Abstract
Background:
The aim of this study was to evaluate the prognostic role of neutrophil–lymphocyte ratio (NLR) in patients with acute ischemic stroke (AIS).
Methods:
PubMed, Embase, Web of Science, Cochrane Library, and China National Knowledge Infrastructure were searched for potential eligible literature. The study characteristics and relevant data were extracted. Odds ratios (ORs) with 95% confidence intervals (CIs) were pooled to estimate the prognostic role of NLR in patients with AIS. Poor functional outcome was defined as modified Rankin Scale ≥ 3.
Results:
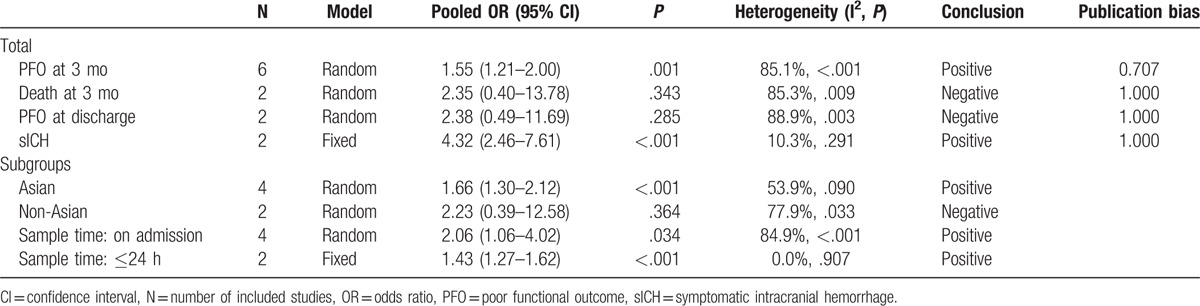
Nine studies with 2947 patients were included. The pooled OR of higher NLR for poor functional outcome at 3 months was 1.55 (95% CI, 1.21–2.00). The pooled ORs for death at 3 months, poor functional outcome at discharge, and symptomatic intracranial hemorrhage (sICH) were 2.35 (95% CI, 0.40–13.78), 2.38 (95% CI, 0.49–11.69), and 4.32 (95% CI, 2.46–7.61), respectively.
Conclusion:
For patients with AIS, higher NLR was associated with poorer functional outcome at 3 months and may be associated with a higher risk of developing sICH. This readily available and inexpensive marker may be helpful in future clinical and research work. However, due to the limited number of included studies, more well-designed studies are warranted to further clarify this issue.
Keywords: acute ischemic stroke, neutrophil–lymphocyte ratio, prognosis, survival
1. Introduction
Stroke is the second-leading cause of death and the third-leading cause of morbidity worldwide.[1,2] In China, the annual stroke mortality rate is approximately 157 per 100,000 people, making it the leading cause of mortality and adult disability.[3] Stroke also has a considerable impact on healthcare expenditures.[3,4] Among all strokes, ischemic stroke accounts for approximately 80% to 85%, and is characterized by the disruption of cerebral blood flow.[5] Current treatment strategies for acute ischemic stroke (AIS) include intravenous or intraarterial recombinant tissue plasminogen activator (rt-PA) and mechanical endovascular therapies.[6] Besides the already known unfavorable prognostic factors in AIS, it is worthwhile to detect new ones and control them at an early stage.[7–9]
It has been suggested that inflammatory response is implicated in all stages of AIS.[10] Ischemic tissues release chemokines and cytokines, and recruit peripheral circulating leukocytes.[11] Among the leukocytes, neutrophils were found to be an important mediator and exacerbate ischemic brain injury.[12] And early neutrophilia has been identified to be associated with larger infarct volumes and increased stroke severity.[13] Lymphocytes also infiltrate the ischemic tissues and mediate inflammatory responses.[11] Some researchers have demonstrated that lower lymphocyte counts were associated with poor functional outcome at 3 months.[14] Neutrophil–lymphocyte ratio (NLR), as a systemic inflammation marker, has been reported to be correlated with poor prognosis in patients with various cancers and acute coronary syndrome.[15,16] Recently, NLR was shown to predict short-term outcome in patients with AIS.[17,18]
The aim of this study was to systematically summarize the existing evidence on the prognostic role of NLR in patients with AIS through performing a meta-analysis.
