Abstract
What do infants learn when they learn to sit upright? We tested behavioral flexibility in learning to sit—the ability to adapt posture to changes in the environment—in 6- to 9-month-old infants sitting on forward and backward slopes. Infants began with slant at 0°; then slant increased in 2° increments until infants lost balance. Infants kept balance on impressively steep slopes, especially in the forward direction, despite the unexpected movements of the apparatus. Between slant adjustments while the slope was stationary, infants adapted posture to the direction and degree of slant by leaning backward on forward slopes and forward on backward slopes. Postural adaptations were nearly optimal for backward slopes. Sitting experience predicted greater postural adaptations and increased ability to keep balance on steeper changes of slant, but only for forward slopes. We suggest that behavioral flexibility is integral to learning to sit and increases with sitting experience.
Keywords: infant, sitting, flexibility, sloping surface, postural control
Introduction
What Do Infants Learn When They Learn To Sit?
Sitting is trivially easy for adults, but it is not easy for infants. Most infants do not learn to sit until 6 to 8 months of age—indexed by sitting on a flat surface with hands free for 10 seconds or longer (Adolph & Avolio, 2000; Karasik, Tamis-LeMonda, Adolph, & Bornstein, 2015; Martorell et al., 2006; Wijnhoven et al., 2004). And infants only achieve this milestone after many weeks of practice sitting with support and attempting to sit independently. So what do infants learn as they learn to sit? The obvious answer is that infants learn to stay upright (Johnson & Blasco, 1997; Piper & Darrah, 1994). However, upright balance involves several non-obvious components, including control of the torso, attainment of a stable position, compensatory postural sway, and perhaps most important, behavioral flexibility.
Infants’ bodies are poorly built for balance. They have large heads, weak necks, and floppy, multi-segmented trunks. Thus, infants must learn to manage the forces acting on their bodies. Postural control slowly progresses from head to hips, as if infants are mastering the exigencies of balance one downward vertebrae at a time (Butler, Saavedra, Sofranac, Jarvis, & Woollacott, 2010; Rachwani, Santamaria, Saavedra, & Woollacott, 2015; Saavedra, van Donkelaar, & Woollacott, 2012). Ultimately, control of the torso is so well orchestrated that for adult sitters, it is accepted practice to model the trunk biomechanically as a single segment (Kaminski, Bock, & Gentile, 1995; Nashner, Shupert, & Horak, 1988; Winter, Mackinnon, Ruder, & Wieman, 1993).
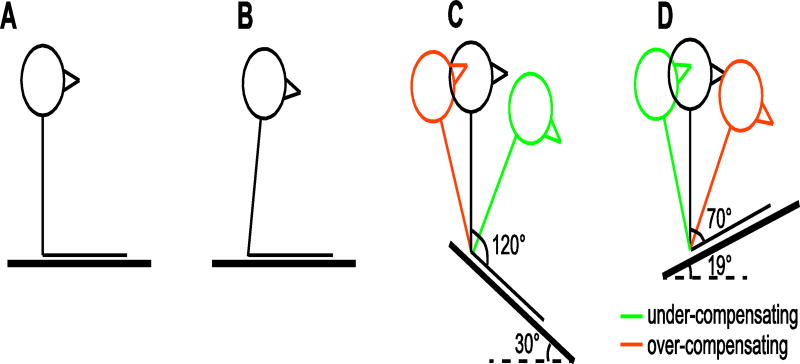
Even after infants can control their head-trunk-hip region, they must discover which upright positions are stable. Most people assume that stability is achieved with the trunk vertical and the thighs horizontal, so the angle between trunk and thighs is 90° as in Figure 1A (Massion, Alexandrov, & Frolov, 2004). The assumption of a vertical torso, however, turns out to be incorrect. Infants, children, and adults actually lean slightly forward while sitting on flat ground as in Figure 1B, with a trunk-thigh angle of 75°–80° (Curtis et al., 2015; Hirschfeld & Forssberg, 1994; Korakakis, Sideris, & Giakas, 2014; Saavedra et al., 2012). A hunched posture may be aesthetically unpleasing, but it increases stability. With the legs in front of the torso, the base of support is larger to the front than to the back (Figure 1A–B). When the trunk leans forward, the center of mass is well within the base of support, so posture is relatively stable and resistant to perturbations. But with the trunk at 90°, the torso is near the edge of the base of support, so a slight backward force would cause the sitter to lose balance.
Figure 1.
