Opinion statement
Merkel cell carcinoma (MCC) is a rare and aggressive neuroendocrine tumor of the skin. Early-stage disease can be cured with surgical resection and radiotherapy (RT). Sentinel lymph node biopsy (SLNB) is an important staging tool, as a microscopic MCC is frequently identified. Adjuvant RT to the primary excision site and regional lymph node bed may improve locoregional control. However, newer studies confirm that patients with biopsy-negative sentinel lymph nodes may not benefit from regional RT. Advanced MCC currently lacks a highly effective treatment as responses to chemotherapy are not durable. Recent work suggests that immunotherapy targeting the programmed cell death receptor 1/programmed cell death ligand 1 (PD-1/PD-L1) checkpoint holds great promise in treating advanced MCC and may provide durable responses in a portion of patients. At the same time, high-throughput sequencing studies have demonstrated significant differences in the mutational profiles of tumors with and without the Merkel cell polyomavirus (MCV). An important secondary endpoint in the ongoing immunotherapy trials for MCC will be determining if there is a response difference between the virus-positive MCC tumors that typically lack a large mutational burden and the virus-negative tumors that have a large number of somatic mutations and predicted tumor neoantigens. Interestingly, sequencing studies have failed to identify a highly recurrent activated driver pathway in the majority of MCC tumors. This may explain why targeted therapies can demonstrate exceptional responses in case reports but fail when treating all comers with MCC. Ultimately, a precision medicine approach may be more appropriate for treating MCC, where identified driver mutations are used to direct targeted therapies. At a minimum, stratifying patients in future clinical trials based on tumor viral status should be considered as virus-negative tumors are more likely to harbor activating driver mutations.
Keywords: Merkel cell carcinoma, Neuroendocrine, Polyomavirus, MCV, CM2B4, VP1, PI3K, AKT, FDG-PET, mTOR, MLN0128, PD-L1, Avelumab, Pembrolizumab, Idelalisib, Cabozantinib, Pazopanib, Imatinib, Octreotide, Vaccine
Introduction
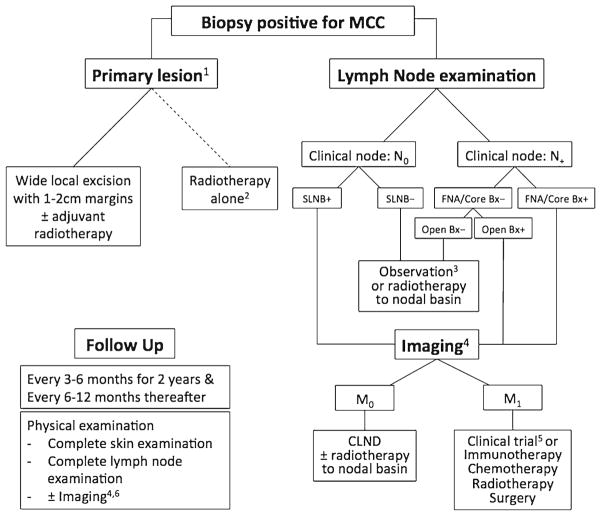
Merkel cell carcinoma (MCC) is a rare neuroendocrine skin cancer. MCC is most common on body areas with higher ultraviolet exposure in Caucasian patients of advanced age [1]. There is also a subset of cases associated with immunocompromised states and a worse prognosis [2, 3•]. The overall 5-year survival for node-negative disease is 64 %, but in those with regional nodal disease or metastatic disease at presentation, the 5-year survival drops to 39 and 18 %, respectively [4]. Factors important in staging MCC are the following: size (greater or less than 2 cm); invasion to underlying structures; lymph node involvement, with a distinction between clinical and pathological node status; and the presence of metastases [5]. Changes in the forthcoming eighth edition AJCC cancer staging handbook may also incorporate the recent observations that nodal MCC with an unknown primary has a better prognosis than comparable stage III disease with a known primary tumor [3•, 6]. In 2008, the discovery of Merkel cell polyomavirus (MCV), believed to be involved in malignant transformation of most cases of MCC, prompted many new directions of research [7]. With an estimated annual incidence of only 0.79 per 100,000 persons (~1600 cases in the USA) in 2011 [8], large prospective clinical trials are difficult to perform and are widely lacking in the literature. As a consequence, the evidence supporting management recommendations for MCC (Fig. 1) relies heavily on small case series and expert opinion [9].
Fig. 1.
Merkel cell carcinoma general treatment algorithm. 1 Definitive treatment of primary lesion should not occur prior to SLNB. 2 Reserved for patients who are poor surgical candidates. 3 The benefit of RT to SLNB-negative basin is unclear- consider observation for low-risk patients with a small primary tumor (<1 cm); in head and neck disease, the risk of false-negative SLNB is higher. 4 PET-CT is the preferred modality; when unavailable, CT and MRI can be used. 5 NCCN guidelines recommend a clinical trial as a first-line treatment for M1 disease. 6 As clinically indicated in high-risk patients. SLNB sentinel lymph node biopsy, FNA fine needle aspiration, CLND completion lymph node dissection.
Diagnosis and work-up
Histological diagnosis
Applying proper immunostains is critical for accurate diagnosis.
The accurate diagnosis of MCC often requires the evaluation of histology and immunostaining by an expert dermatopathologist. Although hematoxylin and eosin (H&E) staining is often diagnostic, a diagnosis of MCC should be confirmed with neuroendocrine and epithelial makers such as neuron-specific enolase (NSE), synaptophysin, chromogranin, neurofilament (NF), epithelial membrane antigen (EMA), cytokeratin (CK) AE1/AE3, and CK20 [10•]. Of these markers, CK20 is the most commonly used for MCC and typically demonstrates a characteristic paranuclear punctate positivity [11]. However, not every MCC tumor stains positively for CK20. As metastatic small cell lung cancer, melanoma, and lymphoma can resemble MCC on H&E, confirming negative staining for thyroid transcription factor 1 (TTF-1), S-100, and leukocyte common antigen (LCA), respectively, is advisable in most cases.
Sentinel lymph node biopsy
Lymph nodes should be pathologically evaluated in almost every case of MCC.
In the past, sentinel lymph node biopsy (SLNB) was more controversial for patients with MCC. Now, it is clear that pathologically node-negative disease has a survival benefit over clinically node-negative disease, a fact that should be considered when offering SLNB [4, 12]. Micrometastatic lymph node disease was present in nearly 30 % of 721 cases included in a recent literature review, reinforcing the utility of SLNB [10•]. Ultimately, SLNB evaluated with immunostains may be beneficial for prognostication and directing treatment in practically all patients with clinically node-negative MCC regardless of tumor size, as is recommended in the most recent National Comprehensive Cancer Network (NCCN) guidelines [4, 9, 13]. More recent work suggests that when evaluating nodal positivity, the number of positive lymph nodes [14•] and the histological presence of sheets of tumor cells [15] may correlate with worse prognosis.
Imaging
Consider imaging for staging in all patients with high-risk disease.
Consider imaging in patients with negative pathological lymph nodes if the primary tumor possesses aggressive features.
There are no head-to-head studies comparing 2-fluoro-[18F]-deoxy-2-D-glucose (FDG)-positron emission tomography (PET) with somatostatin receptor (SSTR)-PET, but both are superior to computed tomography (CT) alone and may be complementary.
