Supplemental Digital Content is available in the text
Keywords: circulating 25-hydroxyvitamin D, dose–response relationship, lung cancer, meta-analysis
Abstract
Lower serum level of 25-hydroxyvitamin D is associated with several negative outcomes. However, previous studies have indicated that 25-hydroxyvitamin D is associated with lung cancer risk and survival, but presented controversial results.
PubMed and Embase databases were searched update to August 2017 to identify and quantify the potential association between 25-hydroxyvitamin D and lung cancer risk and survival.
Seventeen eligible studies involving a total of 138,858 participants with 4368 incident cases were included in this meta-analysis. Our results showed statistically significant association between 25-hydroxyvitamin D and lung cancer risk and mortality. However, circulating 25-hydroxyvitamin D was not associated with overall lung cancer survival. Furthermore, compared with the lowest circulating 25-hydroxyvitamin D, the highest circulating 25-hydroxyvitamin D is significantly decreased risk of lung cancer risk in male and female. In addition, the highest circulating 25-hydroxyvitamin D was significantly associated with a lower risk in Caucasian and Asian. We also obtained the best fit at an inflection point of 10 nmol/L in piecewise regression analysis, increasing 10 nmol/L dose of circulating 25-hydroxyvitamin D was associated with an 8% reduction in the risk of lung cancer risk and an 7% reduction in the risk of lung cancer mortality. Subgroup meta-analyses in study quality, number of participants, and number of cases showed consistent with the primary findings.
The highest circulating 25-hydroxyvitamin D was associated with decreased lung cancer risk and mortality but not overall survival
1. Introduction
Lung cancer is one of the most serious and malignant diseases with the highest morbidity and mortality, and one of the most serious malignancies for population health and life.[1] It accounts for the first incidence of all malignancies in male, with the second highest incidence in female, and the mortality rate account for the second of all malignancies.[2] According to the American Cancer Association statistics, lung cancer mortality gradually increased, the overall cure rate for lung cancer has not improved significantly over the past decade.[3] These data reveal the poor prognosis of lung cancer, and thus to prevent the occurrence of lung cancer is essential. Compared with many other cancers, there are a few identified risk factors for lung cancer, including asthma, chronic obstructive emphysema, pneumonia, tuberculosis, and atmospheric pollution.[4,5] Meanwhile, the role of vitamin D has been recognized as an independently risk in several cancers, including lung cancer.[6] Vitamin D mainly exists in the body in the form of circulating vitamin D, which is hydroxylated into 25-hydroxyvitamin D in the liver.[7]
Vitamin D is a fat-soluble vitamin, and the human body is mainly vitamin D2 and vitamin D3. The body's vitamin D is mainly derived from the body's own synthesis and animal food. The main function of vitamin D is to maintain the metabolism balance of human calcium and the formation of bone. In addition, vitamin D deficiency is closely related to abnormal immune function, cardiovascular disease, metabolic diseases, and tumors.[8] Lower circulating 25-hydroxyvitamin D is a common condition in lung cancer. Also, lower vitamin D level is a potential reversible/modifiable risk factors for lung cancer.[9]
Previous studies have examined the relationship between circulating 25-hydroxyvitamin D and risk of cardiovascular disease, type 2 diabetes, and all-cause mortality, and have found higher circulating 25-hydroxyvitamin D is significantly reduce disease risk.[8,10] Even though some studies supported higher circulating 25-hydroxyvitamin D significantly decrease lung cancer risk and survival. However, the result remains controversial. In addition, no study to quantitative assessed circulating 25-hydroxyvitamin D and lung cancer risk and survival. Thus, we performed this comprehensive dose–response meta-analysis to clarify and quantitative assessed the correlation between circulating 25-hydroxyvitamin D and lung cancer.
2. Methods
Our meta-analysis was conducted according to the Meta-analysis Of Observational Studies in Epidemiology checklist.[11] There are no ethical issues involved in our study for our data were based on published studies.
2.1. Search strategy
Eligible studies were systematically searched of PubMed and Embase update to August 2017 examining the association between circulating 25-hydroxyvitamin D and lung cancer risk and survival, with keywords including “25-hydroxyvitamin D” [MeSH] OR “vitamin D” [MeSH] AND “lung cancer” [MeSH] OR “lung tumor” [MeSH]. We refer to the relevant original essays and commentary articles to determine further relevant research.
