Abstract
The aim of the study was to determine whether thyroid hormones level on admission in patients with ischemic stroke, treated with intravenous recombinant tissue type plasminogen activator (rtPA), was associated with symptomatic intracranial hemorrhage (sICH) and worse outcomes at 3 months.
Patients with acute ischemic stroke (AIS) receiving intravenous rtPA thrombolytic treatment on our stroke unit between January 2015 and June 2016 were included in this study. Serum-free triiodothyronine (fT3), free thyroxine (fT4), total triiodothyronine (tT3), total thyroxine (tT4), and thyroid-stimulating hormone (TSH) were detected on admission. The endpoints were sICH, and poor functional outcomes at 3 and 6 months.
In all, 159 patients (106 males; mean age 65.36 ± 10.02 years) were included. FT3 was independently associated with sICH (odds ratio [OR] 0.204, 95% confidence interval [CI] 0.065–0.642) and poor outcomes at 3 months (OR 0.396, 95% CI 0.180–1.764). The cut-off values of fT3 for sICH was 3.54 pg/mL (sensitivity 83%; specificity 83%; area under the curve 0.88). FT3 values ≤3.54 pg/mL increased risk for sICH by 3.16-fold (95% CI 0.75–1.0) compared with fT3 values >3.54 pg/mL.
Low fT3 levels at admission were independently associated with sICH and worse outcomes at 3 months in AIS patients receiving rtPA thrombolytic therapy.
Keywords: acute ischemic stroke, free triiodothyronine, recombinant tissue type plasminogen activator, symptomatic intracranial hemorrhage, thrombolysis
1. Introduction
Stroke is 1 of the main causes of death and the primary cause of adult disability all over the world. Intravenous (IV) recombinant tissue type plasminogen activator (rtPA) thrombolysis within 4.5 hours after the onset of symptoms is the most effective treatment for acute ischemic stroke (AIS), to increase survival and reduce morbidity.[1,2] The most fatal complication of IV rtPA thrombolysis for treatment of AIS is symptomatic intracranial hemorrhage (sICH), which leads to poor consequences. Although it does not hide overall benefit of thrombolysis, sICH influences the wide use of rtPA thrombolytic therapy. There have been many attempts to seek predictors and risk prediction model of sICH, but the predictive value is too modest to make a treatment decision.[3] Therefore, it is worthwhile to detect new ones to improve the stroke prognostic model.
Studies on cell culture and animal models have suggested mechanisms underlying the neuroprotection of triiodothyronine (T3) and other hypothalamic-pituitary-thyroid (HPT) axis hormones in ischemic stroke.[4] Thyroxine (T4) and T3 administration was associated with reduced infarct volume, relieved brain edema, increasing cerebral blood flow, and better neurologic function.[5,6] In human studies, T3 was significantly associated with infarct volume, severity of stroke, and short-term and long-term outcomes in patients with AIS not treated by rtPA.[7–11] Recently, a small sample study on patients with AIS, treated by IV rtPA, suggested low fT3 levels were significantly associated with sICH and poor neurologic function at discharge,[12] but long-term studies after thrombolysis treatment are lacking.
Here we studied the relationship between thyroid hormones and outcomes in patients with AIS, treated by IV rtPA, attempting to seek a new predictor for sICH and poor outcomes after thrombolysis therapy in patients with AIS.
2. Material and methods
2.1. Patient selection
Consecutive AIS patients who received IV rtPA thrombolytic treatment on Shanghai 10th People's Hospital Stroke Center between January 2015 and June 2016 were included in this study (N = 159). All patients underwent history-taking, complete neurological examination and brain computed tomography (CT) scan upon arriving at our stroke unit; based on these data, the neurologists working in the stroke unit, made the diagnosis of AIS. Patients were treated with rtPA according to the written institutional guidelines. Other standardized treatments were given to every patient. Another brain CT scan was performed 24 hours after treatment, or before clinical deterioration. Fasting venous blood was collected on the morning of the second day. Patients with thyroid disease or taking medication that affects TH levels, or with a discharge diagnose of nonstroke, or with liver and renal failure, or with infections, or with malignancies or short of thyroid hormone detection, were excluded.
