Abstract
Rationale:
In clinical work, neuroendocrine synchronous multiplicity carcinoma was relatively rare. Most were confirmed by the pathological diagnosis of a certain part of the body combined with the imaging of the whole body, while cases that had both pathological and immunohistochemistry diagnosis were few.
Patient concerns:
A patient who presented with abdominal pain visited our hospital, and was diagnosed with lesions in both the small intestine and lung.
Diagnoses:
Both were considered primary tumors by imaging, and diagnosed as neuroendocrine carcinomas by pathology.
Interventions:
The intestinal lesion was surgically resected, and the lung tumor treated by chemoradiotherapy.
Outcomes:
The survival time of this patient exceeded 24 months.
Lessons:
The diagnosis relied on clinical, imaging, pathological, and immunohistochemical features, which confirmed a synchronous multiple carcinoma. Treatment was based on the pathological types. Through this case report, the clinical and pathological data of neuroendocrine synchronous multiplicity carcinoma could be enriched.
Keywords: neuroendocrine, pulmonary, small intestine, synchronous multiple carcinoma
1. Introduction
This case is based on the diagnostic criteria of multiple carcinoma were proposed by Warren and Gales in 1932 as each tumor being malignant, with the possibility of metastasis from one another excluded. The imaging features of abdominal computed tomography (CT) and chest CT were considered to reflect primary tumors at the initial patient assessment, and were not consistent with the characteristics of metastatic tumors, after obtaining the tissue samples separately. Neuroendocrine synchronous multiplicity carcinoma was diagnosed clearly according to the pathologic features and specific immune markers. The diagnosis is clear, neuroendocrine synchronous multiplicity carcinoma was relatively rare. Literature shows that neuron-specific enolase (NSE) and pro-gastrin-releasing peptide (ProGRP) play important roles in neuroendocrine tumors. Literature reported such diseases have genetic abnormalities, provide us with the possibility of detection of gene, genetic testing can be performed to monitor the development of disease in patients with recurrent progression.
2. Case report
A 47-year-old female patient presented with dull abdominal pain without nausea, vomiting, melena, cough, or expectoration for >20 days, at the Special Unit of Surgical Department of Shanxi Provincial Cancer Hospital, on May 4, 2015. Abdominal CT in Shanxi Provincial People's Hospital (April 28, 2015 Fig. 1) showed localized intestinal wall thickening of the left lower small intestine accompanied with multiple peripheral lymph nodes. Admission diagnosis was small intestine occupying tumor. In the past, the patient was healthy with specialized examinations performed without remarkable signs. Blood, urine, and stool tests were basically normal. As for tumor markers, NSE levels were 88.48 μg/L (<12 μg/L) and serum ProGRP amounts 1475.35 pg/mL (<45 pg/mL). Immune function was mildly low. Colonoscopy showed no abnormality. On May 4, 2015 (Fig. 2), Chest CT scan showed a giant multinodular fusion-like occupying tumor near the mediastinum in the upper lobe of left lung, indicating lung cancer. The chest mass underwent chest CT guided biopsy. Pathologic assessment suggested a malignant tumor, and immunohistochemistry showed poorly differentiated neuroendocrine carcinoma (Fig. 3). On May 19, 2015, a senior chief physician performed abdominal mass resection under general anesthesia, for 2 hours. Intraoperative blood loss was about 100 mL. During the operation, the mass was found in the jejunum and adhered to the sigmoid colon, with a size of 8 × 7 × 5 cm and high hardness. Resection of the small intestinal mass was performed. The jejunum was cut off at 5 cm away from both ends of the mass, and end-to-end anastomosis of the jejunum was carried out. Partial sigmoid colon adhering to the mass was resected, followed by end-to-end anastomosis. Postoperative pathologic examination suggested type G3 neuroendocrine carcinoma (Fig. 4). The pulmonary lesion had invaded large vessels, and could not be treated surgically. The patient was admitted to Unit One of Department of Respiration of Shanxi Provincial Cancer Hospital on June 8, 2015, and administered the etoposide cisplatinum (EP) regimen (100 mg/m2 Etoposide and 75 mg/m2 Cisplatin) for 6 cycles of systemic chemotherapy. After the 5th EP cycle, the left lung lesion sequentially underwent radiotherapy with 60 Gy for 30 times. The 6th chemotherapy cycle ended on December 9, 2015, and RECIST 1.1 evaluation criteria were used for efficacy evaluation as complete remission (CR) (Figs. 5 and 6). Adverse reactions included grade II myelosuppression and grade II digestive tract reactions. Afterwards, the patient visited Unit One of Department of Respiration quarterly for follow-up by chest-abdomen-pelvis enhanced CT or whole body PET-CT, as well as NSE, ProGRP, and immune function detection. The last follow-up was April 2017, with NSE of 1.48 μg/L (−) and ProGRP of 2.35 pg/mL (−). Efficacy evaluation indicated maintenance of CR.