2.2. Study selection
Two independent researchers investigate information: outcome was lung cancer incidence and mortality. Moreover, we precluded nonhuman studies, reviews, editorials, and published letters. To ensure the correct identification of qualified research, the 2 researchers read the reports independently.
2.3. Data extraction
Use standardized data collection tables to extract data. Each eligible article information was extracted by 2 independent researchers. The following information was extracted: first author; publication year; age; country; sex; cases and participants; and relative risk or odds ratio. We collect the risk estimates with multivariable-adjusted. According to the Newcastle–Ottawa scale,[12] quality assessment was performed for nonrandomized studies.
2.4. Statistical analysis
We pooled relative risk estimates to measure the association between circulating 25-hydroxyvitamin D and lung cancer risk and survival. Results in different subgroup of circulating 25-hydroxyvitamin D and lung cancer risk and survival were treated as 2 separate reports.
Due to different definitions cut-off points in the included studies for categories, using the method recommended by Greenland, Longnecker and Orsini et al[13] by increase per 10 nmol/L circulating 25-hydroxyvitamin D. A flexible meta-regression based on restricted cubic spline function was used to fit the potential nonlinear trend, and generalized least-square method was used to estimate the parameters. This procedure treats circulating 25-hydroxyvitamin D (continuous data) as an independent variable and logRR of diseases as a dependent variable, with both tails of the curve restricted to linear.[14]
The between-study heterogeneity was assessed by Q-statistic (significance level at P ≤ .10) and the I2 statistic.[15] STATA software 12.0 (STATA Corp, College Station, TX) was used in all analyses. P < .05 was considered significant for all tests.
3. Results
3.1. Literature search results
Figure 1 shows literature research and selection. A total of 1685 studies were retrieved (PubMed: 1634, Embase: 1862). After exclusion of studies, 17 studies were chosen, among the selected studies, 9 studies about the relationships between circulating 25-hydroxyvitamin D and lung cancer risk,[16–24] 3 studies about the relationships between circulating 25-hydroxyvitamin D and lung cancer mortality,[25–27] 5 studies about the relationships between circulating 25-hydroxyvitamin D and lung cancer survival,[28–32] and the data were extracted. These studies were published update to August 2017.
Figure 1.
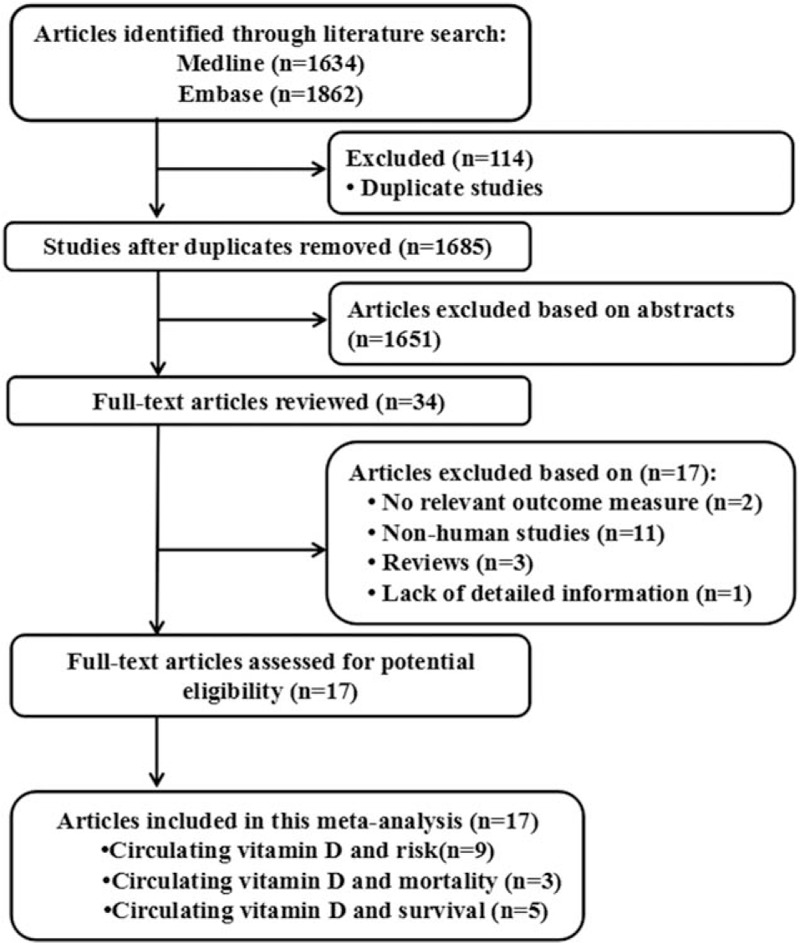
Flow diagram of the study selection process.