Sitting on various surfaces. (A) Sitting with legs outstretched on a rigid, horizontal surface. Stick figure shows a perfectly erect posture with a trunk-thigh angle of 90°. Figure shows that with the trunk perfectly upright, the body is at the edge of the base of support. (B) Infants, like older children and adults, sit with their torso leaning slightly forward, keeping the trunk well within the base of support. Trunk-thigh angle of 81.2° represents the average across infants in the current study. (C) Sitting on a forward slope. To keep the trunk perfectly upright as in A infants would need to increase their trunk-thigh angle by leaning backward (black stick figure shown with trunk-thigh angle of 120°). Green stick figure shows under-compensation (with a trunk-thigh angle of 90°, the infant would be pulled off balance and fall forward) and orange stick figure shows over-compensation (e.g., with a trunk-thigh angle of 140°, infants would need to recruit abdominal muscles to hold themselves stationary and risk losing balance). (D) Sitting on a backward slope. To keep the trunk perfectly upright, infants would need to decrease their trunk-thigh angle by leaning forward (black stick figure shown with trunk-thigh angle of 70°). Green stick figure shows under-compensation (with a trunk-thigh angle of 90°, the infant would be pulled off balance and topple backward) and orange stick figure shows over-compensation (e.g., with a trunk-thigh angle of 40° bringing the trunk close to the knees, infants would also need to recruit abdominal muscles). Note that C-D also give a visual representation of the steepest “sitable” slopes that infants achieved, on average, in the current study in the forward (M = 30°) and backward directions (M = 19°).
Even when sitting is stable, it is never stationary. Learning to sit entails learning to control postural sway. Every breath, every slight movement of the head and arms, creates destabilizing forces that cause the body to sway within the base of support. A destabilizing force in one direction must be met with a compensatory sway in the opposite direction (Deffeyes, Harbourne, Stuberg, & Stergiou, 2011; Kyvelidou, Harbourne, Willett, & Stergiou, 2013). At first, infants’ swaying motions are overly large and wildly variable (Cignetti, Kyvelidou, Harbourne, & Stergiou, 2011); they frequently over-compensate so that attempts to recover balance in one direction cause them to fall in the opposite direction (Saavedra et al., 2012). Over the same period when infants transform their bendable bodies into a head-trunk-hip synergy (Assaiante & Amblard, 1995), they learn to use information from optic flow and muscle-joint receptors to perceive sway and guide the compensatory swaying movements of their torso (Barela, Godoi, Freitas, & Polastri, 2000; Bertenthal, Rose, & Bai, 1997; Schmuckler, 1993). Sensitivity to swaying motions and to optic flow continues to increase with sitting experience (Bertenthal & von Hofsten, 1998; Saavedra et al., 2012).
Acquiring Behavioral Flexibility in Learning to Sit
Perhaps the most important component of sitting skill is behavioral flexibility—the ability to tailor movements to changing body-environment relations and task demands (Adolph & Robinson, 2015). Although sitting is an important developmental milestone, everyday sitting bears little resemblance to the prototypical posture in textbook milestone charts or the archetypal vertical posture shown in Figure 1A. It is far more variable in terms of body position, environmental context, and task (Adolph & Robinson, 2015). In everyday life, infants sit in a variety of positions (legs out in a “V”, bent back in a “W”, etc.) on a variety of surfaces (hard floor, soft mattress, parent’s lap) while performing a variety of tasks (looking around, interacting with objects and people). They must anticipate destabilizing forces caused by self-initiated movements, avoid loss of balance for planning actions, respond adaptively to unanticipated perturbations, and keep balance on a variety of surfaces.
Self-initiated movements of the head and limbs create destabilizing forces that pull the torso off-balance. Thus, infants must activate muscles in their torso or adjust their sitting posture before moving their arms to explore a toy, leaning forward to reach for an object, tilting their face to receive a kiss, or twisting their head to watch an interesting event. Infants must also use reactive strategies to compensate for unanticipated perturbations to balance while being bounced on a caregiver’s knee or jostled in a stroller. In the laboratory, infants show directionally-specific muscle responses when experimenters unexpectedly jerk the floor forward or backward (Hedberg, Carlberg, Forssberg, & Hadders-Algra, 2005; Woollacott, Debu, & Mowatt, 1987) or suddenly tilt the floor 4° up and down and then quickly back to horizontal (Hirschfeld & Forssberg, 1994). Indeed, infants show evidence of these aspects of flexibility—anticipatory muscle actions, postural adjustments, and reactive muscle responses—around the time that they pass criterion for independent sitting (Bertenthal & von Hofsten, 1998; Hopkins & Ronnqvist, 2002; Rachwani et al., 2013; Rachwani et al., 2015; Rochat & Goubet, 1995; Yonas & Hartman, 1993). However, new sitters still have much to learn. They frequently lean too far while reaching for a distant toy, and would fall if not strapped into their seat (Rochat, Goubet, & Senders, 1999; Yonas & Hartman, 1993). They accurately distinguish possible from impossible actions only after many weeks of sitting experience (Adolph, 2000). Similarly, despite reactive muscle responses, new sitters frequently fall due to unexpected perturbations to balance (Hirschfeld & Forssberg, 1994).