The identification of distant metastases during staging can alter the risk-to-benefit assessment of highly aggressive local and regional treatment plans. According to the NCCN guidelines, patients with advanced MCC, including those with grossly positive lymph nodes, should be evaluated with imaging studies. Imaging can also be considered in early-stage MCC for patients whose tumors possess features suggesting poor prognosis and where surgery is expected to have high morbidity.
The ideal imaging modality for MCC has yet to be determined. There are many retrospective studies, as well as meta-analyses, supporting the use of FDG-PET/CT, citing high specificity and sensitivity, and often upstaging patients [16, 17]. CT alone has varying sensitivities for distant and nodal metastases [18, 19] and may be inadequate for sites such as bone and bone marrow [16]. The ability of FDG-PET/CT to detect low-tumor burden metastases is due to the high metabolic activity of MCC. However, this strength is also its weakness as it may fail to identify well-differentiated tumors and, conversely, lead to false-positive results by detecting unrelated foci of non-specific inflammation [20]. Despite advances in imaging, SLNB with immunostains remains the standard for detecting occult nodal metastases [16, 21].
More recently, there has been investigation on the use of somatostatin analogues as imaging modalities, which can be useful because neuroendocrine tumors such as MCC have been found to express SSTRs [22]. Classically performed using 111In-diethylenetriaminepentaacetic acid (DTPA)-octreotide, SSTR-PET is evolving to include newer radiolabeled tracers bound to octreotide, including 68Ga-DOTATATE and 68Ga-DOTATOC, which, when combined with CT, can have high sensitivity for metastases [23]. Cost and availability may factor into the choice between FDG-PET/CT and SSTR-PET/CT, and further comparison studies of the two techniques are needed [24]. An advantage of SSTR is that it also identifies tumors that may be targeted with somatostatin analogues for treatment. One notable drawback, however, is that because of higher background uptake in certain organs, it has lower sensitivity in identifying disease in the liver, adrenal glands, pancreas, thyroid, and spleen [20].
The timing of imaging during post-treatment follow-up also lacks clear evidence. Since nearly all recurrences happen within the first 2 years, with a median of 9 months [12], early and repeated scans are recommended for patients at increased risk of recurrence every 3 to 6 months for the first 2 years [16]. While there are no prospective studies to evaluate imaging frequency, the benefits of more frequent imaging should be weighed against the cost and risk to the patient of repeated scans, including false-positive results. According to current NCCN guidelines, routine imaging should be considered for high-risk patients, which may include patients with clinically node-positive disease and higher. Until treatments for advanced MCC are able to improve disease-specific survival, early detection of distant metastatic disease in asymptomatic patients with MCC may be of limited value. The morbidity associated with conventional systemic therapy and the consequences of false-positive scans should be given consideration when ordering follow-up imaging.
Viral status
Of MCCs, ~80 % are MCV positive.
MCV-negative tumors are associated with more genetic mutations, some of which are potential targets of therapy.
Establishing a standard clinical test for MCV status is needed.
MCV is a double-stranded, non-enveloped DNA virus that has been reported in roughly 80 % of MCC tumors [7], although perhaps significantly less common in tumors from Australian patients [25]. The virus is ubiquitous in the general population and is believed to be acquired in childhood as an asymptomatic primary infection [26]. In contrast to the common episomal infections, MCV DNA in MCC is clonally integrated into the tumor genome [7]. The current lack of a Clinical Laboratory Improvement Amendments (CLIA)-approved test for MCV presents a challenge when comparing and validating studies involving MCC viral status. Detecting MCV in tumor samples is most commonly done by quantitative PCR or immunostaining. There is currently no standard protocol for the PCR detection of MCV DNA in patient samples, and the appropriate threshold for calling a sample positive is a matter of debate. Antibodies have been raised against various viral proteins including small tumor antigen (sTAg), large tumor antigen (LTAg), and the capsid protein VP1. However, only CM2B4, a monoclonal antibody against LTAg, is commercially available. A study using Ab3, a novel LTAg antibody purported to be more sensitive than other antibodies, reported MCV positivity in an unexpected 97 % of MCC tumors [27]; however, these findings have not been replicated by other laboratories. Newer methods have recently been developed for MCV detection, including in situ hybridization and an antibody to sTAg, which may be more sensitive than CM2B4 staining alone [28]. With many test options having varying claims of sensitivity and specificity, comparative studies and establishing a gold standard for MCV detection are needed to more accurately stratify patients for clinical investigations.
Measuring levels of circulating antibodies against MCV proteins is a way to assess humoral immune responses against the virus. Antibodies against VP1 are prevalent in the general population, whereas antibodies against T antigens (TAgs) are more specific to patients with MCC [29]. Interestingly, high levels of VP1 antibodies at the time of MCC diagnosis may portend a better prognosis [30, 31]. In the patients that have them, antibodies against TAgs appear to reflect disease burden, falling after treatment and rising with known or occult recurrent disease [29, 30]. Future directions may see these titers being monitored, much like prostate-specific antigen in prostate cancer, and are already commercially available (http://www.merkelcell.org/sero/).
Recent studies using panel sequencing or whole exome sequencing have provided insights into the biological differences between MCV-positive and MCV-negative MCC. In MCV-positive MCCs, p53 mutations, which are often associated with ultraviolet damage, are uncommon [32]. In contrast, MCV-negative MCCs are much more likely to contain p53 mutations. Additionally, virus-negative MCCs contain, with varying frequencies, mutations in NOTCH, NF1, FGF receptor 2 (FGFR2), and the PI3K/AKT pathway, among others [33, 34•, 35•]. MCV-positive MCCs, however, carry a relatively low mutational burden and lack enrichment of UV signature mutations.
Established treatments
Surgery
Excision should be done with 1- to 2-cm margins for local disease.
SLNB should be done at the time of surgery for patients with clinically negative nodes.
Surgical management of early local disease is primarily by wide local excision with 1–2-cm margins down to fascia, with the goal to achieve clear surgical margins. However, no prospective data is available to correlate the margin size with recurrence risk. When tissue sparing is critical due to the anatomic location of the tumor, techniques with complete peripheral and deep margin control (e.g. Mohs micrographic surgery) may be considered, provided they do not interfere with SLNB when indicated. Positive SLNB, fine needle aspiration, or core needle biopsy should be followed by completion lymph node dissection (CLND) and/or RT, although prospective studies are lacking. Location of the lymph node bed is an important consideration when recommending treatment. RT to the inguinal nodal basin may have lower morbidity than CLND, whereas axillary CLND may be better tolerated than RT [36]. Advances in intensity-modulated radiation therapy (IMRT) may be able to reduce adverse events associated with RT; however, prospective trials will be needed to assess the efficacy and safety of IMRT in MCC.
Radiation therapy
Adjuvant RT improves locoregional control with reduced recurrence rate but may not affect overall survival.
RT does not offer an advantage in the setting of negative SLNB.
RT may be helpful for locoregional control in inoperable cases.
RT is a useful treatment adjunct as MCC tumors are known to be radiation sensitive [37]. Adjuvant RT to the tumor bed for local control may be associated with lower rates of local recurrence, though the impact on survival is unclear. Regional directed RT is also used to improve MCC recurrence-free survival [3•, 36]. To optimize locoregional control, 5-cm RT field margins are recommended, with a minimum of 2 cm when anatomy is constraining [9, 38].
