Abstract
To evaluate indications and outcomes of pediatric keratoplasty in a tertiary eye center, and identify factors that affect visual outcomes.
We performed a retrospective review of penetrating keratoplasty in children aged 0 to 18 years between 1995 and 2011 in the Asociación para Evitar la Ceguera en México IAP, Hospital “Dr. Luis Sánchez Bulnes”.
A total of 574 penetrating keratoplasties were performed during the study interval. Median follow-up was 5.0 years. Main indications included keratoconus (55.58%), postherpetic scarring (9.58%), traumatic opacities (7.49%), and bullous keratopathy (6.09%). Rejection rates at 5 years were 27% overall, and among indications, keratoconus showed the best graft survival at 60-months follow-up (85%). The percentage of patients with best corrected visual acuity (BCVA) posttransplant >20/400 at 5 years in the nonrejection group was 81.25% and 82.74% in < and > 10 years of age (YOA) groups, respectively, versus a BCVA posttransplant > 20/400 at 5 years in the rejection group of 53.68% and 51.72% in < and > 10 YOA groups, respectively. There was a statistically significant reduced rejection rate between genders at 18 months of follow-up, favoring males.
Despite being considered a high-risk procedure in children, penetrating keratoplasty can achieve good results, especially in patients with keratoconus. It can achieve significative improvements of visual acuity, provided there is an adequate follow-up and treatment adherence.
Keywords: cornea, corneal transplant, keratoplasty in children, pediatric corneal transplant, pediatric keratoplasty
1. Introduction
Among the most frequent ocular pathologies, there are corneal disorders secondary to trauma, chemical burns and infectious diseases, as well as congenital entities. Most of these disorders require surgical treatment, mainly with penetrating keratoplasty. These corneal alterations are prevalent in the pediatric population, which is a challenging one due to tissue characteristics—reduced ocular[1] and scleral rigidity that increase the likelihood of refractive errors after corneal transplantation,[2] severe inflammation after surgery, among other factors like rehabilitation issues due to poor patient cooperation.
Besides, there is a well-known increased incidence of graft failure in pediatric patients, reported more than 30 years ago,[3] and some studies suggest it is secondary to involvement of innate immunity,[4] the primary defense system in children. Since then, there has been a widespread notion that outcomes of pediatric keratoplasty are not as good as in adults,[5] and although success rates have improved over the years, there is controversy about the indications for keratoplasty and postsurgical treatment in this group of patients. Although some authors recognize that anatomic success of pediatric keratoplasty is increasing,[6] there are still several factors to take into consideration.
To date, several efforts have been made to improve visual and functional outcomes in children, and although keratoplasty remains the surgery of choice for the management of pediatric corneal disease incurable by medical treatment, some authors have proposed other approaches. Among these, contact lens wear in selected corneal opacities,[7] use of keratoprosthesis,[8] as well as different surgical techniques like rotational autokeratoplasty[9,10] and deep anterior lamellar keratoplasty (DALK)[11–13] have been proposed; the former restricted only for specific cases, and the latter with unclear long-term results and not suitable for all patients.[14] Regarding keratoplasty, most authors agree that smaller grafts and interrupted single sutures may improve postoperative outcome in children.[15,16] Nevertheless, these efforts have not been limited to the selection and performance of the most adequate technique. Different options have been proposed to improve graft survival, as human leukocyte antigens (HLA) HLA-A, -B, and -DR antigen match. But results have been conflicting: some authors report there is no effect of HLA antigen matching on overall graft survival, and other authors also report that ABO blood group matching may be effective to reduce the risk of graft failure.[17,18] On the other hand, other authors state that HLA-A and HLA-B antigen match improve graft survival and reduce the risk of rejection.[19–21] Besides, to achieve better results, postoperative pharmacologic treatment as topical cyclosporine[22] and antiangiogenic therapy[23] have been advocated as useful adjunctive treatments in addition to topical corticosteroids.