Despite previous work showing the importance of behavioral flexibility for the development of sitting, several critical aspects of flexibility have not been studied. First, in all prior work, researchers observed infants sitting on a rigid, horizontal surface. However, a hallmark of behavioral flexibility is adapting movements to changes in environmental properties (Adolph & Robinson, 2015). Variations in surface properties create different forces, induce different swaying motions, and require different control strategies. Second, the perturbations in previous work were fleeting (quick jolts and tilts of the surface of support) or self-initiated (head, trunk, or arm movements). Researchers never parametrically varied the challenge to balance control. Third, researchers have limited understanding about which aspects of behavioral flexibility are available at the onset of independent sitting and which require extensive sitting experience. Put another way, which aspects of behavioral flexibility are part and parcel of learning to sit?
Current Study
The current study addressed the limitations of previous work. We tested infants on an adjustable slope apparatus to examine behavioral flexibility in response to variations in the support surface; we parametrically varied the degree of slant to obtain precise measures of flexible responding and to create a continually increasing challenge to balance control; and we tested infants across a range of sitting experience to ask whether adaptive responding to a novel and varying support surface requires extensive experience after the onset of independent sitting.
Sitting on a slope is a particularly useful task to test behavioral flexibility for several reasons. First, steep slopes are novel, allowing researchers to test infants’ ability to adapt to a new situation. Second, slant can be adjusted in continuous increments to obtain a continuous metric of behavioral flexibility. Slopes require adaptation of the trunk-thigh angle to stay upright, with steeper slants requiring more adaptation. Third, forward and backward slopes present very different biomechanical challenges for balance (Hadders-Algra et al., 2007). A forward slope tilts the pelvis forward, pulling the torso off balance (Figure 1C). To compensate, sitters must lean backward, increasing the trunk-thigh angle. The steeper the slant, the larger the trunk-thigh angle needed to avoid falling face first. Conversely, a backward slope tilts the pelvis backward, pushing the trunk toward the outer edges of the base of support (Figure 1D). Because of the front-to-back asymmetry in the base of support, a backward slope presents a greater, more immediate challenge to balance compared with a forward slope (Forssberg & Hirschfeld, 1992; Hirschfeld & Forssberg, 1994). To compensate for the backward tilt, sitters must lean forward, decreasing the trunk-thigh angle. The steeper the slant, the smaller the trunk-thigh angle required to avoid toppling backward.
In the present study, all infants could sit independently on a horizontal surface, but none had experience keeping balance independently on steep slopes. Infants began with the slant set to horizontal. Then, for forward and backward directions, we increased slant in 2° increments until infants lost balance. Each incremental increase in slant made the task more difficult and more novel because steeper slants made balance more precarious and required more extreme, unfamiliar positions. Thus, our slope task posed two types of novel challenges for sitting balance—responding adaptively to the unexpected movements of the slope and adjusting posture to the new degree of slant when the slope was stationary between movements.
We calculated infants’ baseline trunk-thigh angle with the slope stationary and the slant set to 0° to determine their habitual posture. The sudden changes in slant presented a dynamic perturbation. Repeated success at keeping balance while the angle of slant was changing would be evidence of flexibility in reactive control. To this end, we noted the steepest degree of slant at which infants maintained balance—their steepest sitable slope. The steepest sitable slope reflected the number of increasingly difficult perturbations infants withstood. Based on previous work in which sudden upward tilts of only 4° caused most infants to fall (Hirschfeld & Forssberg, 1994), we expected the backward direction to be more difficult than forward and infants with more sitting experience to withstand more perturbations and thereby achieve steeper sitable slopes.
We were also interested in infants’ behavior when the slope was stationary between adjustments in slant. We considered evidence of flexibility to be differential modifications of the trunk-thigh angle based on the direction and degree of slant. We predicted that successful sitters would lean backward for forward slopes and lean forward for backward slopes. Most important was whether infants would continually adjust their trunk-thigh angle in accordance with incremental changes in the degree of slant. We reasoned that the optimal strategy for coping with the continual change in slant would be to increase or decrease the trunk-thigh angle by 2° for every 2° change in slant. And so for each infant we calculated delta—the linear change in trunk-thigh angle across changes in slant. Delta values close to 2 for forward slopes and −2 for backward slopes would be optimal. Higher delta values would reflect over-compensation, resulting in unnecessary effort. Lower delta values would reflect under-compensation, creating more rotational force on the head and torso, and increasing the likelihood of falling (Figure 1C–D). We predicted that more optimal delta values would be correlated with steeper sitable slopes, and more experienced sitters would display more optimal delta values.
