimstra DS, Hruban RH, Klöppel G, et al. Acinar Cell Neoplasms of the Pancreas. In: Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumors of the Digestive System. 4th ed. Lyon: IARC, 2010: 314–318. [Google Scholar]
- [4].Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- [5].Liu Z, Dong C, Wang C, et al. Mixed acinar-endocrine carcinoma of pancreas: a case report and brief review of the literature. Onco Targets Ther 2015;8:1633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Imaoka H, Amano Y, Moriyama I, et al. Endoscopic ultrasound-guided fine-needle aspiration of a mixed acinar-endocrine carcinoma: a case report. Am J Gastroenterol 2008;103:2659–60. [DOI] [PubMed] [Google Scholar]
- [7].Holen KD, Klimstra DS, Hummer A, et al. Clinical characteristics and outcomes from an institutional series of acinar cell carcinoma of the pancreas and related tumors. J Clin Oncol 2002;20:4673–8. [DOI] [PubMed] [Google Scholar]
- [8].Seki Y, Okusaka T, Ikeda M, et al. Four cases of pancreatic acinar cell carcinoma treated with gemcitabine or S-1 as a single agent. Jpn J Clin Oncol 2009;39:751–5. [DOI] [PubMed] [Google Scholar]
- [9].Abraham SC, Wu TT, Hruban RH, et al. Genetic and immunohistochemical analysis of pancreatic acinar cell carcinoma: frequent allelic loss on chromosome 11p and alterations in the APC/beta-catenin pathway. Am J Pathol 2002;160:953–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Moertel CG, Kvols LK, O’Connell MJ, et al. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer 1991;68:227–32. [DOI] [PubMed] [Google Scholar]
- [11].Mitry E, Baudin E, Ducreux M, et al. Treatment of poorly differentiated neuroendocrine tumours with etoposide and cisplatin. Br J Cancer 1999;81:1351–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pavel M, Baudin E, Couvelard A, et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2012;95:157–76. [DOI] [PubMed] [Google Scholar]
- [13].Yamamoto M, Miyagawa K, Hiura M, et al. Poorly differentiated neuroendocrine carcinoma of the pancreas responsive to combination therapy with gemcitabine and S-1. Intern Med 2012;51:727–32. [DOI] [PubMed] [Google Scholar]
- [14].Masuda T, Kuwahara A. [A long-term survival case of unresectable malignant pancreatic endocrine tumor successfully treated with systemic chemotherapy]. Gan To Kagaku Ryoho 2008;35:833–5. [Japanese]. [PubMed] [Google Scholar]
- [15].Sato I, Ueda N, Kinoshita E, et al. [Curatively resected case of non-functioning pancreatic neuroendocrine carcinoma with multiple liver metastases after downstaging with S-1 monotherapy]. Gan To Kagaku Ryoho 2010;37:1341–4. [Japanese]. [PubMed] [Google Scholar]
- [16].Yoshida Y, Sugawara N, Minami T, et al. [A case of pancreatic neuroendocrine tumor with excessively-advanced liver metastasis treated with S-1/GEM combination chemotherapy plus the long-acting somatostatin analogue octreotide]. Nihon Shokakibyo Gakkai Zasshi 2013;110:660–8. [Japanese]. [PubMed] [Google Scholar]
- [17].Takeuchi H, Fujita H, Kondo A, et al. A case of recurrent islet cell carcinoma of the pancreas 17 years after the initial surgery. Nihon Rinsho Geka Gakkai Zasshi 2008;69:1514–8. [Japanese]. [Google Scholar]
- [18].Kanemasa Y, Kamisawa T, Tabata T, et al. Mixed acinar-endocrine carcinoma of the pancreas treated with S-1. Clin J Gastroenterol 2013;6:459–64. [DOI] [PubMed] [Google Scholar]