Nevertheless, there are marked differences in outcomes depending on the preoperative diagnosis.[24] For example, Peters anomaly presents a high risk of graft failure: 39% at 1 year,[25] 38% at 3 years,[26] and 70% at 5 years,[27] with high consistency in graft failure rates between publications. Children with glaucoma[28] and those subjected to regrafting show poorer prognosis.[29] On the other hand, although there are country differences between the main indications for pediatric keratoplasty, like infectious keratitis in India[30,31] or mechanical trauma and infectious keratitis in North China,[32] one of the most frequent indications for keratoplasty in children worldwide is keratoconus, which shows the best outcomes within subgroups.[33] Even in children with Down syndrome and keratoconus, where we would expect less patient cooperation, 5-year graft survival rates are good.[34] Finally, congenital hereditary endothelial dystrophy (CHED), another relatively frequent indication for early keratoplasty, has been reported as an entity with a moderate postoperative visual success.[35,36]
Due to the complexity of this subject from preoperative decision making to postsurgical management, we aimed to contribute with our 17-year experience in pediatric keratoplasty in a tertiary eye center; exhaustive reviews about keratoplasty in children can be found elsewhere,[37–39] with comprehensive data about the main studies published regarding this subject.[40]
2. Materials and methods
2.1. Study design
This is a retrospective and analytic study approved by the Internal Review Board of the Asociación para Evitar la Ceguera en México I.A.P., “Hospital Dr. Luis Sánchez Bulnes”, in Mexico City, Mexico. All the procedures conformed to the tenets of the Declaration of Helsinki. All participants (or their legally acceptable representatives) signed a written informed consent before the surgical procedure.
2.2. Population and measurements
We performed a retrospective review from 1995 to 2011 of the medical records of all cases of keratoplasty in patients aged ≤ 18 years. We extracted the following data for our database: patient demographics, initial BCVA, yearly BCVA during the 5-year postprocedural period, examination findings, ocular diagnoses, performed surgeries, graft clarity during 5-year follow-up, time to graft failure. Medical records with incomplete data were excluded. Main outcome measurements were BCVA at 5 years, and presence/ absence of rejection as defined in previous publications.[41]
2.3. Statistical analysis
A paired Student t test was used to assess statistical significance between groups in normally distributed data, whereas the Wilcoxon matched pairs signed-rank test was employed for non-normal distributed data. In addition, the Kaplan–Meier method with the Log-rank (Mantel–Cox test) was used to assess survival rates among keratoplasty groups. Pearson correlation coefficient and linear regression analyses were used for rejection graft data as well as age. Normal and non-normal distributions were determined by Shapiro–Wilk tests for all variables. BCVA analyses used logMAR notation, but BCVA is presented as Snellen values within the text and in tables. Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) software (version 20, SPSS Inc, Chicago, IL). Graphs and layouts depicted in all figures included were elaborated using the 2015 GraphPad software Inc. Prism version 6.0.
3. Results
Five hundred seventy-four penetrating keratoplasties were performed during the study interval with complete medical records. Mean age was 11.91 ± 4.35 years. Mean follow-up was 5.0 years. Main indications (by frequency) included keratoconus in 319 eyes (55.58%), postherpetic scarring in 55 eyes (9.58%), traumatic opacities in 43 eyes (7.49%), and bullous keratopathy in 35 eyes (6.09%) (Table 1).
Table 1.
Indications by frequency.
Our results indicated that there was no difference in gender distribution (P = .119, Fig. 1). Rejection rate at 60 months was 27% overall, and among indications, keratoconus showed the best graft survival at 60-months follow-up (85%), with a statistically significant difference compared with the rest of indications (P = .0001, Fig. 2). Interestingly, we found a statistically significant difference between genders in keratoconus patients regarding graft survival in the first 3 years, favoring males (Fig. 3).
Figure 1.