Method
Participants
We tested 22 sitting infants (11 boys, 11 girls), ranging from 6.4 to 8.8 months of age (M = 7.44 months). Families were recruited through commercial mailing lists. Most were White and middle class. All infants were healthy and born at term. Before testing began, an experimenter verified that infants could sit independently without using their hands for support: Before starting the experiment, all infants sat on the floor with their legs outstretched in a “V” for 30 seconds; infants held small toys to keep their hands off the floor. Parents reported the first day they saw infants sit independently, consulting baby books, calendars, or photos/videos to aid their memory (see Adolph, Vereijken, & Shrout, 2003). Sitting experience ranged from 7 to 79 days (M = 36.0 days, SD = 21.7) and was correlated with infants’ age, r(20) = .50, p < .01. As souvenirs of participation, families received diplomas and photographs of the session. Six additional infants (4 boys) did not contribute data, because they cried as soon as we moved the slope and we had to end the experiment.
Slope Apparatus and Procedure
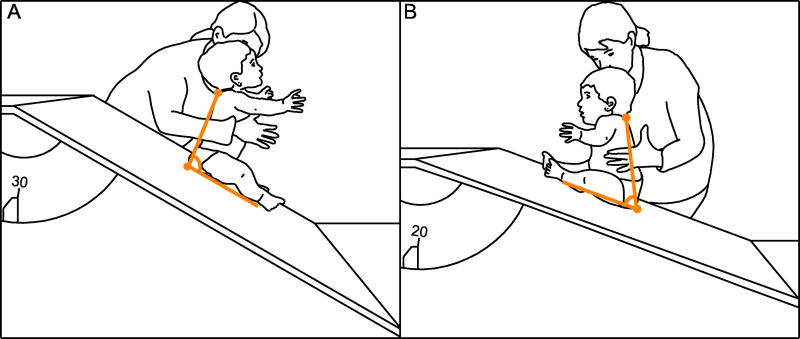
Infants wore only diapers for testing. We observed infants on an adjustable wooden slope (86-cm wide × 91-cm long) covered with high-friction rubber to provide traction. Because the surface only slanted in one direction, we induced forward slants by placing infants facing down the slope and backward slants by facing infants up the slope (Figure 2).
Figure 2.
Line drawing of a typical infant in the (A) forward and (B) backward test directions at the average steepest “sitable” slopes that infants achieved. Because the slope only slanted in one direction, we created the two conditions by facing infants down or up the slope. Orange lines represent trunk-thigh angles derived from manual digitization of videos. (A) Forward: The trunk-thigh angle is 98°, so the infant is under-compensating (not leaning sufficiently backward). (B) Backward: The trunk-thigh angle is 63°, so the infant is slightly over-compensating (leaning slightly more forward than necessary).
Each infant received two slope sequences per direction (two sequences of forward slants and two sequences of backward slants), with direction order counterbalanced across infants. Each sequence began at baseline with slant set to 0° and involved a subsequent series of adjustments of consecutive 2° increases in slant (2°, 4°, 6°, etc.) until the infant lost balance. Throughout the whole procedure, infants sat on the slope with legs outstretched in a “V”, hands up in the air, and were not removed from the slope until they lost balance. An experimenter stood behind infants to ensure their safety if they lost balance. The experimenter straightened infants’ legs if they tried to use their feet to brace themselves and lifted infants’ arms if they placed them on the slope. Caregivers and an assistant stood at the end of the apparatus and kept infants entertained by playing peek-a-boo games and showing them toys and stuffed animals. Infants sat for M = 4 s prior to the first and each subsequent adjustment of the slope. A large protractor displayed the degree of slant. Infants were video recorded from a side view to capture their trunk-thigh angle.
A second assistant adjusted slant in approximate 2° increments (~0.10°/s) by pressing and releasing a push button remote that operated a drive screw beneath the surface of the apparatus. As the slope moved, infants’ balance was disrupted, moving their torsos in the direction of the slope and then in the opposite direction. After the experimenter ensured that infants had again stabilized themselves and would not fall at the new slant (with their legs outstretched and hands up in the air), the assistant increased the slant by another 2°. The process continued until infants lost balance or fussed, ending the sequence.
Data Coding
To ensure that analyses focused on infants’ best performance, we selected the sequence for each infant with the steepest sitable slope in that direction—the steepest slant at which infants maintained balance. Thus, the final dataset included 44 slope sequences, one from each infant in each slope direction.