RT may also be useful in specific situations where tissue-sparing techniques are required. A single-institution retrospective study of 179 patients showed a reduced local recurrence rate after RT to excision sites with <10-mm surgical margins, even with microscopically positive margins, although overall survival was unchanged. They recommend at least 50 Gy following an excision with narrow margins, suggesting that narrow margins plus RT may have similar outcomes to wide margins without RT and may be potentially useful in patients for whom a wide excision may carry higher morbidity [39]. Even RT alone to an unresectable tumor can be beneficial for locoregional control [40, 41].
To date, most of the studies on the adjuvant use of RT for MCC remain retrospective with shortcomings including missing or mixed SLN status. A large prospective trial comparing adjuvant RT to observation in stage I patients did not characterize pathological node status and actually had to be stopped early due to declining enrollment because of the gaining popularity of SLNB [42]. They concluded that adjuvant RT decreased regional recurrence when pathological node status is unknown. A similar argument was made by Bichakjian et al. regarding another large retrospective review of adjuvant RT for head and neck MCC [43]. However, the evidence supporting the general use of regional RT in MCC antedates the routine use of SLNB. Recently, the premise that nodal RT is beneficial in SLNB-negative patients has come under scrutiny. A large retrospective study of 111 SLNB-negative patients found no recurrence or survival benefit from RT of the nodal basin, including head and neck sites [44•], despite a reported 17 % false-negative SLNB rate [10•]. With NCCN recommendations now including SLNB on all patients, additional studies are needed to confirm if sentinel node status should direct RT use.
Chemotherapy
It is most commonly platinum-based ± etoposide.
There is short-term control but no durable response, with high toxicity.
Most regimens employing chemotherapeutics are in the setting of advanced MCC. The literature is flush with case reports, retrospective series, and reviews, but a dearth of prospective studies. MCC is very sensitive to chemotherapy, but responses are not durable and tumors often recur within 4–15 months. Furthermore, the associated toxicity may actually decrease overall survival [45]. The most common regimens, recommended in the NCCN guidelines, employ a platinum-based product, such as carboplatin or cisplatin, often adding etoposide. The next most common regimen is cyclophosphamide, doxorubicin, and vincristine (CAV). In the largest most pertinent review of chemotherapeutics, 204 cases between 1989 and 1997 were reported to have response rates of 76 % to CAV and 60 % to carboplatin and etoposide regimens, though significant toxicities including death were noted [46]. Given the toxicity and lack of durable response, the potential short-term benefits should be weighed against the risks.
Palliation
Single-fraction RT is a promising new technique.
Brachytherapy in high dose has exceptional locoregional control but no change in overall survival.
Currently, treatment for advanced MCC remains largely palliative. One palliative option that was recently described as well tolerated for symptomatic tumor masses is single-fraction radiotherapy. Although retrospective in nature with many confounding variables, such as chemotherapy and immunotherapy, the authors reported 93 tumors treated with objective responses in 94 % and complete responses in 45 % [47•].
Another new and promising treatment for palliation of in-transit disease is high-dose brachytherapy. Of 152 tumors treated in 10 patients, locoregional control at a median of 34 months was 99 % [48]. Despite these impressive results, they reported no change in overall survival as 7 out of the 10 patients developed further metastatic disease outside of the field treated.
Emerging treatments
Immunotherapy
Immunotherapy is one of the most recent and rapidly expanding treatment modalities for all cancers, including MCC. Immune checkpoint inhibitors, in particular, have been able to achieve durable cures in patients with certain tumor types by reactivating antitumor cellular immune responses [49]. When studying the application of immunotherapy to MCC, it may be important to establish the viral status of the tumor. MCV-negative MCCs typically have more mutations and tumor neoantigens than MCV-positive tumors, all of which are potential targets for antitumor immune responses [34•], whereas MCV-positive MCCs express viral peptides that can be targeted by cellular immune responses [50]. Currently, it is unclear how these differing target immune landscapes will impact the use of immunotherapy for MCC.
Anti-programmed cell death receptor 1/programmed cell death ligand 1 antibodies
Monoclonal antibodies targeting programmed cell death receptor 1/programmed cell death ligand 1 (PD-1/PD-L1) interaction show great promise for MCC.
The binding of PD-L1 to PD-1 on cytotoxic T cells inhibits their tumor-killing activity. PD-L1 is frequently expressed on MCC tumor cells and peritumoral immune cells [51]. There is currently an ongoing phase II trial with avelumab, an anti-PD-L1 antibody, as a second-line treatment for patients with advanced MCC who have failed chemotherapy [52]. Although the study’s results have not yet been reported, avelumab as a second-line treatment of metastatic MCC recently received orphan drug designation, fast-track designation, and breakthrough therapy designation from the Food and Drug Administration.
Pembrolizumab is an anti-PD-1 antibody approved for the treatment of melanoma and non-small cell lung cancer. At least one patient with MCC showed a response to pembrolizumab during a phase I trial [53]. A small ongoing phase II trial of pembrolizumab as a first-line treatment for advanced MCC recently reported a 71 % initial response rate among 17 evaluable patients [54••, 55]. It remains to be seen if this response rate will be generalizable and if responses will be durable. Nonetheless, it appears that MCC is exceptionally sensitive to PD-1 checkpoint inhibition.
Other immunotherapies
Adjuvant ipilimumab is in an ongoing phase II trial.
A phase I/II trial of tremelimumab/durvalumab will open soon.
Adoptive T cell transfer is in an ongoing phase I/II trial.
Intratumoral IL-12 plasmid vaccine is in an ongoing phase II trial.
Ipilimumab inhibits the checkpoint molecule cytotoxic T lymphocyte antigen 4 (CTLA-4) and is an approved immunotherapy for metastatic melanoma. Recently, adjuvant ipilimumab was reported to improve recurrence rates for resected melanoma [56]. To test if the drug will show similar benefit in MCC, an ongoing controlled phase II trial in Europe is investigating ipilimumab versus observation as an adjuvant therapy for patients with resected MCC [57].
Combining immunotherapies has improved efficacy in other cancers while also increasing the risk of immune-related adverse events [58, 59]. A new phase I/II trial may open soon, treating metastatic solid tumors, including MCC, with the CTLA-4 inhibitor tremelimumab plus durvalumab, an anti-PD-L1 antibody, in combination with poly-ICLC, a Toll-like receptor-3 ligand [60].
Tumor-infiltrating lymphocytes (TILs), particularly CD8+, are associated with better MCC survival [61–63]. Adoptive cell transfer attempts to increase the available antitumor T cells by harvesting, growing, and reinjecting the patient’s own cells. A phase I/II trial is currently underway using this method in conjunction with an interleukin-2, aldesleukin [64], to stimulate the host’s immune system [65]. This treatment has shown great promise in patients with metastatic melanoma, but it remains to be seen if the same results can be achieved in MCC [66]. At least one patient has shown regression in their MCC by combining local and systemic immunotherapies with autologous T cell transfer [67].
Intratumoral injection, to avoid peripheral toxicity, of an interleukin-12 (IL-12) plasmid vaccine followed by electroporation in mouse melanomas demonstrated increased IL-12, interferon-gamma, and tumor lymphocytes, as well as reduced tumor vascularity [68]. This was followed by a phase I trial of the same method in patients with metastatic melanoma with nearly half of the patients showing stabilization and partial or complete response with few side effects [69]. A phase II trial for this approach is ongoing for MCC with imminent anticipated completion [70].