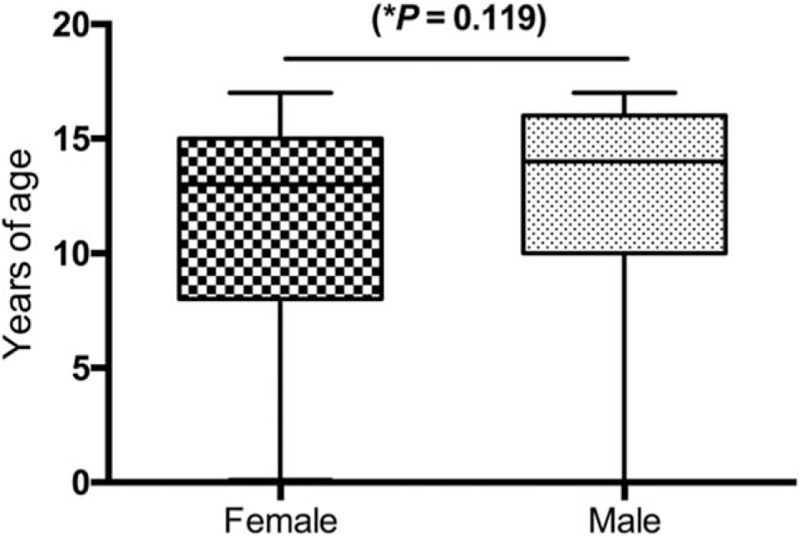
Gender comparison. ∗Unpaired t test (P = .119).
Figure 2.
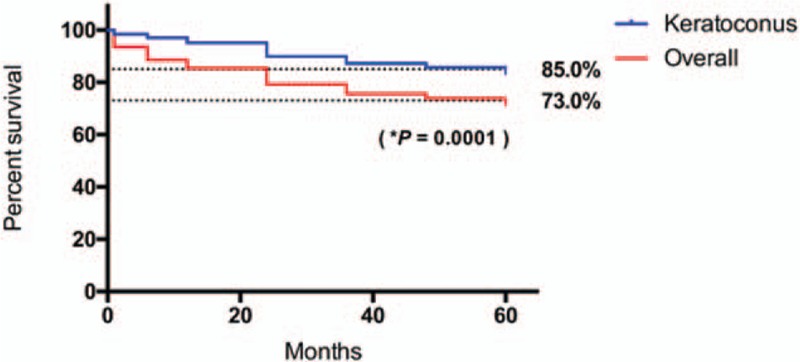
Graft survival in keratoconus patients compared with the overall keratoplasty population. ∗Log-rank (Mantel–Cox) test.
Figure 3.
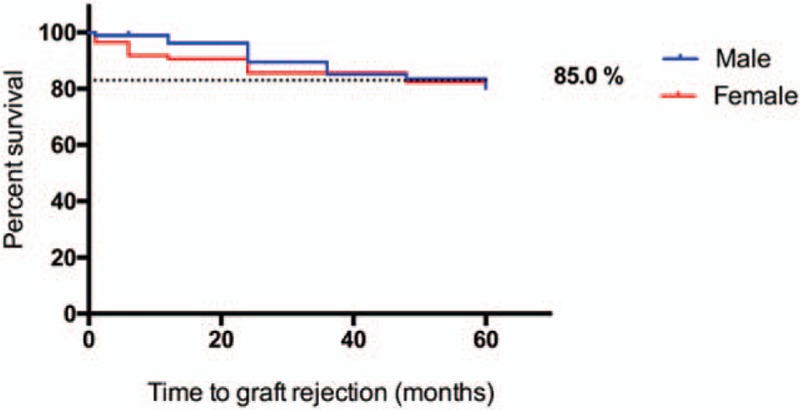
Survival proportions: graft survival in keratoconus: male/female. ∗Log-rank (Mantel–Cox) test.