Figure 1.
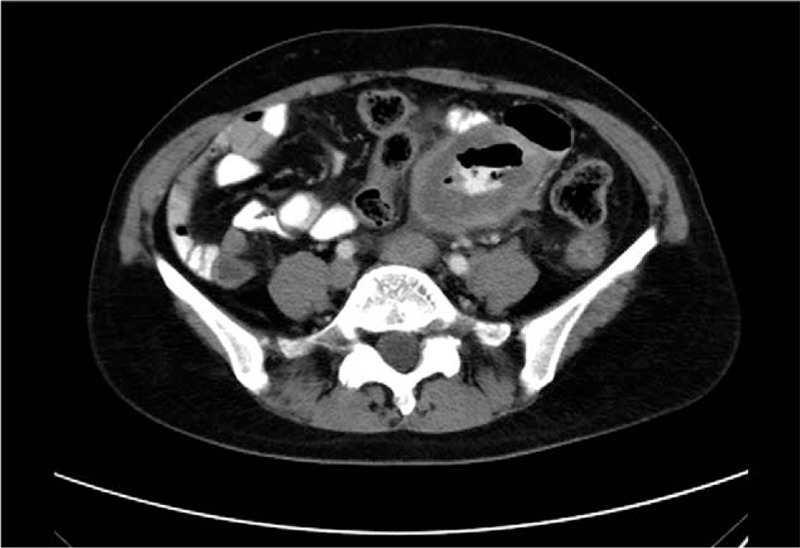
Abdominal enhanced CT (Shanxi Provincial People's Hospital on April 28, 2015) showing localized intestinal wall thickening of the left lower small intestine, and inhomogeneous enhancement after CT enhancement. Finding: localized intestinal wall thickening of the left lower small intestine accompanied with multiple peripheral lymph nodes. CT = computed tomography.
Figure 2.
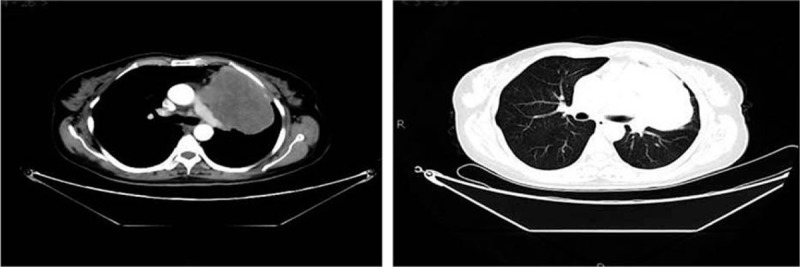
Chest enhanced CT (May 4, 2015) showed giant multinodular fusion-like masses near the mediastinum in the upper lobe of left lung, with incomplete margin and a size of 10.8 × 7.0 × 7.0 cm. Plain scan showed uneven density with inhomogeneous enhancement after enhancement. The mass was near the pulmonary trunk, left pulmonary artery, and left superior pulmonary vein. The left atrium was compressed, and filling defects were found in the left atrium and left upper pulmonary vein, with partial unclear boundaries. CT = computed tomography.
Figure 3.