A primary coder manually digitized trunk-thigh angles from videos. Our digitization process was designed to record infants’ posture for each degree of slant during periods when the slope had stopped moving and infants had stabilized their posture. To minimize the cost of digitizing, we selected a single, representative video frame for each slant. To eliminate the possibility of bias in frame selection, we digitized the video frame prior to each slope movement. A primary coder used Datavyu, a computerized video coding tool (www.datavyu.org) that allows precise frame-by-frame analysis of the recording, to identify the appropriate video frame. The coder watched the video in slow motion to locate the onset of each slope movement, and then jogged backward to select the frame just prior to the slope movement. To ensure inter-observer reliability, a second coder repeated this process for 25% of each infant’s data. The coders were in exact frame agreement for 85% of the data and were within 1 video frame for 97.4% of the data. For each selected video frame, the primary coder noted the degree of slant from the protractor. On 13.4% of slant adjustments, the slope failed to move a full 2° or moved slightly more than 2°. In those cases, the coder rounded the slant to the nearest 2° increment to facilitate comparisons across infants.
Then, the primary coder manually digitized each selected video frame using a customized MATLAB tool for digitizing video files (Hedrick, 2008) by clicking on two anatomical landmarks to mark the angle of the trunk: a point on the neck (seventh cervical vertebra) and the buttock (sacral bone). We estimated trunk-thigh angle based on the trunk angle relative to the slope angle because infants’ thighs were parallel to the slope. Given that trunk-thigh angle is conceptually more meaningful than trunk-slope angle or trunk angle relative to horizontal, we used the term trunk-thigh angle. A second coder, blind to the experimental question, manually digitized 25% of each infant’s data to confirm reliability of the trunk-thigh angle, r(150) = .96, p < .001.
Results
Analyses focused on three measures: (1) baseline trunk-thigh angle when the slope was horizontal; (2) steepest sitable slope at which infants kept balance while the slope was moving; and (3) changes in trunk-thigh angle (as a group and individually) when the slope was stationary between adjustments in slant. We show descriptive data for every measure and used the Pearson’s correlation coefficient (r) to characterize the relations among measures. We used paired-sample t-tests to compare the effects of forward and backward directions. We used a Generalized Estimating Equation (GEE) to analyze the effect of increasing slant on group averaged trunk-thigh angles between forward and backward directions. We chose a GEE approach rather than analyses of variance (ANOVA) because infants lost balance at different degrees of slant (and thus contributed different numbers of observations), and a GEE can account for the unequal observations across slant. Based on goodness of fit statistics (QIC), we chose an identity link function and an independent covariance structure to best fit our model. Preliminary analyses showed no effects of gender or direction order (ts < 0.89, ps > .38), so these factors were collapsed for subsequent analyses.
Sitting on a Horizontal Surface: Baseline Trunk-Thigh Angle
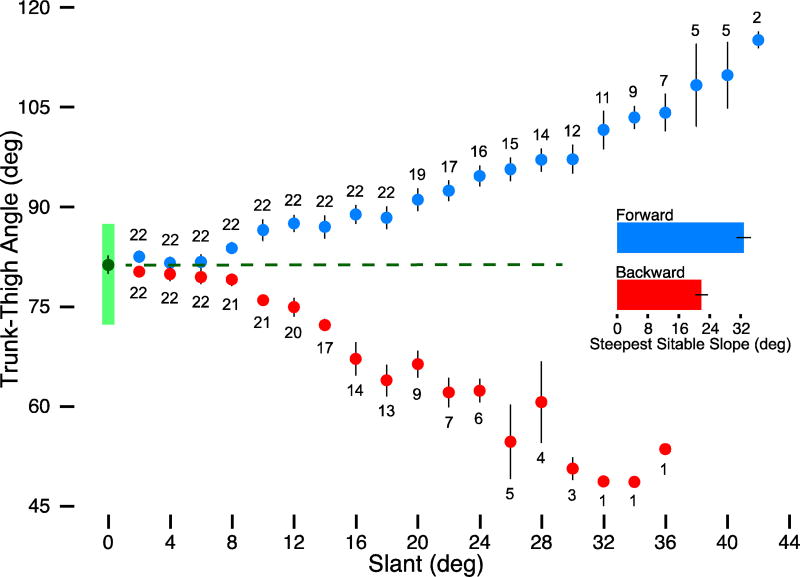
At baseline, with slant set to 0°, infants leaned slightly forward with trunk-thigh angles of 70.3° to 88.6° (see Figure 1B and green bar in Figure 3). Baseline trunk-thigh angles did not differ between the forward (M = 80.8°, SD = 4.0) and backward directions (M = 81.6°, SD = 5.0), t(21) = .77, p = .449, and baseline angles were correlated, r(20) = .48, p = .024. Thus, there were no biases in trunk-thigh angle before changing the degree of slant. Most infants (17/22) differed by less than 5° between forward and backward directions. Infants’ age predicted baseline trunk-thigh angles on the horizontal surface: Older infants sat with their trunks closer to 90°, r(20) = .59, p = .004. Sitting experience, however, was not related to baseline trunk-thigh angle, r(20) = .11, p = .630.