Study design was approved by the institutional ethics committee. A signed informed consent was obtained from each patient or guardian, and potential complications of IV rtPA thrombolytic treatment were discussed
2.2. Data collection
Neurologic impairment was assessed by the National Institute of Health Stroke Scale (NIHSS) Score. The outcomes at 3 or 6 months were assessed by modified Rankin scale (mRS) scoring using telephone follow-up, with a response rate of 95.78% (N = 159).
The following data were collected: demographics (sex, age); past medical history (hypertension, diabetes mellitus, atrial fibrillation, chronic antiplatelet therapy, smoking status); clinical characteristics (baseline NIHSS score, body mass index, systolic and diastolic blood pressure, onset to needles time); laboratory examination (glutamic oxaloacetic transaminase, glutamic pyruvic transaminase, creatinine, total cholesterol, triacylglycerol, high-density lipoprotein, low-density lipoprotein, glycosylated hemoglobin, free thyroxine, total triiodothyronine); post-thrombolytic hemorrhagic transformation and sICH on the basis of the European Cooperative Acute Stroke Study[13,14]; and outcomes at 3 or 6 months (we defined mRS 0–2 as good outcome and mRS more than 2 as bad outcome).
2.3. Statistical analysis
Statistical analyses were conducted using the Statistical Package for the Social Sciences (SPSS 20.0) for Windows (SPSS Inc, Chicago, IL) and GraphPad Prism 5 software package (GraphPad software, San Diego, CA). The Kolmogorov-Smirnov test was used to evaluate whether the distribution of continuous variables was normal. Continuous data were expressed as mean ± standard deviation (SD) or median (interquartile range). Patients were divided by quartiles of fT3, fT4, and fT3/fT4, and 1-way analysis of variance was used to compare the 4 groups. Differences in continuous variables between 2 groups were determined by t test. Non-normally distributed data were compared using Mann-Whitney U test and Kruskal-Wallis test. Noncontinuous and categorical data were expressed as number (%) and compared with the chi-square test or Fisher exact test. To adjust for the traditional risk factors, multivariable logistic regression analysis