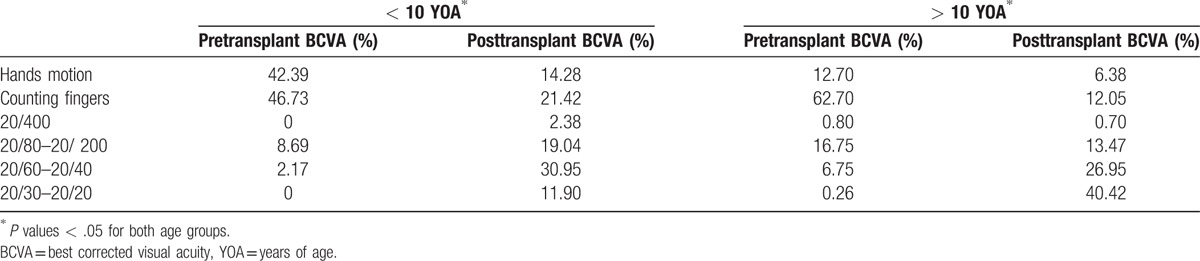
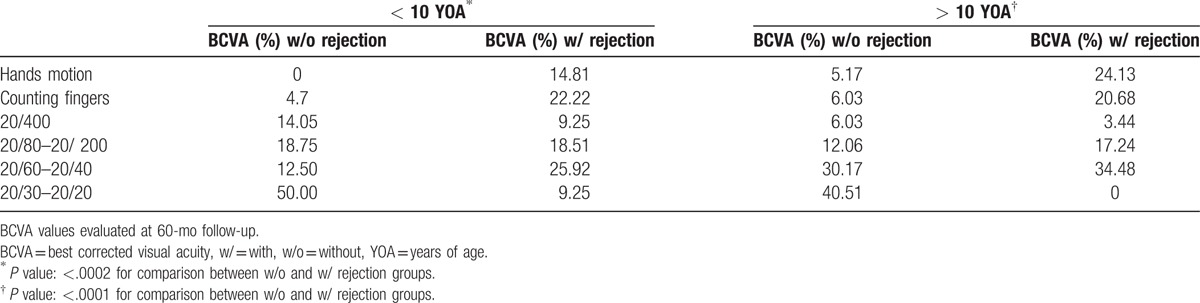
Overall, the percentage of patients with best corrected visual acuity (BCVA) posttransplant >20/400 at 5 years in the nonrejection group was 81.25% and 82.74% in < and > 10 YOA groups, respectively, versus a BCVA posttransplant > 20/400 at 5 years in the rejection group of 53.68% and 51.72% in < and > 10 YOA groups, respectively (Table 2).
Also, the mean time of graft-rejection after surgery was 20.75 ± 18.21 months. In addition, we found a statistically significant difference when comparing pre-surgical BCVA values to those obtained at 60-months follow-up for both groups of age (Table 3).
Table 3.
BCVA pre- and posttransplant per group of age.
4. Discussion
Penetrating keratoplasty in children and adolescents is a challenging surgery, not only regarding the surgical procedure per se, but also during follow-up and rehabilitation. In addition, it poses a higher risk of graft rejection compared with adults, due to a stronger immunological response in young patients. The graft survival rate reported 1 and 2 years after surgery is around 80% and 67%, respectively,[42] but reports on 5-year graft survival range from 50% to 91%, depending on the series.[43–45] In our study, 2-year graft survival was 79.09%, and 5-year graft survival was 73% in the whole population of the study, which is consistent with the graft survival rates reported in other tertiary eye care centers.[30] Similarly, our graft survival rate for keratoconus was higher compared with the rest of indications (P = .0001, Fig. 2). Of note, graft survival rates for keratoconus patients varied between genders in the first 2 years: females presented a higher graft rejection with a peak at 18 months after the procedure; nevertheless, at 24 months this difference was no longer observed.
To the best of our knowledge, it has not been previously reported a gender difference regarding graft survival in children; but there are some studies showing significant differences in adults. The Australian Corneal Graft Registry and The Canadian Corneal Graft Outcome study reported statistically significant gender differences in adults, showing that females were more likely to have a rejection event compared with males. However, the causes of these differences were not discussed.[46–49] Another proposed explanation is the augmented activity of the immune system in females that increases the incidence of autoimmune conditions.[50]
One possible mechanism to explain that young females present higher numbers of rejection events, may be the mismatching between the gender of the donor and the recipient. A study published in the American Journal of Transplantation indicated poorer outcomes in women who received corneas from males.[51] This study explains that this effect, only observed in females, is a consequence of H-Y antigen incompatibility related to the Y chromosome; the lack of Y chromosome allows compatibility from female donors to male recipients.[52] In addition, steroid hormones are involved in female graft rejection susceptibility.[53] Specifically, estrogens may be immunostimulatory by regulating lymphocyte development and function,[54] whereas some androgens are capable of inducing an immunosuppressive response by reducing lymphocyte proliferation and differentiation;[55] the latter could be the reason for the apparent protection observed in males in this study.
Nevertheless, since the effects of androgens vary considerably due to the level of exposure and diverse factors may contribute to the immune profile in females, the potential role of hormones in gender-specific immune function related to graft survival remains to be elucidated.