2. Methods
2.1. Search strategy
Since this is a meta-analysis, ethical approval was not necessary. We followed the developed guidelines for systematic reviews and meta-analyses in performing our study.[19] Databases, including PubMed, Embase, Web of Science, Cochrane Library, and China National Knowledge Infrastructure, were searched for relevant literature (last search ran on May 5, 2017). The following search terms were used: “neutrophil lymphocyte ratio” AND (“Stroke” OR “Brain Ischemia” OR “Brain Infarction” OR “Cerebral Infarction”). Reference lists of relevant articles were also screened for additional studies. Languages were restricted to English and Chinese.
2.2. Study selection
The study selection process was performed independently by 2 reviewers (JZ and YS), with any disagreements being discussed. Studies were considered eligible according to the following inclusion criteria: the patients were diagnosed with AIS; white blood cell counts were assessed after admission, and NLR was calculated; patients were followed up for survival outcomes or functional outcomes; and enough data were reported to estimate the prognostic role of NLR in patients with AIS. Conference abstracts, letters, case reports, reviews, unrelated articles, and studies without enough data were excluded.
2.3. Data extraction
Two researchers (JZ and QR) independently extracted relevant data from the eligible studies and disagreements were resolved by consensus. The primary data were odds ratio (OR) for poorer outcomes with 95% confidence interval (CI), or the data that could be used to calculate the OR and 95% CI. OR calculated form multivariate analysis was extracted if both univariate and multivariate analyses were provided. The study and patients’ characteristics included first author, publication year, patient source, number of patients, median or mean age of patients, interval between stroke onset and admission, sampling time of the blood, and the cut-off value of NLR.
2.4. Statistical analysis
We define poor functional outcome as modified Rankin Scale (mRS) ≥ 3. The logOR and variance were calculated from the OR and 95% CI, and were used for aggregation. Forest plots were used to estimate the pooled prognostic role of NLR in patients with AIS. The pooled OR was considered significant if the P value was <.05 and the 95% CI did not overlap 1. Subgroup analyses were performed based on patient source, and sampling time of the blood. The between-study heterogeneity was also assessed, with P < .10 or I2 > 50% implying significant heterogeneity.[20] A random-effects model was used if heterogeneity was present. Furthermore, sensitivity analysis was performed to test the contribution of each study to heterogeneity by excluding individual studies one at a time. Publication bias was assessed by Begg test with P > .05 indicating no significant publication bias. All the statistical analyses above were performed by STATA 11.0 (STATA Corporation, College Station, TX).
3. Results
3.1. Literature research
The initial literature search retrieved 412 studies. After removing duplicates, 289 studies were screened by titles and abstracts. Then, 262 studies were excluded according to the predefined criteria. The rest 27 studies were assessed in full text and 18 were further excluded due to unrelated or lack of enough data. Eventually, 9 articles[7–9,21–26] met the inclusion criteria and were included. The study selection process was shown in Fig. 1.
Figure 1.

Selection process of studies.
3.2. Study characteristics
The basic characteristics of the 9 included studies were shown in Table 1. Among them, 6 were from China, 1 was from Korea, 1 was from the United States, and 1 was from France and Finland. A total of 2947 patients were included (mean 327). Blood samples were drawn on admission or in the first 24 h after admission. The cut-off values of NLR varied between studies. The outcome measures included poor functional outcome at discharge, poor functional outcome at 3 months, death at 3 months, and symptomatic intracranial hemorrhage (sICH). All the ORs were adjusted except one (not reported).
Table 1.
Characteristics of the included studies.

3.3. Overall analysis
Six studies used the outcome measure of poor functional outcome at 3 months. The pooled OR of the 6 studies was 1.55 (95% CI, 1.21–2.00) (Fig. 2), indicating that higher NLR was associated with higher risk of poor functional outcome at 3 months in patients with AIS. Significant between-study heterogeneity was observed (I2 = 85.1%, P < .001). Sensitivity analysis identified that the study by Maestrini et al[8] was a significant contributor to the heterogeneity. After excluding this study, the heterogeneity shrinked to 58.3% and the pooled OR remained statistically significant (OR 1.76; 95% CI, 1.33–2.33).
Figure 2.