was performed while evaluating the relationship between outcomes and thyroid hormone levels. The variables for which the unadjusted P was <.15 in a logistic regression analysis were identified as potential risk markers and were included in the multivariate model. Forward elimination multivariate logistic regression analyses were performed using likelihood ratio tests to eliminate variables. A receiver-operating characteristic curve was used to determine the sensitivity and specificity of fT3. Optimal cut-off value of fT3 in the receiver-operating characteristic curve was determined at the level with the highest Youden index (sensitivity + specificity − 1). A 2-tailed P value less than .05 was considered statistically significant.
3. Results
3.1. General data
In all, 159 patients (106 males; mean age 65.36 ± 10.02 years) with AIS receiving thrombolytic therapy were included in the analysis. Among these patients, 6 (3.77%) presented sICH, 70 (44.03%), and 118 (74.21%) presented a good outcome at 3 and 6 months, respectively.
3.2. Clinical characteristics by quartiles of fT3 level
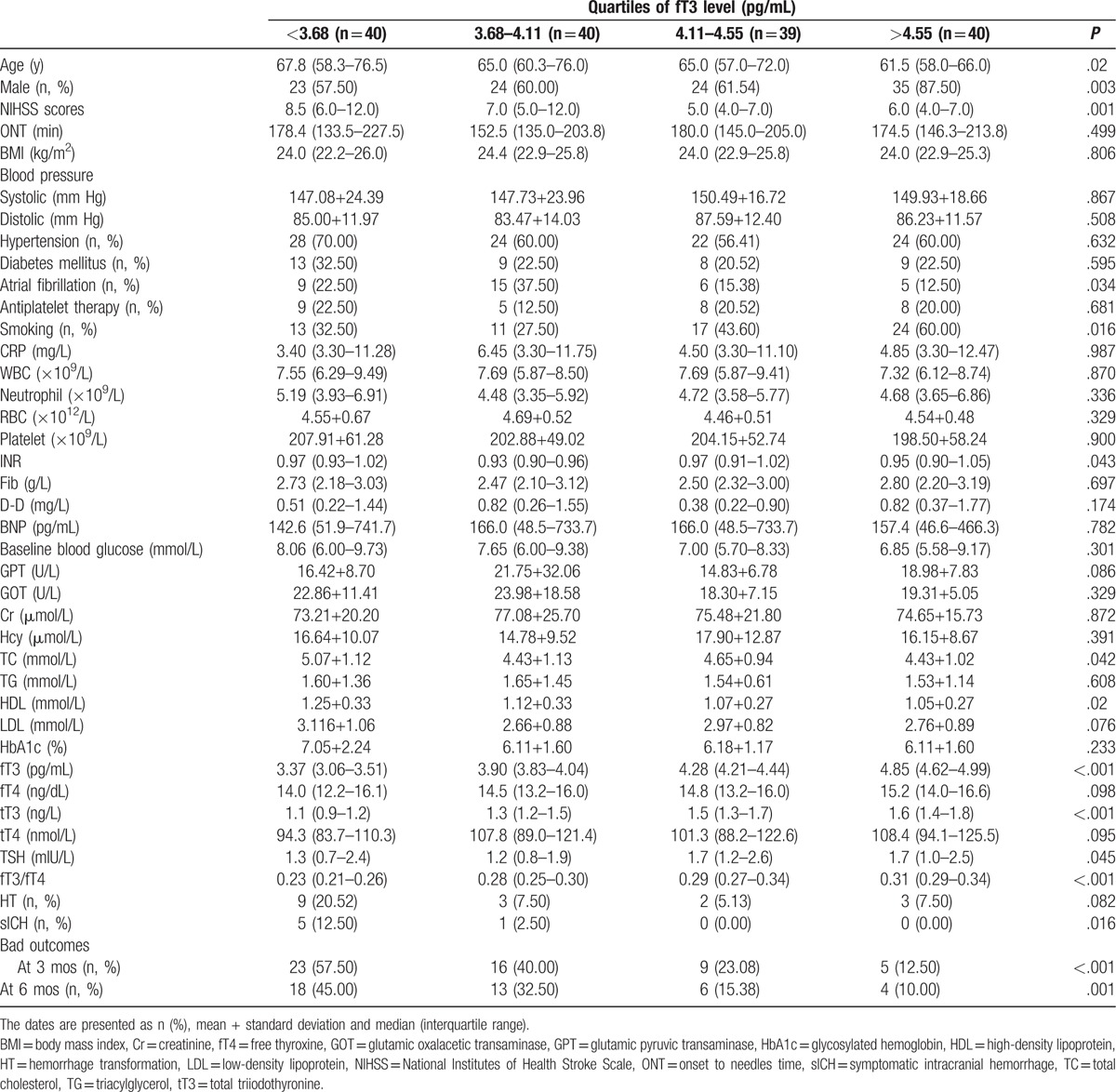
Table 1 shows baseline characteristics of patients divided by quartiles according to fT3. A significant decreasing trend could be detected with age across the listed fT3 quartiles. The quartile 4 group had the highest percentage of male and smoking. Patients in the former 2 groups, in which higher NIHSS scores were present, had higher percentages of atrial fibrillation than the latter 2 groups.
Table 1.
Baseline characteristics of study population by quartiles of fT3 level.
3.3. Unadjusted association of fT3, fT4, tT3, tT4, thyroid-stimulating hormone, and fT3/fT4 with outcomes
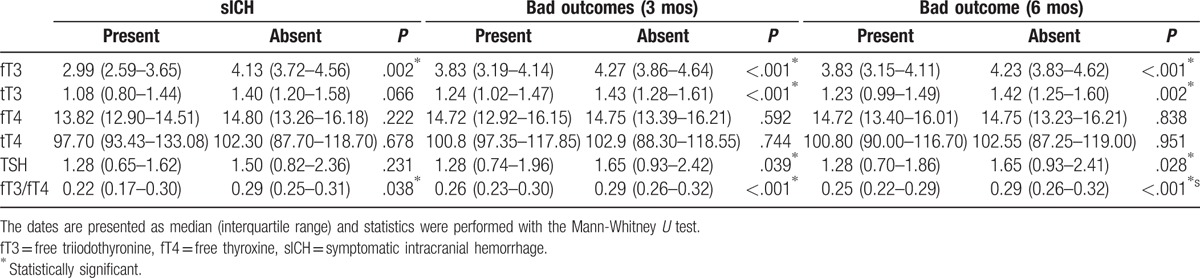
The unadjusted comparison of fT3, fT4, tT3, tT4, thyroid-stimulating hormone (TSH), and fT3/fT4 according to each outcome category are presented in Table 2. Patients who developed sICH presented lower fT3 (P = .002) and fT3/fT4 (P = .038). Patients with mRS 3 to 6 at 3 and 6 months, defined as bad outcomes, showed lower fT3 (P < .001) and fT3/fT4 (P < .001). However, no statistically significant differences were found between fT4 levels in each outcome category.