3.2. Study characteristics
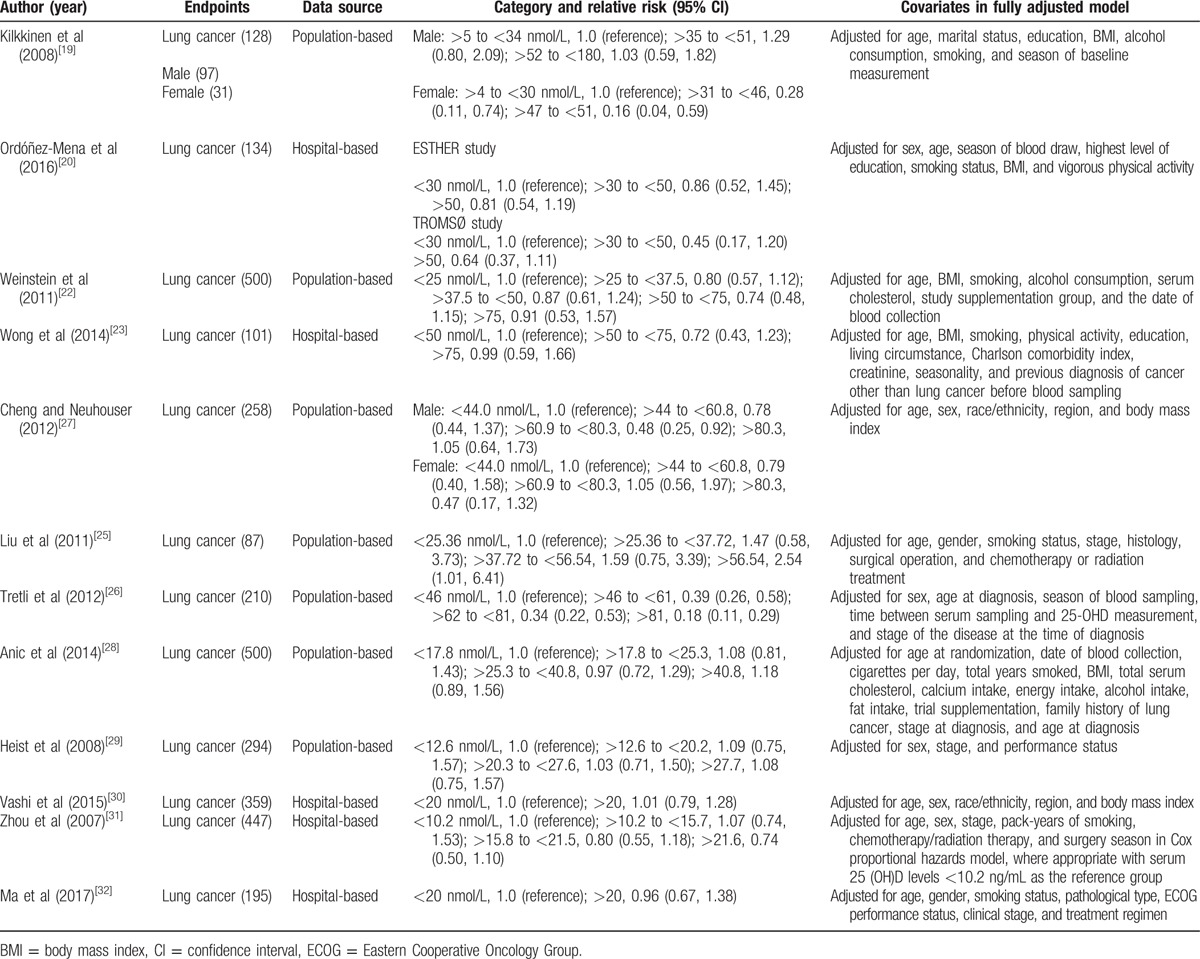
The characteristics of the included studies are shown in Tables 1 and 2. A total of 138,858 participants with 4368 incident cases were included in this meta-analysis.