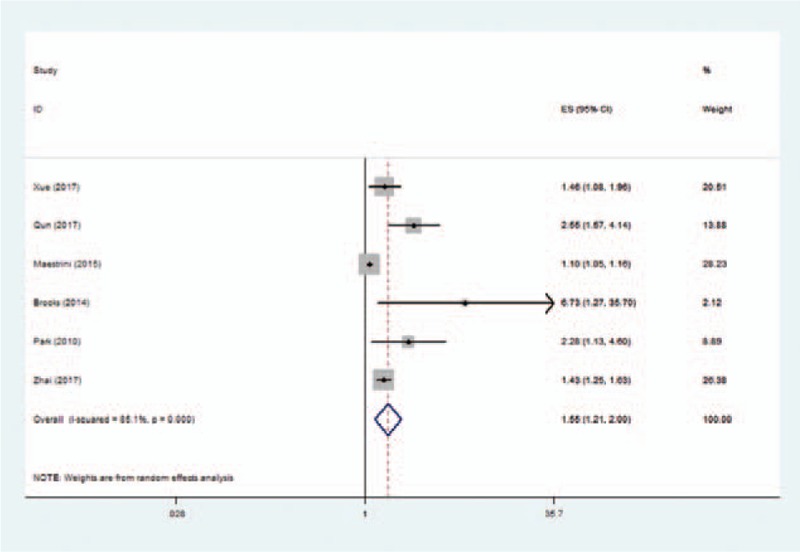
Pooled odds ratio of higher neutrophil–lymphocyte ratio for poor functional outcome at 3 months in patients with acute ischemic stroke.
Two studies used the outcome measure of death at 3 months, and the pooled OR of higher NLR was 2.35 (95% CI, 0.40–13.78). Another 2 studies used the outcome measure of poor functional outcome at discharge, and the pooled OR of higher NLR was 2.38 (95% CI, 0.49–11.69). Two studies examined the risk of sICH in patients with AIS, and the pooled OR of higher NLR was 4.32 (95% CI, 2.46–7.61).
3.4. Subgroup analysis
We subdivided the 6 studies using the outcome measure of poor functional outcome at 3 months. The pooled OR was 1.66 (95% CI, 1.30–2.12) for the 4 studies from Asia, and 2.23 (95% CI, 0.39–12.58) for the 2 non-Asian studies. Besides, 4 studies used blood samples on admission, and the pooled OR was 2.06 (95% CI, 1.06–4.02). The rest 2 studies using blood samples in 24 h after admission had a pooled OR of 1.43 (95% CI, 1.27–1.62).
All the pooled results above were shown in Table 2.
Table 2.
Summary of meta-analysis results.
3.5. Publication bias
No significant publication bias was found in the meta-analysis (Table 2). The Begg plot of publication bias of the 6 studies (using the outcome measure of poor functional outcome at 3 months) was shown in Fig. 3 (P = .707).
Figure 3.

The Begg publication bias plot of the 6 studies using the outcome measure of poor functional outcome at 3 months.
4. Discussion
This study aimed to evaluate the prognostic role of NLR in patients with AIS. We performed a meta-analysis of the relevant literature. To our best knowledge, this is the first meta-analysis on this topic. Our results showed that higher NLR was associated with poorer functional outcome at 3 months and may be associated with a higher risk of developing sICH.
Subgroup analyses were performed to examine the role of NLR in predicting functional outcome at 3 months in different settings. In Asian patients, higher NLR was found to be associated with worse functional outcome, but the association was not significant among non-Asian participants. However, the conclusion should be treated with caution, since the number of studies in each subgroup was limited, especially in the non-Asian group. We also divided the studies according to sampling time after admission. The pooled OR of the 4 studies using blood sample on admission was higher than that of the 2 studies using blood sample in 24 h after admission (2.06 vs 1.43). A possible explanation might be due to the dynamic change of leukocytes after stroke. So, exploring dynamic change of NLR in patients with AIS and the role of NLR at different time is a future direction, just like Guo et al[22] did.
As we mentioned above, inflammatory response is important in ischemic stroke. And elevated white blood cell count is associated with worse outcome in patients with AIS.[27] Neutrophils are recruited rapidly in cerebral vessels within hours.[28] Higher neutrophil counts are shown to be related with more severe stroke at admission.[14] Neutrophils infiltrated in ischemic brain may lead to the release of inflammatory mediators, thus damaging the tissues.[29] Besides, some investigators suggested that the inverse effect of higher neutrophil counts may be due to the release of matrix metalloproteinase-9 by neutrophils.[8] Lymphocytes accumulate in the ischemic brain 3 to 6 days after stroke, which is later than neutrophils.[30] The role of lymphocytes in AIS is controversial. Some studies proved that lymphocytes could repair the injury by inflammation.[7,31] However, some studies demonstrated that lymphocytes could cause the release of proinflammatory cytokines and cytotoxic substances which damage the ischemic brain.[32] Kim et al[14] found that, in patients with AIS, lower lymphocyte counts were associated with poor functional outcome at 3 months.