Table 2.
Unadjusted comparison of fT3 (pg/mL), fT4 (ng/dL), and fT3/fT4 in each outcome category.
When divided by quartiles of fT3, fT4, and fT3/fT4, the patients showed less percentages of sICH and better outcomes at 3 and 6 months in higher quartiles for fT3 and fT3/fT4. FT4 was not associated with either sICH or good outcomes (Fig. 1).
Figure 1.
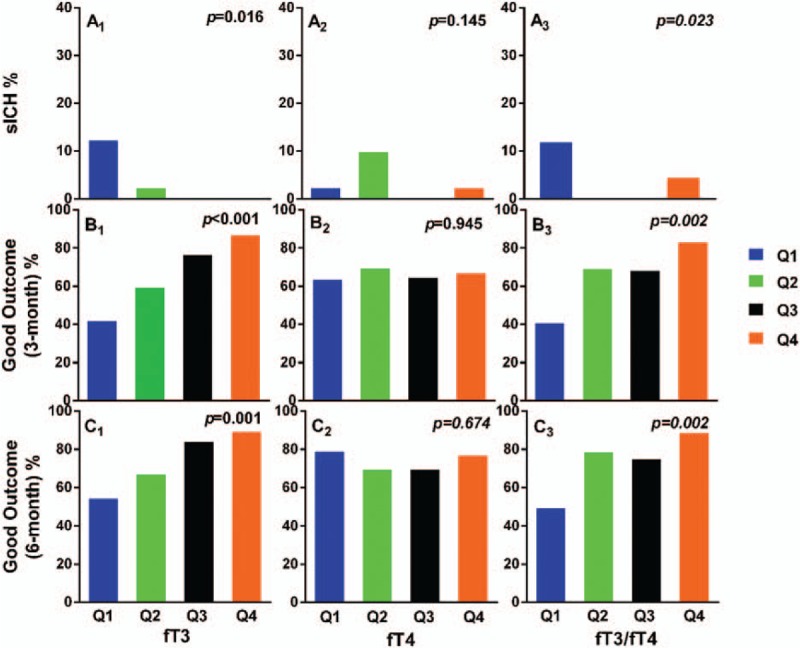
Comparison of outcomes between patients divided by quartiles of fT3, fT4, fT3/fT4. Comparison of outcomes between patients from the lowest (Q1) to the highest (Q4) quartiles for fT3, fT4, fT3/fT4. sICH = symptomatic intracranial hemorrhage.
3.4. Adjusted association of fT3 and outcomes
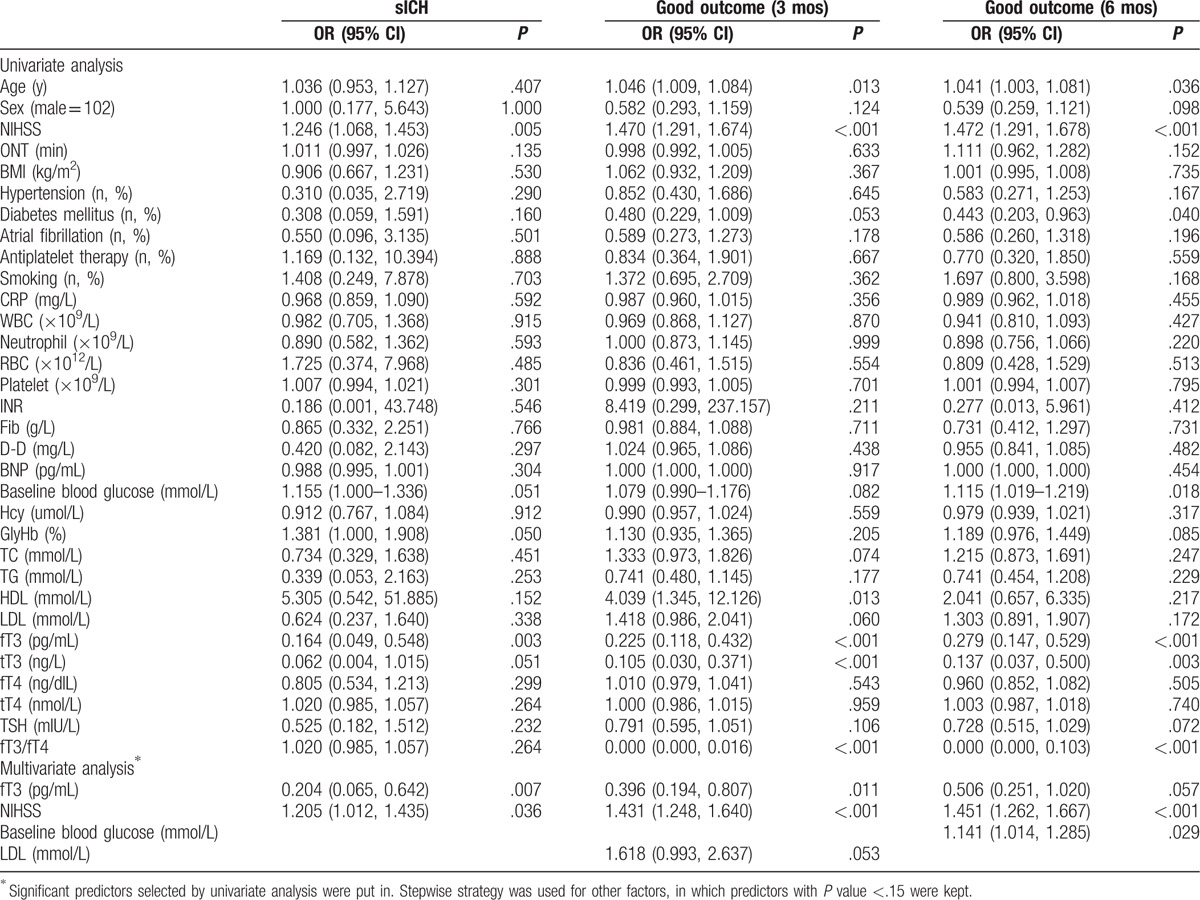
To adjust for the potential confounding effects of other risk factors, we used multivariable logistic regression to evaluate outcomes and fT3 (Table 3). For patients who developed sICH after rtPA thrombolytic treatment, NIHSS score was an independent risk factor for sICH (adjusted odds ratio [ORadj] 1.205, 95% confidence interval [CI] 1.012–1.435), whereas fT3 was a significant protective factor. ORadj for fT3 was 0.204 (95% CI 0.065–0.642). For patients