Targeted therapies
Targeted therapies use drugs that inhibit molecules required for tumor growth and progression. With a few exceptions, disease progression due to drug resistance is a limitation of targeted therapies. Nonetheless, when activating mutations in a specific receptor, pathway, or oncogene can be targeted in a cancer, responses can be rapid and there can be fewer toxicities than conventional chemotherapy. In the case of MCC, the majority of tumors are MCV-positive and lacking in druggable driver mutations. In contrast, MCV-negative MCCs frequently have mutations predicted to be oncogenic. Thus far, no recurrent activated pathway has been identified in the majority of MCV-negative MCCs, making it unlikely that a single targeted therapy will be highly effective in treating MCC. However, exceptional responders are likely if drugs can be matched to the targets present in a given tumor.
PI3K/AKT inhibition
Mutations are uncommon, mostly found in MCV-negative MCC.
Idelalisib in a case report resulted in a complete response.
PI3K/AKT-activating mutations have been found independent of viral status [71] or nearly entirely in MCV-negative patients [72], although each study reported extremely small sample sizes as these mutations are uncommon with a prevalence of 4 and 10 %, respectively. Since these mutations are commonly found in other cancers, targeted inhibitors already exist. In a recent case report, a patient with metastatic MCC carrying a known PI3K mutation was treated with the PI3Kδ inhibitor, idelalisib, resulting in a rapid and complete response. Unfortunately, the patient died from other causes before long-term response could be measured [73]. This case is a good example of precision medicine, where patient’s unique tumor mutations are treated specifically with a targeted therapy. As the costs and delays of genotyping fall, this approach may become more common in MCC clinical trials and in patient care.
Mammalian target of rapamycin inhibitors
Pathway activation is more common in MCV-negative MCC.
MLN0128 is an ongoing dual mammalian target of rapamycin (mTOR) inhibitor phase II trial.
Also in the PI3K-related kinase pathway, AKT and mTOR have been found to be inappropriately activated in MCC, more commonly in MCV-negative tumors [74]. Specifically targeting both mTOR complex 1 and complex 2, the dual mTOR inhibitor, MLN0128, was able to inhibit MCC xenograft tumor growth in vivo [75]. A phase I dose escalation protocol of MLN0128 is expected to complete imminently [76], and a phase II trial for patients with advanced MCC has just begun recruiting patients [77].
Tyrosine kinase inhibitors
Targets include VEGF receptor (VEGFR), PDGF receptor (PDGFR), and c-kit.
Pazopanib case reported a partial response.
Cabozantinib is in an ongoing phase II trial.
Imatinib has likely no benefit.
Other potential therapeutic targets found in varying percentages of MCC tumors include kinases such as VEGFR, PDGFR, and c-kit [78]. Pazopanib is a multi-tyrosine kinase inhibitor that targets VEGFR, PDGFR, FGFR, and c-kit. There is one case report of a patient with MCC with a partial response to pazopanib [79], and there is an ongoing trial in the UK [80]. Another phase II trial is actively recruiting patients with advanced MCC for treatment with cabozantinib, which has activity against c-Met and VEGFR2, blocking angiogenesis [81]. In theory, this mechanism might be expected to have an effect on such a vascular tumor as MCC that is known to express VEGFR2 [78]. Based on the expression of c-kit in MCC, a phase II trial using imatinib, a tyrosine kinase inhibitor active against c-kit, as well as abl and PDGFR, was completed but failed to show benefit [82]. It appears that although MCC may express c-kit, activating mutations in the gene are generally absent. However, there is one case report of clinical remission with imatinib in a patient with MCV-positive MCC [83].
Octreotide
Peptide receptor radionuclide therapy (PPRT) with 177Lu-DOTATATE is in an ongoing phase II trial.
Several other studies are ongoing for other somatostatin analogues.
An extension of using SSTR ligands for imaging is to use the same mechanism to target treatment. Neuroendocrine tumors found to express SSTRs are good candidates for treatment with PPRT. This method uses a radiolabeled somatostatin analogue, such as 177Lu-DOTATATE, 68Ga-DOTA-Tyr3-octreotide, or 68Ga-DOTA-Tyr3-octreotate, that is taken up by the tumor and metabolized to slowly emit targeted localized radiation [84–86]. Earlier treatments used DTPA-octreotide, which lacked significant tumor response but was then replaced by 90Y-DOTA-Tyr3-octreotide (90Y-DOTATOC), which had better tumor responses, but more myelosuppression and renal toxicity. This was further refined and replaced by DOTA-Tyr3-octreotate (DOTATATE), which was attached to 177lutetium as opposed to 90yttrium, resulting in less penetration (2 versus 11 mm) into normal tissue and fewer side effects such as renal failure [87]. A phase II study of 177Lu-DOTATATE to treat neuroendocrine tumors has been completed, although no results are available yet [88]. Other non-radioactive somatostatin analogues, such as pasireotide [89] and lanreotide [90], are also being investigated in trials to treat MCC.
Future directions
Stratifying clinical trials by viral status for precision medicine should be considered.
Circulating biomarkers to track disease status and monitor for occult disease is a growing trend.
Tumor vaccines could be used to stimulate host immune responses.
RT is being examined in combination with checkpoint inhibitors.
It is now clear that the mutational profile of MCV-positive and MCV-negative MCC is vastly different. Going forward, it would be prudent to stratify clinical trials by tumor viral status, especially for therapies that target driver mutations more likely to be found in MCV-negative tumors. For this reason, developing a validated test for tumor viral status is a priority to help guide treatment and allow for more accurate interpretation of clinical trials. Of course, high-throughput sequencing could be applied to simultaneously identify viral status and the mutational profile of MCC tumors to better direct therapy. Tumors like MCC that lack highly recurrent oncogene mutations are candidates for this type of precision medicine approach.
Circulating biomarkers for the diagnosis, prognosis, and monitoring of cancer is a growing trend. Based on their use in other neuroendocrine tumors, some institutions have attempted to use serum levels of neuron-specific enolase and chromogranin A to monitor disease activity in MCC, but a recent paper has questioned the utility of these tests in determining outcomes, disease progression, and MCC tumor burden [91]. In contrast, circulating tumor cells appear to hold promise as biomarkers for MCC [91, 92]. The further development of circulating tumor cells, MCV serology, or other biomarkers could potentially identify patients with the highest risk of recurrence, who would benefit the most from adjuvant therapies.
Immunotherapy with checkpoint blockade or with cytokine therapy can be effective in reversing immune tolerance and stimulating antitumor immune responses. Since ~80 % of MCCs express viral proteins, adding an MCV vaccine to other immunotherapies may further enhance the efficacy of these approaches. This approach could be effective in treating advanced MCC as well as preventing recurrence in the setting of adjuvant immunotherapy. In 2012, a vaccine to the MCV LTAg demonstrated a successful immune response in mice [93]. One year later, the same group published data on a vaccine against MCV sTAg, which was also effective in mice [94]. Thus, a vaccine approach may be feasible to enhance immune responses against MCV-expressing MCC tumors.
Studies have demonstrated a synergistic effect when combining immunotherapy and RT to treat melanoma [95, 96]. Judicious application of RT to tumors can cause the release of tumor antigens, induce inflammation, and reverse immune escape mechanisms such as the downregulation of major histocompatibility complex I (MHC-I). All of these effects can potentially enhance responses to checkpoint blockade immunotherapy. Reduced MHC-I is observed in MCC, particularly in MCV-positive tumors. Multiple treatments such as interferon, etoposide, and RT are being explored as a mechanism to restore MHC-I expression in MCC, and combining these modalities with checkpoint blockade is another potential area of future study [97].