NLR reflects the balance between neutrophils and lymphocytes.[8] Every included study reported a negative impact of higher NLR on patients’ outcome. And our meta-analysis demonstrated that higher NLR was associated with poorer functional outcome at 3 months and higher risk of developing sICH. More work is needed to explore the underlining molecular mechanism. Although we fail to prove the correlation between higher NLR and poorer functional outcome at discharge or higher risk of death at 3 months, the numbers of studies using these outcome measures were both only 2, thus more studies were needed to clarify this.
This readily available and inexpensive test could be used as a predictor of outcome in patients with AIS.[9] NLR could be used to predict outcomes with other markers, and even be used as a criterion for patients’ enrollment in clinical trials.[9] Moreover, these findings also support the anti-inflammatory therapy as a potential treatment for AIS.[21]
Significant between-study heterogeneity was present in this meta-analysis, and sensitivity analysis revealed that the study by Maestrini et al[8] was a major contributor to the heterogeneity. After excluding this study, the heterogeneity shrinked from 85.1% to 58.3% and the pooled OR remained statistically significant. The reason why this study contributed greatly to heterogeneity might be that it was the only study that included patients admitted within the time window of using rt-PA and collected blood samples before the bolus of rt-PA. Other potential sources of heterogeneity may be from different patient sources, different time of blood collection after admission, and different cut-off values NLR.
There were several limitations in our study. First, the meta-analysis was based on a limited number of studies, and the number of studies in each subgroup was even smaller. Therefore, caution should be applied as to the subgroup results. Second, some characteristics differed between the studies, such as the blood sampling time and cut-off values of NLR. So, more studies are needed to validate the prognostic role of NLR in patients with AIS. In addition, most of the included studies were from Asia (and most from China), the overall conclusions may need to be treated with caution. Furthermore, significant between-study heterogeneity was present and random-effects model was used. Besides, publication bias was a major concern for all meta-analyses. Although no publication bias was found in our study, it should not be completely excluded.
In conclusion, our results suggested that, for patients with AIS, higher NLR was associated with poorer functional outcome at 3 months and may be associated with a higher risk of developing sICH. This readily available and inexpensive marker may be a helpful tool in clinical work. These findings also suggest further exploration on modulating immune response to treat AIS. However, due to the limited number of included studies, more well-designed studies are warranted to further clarify this issue.
Acknowledgment
The authors thank the reviewers for their constructive comments.
Footnotes
Abbreviations: AIS = acute ischemic stroke, CI = confidence interval, mRS = modified Rankin Scale, NLR = neutrophil–lymphocyte ratio, OR = odds ratio, rt-PA = recombinant tissue plasminogen activator, sICH = symptomatic intracranial hemorrhage.
JZ, QR, and YS have contributed equally to this work.
This work was supported by the National Key Technology Research and Development Program of the Ministry of Science and Technology of China; the National Brain Tumor Registry of China (NBTRC) (the Key Control Technology Research for Ischemic Cerebrovascular Disease and Brain Tumor, 2015BAI12B04).
The authors have no conflicts of interest to disclose.
References
- [1].Goldstein LB, Bushnell CD, Adams RJ, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:517–84. [DOI] [PubMed] [Google Scholar]
- [2].Powers WJ, Derdeyn CP, Biller J, et al. 2015 American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2015;46:3020–35. [DOI] [PubMed] [Google Scholar]
- [3].Liu L, Wang D, Wong KS, et al. Stroke and stroke care in China: huge burden, significant workload, and a national priority. Stroke 2011;42:3651–4. [DOI] [PubMed] [Google Scholar]
- [4].Zhao L, Dai Q, Chen X, et al. Neutrophil-to-lymphocyte ratio predicts length of stay and acute hospital cost in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis 2016;25:739–44. [DOI] [PubMed] [Google Scholar]
- [5].Allen CL, Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke 2009;4:461–70. [DOI] [PubMed] [Google Scholar]