Regarding indications for keratoplasty, similar to other series,[56] keratoconus was the leading indication for transplant in over half of the patients (55.58%). Surprisingly, we only found 33 cases (5.74%) of infectious keratitis in children who underwent corneal transplant, which is a low number compared with other authors who report infectious keratitis as the primary indication for pediatric keratoplasty in developing countries.[30,31] This apparent low incidence of keratoplasty is due to our preferred surgical choice in these cases: conjunctival flap. The election of conjunctival flap over keratoplasty in children is due to the following reasons: higher risk of rejection, higher risk of an early second procedure that increases the risk of complications, and because children sometimes are noncompliant with treatment. In addition, conjunctival flap does not increase the risk of rejection in the long term.
Consistent with the literature, our patients with less frequent pathologies like Peters anomaly, sclerocornea, and Axenfeld–Rieger syndrome, which usually present several ocular comorbidities and undergo combined procedures, had the worst outcomes with high rejection rates. For example, despite other authors reporting good outcomes for CHED, we had 6 cases with very bad outcomes: all of them were in the graft rejection group.[57] This was also the case for congenital glaucoma, with 13 cases in our series, all of them with a poor outcome, as reported by other authors.[58] Nevertheless, other authors report similar graft survival rates irrespective of etiology.[59–61]
Age is another important factor related to the success of penetrating keratoplasty. It has been established that older children have better prognosis,[62,63] and this is consistent with our findings. As depicted in Table 2, corneal transplantation improved overall posttransplant BCVA regardless of age (P = .002 and P = .0001, respectively), but the > 10 YOA group showed better BCVA postsurgery. A significant correlation was observed between graft-rejection and age in months (r = 0.153; R2 = 0.023; P = .048), with a mean age at rejection of 10.84 + 4.80 years.
Table 2.
BCVA with and without graft rejection per group of age.
Finally, we can conclude that overall, penetrating keratoplasty is a procedure that can achieve good results in children with keratoconus,[64] provided there is an adequate follow-up and treatment, as in the case of the patients included in this study.
Footnotes
Abbreviations: BCVA = best corrected visual acuity, CHED = congenital hereditary endothelial dystrophy, DALK = deep anterior lamellar keratoplasty, HLA = human leukocyte antigens, YOA = years of age.
The authors have no conflicts of interest to disclose.
References
- [1].Reidy JJ. Penetrating keratoplasty in infancy and early childhood. Curr Opin Ophthalmol 2001;12:258–61. [DOI] [PubMed] [Google Scholar]
- [2].Pan ZQ. Pay attention to specialization of pediatric penetrating keratoplasty. Zhonghua Yan Ke Za Zhi 2008;44:101–3. [PubMed] [Google Scholar]
- [3].Beauchamp GR. Pediatric keratoplasty: problems in management. J Pediatr Ophthalmol Strabismus 1979;16:388–94. [DOI] [PubMed] [Google Scholar]
- [4].Schwartzkopff J, Berger M, Birnbaum F, et al. Accelerated corneal graft rejection in baby rats. Br J Ophthalmol 2010;94:1062–6. [DOI] [PubMed] [Google Scholar]
- [5].Bradley BA, Vail A, Gore SM, et al. Penetrating keratoplasty in the United Kingdom: an interim analysis of the corneal transplant follow-up study. Clin Transpl 1993;293–315. [PubMed] [Google Scholar]