Table 1.
Characteristics of participants in included studies of circulating 25-hydroxyvitamin D in relation to lung cancer.

Table 2.
Outcomes and covariates of included studies of circulating 25-hydroxyvitamin D in relation to lung cancer.
3.3. Circulating 25-hydroxyvitamin D and lung cancer risk
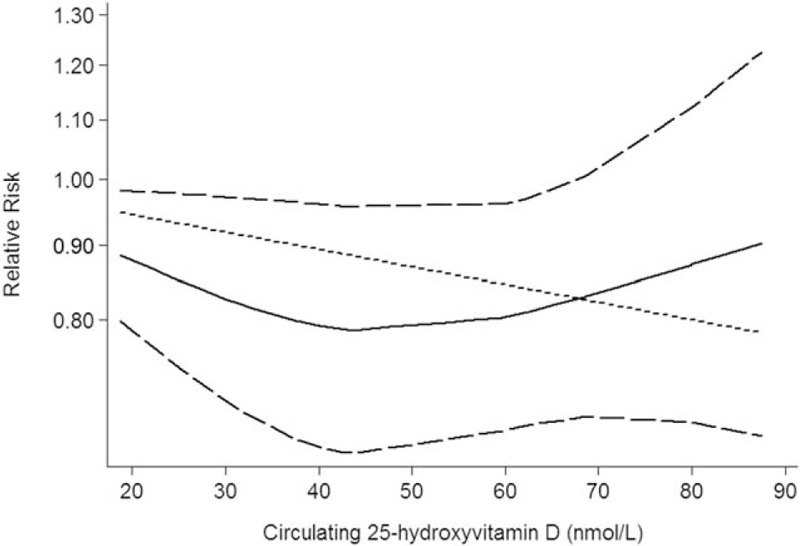
Nine studies including 11 independent reports investigated the association between circulating 25-hydroxyvitamin D and lung cancer risk. Higher circulating 25-hydroxyvitamin D was significantly decreased risk of lung cancer (relevant risk [RR]: 0.84; 95% confidence interval (CI): 0.74–0.95; P = .006; Table 3). Furthermore, higher circulating 25-hydroxyvitamin D was associated with a significantly decrement risk of lung cancer in female (odds ratio [OR] = 0.16, 95% CI: 0.04–0.59, P < .001; Table 3) and in male (OR = 0.82, 95% CI: 0.71–0.91, P < .001; Table 3). In addition, higher circulating 25-hydroxyvitamin D was significantly decreased risk in Caucasian (RR: 0.92; 95% CI: 0.88–0.95; P < .001; Table 3) and Asian (RR: 0.41; 95% CI: 0.19–0.91; P < .001; Table 3). We also obtained the best fit at an inflection point of 10 nmol/L in piecewise regression analysis, increasing 10 nmol/L of circulating 25-hydroxyvitamin D was associated with a 8% reduction in the risk of lung cancer, the summary relative risk of lung cancer risk for an per 10 nmol/L of circulating 25-hydroxyvitamin D was 0.92 (95% CI: 0.87–0.96, P < .001; Fig. 2).
Table 3.
Stratified analyses of relative risk of lung cancer.