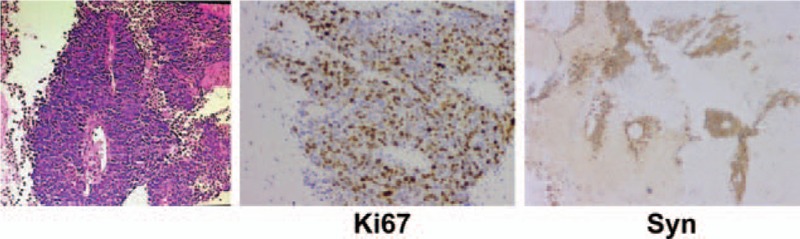
Biopsy of the upper lobe of left lung (May 8, 2015) showing a malignant tumor accompanied with necrosis. Immunohistochemistry revealed AE1/AE3 (+/−), P63 (−), TTF (weak + in focal lesion), Napsin A (−), Syn (+), CgA (+), Ki67 (approximately 60% +), CD56 (+), CD20 (partial +), and CDX2 (−), in accordance with poorly differentiated neuroendocrine carcinoma.
Figure 4.
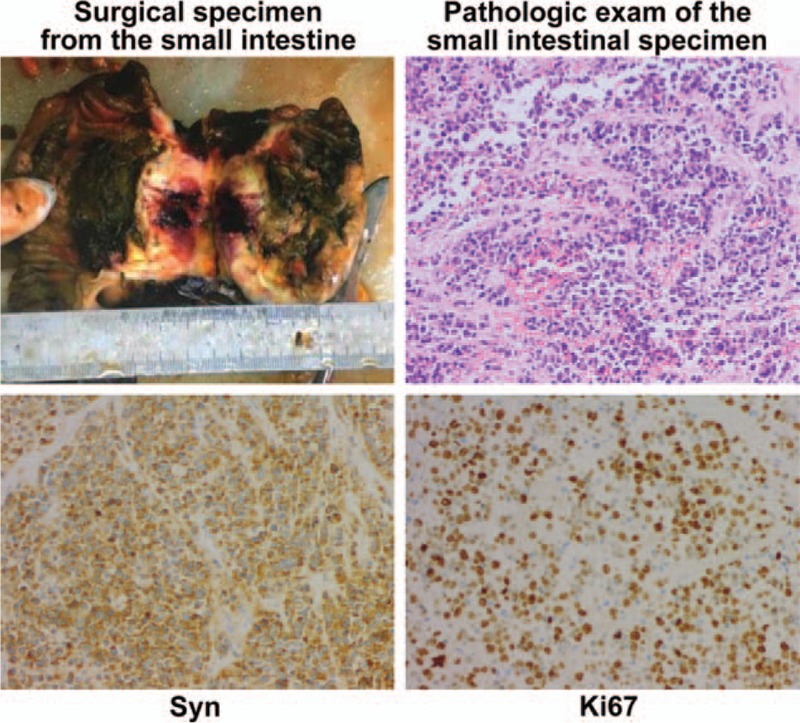
Surgical specimen from the small intestine and pathologic exam (Shanxi Provincial Cancer Hospital on May 19, 2015). Results indicated poorly differentiated carcinoma, AE1/AE3 (−), CAM5.2 (−), CDX2 (−), P63 (−), Syn (+), CgA (+), Ki67 (approximately 80% +), and HER2 (0), consistent with type G3 neuroendocrine carcinoma. The mass size was approximately 8 × 7 × 5 cm. Tumor thrombus was seen in the vessels, with no carcinoma in both surgical margins of the small intestine and colon. Metastatic carcinoma and carcinoma nodules of small mesenteric lymph nodes: 5/14.
Figure 5.