Figure 3.
Summary of major findings. Trunk-thigh angles and slants are measured in degrees. Blue circles represent the average trunk-thigh angles for the forward direction, and the red circles show the average trunk-thigh angles for the backward direction at each increment of slant. Error bars denote ±1 SE. Light green bar shows the range of baseline trunk-thigh angles with slant set to 0°. The dark green circle and dashed line denote the average baseline trunk-thigh angle. The increasing distance between circles and dashed line emphasizes the faster rate of change in trunk-thigh angle in the backward direction compared with the forward direction. Ns represent the number of infants contributing data at each slant in each direction. All 22 infants contributed data to 2°-18° slants in the forward direction and to 2°–6° in the backward direction. Two infants achieved steepest sitable slopes of 42° in the forward direction and 1 achieved 36° in the backward direction. Bar graph shows the group average for steepest sitable slope for each direction. Error bars denote ±1 SE.
Keeping Balance on a Moving Slope: Steepest “Sitable” Slope
To our surprise, all infants could keep balance on the moving slope. All kept balance on at least 18° in the forward direction, and all managed at least 6° in the backward direction (see Ns in Figure 3). Slope direction affected infants’ ability to maintain a sitting posture. On average, the steepest sitable slope was 30° (SD = 8°) in the forward direction, meaning that infants withstood 15 perturbations to balance. On average, the steepest sitable slope was lower, but still a sizable 19° (SD = 8°) in the backward direction (see bar graph in Figure 3). Figure 1C–D and Figure 2 show pictorial representations of the average steepest sitable slopes.
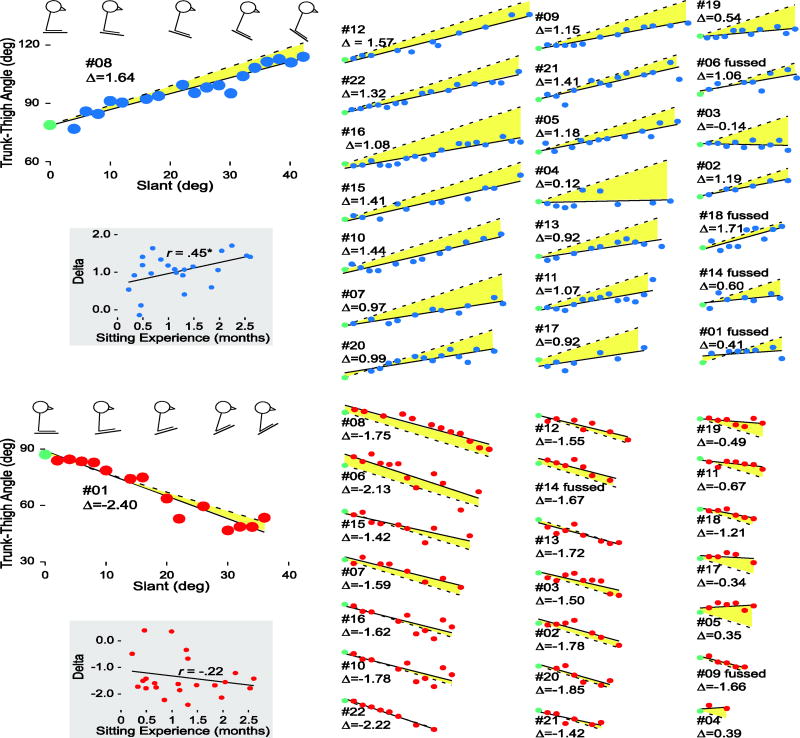
In the forward direction, 6/22 sequences ended because infants fell forward, 12 sequences ended because infants fell backward, and the experimenter ended 4 sequences because infants became fussy (see marked infants in Figure 4, top panel). In the backward direction, 20/22 sequences ended with infants toppling over backward and 2 because they began to fuss, including one who also fussed in the forward direction (see marked infants in Figure 4, bottom panel). Indeed, the advantage for the forward direction would have been even more pronounced had the 4 fussy infants been able to demonstrate their true abilities. On average, infants achieved steeper sitable slopes in the forward than backward direction, t(21) = 4.83, p < .001, d = 1.38, indicating that backward slopes were more challenging. Most infants (19/22) showed this pattern; one infant had the same steepest sitable slope in both directions, and two of the forward-fussers had steeper sitable slopes in the backward than forward direction.