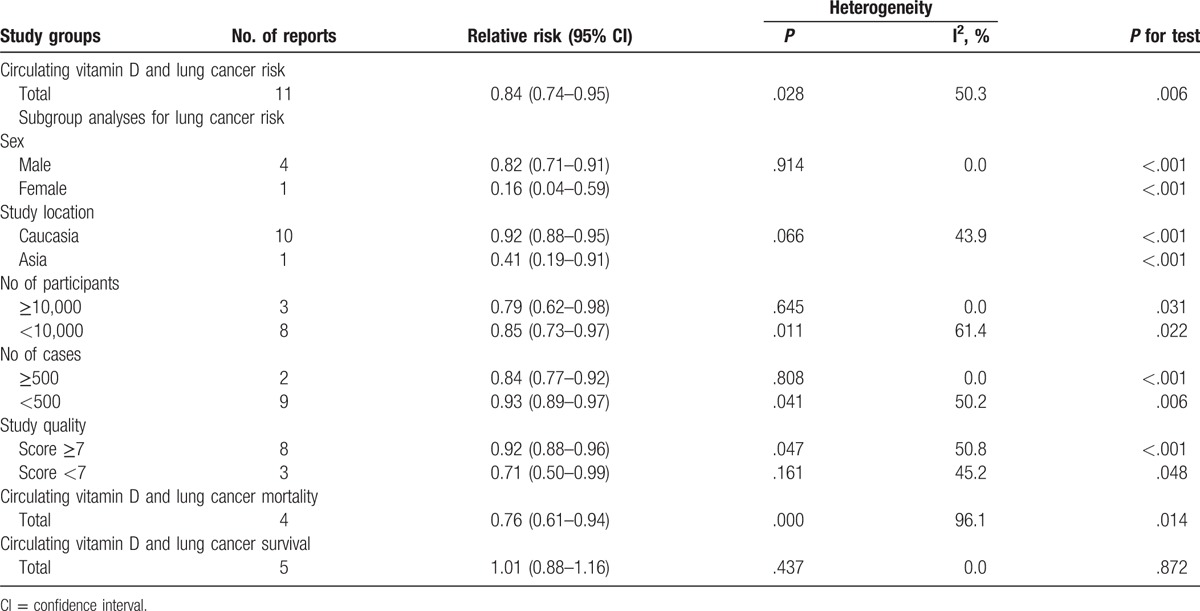
Figure 2.
Circulating 25-hydroxyvitamin D is associated with lung cancer risk.
3.4. Circulating 25-hydroxyvitamin D and lung cancer mortality
Three studies including 4 independent reports investigated the association between circulating 25-hydroxyvitamin D and lung cancer mortality. Higher circulating 25-hydroxyvitamin D was significantly decreased risk of lung cancer mortality (RR: 0.76; 95% CI: 0.61–0.94; P = .014; Table 3). We also obtained the best fit at an inflection point of 10 nmol/L in piecewise regression analysis, increasing 10 nmol/L of circulating 25-hydroxyvitamin D was associated with a 7% reduction in lung cancer mortality, the summary relative risk of lung cancer mortality for an per 10 nmol/L of circulating 25-hydroxyvitamin D was 0.93 (95% CI: 0.88–0.96, P < .001; Fig. 3).
Figure 3.
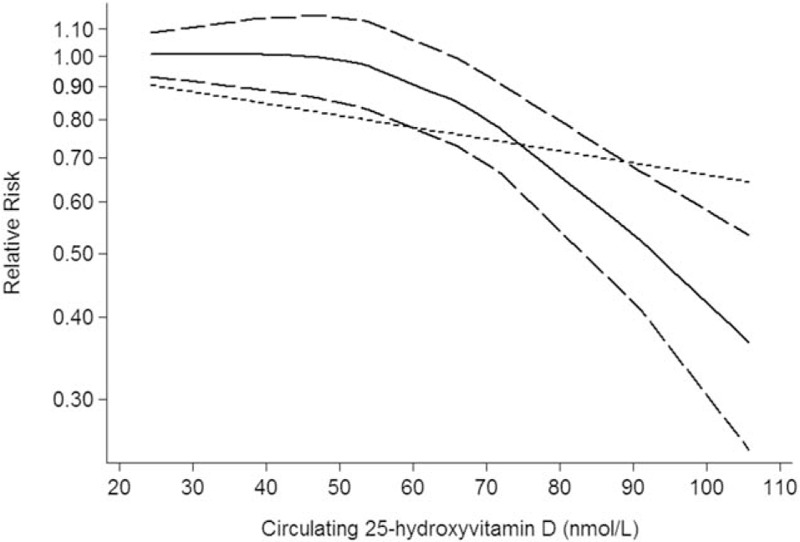
Circulating 25-hydroxyvitamin D is associated with lung cancer mortality.