Conclusions
As with any rare disease, future progress in the management of MCC will require multi-institutional collaborative efforts, as no single institution treats enough patients to effectively analyze the natural history of the disease or effectively compare treatments. As we learn more about MCC, treatment protocols can be refined and standardized. It seems likely that immunotherapy with checkpoint inhibitors will be found to benefit patients with metastatic MCC in the near future, and adjuvant immunotherapy may eventually prove useful. However, not every patient will respond to immunotherapy, and hence appropriate targeted therapies are also needed. Until a common therapeutic target or predictive biomarkers can be identified in MCC, it is likely that targeted therapies will require a precision medicine approach to be effective.
Footnotes
Compliance with Ethical Standards
Disclosure
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the US Navy, the Department of Defense, or the National Institutes of Health.
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Heath M, Jaimes N, Lemos B, Mostaghimi A, Wang LC, Peñas PF, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58(3):375–81. doi: 10.1016/j.jaad.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paulson KG, Iyer JG, Blom A, Warton EM, Sokil M, Yelistratova L, et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J Invest Dermatol. 2013;133(3):642–6. doi: 10.1038/jid.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3•.Asgari MM, Sokil MM, Warton EM, Iyer J, Paulson KG, Nghiem P. Effect of host, tumor, diagnostic, and treatment variables on outcomes in a large cohort with Merkel cell carcinoma. JAMA Dermatol. 2014;150(7):716–23. doi: 10.1001/jamadermatol.2013.8116. A single-institution study describing a negative prognostic impact of immune compromise and confirming prior observations that nodal MCC with unknown primary has a better prognosis than nodal disease with known primary. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemos BD, Storer BE, Iyer JG, Phillips JL, Bichakjian CK, Fang LC, et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5823 cases as the basis of the first consensus staging system. J Am Acad Dermatol. 2010;63(5):751–61. doi: 10.1016/j.jaad.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 6.Foote M, Veness M, Zarate D, Poulsen M. Merkel cell carcinoma: the prognostic implications of an occult primary in stage IIIB (nodal) disease. J Am Acad Dermatol. 2012;67(3):395–9. doi: 10.1016/j.jaad.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319(5866):1096–100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitzgerald TL, Dennis S, Kachare SD, Vohra NA, Wong JH, Zervos EE. Dramatic increase in the incidence and mortality from Merkel cell carcinoma in the United States. Am Surg. 2015;81(8):802–6. doi: 10.1177/000313481508100819. [DOI] [PubMed] [Google Scholar]
- 9.Network NCC. [Accessed Jan 2016];Merkel cell carcinoma (version 1.2016) http://www.nccn.org/professionals/physician_gls/PDF/mcc.pdf.
- 10•.Gunaratne DA, Howle JR, Veness MJ. Sentinel lymph node biopsy in Merkel cell carcinoma: a 15 year institutional experience and statistical analysis of 721 reported cases. Br J Dermatol. 2015 doi: 10.1111/bjd.14240. This large retrospective review demonstrated no increased recurrence with adjuvant RT in the setting of negative SLNB. [DOI] [PubMed] [Google Scholar]
- 11.Chan JK, Suster S, Wenig BM, Tsang WY, Chan JB, Lau AL. Cytokeratin 20 immunoreactivity distinguishes Merkel cell (primary cutaneous neuroendocrine) carcinomas and salivary gland small cell carcinomas from small cell carcinomas of various sites. Am J Surg Pathol. 1997;21(2):226–34. doi: 10.1097/00000478-199702000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Allen PJ, Bowne WB, Jaques DP, Brennan MF, Busam K, Coit DG. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23(10):2300–9. doi: 10.1200/JCO.2005.02.329. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz JL, Griffith KA, Lowe L, Wong SL, McLean SA, Fullen DR, et al. Features predicting sentinel lymph node positivity in Merkel cell carcinoma. J Clin Oncol. 2011;29(8):1036–41. doi: 10.1200/JCO.2010.33.4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14•.Iyer JG, Storer BE, Paulson KG, Lemos B, Phillips JL, Bichakjian CK, et al. Relationships among primary tumor size, number of involved nodes, and survival for 8044 cases of Merkel cell carcinoma. J Am Acad Dermatol. 2014;70(4):637–43. doi: 10.1016/j.jaad.2013.11.031. This study reinforced the utility of SLNB for tumors of any size and correlated the number of positive nodes with survival. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ko JS, Prieto VG, Elson PJ, Vilain RE, Pulitzer MP, Scolyer RA, et al. Histological pattern of Merkel cell carcinoma sentinel lymph node metastasis improves stratification of stage III patients. Mod Pathol. 2015 doi: 10.1038/modpathol.2015.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawryluk EB, O’Regan KN, Sheehy N, Guo Y, Dorosario A, Sakellis CG, et al. Positron emission tomography/computed tomography imaging in Merkel cell carcinoma: a study of 270 scans in 97 patients at the Dana-Farber/Brigham and Women’s Cancer Center. J Am Acad Dermatol. 2013;68(4):592–9. doi: 10.1016/j.jaad.2012.08.042. [DOI] [PubMed] [Google Scholar]
- 17.Treglia G, Kakhki VR, Giovanella L, Sadeghi R. Diagnostic performance of fluorine-18-fluorodeoxyglucose positron emission tomography in patients with Merkel cell carcinoma: a systematic review and meta-analysis. Am J Clin Dermatol. 2013;14(6):437–47. doi: 10.1007/s40257-013-0040-x. [DOI] [PubMed] [Google Scholar]
- 18.Colgan MB, Tarantola TI, Weaver AL, Wiseman GA, Roenigk RK, Brewer JD, et al. The predictive value of imaging studies in evaluating regional lymph node involvement in Merkel cell carcinoma. J Am Acad Dermatol. 2012;67(6):1250–6. doi: 10.1016/j.jaad.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Peloschek P, Novotny C, Mueller-Mang C, Weber M, Sailer J, Dawid M, et al. Diagnostic imaging in Merkel cell carcinoma: lessons to learn from 16 cases with correlation of sonography, CT, MRI and PET. Eur J Radiol. 2010;73(2):317–23. doi: 10.1016/j.ejrad.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 20.Kritikos N, Priftakis D, Stavrinides S, Kleanthous S, Sarafianou E. Nuclear medicine techniques in Merkel cell carcinoma: a case report and review of the literature. Oncol Lett. 2015;10(3):1610–6. doi: 10.3892/ol.2015.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Larcos G, Howle J, Veness M. Lack of clinical impact of (18) F-fluorodeoxyglucose positron emission tomography with simultaneous computed tomography for stage I and II Merkel cell carcinoma with concurrent sentinel lymph node biopsy staging: a single institutional experience from Westmead Hospital, Sydney. Australas J Dermatol. 2015 doi: 10.1111/ajd.12400. [DOI] [PubMed] [Google Scholar]