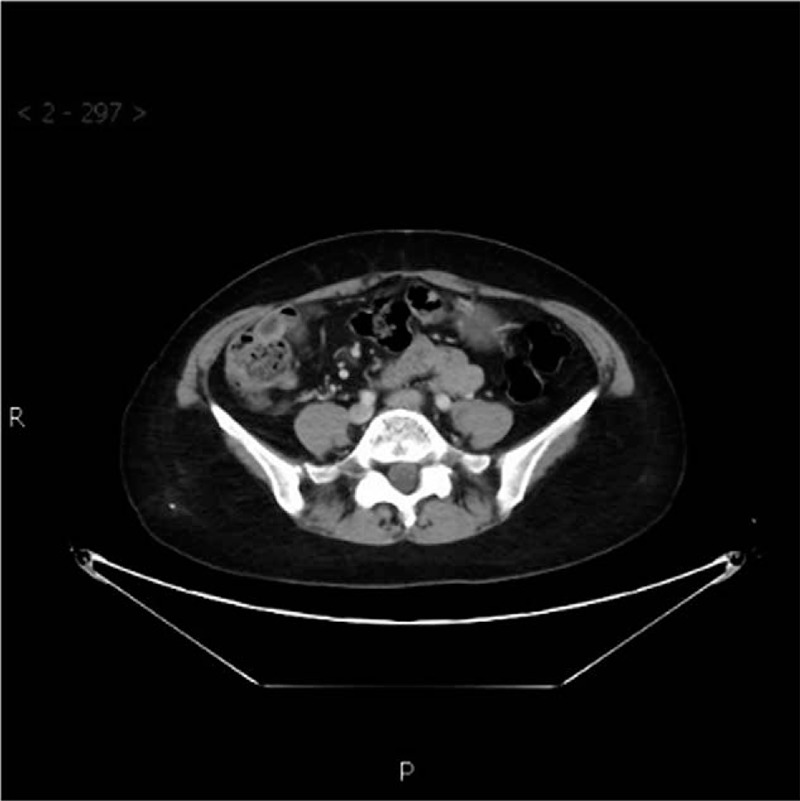
Abdominal enhanced CT (Shanxi Provincial Cancer Hospital on October 19, 2016) showing high density shadow in the lower abdominal intestine, with no overt anastomotic wall thickening and clear peripheral space. CT = computed tomography.
Figure 6.
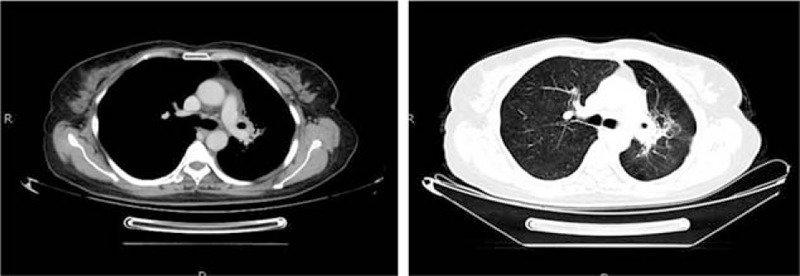
Chest enhanced CT (October 19, 2016), showing a mass shadow with irregular soft tissue density and incomplete margin in the upper lobe of left lung after radiochemotherapy for the left lung lesion. Plain scan showed a patchy area, with a value of 25 HU. After enhancement, the solid part of the lesion was inhomogeneously enhanced, indicating inflammation. CT = computed tomography.
3. Discussion
The diagnostic criteria of multiple carcinoma were proposed by Warren and Gales in 1932[1] as each tumor being malignant, with the possibility of metastasis from one another excluded. In the current patient, the lesions in the lung and small intestine were both malignant tumors, and imaging excluded the possibility of metastasis from one another, reflecting multiple carcinoma.
Neuroendocrine tumors have typical neuroendocrine morphological features, and could be detected by multiple immune markers. Among these, CD56 has the highest sensitivity, while CgA and Syn have highest specificity.[2–4] Of the tumor markers, NSE and ProGRP play important roles in neuroendocrine tumors.[5] Based on pathological morphologies of the small lung biopsy and large small intestine resection specimens, malignancy was considered for both. In combination with immunohistochemical data, neuroendocrine carcinoma was considered.
The CT features of small cell lung cancer were central with pulmonary hilar mass shadow. Cases of small intestine metastases of lung cancer are rare, and the clinical manifestations and imaging data nonspecific.[6] Meanwhile, lung metastases are common, and images are characterized by typical multiple nodules. The imaging features of abdominal CT and chest CT were considered to reflect primary tumors at the initial patient assessment, and were not consistent with the characteristics of metastatic tumors.