3.5. Circulating 25-hydroxyvitamin D and lung cancer survival
Five studies including 5 independent reports investigated the association between circulating 25-hydroxyvitamin D and lung cancer survival. Higher circulating 25-hydroxyvitamin D did not significantly decreased risk of lung cancer survival (RR: 1.01; 95% CI: 0.88–1.16; P < .001; Table 3). We also obtained the best fit at an inflection point of 10 nmol/L in piecewise regression analysis, increasing 10 nmol/L of circulating 25-hydroxyvitamin D was not increase lung cancer survival, the summary relative risk of lung cancer survival for an per 10 nmol/L of circulating 25-hydroxyvitamin D was 1.04 (95% CI: 0.91–1.17, P = .827; Fig. 4).
Figure 4.
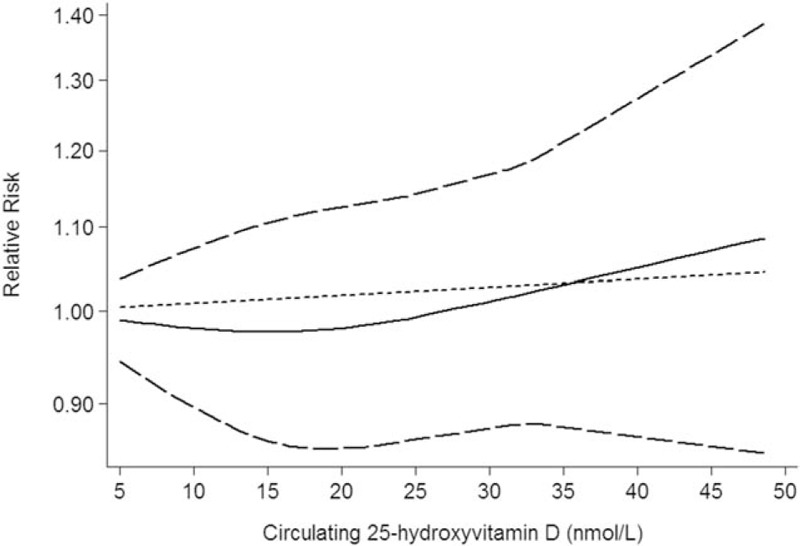
Circulating 25-hydroxyvitamin D is associated with lung cancer survival.
3.6. Subgroup analyses
Subgroup analysis was performed to check the stability of the primary outcome. Subgroup meta-analyses in study quality, number of participants, and number of cases showed consistent findings (Table 3).
3.7. Publication bias
The results show that no obvious evidence of publication bias was found in the associations between circulating 25-hydroxyvitamin D and lung cancer risk (Supplementary Table 1). P > .05 was considered no publication bias. A funnel plot for publication bias assessment is shown in Supplementary Figures 1 to 3.
4. Discussion
Vitamin D is an important vitamin, mainly from fat-rich fish, butter, cheese, and fortified milk. The body itself can produce vitamin D in the sun. However, vitamin D deficiency is a common phenomenon.[8] It can maintain the stability of serum calcium and phosphorus levels, when the serum calcium concentration is low, it induced parathyroid hormone secretion, release it to the kidney and bone cells.[33] Also, vitamin D participates in critical cell functions such as cell proliferation, apoptosis, differentiation, metastasis, and angiogenesis. Vitamin D is one of the indispensable elements of health and disease prevention. Previous studies supported higher circulating 25-hydroxyvitamin D significantly decrease lung cancer risk and survival. However, the result remains controversial.
In the current, meta-analysis was based on 17 prospective cohort studies, with 138,858 participants with 4368 incident cases. Thus, this meta-analysis provides the most up-to-date epidemiological evidence supporting higher circulating 25-hydroxyvitamin D is helpful for lung cancer. A dose–response analysis revealed that increasing 10 nmol/L dose of circulating 25-hydroxyvitamin D was associated with an 8% reduction in the risk of lung cancer risk and a 7% reduction in the risk of lung cancer mortality. Subgroup meta-analyses in study quality, number of participants, and number of cases showed consistent with the primary findings.