Figure 4.
Individual differences in infants’ ability to cope with forward (top panel) and backward (bottom panel) slopes. Trunk-thigh angles and slants are measured in degrees. Each infant is represented by the same participant number in each direction; infants who fussed out in each direction are noted. Data are ordered in columns by the steepest sitable slope in each direction (steepest sitable slope is also represented by the width of each graph). Large graphs on left side of each panel show the most successful infant in each direction and the extent of the axes. Stick figures above each graph illustrate (A) the increase in infant #08’s trunk-thigh angle from baseline to steeper slants and (B) the decrease in infant #01’s trunk-thigh angle from baseline to steeper slants. The legs-down posture in A and legs-up posture in B reflect the thigh angle relative to horizontal; the trunk position shows infant’s trunk relative to vertical. Circles represent trunk-thigh angle at each observed degree of slant. Delta values (Δ), the change in trunk-thigh angle across slants, are denoted for each infant in each direction based on the fitted regression line through each infant’s data (solid lines). Dashed lines represent the optimal regression line if infants adjusted their trunk-thigh angle from baseline by 2° for each 2° increase in slant. Size of the yellow colored region between the solid and dashed lines reflects infants’ deviation from optimal responding. Scatterplots in gray boxes on left side of each panel show delta values across months of sitting experience.
For correlational analyses involving the steepest sitable slopes, we removed the fussy infants from each direction because they were not able to demonstrate the limits of their ability. Steepest sitable slopes for forward and backward directions were correlated, r(15) = .60, p = .010. Sitting experience predicted the steepest sitable slope for the forward direction, r(16) = .51, p < .032, but not backward, r(18) = .29, p = .209. Age marginally predicted the steepest sitable slope for forward, r(16) = .46, p = .056, but not for backward, r(18) = .19, p = .420.
Adaptation to Increasing Slant: Change in Trunk-Thigh Angle
The critical question was whether and how infants tailored their trunk-thigh angle to the challenges posed by increasingly steeper slants when the slope was stationary. Figure 3 shows that in both directions, infants systematically adapted their trunk-thigh angle to incremental changes in slant. Infants adapted to increasing slant differentially in each direction—by increasing trunk-thigh angle (leaning backward) in the forward direction and by decreasing trunk-thigh angle (leaning forward) in the backward direction, and these changes in trunk-thigh angle became more distinguishable beyond 8° of slant.
The GEE assessed the interactive effects of direction (forward, backward) and slant (2°-42° in 2° increments) as fixed-effects factors on trunk-thigh angle. The GEE confirmed an interaction between direction and slant, Wald χ2(17) = 3379.77, p < .001. Follow-up paired-t tests (at α = .002 to control for multiple comparisons) indicated that trunk-thigh angle did not significantly differ between directions at slants from 2°–6° (Wald χ2s < 2.49, ps > .066) but did start to significantly differ from 8° and beyond (Wald χ2s > 10.17, ps < .001).
Figure 4 depicts data from individual infants and corroborated the group averages. For each sequence, we computed the best fitting regression line (solid lines in the figure) between trunk-thigh angle and degree of slant (circles in the figure). The unstandardized beta value represents the change in trunk-thigh angle for every unit change in slant. Because every unit change of slant in the regression equation is actually a 2° change in the slant of the apparatus, we multiplied the unstandardized beta value by 2 to create a delta value for each sequence. This strategy puts the delta value into the same unit space as the slant adjustment for a more meaningful interpretation.
Delta values were positive for 21/22 infants in the forward direction (M = 1.03, SD = 0.48) and negative for 20/22 infants in the backward direction (M = −1.36, SD = 0.75). All infants showed directionally-specific adaptations in at least one direction and 19 showed it in both directions. Infant #15, for example displayed a maximum trunk-thigh angle of 119° in the forward direction (see last point in Figure 4), an increase of 35° from baseline. Infant #06 displayed a minimum trunk-thigh angle of 38° in the backward direction (see second to last point in Figure 4), a decrease of 40° from baseline. The infants who failed to show directionally-specific leaning had delta values close to 0, rather than in the opposite direction. Note, however, that directionally-specific leaning, while beneficial, was not required for keeping balance. In the forward direction, infant #04 kept balance until 30° with a delta of only 0.12, and infant #03 kept balance until 20° with a negative delta of −0.14. Similarly, in the backward direction, infant #05 achieved a 12° steepest sitable slope with a positive delta of 0.35, meaning that he maintained balance while barely changing posture.
