Abstract
Introduction:
This is a unique case of nonketotic hyperglycemic (NKH) chorea in 84-year-old Asian woman. The patient had a history of type 2 diabetes mellitus more than 30 years, but had a poor control of blood sugar. She complained of acute onset of bilateral limb involuntary activities, and being easy to fall within a week. Laboratory testing disclosed hyperglycemia (669 mg/dL), glycated hemoglobin (14%), and normal ketones. The brain computed tomography scan and magnetic resonance imaging did not disclose any abnormality in the basal ganglion unlike most cases. The patient was then diagnosed with NKH chorea. Her symptoms improved quickly.
Conclusions:
NKH chorea with normal imaging may represent a new subtype.
Keywords: chorea, diabetes mellitus, nonketotic hyperglycinemia
1. Introduction
Chorea is an abnormal involuntary movement disorder characterized by brief, abrupt, irregular movements. Various conditions such as neurodegenerative diseases, cerebrovascular disease, immunological diseases, neoplastic diseases, infectious diseases, and metabolic diseases are known as secondary causes of chorea.[1,2] Nonketotic hyperglycemic (NKH) chorea is mainly seen in elderly patients, especially females from East Asian origin.[3,4] The general treatment measures include improving control of blood glucose and the use of neuroleptic drugs. We report a unique case of chorea secondary to nonketotic hyperglycemia with negative imaging changes.
2. Case
An 84-year-old woman came to our attention for acute involuntary movements before a week. She began bilateral limb involuntary, dance-like activities, also accompanied by abnormal facial and tongue muscle activities. These activities occurred while awake, and disappeared during sleep. She had a history of type 2 diabetes mellitus (DM) for more than 30 years with poor glycemic control. Neurological examination found that patient has a clear verbal ability, sanity, present involuntary dance-like movements of limbs, facial, jaw, and tongue. She had normal muscle strength and mild hypotonia. Other physical examination found no abnormalities. A computed tomography (CT) scan of the head (Fig. 1A) showed no abnormal findings taken in the emergency department. Laboratory tests showed that random blood sugar was 669 mg/dL, urine sugar (4+), and urine ketone (−). Glycated hemoglobin was 14%. The patient's subsequent thyroid function tests, autoantibodies, liver and kidney function tests, anti-neutrophilic cytoplasmic antibodies, ceruloplasmin, vitamins D and B12, and folic acid found no abnormal findings. The brain 1.5 T magnetic resonance imaging (MRI) (Fig. 1B–C) showed no abnormalities in the basal ganglia. She was diagnosed with chorea associated with NKH. The treatments included improving blood glucose with insulin and symptomatic treatment of chorea with tiapride 100 mg 3 times a day and clonazepam 1 mg every night. On the fourth day of admission, patient's involuntary movements improved obviously. Blood sugar levels become normalized after a week. After 10 days, her dance-like symptoms disappeared completely. She tapered off tiapride and clonazepam within 3 months and continued to control blood sugar with premixed insulin after discharge. In 1 year of follow-up, involuntary movements did not arise again, and the patient refused to have a follow-up brain MRI or CT scans.
Figure 1.
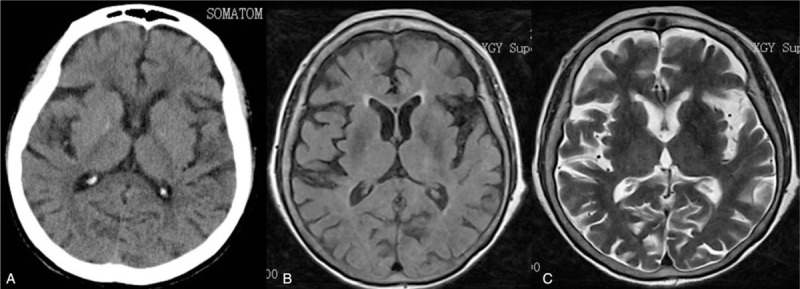
A, A brain computed tomography (CT) scan. B, T1-weighted imaging. C, T2-weighted imaging.
3. Discussion
Typical patient with NKH chorea show triad: nonketotic hyperglycemia, hemichorea, and basal ganglia in MRI T1 showed high signal or high density in CT scan.[5] This is an interesting case because of its negative imaging and bilateral chorea, which may contribute to a better understanding of this disease.
NKH chorea is a rare clinical syndrome, first reported by Bedwell in 1960.[6] Oh et al found that, male to female ratio was 1: 1.76, the average age was 71.1 years, and 48 patients (91%) of Asians, through reviewing 53 cases reported in the MEDLINE database from 1985 to 2001. This revealed that the disease commonly occurs in elderly Asian women with poor control of DM more.[3] Recently, case series about patients of other ethnic background have been reported.[7] The differences between racial and sex may be related to genetic factors and estrogen. The decline of estrogen concentration in postmenopausal women results in nigrostriatal dopamine system receptors become hypersensitive, so that striatal dopamine system function is relatively enhanced.