- 22.Fantini F, Johansson O. Neurochemical markers in human cutaneous Merkel cells. An immunohistochemical investigation. Exp Dermatol. 1995;4(6):365–71. doi: 10.1111/j.1600-0625.1995.tb00061.x. [DOI] [PubMed] [Google Scholar]
- 23.Buder K, Lapa C, Kreissl MC, Schirbel A, Herrmann K, Schnack A, et al. Somatostatin receptor expression in Merkel cell carcinoma as target for molecular imaging. BMC Cancer. 2014;14:268. doi: 10.1186/1471-2407-14-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epstude M, Tornquist K, Riklin C, di Lenardo F, Winterhalder R, Hug U, et al. Comparison of (18)F-FDG PET/CT and (68)Ga-DOTATATE PET/CT imaging in metastasized Merkel cell carcinoma. Clin Nucl Med. 2013;38(4):283–4. doi: 10.1097/RLU.0b013e318281658e. [DOI] [PubMed] [Google Scholar]
- 25.Garneski KM, Warcola AH, Feng Q, Kiviat NB, Leonard JH, Nghiem P. Merkel cell polyomavirus is more frequently present in North American than Australian Merkel cell carcinoma tumors. J Invest Dermatol. 2009;129(1):246–8. doi: 10.1038/jid.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spurgeon ME, Lambert PF. Merkel cell polyomavirus: a newly discovered human virus with oncogenic potential. Virology. 2013;435(1):118–30. doi: 10.1016/j.virol.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodig SJ, Cheng J, Wardzala J, DoRosario A, Scanlon JJ, Laga AC, et al. Improved detection suggests all Merkel cell carcinomas harbor Merkel polyomavirus. J Clin Invest. 2012;122(12):4645–53. doi: 10.1172/JCI64116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsushita M, Nonaka D, Iwasaki T, Kuwamoto S, Murakami I, Kato M, et al. A new in situ hybridization and immunohistochemistry with a novel antibody to detect small T-antigen expressions of Merkel cell polyomavirus (MCPyV) Diagn Pathol. 2014;9:65. doi: 10.1186/1746-1596-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paulson KG, Carter JJ, Johnson LG, Cahill KW, Iyer JG, Schrama D, et al. Antibodies to Merkel cell polyomavirus T antigen oncoproteins reflect tumor burden in Merkel cell carcinoma patients. Cancer Res. 2010;70(21):8388–97. doi: 10.1158/0008-5472.CAN-10-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samimi M, Molet L, Fleury M, Laude H, Carlotti A, Gardair C, et al. Prognostic value of antibodies to Merkel cell polyomavirus T-antigens and VP1 protein in Merkel cell carcinoma patients. Br J Dermatol. 2015 doi: 10.1111/bjd.14313. [DOI] [PubMed] [Google Scholar]
- 31.Touzé A, Le Bidre E, Laude H, Fleury MJ, Cazal R, Arnold F, et al. High levels of antibodies against Merkel cell polyomavirus identify a subset of patients with Merkel cell carcinoma with better clinical outcome. J Clin Oncol. 2011;29(12):1612–9. doi: 10.1200/JCO.2010.31.1704. [DOI] [PubMed] [Google Scholar]
- 32.Sihto H, Kukko H, Koljonen V, Sankila R, Böhling T, Joensuu H. Merkel cell polyomavirus infection, large T antigen, retinoblastoma protein and outcome in Merkel cell carcinoma. Clin Cancer Res. 2011;17(14):4806–13. doi: 10.1158/1078-0432.CCR-10-3363. [DOI] [PubMed] [Google Scholar]
- 33.Wong SQ, Waldeck K, Vergara IA, Schröder J, Madore J, Wilmott JS, et al. UV-associated mutations underlie the etiology of MCV-negative Merkel cell carcinomas. Cancer Res. 2015;75(24):5228–34. doi: 10.1158/0008-5472.CAN-15-1877. [DOI] [PubMed] [Google Scholar]
- 34•.Goh G, Walradt T, Markarov V, Blom A, Riaz N, Doumani R, et al. Mutational landscape of MCPyV-positive and MCPyV-negative Merkel cell carcinomas with implications for immunotherapy. Oncotarget. 2015 doi: 10.18632/oncotarget.6494. Whole exome sequencing identifies that MCV-negative MCCs have a higher mutational burden than MCV-positive tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Harms PW, Vats P, Verhaegen ME, Robinson DR, Wu YM, Dhanasekaran SM, et al. The distinctive mutational spectra of polyomavirus-negative Merkel cell carcinoma. Cancer Res. 2015;75(18):3720–7. doi: 10.1158/0008-5472.CAN-15-0702. Whole exome sequencing identifies that MCV-negative MCCs have a higher mutational burden than MCV-positive tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rush Z, Fields RC, Lee N, Brownell I. Radiation therapy in the management of Merkel cell carcinoma: current perspectives. Expert Rev Dermatol. 2011;6(4):395–404. doi: 10.1586/edm.11.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leonard JH, Ramsay JR, Kearsley JH, Birrell GW. Radiation sensitivity of Merkel cell carcinoma cell lines. Int J Radiat Oncol Biol Phys. 1995;32(5):1401–7. doi: 10.1016/0360-3016(94)00610-W. [DOI] [PubMed] [Google Scholar]
- 38.Lok B, Khan S, Mutter R, Liu J, Fields R, Pulitzer M, et al. Selective radiotherapy for the treatment of head and neck Merkel cell carcinoma. Cancer. 2012;118(16):3937–44. doi: 10.1002/cncr.26738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harrington C, Kwan W. Radiotherapy and conservative surgery in the locoregional management of Merkel cell carcinoma: the British Columbia Cancer Agency experience. Ann Surg Oncol. 2015 doi: 10.1245/s10434-015-4812-9. [DOI] [PubMed] [Google Scholar]
- 40.Veness M, Foote M, Gebski V, Poulsen M. The role of radiotherapy alone in patients with Merkel cell carcinoma: reporting the Australian experience of 43 patients. Int J Radiat Oncol Biol Phys. 2010;78(3):703–9. doi: 10.1016/j.ijrobp.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 41.Mortier L, Mirabel X, Fournier C, Piette F, Lartigau E. Radiotherapy alone for primary Merkel cell carcinoma. Arch Dermatol. 2003;139(12):1587–90. doi: 10.1001/archderm.139.12.1587. [DOI] [PubMed] [Google Scholar]
- 42.Jouary T, Leyral C, Dreno B, Doussau A, Sassolas B, Beylot-Barry M, et al. Adjuvant prophylactic regional radiotherapy versus observation in stage I Merkel cell carcinoma: a multicentric prospective randomized study. Ann Oncol. 2012;23(4):1074–80. doi: 10.1093/annonc/mdr318. [DOI] [PubMed] [Google Scholar]
- 43.Bichakjian CK, Harms KL, Schwartz JL. Selective use of adjuvant therapy in the management of Merkel cell carcinoma. JAMA Oncol. 2015;1(8):1162–3. doi: 10.1001/jamaoncol.2015.1503. [DOI] [PubMed] [Google Scholar]
- 44•.Grotz TE, Joseph RW, Pockaj BA, Foote RL, Otley CC, Bagaria SP, et al. Negative sentinel lymph node biopsy in Merkel cell carcinoma is associated with a low risk of same-nodal-basin recurrences. Ann Surg Oncol. 2015;22(12):4060–6. doi: 10.1245/s10434-015-4421-7. A single-institution study suggesting that regional RT can be avoided if SLNB is negative. [DOI] [PubMed] [Google Scholar]