- [6].Aasuri MK, Garg P, Gokhle N, et al. Penetrating keratoplasty in children. Cornea 2000;19:140–4. [DOI] [PubMed] [Google Scholar]
- [7].Singh K, Jain D, Teli K. Rehabilitation of vision disabling corneal opacities: is there hope without corneal transplant? Cont Lens Anterior Eye 2013;36:74–9. [DOI] [PubMed] [Google Scholar]
- [8].Aquavella JV, Gearinger MD, Akpek EK, et al. Pediatric keratoprosthesis. Ophthalmology 2007;114:989–94. [DOI] [PubMed] [Google Scholar]
- [9].Ramappa M, Pehere NK, Murthy S, et al. Rotational autokeratoplasty in pediatric patients for nonprogressive paracentral corneal scars. Ophthalmology 2012;119:2458–62. [DOI] [PubMed] [Google Scholar]
- [10].Meiser S, Sundmacher R, Greber H. [Keratoplasty in infancy and early childhood with special reference to the auto-rotation technique]. Fortschr Ophthalmol 1991;88:363–7. [PubMed] [Google Scholar]
- [11].Ashar JN, Pahuja S, Ramappa M, et al. Deep anterior lamellar keratoplasty in children. Am J Ophthalmol 2013;155: 570–574.e1. [DOI] [PubMed] [Google Scholar]
- [12].Colby K. Changing times for pediatric keratoplasty. J AAPOS 2008;12:223–4. [DOI] [PubMed] [Google Scholar]
- [13].Harding SA, Nischal KK, Upponi-Patil A, et al. Indications and outcomes of deep anterior lamellar keratoplasty in children. Ophthalmology 2010;117:2191–5. [DOI] [PubMed] [Google Scholar]
- [14].Medsinge A, Nischal KK. Paediatric keratoplasty: choices and conundrums. Br J Ophthalmol 2013;97:1225–7. [DOI] [PubMed] [Google Scholar]
- [15].Seitz B, Hager T, Szentmáry N, et al. [Keratoplasty in children—still a dilemma]. Klin Monbl Augenheilkd 2013;230:587–94. [DOI] [PubMed] [Google Scholar]
- [16].Schonherr U, Naumann GO. Pediatric keratoplasty. Ophthalmology 1996;103:699–700. [DOI] [PubMed] [Google Scholar]
- [17].Fink N, Stark WJ, Maguire MG. Effectiveness of histocompatibility matching in high-risk corneal transplantation: a summary of results from the Collaborative Corneal Transplantation Studies. Cesk Oftalmol 1994;50:3–12. [PubMed] [Google Scholar]
- [18].The collaborative corneal transplantation studies (CCTS). Effectiveness of histocompatibility matching in high-risk corneal transplantation. The Collaborative Corneal Transplantation Studies Research Group. Arch Ophthalmol 1992;110:1392–403. [PubMed] [Google Scholar]
- [19].Bartels MC, Doxiadis II, Colen TP, et al. Long-term outcome in high-risk corneal transplantation and the influence of HLA-A and HLA-B matching. Cornea 2003;22:552–6. [DOI] [PubMed] [Google Scholar]
- [20].Hoffmann F, Pahlitzsch T. Predisposing factors in corneal graft rejection. Cornea 1989;8:215–9. [PubMed] [Google Scholar]
- [21].Sanfilippo F, MacQueen JM, Vaughn WK, et al. Reduced graft rejection with good HLA-A and B matching in high-risk corneal transplantation. N Engl J Med 1986;315:29–35. [DOI] [PubMed] [Google Scholar]
- [22].Cosar CB, Laibson PR, Cohen EJ, et al. Topical cyclosporine in pediatric keratoplasty. Eye Contact Lens 2003;29:103–7. [DOI] [PubMed] [Google Scholar]
- [23].Cursiefen C, Küchle M, Naumann GO, et al. Angiogenesis in corneal diseases: histopathologic evaluation of 254 human corneal buttons with neovascularization. Cornea 1998;17:611–3. [DOI] [PubMed] [Google Scholar]
- [24].Al-Ghamdi A, Al-Rajhi A, Wagoner MD. Primary pediatric keratoplasty: indications, graft survival, and visual outcome. J AAPOS 2007;11:41–7. [DOI] [PubMed] [Google Scholar]
- [25].Comer RM, Daya SM, O’Keefe M, et al. Penetrating keratoplasty in infants. J AAPOS 2001;5:285–90. [DOI] [PubMed] [Google Scholar]