with poor outcomes at 3 months, fT3 was also an independent protective factor (ORadj 0.396, 95% CI 0.194–0.087), whereas NIHSS score was a significant risk factor (ORadj 1.431, 95% CI 1.248–1.640). For patients with poor outcomes at 6 months, fT3 was not an independently protective or risk factor, whereas NIHSS score and baseline blood glucose level were 2 independent risk factors (ORadj 1.451, 95%CI 1.262–1.667; ORadj 1.414, 95% CI 1.014–1.285). FT4, tT3, tT4, TSH, and fT3/fT4 were not independently associated with any of the outcomes.
Table 3.
Predictors of outcomes by logistic regression.
3.5. Predictive values of fT3 for the 3 endpoints
Receiver-operating characteristic curves for fT3 with 3 endpoints are shown in Fig. 2. The best predictive values for fT3 were found with sICH. The cut-off value of fT3 for sICH was 3.54 pg/mL (sensitivity 83%; specificity 83%; area under the curve 0.88; likelihood ratio 4.97). Thirty-one patients (19.5%) had fT3 ≤3.54 pg/mL and 128 patients (80.5%) had fT3 ≥3.54 pg/mL.
Figure 2.
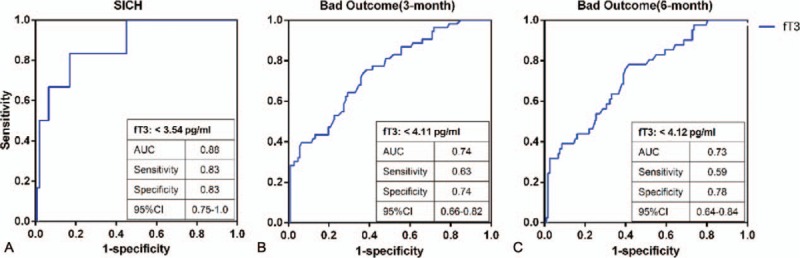
Receiver-operating characteristic analysis (ROC) of fT3 for the 3 endpoints. AUC = area under the curve.