Several plausible pathways may reasonable for the relationship between 25-hydroxyvitamin D and lung cancer. Vitamin D metabolites play a cytostatic effect most dependent on vitamin D receptor. Previous study found that 25-hydroxyvitamin D plays the role of inhibiting lung cancer cells growth in mouse epidermal cells formation.[34] The immunomodulatory function of vitamin D metabolites may be an important mechanism for vitamin D in prevent lung cancer; 25-hydroxyvitamin D can inhibit the activity of mammalian target of rapamycin in lung cancer cells and raise the level of protein expression, which can promote the autophagy of tumor cells.[35] Meanwhile, 25-hydroxyvitamin can induce the expression of major antioxidant protein–superoxide dismutase SOD1 and SOD2, thereby inhibiting the formation of lung cancer to some extent.[36] In addition, vitamin D can regulate immunological function of lung epithelial cells and inhibit cellular proliferation and angiogenesis while promoting cellular differentiation and apoptosis.[34,37,38]
Some limitations must be considered in this meta-analysis. First, we only select literature written in English, which may have resulted in a language or cultural bias, other languages should be chosen in the further. Second, we only select literature from PubMed and Embase databases, other databases should be chosen in the further.
In conclusion, our findings underscore the notion that higher vitamin D was significantly associated with lung cancer decrement. In the future, large-scale and population-based association studies must be performed in the future to validate the risk identified in the current meta-analysis.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, OR = odds ratio, RR = relevant risk.
QF and HZ have contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Jemal A, Bray F, Center M, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- [2].Zeng H, Zheng R, Guo Y, et al. Cancer survival in China, 2003–2005: a population-based study. Int J Cancer 2015;136:1921–30. [DOI] [PubMed] [Google Scholar]
- [3].Miller K, Siegel R, Lin C, et al. Cancer treatment and survivorship statistics. CA Cancer J Clin 2016;66:271–89. [DOI] [PubMed] [Google Scholar]
- [4].Ezzati M, Lopez A. Estimates of global mortality attributable to smoking in 2000. Lancet 2003;362:847–52. [DOI] [PubMed] [Google Scholar]
- [5].Kushi L, Doyle C, McCullough M, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin 2012;62:30–67. [DOI] [PubMed] [Google Scholar]
- [6].van der Rhee H, Coebergh J, de Vries E. Sunlight, vitamin D and the prevention of cancer: a systematic review of epidemiological studies. Eur J Cancer Prev 2009;18:458–75. [DOI] [PubMed] [Google Scholar]
- [7].Zerwekh J. Blood biomarkers of vitamin D status. Am J Clin Nutr 2008;87:1087S–91S. [DOI] [PubMed] [Google Scholar]
- [8].Holick M. Vitamin D deficiency. N Engl J Med 2007;357:266–81. [DOI] [PubMed] [Google Scholar]
- [9].LaPar D, Nagji A, Bhamidipati C, et al. Seasonal variation influences outcomes following lung cancer resections. Eur J Cardiothorac Surg 2011;40:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Marshall Brinkley D, Ali O, Zalawadiya S, et al. Vitamin D and heart failure. Curr Heart Fail Rep 2017. [DOI] [PubMed] [Google Scholar]
- [11].Stroup D, Berlin J, Morton S, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [12].Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [13].Orsini N, Li R, Wolk A, et al. Meta-analysis for linear and nonlinear dose–response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 2012;175:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med 1989;8:551–61. [DOI] [PubMed] [Google Scholar]
- [15].Higgins J, Thompson S, Deeks J, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Afzal S, Bojesen S, Nordestgaard B. Low plasma 25-hydroxyvitamin D and risk of tobacco-related cancer. Clin Chem 2013;59:771–80. [DOI] [PubMed] [Google Scholar]
- [17].Ananthakrishnan A, Cheng S, Cai T, et al. Association between reduced plasma 25-hydroxy vitamin D and increased risk of cancer in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2014;12:821–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Giovannucci E, Liu Y, Rimm E, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst 2006;98:451–9. [DOI] [PubMed] [Google Scholar]