- [26].Dana MR, Schaumberg DA, Moyes AL. Corneal transplantation in children with Peters anomaly and mesenchymal dysgenesis. Multicenter Pediatric Keratoplasty Study. Ophthalmology 1997;104:1580–6. [DOI] [PubMed] [Google Scholar]
- [27].Chang JW, Kim MK, Kim JH, et al. Long-term visual outcomes of penetrating keratoplasty for Peters anomaly. Graefes Arch Clin Exp Ophthalmol 2013;251:953–8. [DOI] [PubMed] [Google Scholar]
- [28].Zaidman GW, Flanagan JK, Furey CC. Long-term visual prognosis in children after corneal transplant surgery for Peters anomaly type I. Am J Ophthalmol 2007;144:104–8. [DOI] [PubMed] [Google Scholar]
- [29].Yalniz-Akkaya Z, Burcu Nurozler A, Yildiz E, et al. Repeat penetrating keratoplasty: indications and prognosis, 1995–2005. Eur J Ophthalmol 2009;19:362–8. [DOI] [PubMed] [Google Scholar]
- [30].Sharma N, Prakash G, Titiyal JS, et al. Pediatric keratoplasty in India: indications and outcomes. Cornea 2007;26:810–3. [DOI] [PubMed] [Google Scholar]
- [31].Dada T, Sharma N, Vajpayee RB, et al. Indications for pediatric keratoplasty in India. Cornea 1999;18:296–8. [DOI] [PubMed] [Google Scholar]
- [32].Shi W, Jin H, Li S, et al. Indications of paediatric keratoplasty in north China. Clin Experiment Ophthalmol 2007;35:724–7. [DOI] [PubMed] [Google Scholar]
- [33].Bishop VL, Robinson LP, Wechsler AW, et al. Corneal graft survival: a retrospective Australian study. Aust N Z J Ophthalmol 1986;14:133–8. [DOI] [PubMed] [Google Scholar]
- [34].Völker-Dieben HJ, Odenthal MT, D’Amaro J, et al. Surgical treatment of corneal pathology in patients with Down's syndrome. J Intellect Disabil Res 1993;37(pt 2):169–75. [DOI] [PubMed] [Google Scholar]
- [35].Schaumberg DA, Moyes AL, Gomes JA. Corneal transplantation in young children with congenital hereditary endothelial dystrophy. Multicenter Pediatric Keratoplasty Study. Am J Ophthalmol 1999;127:373–8. [DOI] [PubMed] [Google Scholar]
- [36].al-Rajhi AA, Wagoner MD. Penetrating keratoplasty in congenital hereditary endothelial dystrophy. Ophthalmology 1997;104:956–61. [DOI] [PubMed] [Google Scholar]
- [37].Chan AS, Colby K. Update on pediatric keratoplasty. Int Ophthalmol Clin 2008;48:25–33. [DOI] [PubMed] [Google Scholar]
- [38].Afshari NA, Azar NF, Afshari MA, et al. Corneal transplantation in children. Int Ophthalmol Clin 2001;41:1–7. [DOI] [PubMed] [Google Scholar]
- [39].O’Hara MA, Mannis MJ. Pediatric penetrating keratoplasty. Int Ophthalmol Clin 2013;53:59–70. [DOI] [PubMed] [Google Scholar]
- [40].Vanathi M, Panda A, Vengayil S. Pediatric keratoplasty. Surv Ophthalmol 2009;54:245–71. [DOI] [PubMed] [Google Scholar]
- [41].Karadag R, Chan TC, Azari AA, et al. Survival of primary penetrating keratoplasty in children. Am J Ophthalmol 2016;171:95–100. [DOI] [PubMed] [Google Scholar]
- [42].Dana MR, Moyes AL, Gomes JA, et al. The indications for and outcome in pediatric keratoplasty. A multicenter study. Ophthalmology 1995;102:1129–38. [DOI] [PubMed] [Google Scholar]
- [43].Thompson RW, Price MO, Bowers PJ. Long-term graft survival after penetrating keratoplasty. Ophthalmology 2003;110:1396–402. [DOI] [PubMed] [Google Scholar]
- [44].Khvatova AV, Pleskova AV. Experience in penetrating keratoplasty in children: graft survival, functional results and risk factors. Vestn Oftalmol 2003;119:3–7. [PubMed] [Google Scholar]
- [45].Price FW, Whitson WE, Collins KS, et al. Five-year corneal graft survival. A large, single-center patient cohort. Arch Ophthalmol 1993;111:799–805. [DOI] [PubMed] [Google Scholar]