3.6. Predictive values of fT3 (dichotomized) for sICH
FT3 was categorized into dichotomy by the cut-off value for sICH. The logistic regression analysis with sICH as dependent variable found an fT3 level of 3.54 pg/mL or lower (ORadj 3.163, 95% CI 2.282–245.147, P = .008) and NIHSS scores (ORadj 0.247, 95% CI 1.043–1.571, P = .018) were independent risk factors for sICH. In patients with fT3 ≤3.54 pg/mL, 5 patients (16.1%) developed sICH (P = .001), whereas in patients with fT3 ≥3.54 pg/mL, 1 patient (0.78%) developed sICH (P < .001).
4. Discussion
Our results have shown that fT3 was significantly associated with sICH, and also outcomes at 3 months in patients with AIS, treated by IV rtPA. We found low fT3 was an independent risk factor for sICH and poor functional outcomes after adjusting for potential confounding factors. An fT3 level of 3.54 pg/mL or lower increased risk for sICH after IV rtPA therapy for patients with AIS by 3.16-fold. In addition, the tT3, fT4, tT4, and TSH were not independently related with sICH or functional outcomes.
The predictive value of thyroid hormone has been shown in various diseases, especially cardiovascular and cerebrovascular diseases. In previous studies, low fT3 levels were reported to be associated with stroke severity, and bad short-term and long-term functional outcomes (ie, severe disability or death),[8,9,11] whereas another study provided support for tT3, not fT3, as an independent predictor for functional outcomes in patients with AIS.[15] In addition, other research showed that patients with higher TSH within the reference range may have a lower risk of stroke, decreased NIHSS score at admission, and a better neurologic outcome at discharge.[16,17] We discussed and analyzed fT3, tT3, fT4, tT4, and TSH at the same time, only finding low fT3 levels have significant predictive value for sICH and poor functional outcomes. A recent small sample study on patients with AIS, treated with IV rtPA, suggested low fT3 levels were significantly associated with sICH and poor neurologic function at discharge.[12] The results of our study confirm and extend the findings.
Studies of thyroid function with AIS reported thyroid function had a complex association with poor functional outcome, independent of AF, suggesting that thyroid hormones might be related to stroke outcome independent of stroke etiology.[9,18,19] Our finding expand on these reports, showed fT3 levels were related to AF in patients with AIS, treated by IV rtPA. However, the association with sICH or poor outcome remained significant after adjustment for AF.
Some underlying mechanisms may explain the results presented. It was well-documented that serious illness has something to do with HPT axis, thyrotropin-releasing hormone (TRH), and TSH secretion, and extrathyroidal T4 conversion to T3 decrease, which are possibly associated with heterogenic changes in brain thyroid hormone receptor (TR) expression.[20,21] These findings imply the possible reasons for the significant relationship between low fT3 levels and stroke severity. Moreover, animal studies show that T3 plays a role in reducing infarct volume and relieving the cerebral edema,[6,8] which is known as closely related to sICH, may be by inhibiting the expression of aquaporin-4 water channels.[22] On the contrary, the protective value of high fT3 levels for good functional outcomes may be due to putative neuroprotective effect. T3 significantly increased glutamate uptake by astrocytes, protecting nervous system from glutamate neurotoxicity.[23] Another study using PET imaging observed that temporary decrease of thyroid hormone may cause impaired glucose metabolism in brain.[24] Furthermore, low fT3 levels may induce the down-regulation of hepatic CYP2B1 in stroke model, which mediates metabolism of exogenous and endogenous substances.[25] All of these can affect recovery of neurological function after stroke.
What is worth mentioning is that the results do not display the predict value of fT3 for sICH in patients with AIS, treated by IV rtPA, is better than NIHSS score at discharge. We also cannot be sure whether fT3 as a new maker added to the existing risk prediction models may improve prediction value. Therefore, further study is needed on thyroid hormone level which is used as a routine evaluation at discharge and used to predict sICH or poor functional outcomes in patients with AIS, treated with IV rtPA.