- [6].Altintas O, Altintas MO, Tasal A, et al. The relationship of platelet-to-lymphocyte ratio with clinical outcome and final infarct core in acute ischemic stroke patients who have undergone endovascular therapy. Neurol Res 2016;38:759–65. [DOI] [PubMed] [Google Scholar]
- [7].Xue J, Huang W, Chen X, et al. Neutrophil-to-lymphocyte ratio is a prognostic marker in acute ischemic stroke. J Stroke Cerebrovasc Dis 2017;26:650–7. [DOI] [PubMed] [Google Scholar]
- [8].Maestrini I, Strbian D, Gautier S, et al. Higher neutrophil counts before thrombolysis for cerebral ischemia predict worse outcomes. Neurology 2015;85:1408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Brooks SD, Spears C, Cummings C, et al. Admission neutrophil-lymphocyte ratio predicts 90 day outcome after endovascular stroke therapy. J Neurointerv Surg 2014;6:578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chamorro A, Hallenbeck J. The harms and benefits of inflammatory and immune responses in vascular disease. Stroke 2006;37:291–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kim JY, Park J, Chang JY, et al. Inflammation after ischemic stroke: the role of leukocytes and glial cells. Exp Neurobiol 2016;25:241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Herz J, Sabellek P, Lane TE, et al. Role of neutrophils in exacerbation of brain injury after focal cerebral ischemia in hyperlipidemic mice. Stroke 2015;46:2916–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Buck BH, Liebeskind DS, Saver JL, et al. Early neutrophilia is associated with volume of ischemic tissue in acute stroke. Stroke 2008;39:355–60. [DOI] [PubMed] [Google Scholar]
- [14].Kim J, Song TJ, Park JH, et al. Different prognostic value of white blood cell subtypes in patients with acute cerebral infarction. Atherosclerosis 2012;222:464–7. [DOI] [PubMed] [Google Scholar]
- [15].Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. [DOI] [PubMed] [Google Scholar]
- [16].Tamhane UU, Aneja S, Montgomery D, et al. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol 2008;102:653–7. [DOI] [PubMed] [Google Scholar]
- [17].Tokgoz S, Keskin S, Kayrak M, et al. Is neutrophil/lymphocyte ratio predict to short-term mortality in acute cerebral infarct independently from infarct volume? J Stroke Cerebrovasc Dis 2014;23:2163–8. [DOI] [PubMed] [Google Scholar]
- [18].Celikbilek A, Ismailogullari S, Zararsiz G. Neutrophil to lymphocyte ratio predicts poor prognosis in ischemic cerebrovascular disease. J Clin Lab Anal 2014;28:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Qun S, Tang Y, Sun J, et al. Neutrophil-to-lymphocyte ratio predicts 3-month outcome of acute ischemic stroke. Neurotox Res 2017;31:444–52. [DOI] [PubMed] [Google Scholar]
- [22].Guo Z, Yu S, Xiao L, et al. Dynamic change of neutrophil to lymphocyte ratio and hemorrhagic transformation after thrombolysis in stroke. J Neuroinflammation 2016;13:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Park JK, Oh HG, Park TH. Neutrophil to lymphocyte ratio at admission: prognostic factor in patients with acute ischemic stroke. J Korean Neurol Assoc 2010;28:172–8. [Google Scholar]
- [24].Zhai M, Wang J, Yu L, et al. Neutrophil and lymphocyte ratios for the predictive analysis of the prognosis in patients with acute cerebral infarction. Chin J Cerebrovasc Dis 2017;14:82–6. [Google Scholar]
- [25].Zhao L, Chen X, Xu X, et al. Predictive value of leukocyte differential count in patients with acute cerebral infarction. J Med Postgra 2015;28:1148–51. [Google Scholar]
- [26].Gao W, Han Z, Du Y, et al. Association between neutrophil lymphocyte ratio and prognosis of acute ischemic stroke. J Clin Pathol Res 2014;34:509–13. [Google Scholar]
- [27].Furlan JC, Vergouwen MD, Fang J, et al. White blood cell count is an independent predictor of outcomes after acute ischaemic stroke. Eur J Neurol 2014;21:215–22. [DOI] [PubMed] [Google Scholar]
- [28].Kleinig TJ, Vink R. Suppression of inflammation in ischemic and hemorrhagic stroke: therapeutic options. Curr Opin Neurol 2009;22:294–301. [DOI] [PubMed] [Google Scholar]
- [29].Hartl R, Schurer L, Schmid-Schonbein GW, et al. Experimental antileukocyte interventions in cerebral ischemia. J Cereb Blood Flow Metab 1996;16:1108–19. [DOI] [PubMed] [Google Scholar]
- [30].Li GZ, Zhong D, Yang LM, et al. Expression of interleukin-17 in ischemic brain tissue. Scand J Immunol 2005;62:481–6. [DOI] [PubMed] [Google Scholar]
- [31].Schwartz M, Moalem G. Beneficial immune activity after CNS injury: prospects for vaccination. J Neuroimmunol 2001;113:185–92. [DOI] [PubMed] [Google Scholar]
- [32].Kim JY, Kawabori M, Yenari MA. Innate inflammatory responses in stroke: mechanisms and potential therapeutic targets. Curr Med Chem 2014;21:2076–97. [DOI] [PMC free article] [PubMed] [Google Scholar]