- [19].Kilkkinen A, Knekt P, Heliövaara M, et al. Vitamin D status and the risk of lung cancer: a cohort study in Finland. Cancer Epidemiol Biomarkers Prev 2008;17:3274–8. [DOI] [PubMed] [Google Scholar]
- [20].Ordóñez-Mena J, Schöttker B, Fedirko V, et al. Pre-diagnostic vitamin D concentrations and cancer risks in older individuals: an analysis of cohorts participating in the CHANCES consortium. Eur J Epidemiol 2016;31:311–23. [DOI] [PubMed] [Google Scholar]
- [21].Skaaby T, Husemoen L, Thuesen B, et al. Prospective population-based study of the association between serum 25-hydroxyvitamin-D levels and the incidence of specific types of cancer. Cancer Epidemiol Biomarkers Prev 2014;23:1220–9. [DOI] [PubMed] [Google Scholar]
- [22].Weinstein S, Yu K, Horst R, et al. Serum 25-hydroxyvitamin D and risk of lung cancer in male smokers: a nested case–control study. PLoS ONE 2011;6:e20796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wong Y, Hyde Z, McCaul K, et al. In older men, lower plasma 25-hydroxyvitamin D is associated with reduced incidence of prostate, but not colorectal or lung cancer. PLoS ONE 2014;9:e99954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang X, Cui J, Gu J, et al. Plasma 25-hydroxyvitamin D deficiency is associated with the risk of non-small cell lung cancer in a Chinese population. Cancer Biomark 2015;15:663–8. [DOI] [PubMed] [Google Scholar]
- [25].Liu Y, Chen W, Hu Z, et al. Plasma vitamin D levels and vitamin D receptor polymorphisms are associated with survival of non-small cell lung cancer. Chin J Cancer Res 2011;23:33–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tretli S, Schwartz G, Torjesen P, et al. Serum levels of 25-hydroxyvitamin D and survival in Norwegian patients with cancer of breast, colon, lung, and lymphoma: a population-based study. Cancer Causes Control 2012;23:363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cheng T, Neuhouser M. Serum 25-hydroxyvitamin D, vitamin A, and lung cancer mortality in the US population: a potential nutrient-nutrient interaction. Cancer Causes Control 2012;23:1557–65. [DOI] [PubMed] [Google Scholar]
- [28].Anic G, Weinstein S, Mondul A, et al. Serum vitamin D, vitamin D binding protein, and lung cancer survival. Lung Cancer 2014;86:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Heist R, Zhou W, Wang Z, et al. Circulating 25-hydroxyvitamin D, VDR polymorphisms, and survival in advanced non-small-cell lung cancer. J Clin Oncol 2008;26:5596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Vashi P, Edwin P, Popiel B, et al. The relationship between circulating 25-hydroxyvitamin D and survival in newly diagnosed advanced non-small-cell lung cancer. BMC Cancer 2015;15:1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhou W, Heist R, Liu G, et al. Circulating 25-hydroxyvitamin D levels predict survival in early-stage non-small-cell lung cancer patients. J Clin Oncol 2007;25:479–85. [DOI] [PubMed] [Google Scholar]
- [32].Ma K, Xu W, Wang C, et al. Vitamin D deficiency is associated with a poor prognosis in advanced non-small cell lung cancer patients treated with platinum-based first-line chemotherapy. Cancer Biomark 2017;18:297–303. [DOI] [PubMed] [Google Scholar]
- [33].Higashimoto Y, Ohata M, Nishio K, et al. 1 alpha, 25-dihydroxyvitamin D3 and all-trans-retinoic acid inhibit the growth of a lung cancer cell line. Anticancer Res 1996;16:2653–9. [PubMed] [Google Scholar]
- [34].Nakagawa K, Sasaki Y, Kato S, et al. 22-Oxa-1alpha,25-dihydroxyvitamin D3 inhibits metastasis and angiogenesis in lung cancer. Carcinogenesis 2005;26:1044–54. [DOI] [PubMed] [Google Scholar]
- [35].Hershberger P, Modzelewski R, Shurin Z, et al. 1,25-Dihydroxycholecalciferol (1,25-D3) inhibits the growth of squamous cell carcinoma and down-modulates p21(Waf1/Cip1) in vitro and in vivo. Cancer Res 1999;59:2644–9. [PubMed] [Google Scholar]
- [36].Skaaby T, Husemoen L, Thuesen B, et al. Vitamin D status and chronic obstructive pulmonary disease: a prospective general population study. PLoS ONE 2014;9:e90654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hansdottir S, Monick MM, Hinde SL, et al. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol (Baltimore, MD: 1950) 2008;181:7090–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Nakagawa K, Kawaura A, Kato S, et al. 1 alpha,25-Dihydroxyvitamin D(3) is a preventive factor in the metastasis of lung cancer. Carcinogenesis 2005;26:429–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