Genetically, some small intestinal neuroendocrine neoplasms (NEN) types have familial inheritance. In 2015, the American National Institutes of Health found that hereditary small intestinal NENs have heterozygous mutations in the inositol polyphosphate multikinase gene,[7] frameshift mutations in the cyclin-dependent kinase inhibitor gene,[8] or heterozygous deletion of chromosome 18.[9] Other studies reported that this cancer is related to miRNA.[10] The current patient had neuroendocrine tumors in different organs simultaneously, and genetic factors could not be excluded. Gene detection can be performed with the tissue specimens to define. Also, the detection of circulating tumor cell (CTC) or circulating tumor DNA (ctDNA) by drawing peripheral blood at fixed period during the follow-up can act as the diagnostic method of cancer progression in patients.
For treatment, radical resection as the primary therapy for multiple carcinoma was used, and the small intestinal lesion was surgically removed. Due to the invasion of large vessels and the presence of tumor thrombus, the pulmonary lesion could not be surgically treated. An EP regimen was used as first-line chemotherapy, followed by sequential radiotherapy. The patient's lesions were significantly reduced after chemoradiotherapy. The patient is currently in CR and still under follow-up.
Footnotes
Abbreviations: CR = complete remission, CTC = circulating tumor cell, ctDNA = circulating tumor DNA, NSE = neuron specific enolase, ProGRP = pro-gastrin-releasing peptide.
BS and QZ have contributed equally to this work.
All authors declare that they have no any conflict of interests.
References
- [1].Warren S, Gates O. Multiple primary malignant tumors: a survery of theliterature and a statistical study. Am J Cancer 1932;16:1358–414. [Google Scholar]
- [2].Viberti L, Bongiovanni M, Croce S, et al. 34betaE12 cytokeratin immunodetection in the differential diagnosis of small cell tumors of lung. Int J Surg Pathol 2000;8:317–22. [DOI] [PubMed] [Google Scholar]
- [3].Ionescu DN, Treaba D, Gilks CB, et al. Nonsmall cell lung carcinoma with neuroendocrine differentiation—an entity of no clinical or prognostic significance. Am J Surg Pathol 2007;31:26–32. [DOI] [PubMed] [Google Scholar]
- [4].Lyda MH, Weiss LM. Immunoreactivity for epithelial and neuroendocrine antibodies are useful in the differential diagnosis of lung carcinomas. Hum Pathol 2000;31:980–7. [DOI] [PubMed] [Google Scholar]
- [5].Petrovic M, Bukumiric Z, Zdravkovic V, et al. The prognostic significance of the circulating neuroendocrine markers chromogranin A, pro-gastrin-releasing peptide, and neuron-specific enolase in patients with small-cell lung cancer. Med Oncol 2014;31:823. [DOI] [PubMed] [Google Scholar]
- [6].Kim SY, Ha HK, Park SW, et al. Gastrointestinal metastasis from primary lung cancer: CT findings and clinicopathologic features. AJR Am J Roentgenol 2009;193:W197–201. [DOI] [PubMed] [Google Scholar]
- [7].Sei Y, Zhao X, Forbes J, et al. A hereditary form of small intestinal carcinoid associated with a germline mutation in inositol polyphosphate multikinase. Gastroenterology 2015;149:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Francis JM, Kiezun A, Ramos AH, et al. Somatic mutation of CDKN1B in small intestine neuroendocrine tumors. Nat Genet 2013;45:1483–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang GG, Yao JC, Worah S, et al. Comparison of genetic alterations in neuroendocrine tumors: frequent loss of chromosome 18 in ileal carcinoid tumors. Mod Pathol 2005;18:1079–87. [DOI] [PubMed] [Google Scholar]
- [10].Li SC, Essaghir A, Martijn C, et al. Global microRNA profiling of well-differentiated small intestinal neuroendocrine tumors. Mod Pathol 2013;26:685–96. [DOI] [PMC free article] [PubMed] [Google Scholar]