- 45.Desch L, Kunstfeld R. Merkel cell carcinoma: chemotherapy and emerging new therapeutic options. J Skin Cancer. 2013;2013:327150. doi: 10.1155/2013/327150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tai PT, Yu E, Winquist E, Hammond A, Stitt L, Tonita J, et al. Chemotherapy in neuroendocrine/Merkel cell carcinoma of the skin: case series and review of 204 cases. J Clin Oncol. 2000;18(12):2493–9. doi: 10.1200/JCO.2000.18.12.2493. [DOI] [PubMed] [Google Scholar]
- 47•.Iyer JG, Parvathaneni U, Gooley T, Miller NJ, Markowitz E, Blom A, et al. Single-fraction radiation therapy in patients with metastatic Merkel cell carcinoma. Cancer Med. 2015;4(8):1161–70. doi: 10.1002/cam4.458. This study used SFRT as a successful alternative to chemotherapy for palliation in advanced disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garibyan L, Cotter SE, Hansen JL, Noell C, Dorosario A, O’Farrell DA, et al. Palliative treatment for in-transit cutaneous metastases of Merkel cell carcinoma using surface-mold computer-optimized high-dose-rate brachytherapy. Cancer J. 2013;19(4):283–7. doi: 10.1097/PPO.0b013e31829e3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lyngaa R, Pedersen NW, Schrama D, Thrue CA, Ibrani D, Met O, et al. T-cell responses to oncogenic Merkel cell polyomavirus proteins distinguish patients with Merkel cell carcinoma from healthy donors. Clin Cancer Res. 2014;20(7):1768–78. doi: 10.1158/1078-0432.CCR-13-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lipson EJ, Vincent JG, Loyo M, Kagohara LT, Luber BS, Wang H, et al. PD-L1 expression in the Merkel cell carcinoma microenvironment: association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol Res. 2013;1(1):54–63. doi: 10.1158/2326-6066.CIR-13-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Avelumab in subjects with Merkel cell carcinoma (JAVELIN Merkel 200) National Institutes of Health; [Accessed Jan 2016]. https://clinicaltrials.gov/ct2/show/NCT02155647. [Google Scholar]
- 53.Patnaik A, Kang SP, Rasco D, Papadopoulos KP, Elassaiss-Schaap J, Beeram M, et al. Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res. 2015;21(19):4286–93. doi: 10.1158/1078-0432.CCR-14-2607. [DOI] [PubMed] [Google Scholar]
- 54••.Nghiem P, Bhatia S, Daud A, Friedlander P, Kluger H, Kohrt H, et al. Activity of PD-1 blockade with pembrolizumab as first systemic therapy in patients with advanced Merkel cell carcinoma [abstract]. The European Cancer Congress; September 27; Vienna, Austria. 2015. This early study demonstrated exceptionally high initial response rates. [Google Scholar]
- 55.Pembrolizumab in treating patients with advanced Merkel cell cancer. National Institutes of Health; [Accessed Jan 2016]. https://clinicaltrials.gov/show/NCT02267603. [Google Scholar]
- 56.Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16(5):522–30. doi: 10.1016/S1470-2045(15)70122-1. [DOI] [PubMed] [Google Scholar]
- 57.Adjuvant therapy of completely resected Merkel cell carcinoma with 3 mg/kg BW ipilimumab (Yervoy®) versus observation (ADMEC) National Institutes of Health; [Accessed Jan 2016]. https://clinicaltrials.gov/ct2/show/NCT02196961. [Google Scholar]
- 58.Valsecchi ME. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(13):1270. doi: 10.1056/NEJMc1509660#SA1. [DOI] [PubMed] [Google Scholar]
- 59.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–17. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.A phase 1/2 study of in situ vaccination with tremelimumab and IV durvalumab plus polyICLC in subjects with advanced, measurable, biopsy-accessible cancers. National Institutes of Health; [Accessed Jan 2016]. https://clinicaltrials.gov/ct2/show/NCT02643303. [Google Scholar]
- 61.Sihto H, Böhling T, Kavola H, Koljonen V, Salmi M, Jalkanen S, et al. Tumor infiltrating immune cells and outcome of Merkel cell carcinoma: a population-based study. Clin Cancer Res. 2012;18(10):2872–81. doi: 10.1158/1078-0432.CCR-11-3020. [DOI] [PubMed] [Google Scholar]
- 62.Paulson KG, Iyer JG, Simonson WT, Blom A, Thibodeau RM, Schmidt M, et al. CD8+ lymphocyte intratumoral infiltration as a stage-independent predictor of Merkel cell carcinoma survival: a population-based study. Am J Clin Pathol. 2014;142(4):452–8. doi: 10.1309/AJCPIKDZM39CRPNC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paulson KG, Iyer JG, Tegeder AR, Thibodeau R, Schelter J, Koba S, et al. Transcriptome-wide studies of Merkel cell carcinoma and validation of intratumoral CD8+ lymphocyte invasion as an independent predictor of survival. J Clin Oncol. 2011;29(12):1539–46. doi: 10.1200/JCO.2010.30.6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schmidinger M, Hejna M, Zielinski CC. Aldesleukin in advanced renal cell carcinoma. Expert Rev Anticancer Ther. 2004;4(6):957–80. doi: 10.1586/14737140.4.6.957. [DOI] [PubMed] [Google Scholar]
- 65.Viral oncoprotein targeted autologous T cell therapy for Merkel cell carcinoma. National Institutes of Health; [Accessed Jan 2016]. https://clinicaltrials.gov/ct2/show/NCT01758458. [Google Scholar]
- 66.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17(13):4550–7. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chapuis AG, Afanasiev OK, Iyer JG, Paulson KG, Parvathaneni U, Hwang JH, et al. Regression of metastatic Merkel cell carcinoma following transfer of polyomavirus-specific T cells and therapies capable of re-inducing HLA class-I. Cancer Immunol Res. 2014;2(1):27–36. doi: 10.1158/2326-6066.CIR-13-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lucas ML, Heller L, Coppola D, Heller R. IL-12 plasmid delivery by in vivo electroporation for the successful treatment of established subcutaneous B16.F10 melanoma. Mol Ther. 2002;5(6):668–75. doi: 10.1006/mthe.2002.0601. [DOI] [PubMed] [Google Scholar]
- 69.Daud AI, DeConti RC, Andrews S, Urbas P, Riker AI, Sondak VK, et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol. 2008;26(36):5896–903. doi: 10.1200/JCO.2007.15.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.IL-12 gene and in vivo electroporation-mediated plasmid DNA vaccine therapy in patients with Merkel cell cancer. National Institutes of Health; [Accessed Jan 2016]. https://clinicaltrials.gov/ct2/show/NCT01440816. [Google Scholar]