Most patients with chorea associated with NKH present acute or subacute onset of limb involuntary activities (more common in unilateral), sometimes involuntary movements also appear in the facial muscle, jaw, and tongue, accompanied by severe increase in blood glucose and negative ketones in urine. There are characteristic radiographic manifestations under normal circumstances: the contralateral striatum in MRI T1-weighted show high signal changes and equal or low signal in MRI T2-weighted, mostly high-density changes in head CT.[8,9] With the improvement of the condition, the image changes can weaken or disappear. In addition, as reported above, there are very few patients with a negative performance in MRI or CT scan. Most patients with this condition have a good prognosis. The control of blood glucose is the most important treatment, and the involuntary movements in some patients can be relieved with the decline in blood glucose level. Dopamine receptor antagonists (haloperidol, risperidone, etc) can be used to control chorea, if necessary plus clonazepam.
At present, the specific mechanism of the NKH chorea remains unclear. The possible mechanisms may be, when hyperglycemia, brain cell metabolism gradually transformed into anaerobic metabolism because of decreased regional cerebral blood flow and glucose metabolism failure. Then gamma-aminobutyric acid (GABA) becomes the main source of energy for brain cells. Acetoacetate in ketosis patients can be used to synthesize GABA. GABA in patients with nonketotic hyperglycemia is rapidly depleted due to lack of acetoacetate, so the normal activities of the basal ganglia are damaged.[3,10,11] Since patients with NKH are easy to suffer from chorea.
To date, only few cases with negative imaging have been reported by other researchers.[7,12] Based on the current reported cases and our case, we argue that, the syndrome can be divided into 2 types: patients with diabetes, high blood glucose, negative ketone, unilateral or bilateral chorea, and typical radiographic changes in MRI or CT scan of the head, are the most common type and patients with diabetes, high blood sugar, negative ketones, unilateral or bilateral chorea, and negative imaging changes, are relatively uncommon type. In conclusion, we wish to highlight that NKH chorea with normal imaging can represent a subtype, although infrequent.
Footnotes
Abbreviations: CT = computed tomography, DM = diabetes mellitus, GABA = gamma-aminobutyric acid, NKH = nonketotic hyperglycemic.
The patient in this case report has given her informed consent for the case report to be published.
This study was supported by Zhejiang Provincial Zhedong Regional Special Disease Center Construction Program (2015-21).
The authors declare that there is no conflict of interests regarding the publication of this article.
References
- [1].Jagota P, Bhidayasiri R, Lang AE. Movement disorders in patients with diabetes mellitus. J Neurol Sci 2012;314:5–11. [DOI] [PubMed] [Google Scholar]
- [2].Hawley JS, Weiner WJ. Hemiballismus: current concepts and review. Parkinsonism Relat Disord 2012;18:125–9. [DOI] [PubMed] [Google Scholar]
- [3].Oh SH, Lee KY, Im JH, et al. Chorea associated with non-ketotic hyperglycemia and hyperintensity basal ganglia lesion on T1-weighted brain MRI study: a meta-analysis of 53 cases including four present cases. J Neurol Sci 2002;200:57–62. [DOI] [PubMed] [Google Scholar]
- [4].Lee SH, Shin JA, Kim JH, et al. Chorea-ballism associated with nonketotic hyperglycaemia or diabetic ketoacidosis: characteristics of 25 patients in Korea. Diabetes Res Clin Pract 2011;93:e80–3. [DOI] [PubMed] [Google Scholar]
- [5].Bizet J, Cooper CJ, Quansah R, et al. Chorea, hyperglycemia, basal ganglia syndrome (C-H-BG) in an uncontrolled diabetic patient with normal glucose levels on presentation. Am J Case Rep 2014;15:143–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bedwell SF. Some observations on hemiballismus. Neurology 1960;10:619–22. [DOI] [PubMed] [Google Scholar]
- [7].Tocco P, Barbieri F, Bonetti B, et al. Hemichorea-hemiballismus in patients with non-ketotic hyperglycemia. Neurol Sci 2016;37:297–8. [DOI] [PubMed] [Google Scholar]
- [8].Vale TC, Freitas Dda S, Maciel RO, et al. Teaching video neuroimages: hemichorea-hemiballismus secondary to nonketotic hyperglycemia. Neurology 2013;80:e178. [DOI] [PubMed] [Google Scholar]
- [9].Zaitout Z. CT and MRI findings in the basal ganglia in non-ketotic hyperglycaemia associated hemichorea and hemi-ballismus (HC-HB). Neuroradiology 2012;54:1119–20. [DOI] [PubMed] [Google Scholar]
- [10].Taskapilioglu O, Hakyemez B, Bora İ. Hyperglycemia as a cause of choreoathetosis: two case reports and review of the literature. Eur J Radiol Extra 2006;58:1–4. [Google Scholar]
- [11].Cheema H, Federman D, Kam A. Hemichorea-hemiballismus in non-ketotic hyperglycaemia. J Clin Neurosci 2011;18:293–4. [DOI] [PubMed] [Google Scholar]
- [12].Branca D, Gervasio O, Le Piane E, et al. Chorea induced by non-ketotic hyperglycaemia: a case report. Neurol Sci 2005;26:275–7. [DOI] [PubMed] [Google Scholar]