In addition, according to most investigators, thyroid function is age-dependent and median serum TSH progressively increases with age.[26–28] Therefore, the results cannot be extrapolated to the patients in pediatric age, in which diagnostic delay is especially high, especially as compared with adult patients.[29]
The strengths of our study are that this is a prospective study on patients with AIS, treated with IV rtPA. Thyroid hormones are significantly associated with sICH and short-term outcomes at the same time. Some limitations of our study should be considered. Firstly, this is a single-center study with small sample size, and the outcome had to be assessed by telephone follow-up. Secondly, we only measure thyroid hormone once without dynamic observation. Thirdly, we did not get the data of thyroid antibodies, which might themselves affect cerebrovascular risk,[18] and higher thyroid antibodies are associated with worse outcomes in patients with AIS according to 1 study, implying immune-mediated vascular damage may raise the risk of bad outcomes.[30] Fourthly, we did not assess the size of stroke, although it can be related to NIHSS scores. Finally, we cannot identify cause-and-effect relations between fT3 and sICH, or outcomes, and our findings require confirmation in additional cohorts.
5. Conclusions
We conclude that lower fT3 levels on admission are independently associated with sICH and poor outcomes at 3 months in patients with AIS treated with rtPA. The underlying mechanisms and prognostic predictor values of fT3 need further studies.
Footnotes
Abbreviations: AIS = acute ischemic stroke, CI = confidence interval, CT = computed tomography, IV = intravenous, mRS = modified Rankin scale, NIHSS = National Institute of Health Stroke Scale, rtPA = recombinant tissue type plasminogen activator, sICH = symptomatic intracranial hemorrhage, T3 = triiodothyronine, T4 = thyroxine, TR = thyroid hormone receptors, TRH = thyrotropin-releasing hormone, TSH = thyroid-stimulating hormone.
The authors report no conflicts of interest.
References
- [1].Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2 suppl):e601S–36S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010;375:1695–703. [DOI] [PubMed] [Google Scholar]
- [3].Karaszewski B, Houlden H, Smith EE, et al. What causes intracerebral bleeding after thrombolysis for acute ischaemic stroke? Recent insights into mechanisms and potential biomarkers. J Neurol Neurosurg Psychiatry 2015;86:1127–36. [DOI] [PubMed] [Google Scholar]
- [4].Bunevicius A, Iervasi G, Bunevicius R. Neuroprotective actions of thyroid hormones and low-T3 syndrome as a biomarker in acute cerebrovascular disorders. Expert Rev Neurother 2015;15:315–26. [DOI] [PubMed] [Google Scholar]
- [5].Genovese T, Impellizzeri D, Ahmad A, et al. Post-ischaemic thyroid hormone treatment in a rat model of acute stroke. Brain Res 2013;1513:92–102. [DOI] [PubMed] [Google Scholar]
- [6].Mdzinarishvili A, Sutariya V, Talasila PK, et al. Engineering triiodothyronine (T3) nanoparticle for use in ischemic brain stroke. Drug Deliv Transl Res 2013;3:309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Alevizaki M, Synetou M, Xynos K, et al. Low triiodothyronine: a strong predictor of outcome in acute stroke patients. Eur J Clin Invest 2007;37:651–7. [DOI] [PubMed] [Google Scholar]
- [8].Ambrosius W, Kazmierski R, Gupta V, et al. Low free triiodothyronine levels are related to poor prognosis in acute ischemic stroke. Exp Clin Endocrinol Diabetes 2011;119:139–43. [DOI] [PubMed] [Google Scholar]
- [9].Forti P, Maioli F, Coveri M, et al. Thyroid function tests and early outcomes of acute ischemic stroke in older euthyroid patients. Exp Gerontol 2015;61:8–14. [DOI] [PubMed] [Google Scholar]
- [10].Ma L, Zhu D, Jiang Y, et al. Low triiodothyronine: a new facet of inflammation in acute ischemic stroke. Clin Chim Acta 2016;458:63–7. [DOI] [PubMed] [Google Scholar]