- 71.Hafner C, Houben R, Baeurle A, Ritter C, Schrama D, Landthaler M, et al. Activation of the PI3K/AKT pathway in Merkel cell carcinoma. PLoS One. 2012;7(2):e31255. doi: 10.1371/journal.pone.0031255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nardi V, Song Y, Santamaria-Barria JA, Cosper AK, Lam Q, Faber AC, et al. Activation of PI3K signaling in Merkel cell carcinoma. Clin Cancer Res. 2012;18(5):1227–36. doi: 10.1158/1078-0432.CCR-11-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shiver MB, Mahmoud F, Gao L. Response to idelalisib in a patient with stage IV Merkel-cell carcinoma. N Engl J Med. 2015;373(16):1580–2. doi: 10.1056/NEJMc1507446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iwasaki T, Matsushita M, Nonaka D, Kuwamoto S, Kato M, Murakami I, et al. Comparison of Akt/mTOR/4E-BP1 pathway signal activation and mutations of PIK3CA in Merkel cell polyomavirus-positive and Merkel cell polyomavirus-negative carcinomas. Hum Pathol. 2015;46(2):210–6. doi: 10.1016/j.humpath.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 75.Kannan A, Lin Z, Shao Q, Zhao S, Fang B, Moreno MA, et al. Dual mTOR inhibitor MLN0128 suppresses Merkel cell carcinoma (MCC) xenograft tumor growth. Oncotarget. 2015 doi: 10.18632/oncotarget.5878. [DOI] [PMC free article] [PubMed]
- 76.Dose escalation study of MLN0128 in subjects with advanced malignancies. National Institutes of Health; [Accessed Jan 2016]. https://clinicaltrials.gov/ct2/show/NCT01058707. [Google Scholar]
- 77.MLN0128 in recurrent/metastatic Merkel cell carcinoma. National Institutes of Health; [Accessed Jan 2016]. https://clinicaltrials.gov/ct2/show/NCT02514824. [Google Scholar]
- 78.Brunner M, Thurnher D, Pammer J, Geleff S, Heiduschka G, Reinisch CM, et al. Expression of VEGF-A/C, VEGF-R2, PDGF-alpha/beta, c-kit, EGFR, Her-2/Neu, Mcl-1 and Bmi-1 in Merkel cell carcinoma. Mod Pathol. 2008;21(7):876–84. doi: 10.1038/modpathol.2008.63. [DOI] [PubMed] [Google Scholar]
- 79.Davids MS, Davids M, Charlton A, Ng SS, Chong ML, Laubscher K, et al. Response to a novel multitargeted tyrosine kinase inhibitor pazopanib in metastatic Merkel cell carcinoma. J Clin Oncol. 2009;27(26):e97–100. doi: 10.1200/JCO.2009.21.8149. [DOI] [PubMed] [Google Scholar]
- 80.A trial of pazopanib for Merkel cell skin cancer (UKMCC-01) Cancer Research; UK: [Accessed Jan 2016]. http://www.cancerresearchuk.org/about-cancer/find-a-clinical-trial/a-trial-of-pazopanib-for-merkel-cell-carcinoma-ukmcc-01. [Google Scholar]
- 81.Cabozantinib in recurrent/metastatic Merkel cell carcinoma. National Institutes of Health; [Accessed Jan 2016]. https://clinicaltrials.gov/ct2/show/NCT02036476. [Google Scholar]
- 82.Samlowski WE, Moon J, Tuthill RJ, Heinrich MC, Balzer-Haas NS, Merl SA, et al. A phase II trial of imatinib mesylate in Merkel cell carcinoma (neuroendocrine carcinoma of the skin): a Southwest Oncology Group study (S0331) Am J Clin Oncol. 2010;33(5):495–9. doi: 10.1097/COC.0b013e3181b9cf04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Loader DE, Feldmann R, Baumgartner M, Breier F, Schrama D, Becker JC, et al. Clinical remission of Merkel cell carcinoma after treatment with imatinib. J Am Acad Dermatol. 2013;69(4):e181–3. doi: 10.1016/j.jaad.2013.03.042. [DOI] [PubMed] [Google Scholar]
- 84.Kratochwil C, Giesel FL, López-Benítez R, Schimpfky N, Kunze K, Eisenhut M, et al. Intraindividual comparison of selective arterial versus venous 68Ga-DOTATOC PET/CT in patients with gastroenteropancreatic neuroendocrine tumors. Clin Cancer Res. 2010;16(10):2899–905. doi: 10.1158/1078-0432.CCR-10-0004. [DOI] [PubMed] [Google Scholar]
- 85.Pool SE, Kam BL, Koning GA, Konijnenberg M, Ten Hagen TL, Breeman WA, et al. [(111)In-DTPA]octreotide tumor uptake in GEPNET liver metastases after intra-arterial administration: an overview of preclinical and clinical observations and implications for tumor radiation dose after peptide radionuclide therapy. Cancer Biother Radiopharm. 2014;29(4):179–87. doi: 10.1089/cbr.2013.1552. [DOI] [PubMed] [Google Scholar]
- 86.Limouris GS, Chatziioannou A, Kontogeorgakos D, Mourikis D, Lyra M, Dimitriou P, et al. Selective hepatic arterial infusion of In-111-DTPA-Phe1-octreotide in neuroendocrine liver metastases. Eur J Nucl Med Mol Imaging. 2008;35(10):1827–37. doi: 10.1007/s00259-008-0779-0. [DOI] [PubMed] [Google Scholar]
- 87.Kwekkeboom DJ, Krenning EP. Peptide receptor radionuclide therapy in the treatment of neuroendocrine tumors. Hematol Oncol Clin North Am. 2016;30(1):179–91. doi: 10.1016/j.hoc.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 88.177Lutetium-DOTA-octreotate therapy in somatostatin receptor-expressing neuroendocrine neoplasms. National Institutes of Health; [Accessed Jan 2016]. https://clinicaltrials.gov/ct2/show/NCT01237457. [Google Scholar]
- 89.A phase I, exploratory, intra-patient dose escalation study to investigate the preliminary safety, pharmacokinetics, and anti-tumor activity of pasireotide (SOM230) National Institutes of Health; [Accessed Jan 2016]. https://clinicaltrials.gov/ct2/show/NCT01652547. [Google Scholar]
- 90.Treatment of unresectable and/or metastatic Merkel cell carcinoma by somatostatine analogues. National Institutes of Health; [Accessed Jan 2016]. https://clinicaltrials.gov/ct2/show/NCT02351128. [Google Scholar]
- 91.Gaiser MR, Daily K, Hoffmann J, Brune M, Enk A, Brownell I. Evaluating blood levels of neuron specific enolase, chromogranin A, and circulating tumor cells as Merkel cell carcinoma biomarkers. Oncotarget. 2015;6(28):26472–82. doi: 10.18632/oncotarget.4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blom A, Bhatia S, Pietromonaco S, Koehler K, Iyer JG, Nagase K, et al. Clinical utility of a circulating tumor cell assay in Merkel cell carcinoma. J Am Acad Dermatol. 2014;70(3):449–55. doi: 10.1016/j.jaad.2013.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zeng Q, Gomez BP, Viscidi RP, Peng S, He L, Ma B, et al. Development of a DNA vaccine targeting Merkel cell polyomavirus. Vaccine. 2012;30(7):1322–9. doi: 10.1016/j.vaccine.2011.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gomez B, He L, Tsai YC, Wu TC, Viscidi RP, Hung CF. Creation of a Merkel cell polyomavirus small T antigen-expressing murine tumor model and a DNA vaccine targeting small T antigen. Cell Biosci. 2013;3(1):29. doi: 10.1186/2045-3701-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Barker CA, Postow MA. Combinations of radiation therapy and immunotherapy for melanoma: a review of clinical outcomes. Int J Radiat Oncol Biol Phys. 2014;88(5):986–97. doi: 10.1016/j.ijrobp.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barker CA, Postow MA, Khan SA, Beal K, Parhar PK, Yamada Y, et al. Concurrent radiotherapy and ipilimumab immunotherapy for patients with melanoma. Cancer Immunol Res. 2013;1(2):92–8. doi: 10.1158/2326-6066.CIR-13-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Paulson KG, Tegeder A, Willmes C, Iyer JG, Afanasiev OK, Schrama D, et al. Downregulation of MHC-I expression is prevalent but reversible in Merkel cell carcinoma. Cancer Immunol Res. 2014;2(11):1071–9. doi: 10.1158/2326-6066.CIR-14-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]