- [46].Williams KA, Roder D, Esterman A, et al. Factors predictive of corneal graft survival. Report from the Australian Corneal Graft Registry. Ophthalmology 1992;99:403–14. [DOI] [PubMed] [Google Scholar]
- [47].Sit M, Weisbrod DJ, Naor J, et al. Corneal graft outcome study. Cornea 2001;20:129–33. [DOI] [PubMed] [Google Scholar]
- [48].Stulting RD, Sugar A, Beck R, et al. Effect of donor and recipient factors on corneal graft rejection. Cornea 2012;31:1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Anshu A, Lim LS, Htoon HM, et al. Postoperative risk factors influencing corneal graft survival in the Singapore Corneal Transplant Study. Am J Ophthalmol 2011;151:442–8. [DOI] [PubMed] [Google Scholar]
- [50].Oertelt-Prigione S. The influence of sex and gender on the immune response. Autoimmun Rev 2012;11:A479–85. [DOI] [PubMed] [Google Scholar]
- [51].Hopkinson CL, Romano V, Kaye RA, et al. The influence of donor and recipient gender incompatibility on corneal transplant rejection and failure. Am J Transplant 2017;17:210–7. [DOI] [PubMed] [Google Scholar]
- [52].Haskova Z, Filipec M, Holan V. The significance of gender incompatibility in donors and recipients and the role of minor histocompatibility antigens in corneal transplantation. Cesk Slov Oftalmol 1997;53:128–35. [PubMed] [Google Scholar]
- [53].Costenbader KH, Gay S, Alarcon-Riquelme ME, et al. Genes, epigenetic regulation and environmental factors: which is the most relevant in developing autoimmune diseases? Autoimmun Rev 2012;11:604–9. [DOI] [PubMed] [Google Scholar]
- [54].Grimaldi CM, Cleary J, Dagtas AS, et al. Estrogen alters thresholds for B cell apoptosis and activation. J Clin Invest 2002;109:1625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sakiani S, Olsen NJ, Kovacs WJ. Gonadal steroids and humoral immunity. Nat Rev Endocrinol 2013;9:56–62. [DOI] [PubMed] [Google Scholar]
- [56].Patel HY, Ormonde S, Brookes NH, et al. The indications and outcome of paediatric corneal transplantation in New Zealand:1991-2003. Br J Ophthalmol 2005;89:404–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Groh MJ, Gusek-Schneider GC, Seitz B, et al. [Outcomes after penetrating keratoplasty in congenital hereditary corneal endothelial dystrophy (CHED). Report on 13 eyes]. Klin Monbl Augenheilkd 1998;213:201–6. [DOI] [PubMed] [Google Scholar]
- [58].Erlich CM, Rootman DS, Morin JD. Corneal transplantation in infants, children and young adults: experience of the Toronto Hospital for Sick Children, 1979–1988. Can J Ophthalmol 1991;26:206–10. [PubMed] [Google Scholar]
- [59].Huang PT. Penetrating keratoplasty in infants and children. J AAPOS 2007;11:5–6. [DOI] [PubMed] [Google Scholar]
- [60].Huang C, O’Hara M, Mannis MJ. Primary pediatric keratoplasty: indications and outcomes. Cornea 2009;28:1003–8. [DOI] [PubMed] [Google Scholar]
- [61].Hong JX, Xu JJ, Sheng MJ, et al. Pediatric penetrating keratoplasty in Shanghai: a retrospective multiple centre study from 2003 to 2007. Chin Med J (Engl) 2008;121:1911–4. [PubMed] [Google Scholar]
- [62].Cowden JW. Penetrating keratoplasty in infants and children. Ophthalmology 1990;97:324–8. [DOI] [PubMed] [Google Scholar]
- [63].Lowe MT, Keane MC, Coster DJ, et al. The outcome of corneal transplantation in infants, children, and adolescents. Ophthalmology 2011;118:492–7. [DOI] [PubMed] [Google Scholar]
- [64].Ganekal S, Gangangouda C, Dorairaj S. Early outcomes of primary pediatric keratoplasty in patients with acquired, atraumatic corneal pathology. J AAPOS 2011;15:353–5. [DOI] [PubMed] [Google Scholar]