- [11].Zhang Y, Meyer MA. Clinical analysis on alteration of thyroid hormones in the serum of patients with acute ischemic stroke. Stroke Res Treat 2010;2010: doi: 10.4061/2010/290678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liu J, Wang D, Xiong Y, et al. Low free triiodothyronine levels are related to symptomatic intracranial hemorrhage and poor functional outcomes after intravenous thrombolysis in acute ischemic stroke patients. Neurol Res 2016;38:429–33. [DOI] [PubMed] [Google Scholar]
- [13].Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet 1998;352:1245–51. [DOI] [PubMed] [Google Scholar]
- [14].Strbian D, Sairanen T, Meretoja A, et al. Patient outcomes from symptomatic intracerebral hemorrhage after stroke thrombolysis. Neurology 2011;77:341–8. [DOI] [PubMed] [Google Scholar]
- [15].Xu XY, Li WY, Hu XY. Alteration of thyroid-related hormones within normal ranges and early functional outcomes in patients with acute ischemic stroke. Int J Endocrinol 2016;2016:3470490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chaker L, Baumgartner C, den Elzen WP, et al. Thyroid function within the reference range and the risk of stroke: an individual participant data analysis. J Clin Endocrinol Metab 2016;101:4270–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Delpont B, Aboa-Eboule C, Durier J, et al. Associations between thyroid stimulating hormone levels and both severity and early outcome of patients with ischemic stroke. Eur Neurol 2016;76:125–31. [DOI] [PubMed] [Google Scholar]
- [18].Karch A, Thomas SL. Autoimmune thyroiditis as a risk factor for stroke: a historical cohort study. Neurology 2014;82:1643–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wollenweber FA, Zietemann V, Gschwendtner A, et al. Subclinical hyperthyroidism is a risk factor for poor functional outcome after ischemic stroke. Stroke 2013;44:1446–8. [DOI] [PubMed] [Google Scholar]
- [20].Lourbopoulos A, Mourouzis I, Karapanayiotides T, et al. Changes in thyroid hormone receptors after permanent cerebral ischemia in male rats. J Mol Neurosci 2014;54:78–91. [DOI] [PubMed] [Google Scholar]
- [21].Warner MH, Beckett GJ. Mechanisms behind the non-thyroidal illness syndrome: an update. J Endocrinol 2010;205:1–3. [DOI] [PubMed] [Google Scholar]
- [22].Sadana P, Coughlin L, Burke J, et al. Anti-edema action of thyroid hormone in MCAO model of ischemic brain stroke: possible association with AQP4 modulation. J Neurol Sci 2015;354:37–45. [DOI] [PubMed] [Google Scholar]
- [23].Losi G, Garzon G, Puia G. Nongenomic regulation of glutamatergic neurotransmission in hippocampus by thyroid hormones. Neuroscience 2008;151:155–63. [DOI] [PubMed] [Google Scholar]
- [24].Jeong HS, Choi EK, Song IU, et al. Differences in brain glucose metabolism during preparation for 131I ablation in thyroid cancer patients: thyroid hormone withdrawal versus recombinant human thyrotropin. Thyroid 2017;27:23–8. [DOI] [PubMed] [Google Scholar]
- [25].Bing Y, Zhu S, Jiang K, et al. Reduction of thyroid hormones triggers down-regulation of hepatic CYP2B through nuclear receptors CAR and TR in a rat model of acute stroke. Biochem Pharmacol 2014;87:636–49. [DOI] [PubMed] [Google Scholar]
- [26].Bremner AP, Feddema P, Leedman PJ, et al. Age-related changes in thyroid function: a longitudinal study of a community-based cohort. J Clin Endocrinol Metab 2012;97:1554–62. [DOI] [PubMed] [Google Scholar]
- [27].Hadlow NC, Rothacker KM, Wardrop R, et al. The relationship between TSH and free T(4) in a large population is complex and nonlinear and differs by age and sex. J Clin Endocrinol Metab 2013;98:2936–43. [DOI] [PubMed] [Google Scholar]
- [28].Waring AC, Arnold AM, Newman AB, et al. Longitudinal changes in thyroid function in the oldest old and survival: the cardiovascular health study all-stars study. J Clin Endocrinol Metab 2012;97:3944–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Suppiej A, Gentilomo C, Saracco P, et al. Paediatric arterial ischaemic stroke and cerebral sinovenous thrombosis. First report from the Italian Registry of Pediatric Thrombosis (R. I. T. I., Registro Italiano Trombosi Infantili). Thromb Haemost 2015;113:1270–7. [DOI] [PubMed] [Google Scholar]
- [30].Cho HJ, Kim SS, Sung SM, et al. Impact of thyroid autoantibodies on functional outcome in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis 2014;23:1915–20. [DOI] [PubMed] [Google Scholar]