Moreover, as shown in Figure 3 by the vertical distance between the circles and the dashed horizontal line at each degree of slant, infants showed greater adjustments in the backward than forward direction. On average, the absolute delta values were larger for backward (M = 1.43°, SD = .61°) than forward slants (M = 1.04°, SD = .45°), t(21) = .39, p = .013, d = 0.73. Greater compensation (larger absolute deltas) for backward compared to forward slants remained true even when statistically controlling for steepest sitable slope (mean-centered in each direction) in an analysis of covariance, F(1, 19) = 10.65, p = .004, partial η2 = .36.
For visualization purposes, we also calculated an “optimal” regression line for each sequence, based on the baseline trunk-thigh angle and a 2° change of trunk-thigh angle for every 2° change in slant (dashed lines in Figure 4). The optimal regression line denotes what infants’ trunk-thigh angle would be for each adjustment in slant if they maintained the same trunk angle relative to horizontal as they displayed during baseline (0°). Thus, a baby with a baseline trunk-thigh angle of 88° would have a different y-intercept than a baby with a baseline trunk-thigh angle of 70°, but their two optimal regression lines would be parallel. The yellow colored region between the obtained and optimal regression lines shows infants’ deviation from optimal responding. The yellow colored regions were smaller in the backward than forward direction, reflecting that infants compensated more for backward than for forward slopes.
As we predicted, greater delta values (closer to 2 and −2) predicted steeper sitable slopes in the forward direction, r(16) = .66, p = .003, and in the backward direction, r(18) = −.75, p < .001 (excluding the infants who fussed). Delta values for forward and backward directions were not correlated, r(20) = −.22, p = .317, meaning that infants were not equally adept at adjusting posture to both directions. In fact, infant #01, who displayed the largest delta value for the backward direction (Δ = −2.40°) showed one of the smallest delta values for the forward direction (Δ = 0.41°), and infant #05, who displayed one of the smallest delta values for the backward direction (Δ = 0.35°) showed one of the largest values for the forward direction (Δ = 1.18°). Age was not related to delta values in either direction, rs(20) < .21, ps > .358. Sitting experience was significantly correlated with delta values in the forward, r(20) = .45, p = .035 but not backward direction, r(20) = −.22, p = .321 (see scatterplots in gray boxes in Figure 4).
Discussion
We showed that behavioral flexibility—the ability to tailor actions to the demands of the current situation—is integral to the development of sitting. Specifically, we focused on infants’ ability to continually adapt posture to changes in the support surface while sitting on an adjustable slope. All infants were proficient at keeping balance with slant set to 0°. To our surprise, every infant—at least to some extent—maintained balance in the novel slope task. They engaged reactive control as the slope moved to compensate for the perturbation, and they adapted sitting posture to the subsequent change in slant while the slope was stationary. As predicted, due to the different biomechanical constraints of slope direction, infants were more successful at keeping balance despite the movement of the slope in the forward than backward direction. Infants adapted to the direction of slant by leaning backward (increasing their trunk-thigh angle) on forward slopes and by leaning forward (decreasing their trunk-thigh angle) on backward slopes. Moreover, on the stationary slopes between adjustments in slant, infants adjusted their posture for each incremental change in slant and were more adept at it in the backward direction. The more closely infants tailored sitting posture to slant (greater delta values), the steeper sitable slope they attained. Sitting experience predicted infants’ ability to cope with the moving and stationary slopes, but only in the forward direction.
Acquiring Flexibility While Learning to Sit
Flexibility is what makes skills truly functional (Adolph & Robinson, 2015). Sitting, like all everyday actions, must be continually modified to suit changes in local conditions. Infants’ growing bodies and variations in the rigidity, friction, extent, and slope of the support surface alter the biomechanical constraints on balance. Unanticipated perturbations (e.g., a caregiver’s moving lap) challenge balance control. Requirements of the task—looking around, interacting with objects and people—create upcoming disruptions to balance. Flexibility is essential for functional sitting because body-environment relations and tasks are always in flux.
Previous work showed that experienced sitting infants demonstrate flexibility by perceiving whether they can lean forward to grasp a toy (Adolph, 2000; Yonas & Hartman, 1993), anticipating disruptions to balance while planning a reach (de Graaf-Peters, Bakker, van Eykern, Otten, & Hadders-Algra, 2007; Rachwani et al., 2015; van Balen, Dijkstra, & Hadders-Algra, 2012), and responding with directionally appropriate compensatory sways and muscle responses to unexpected visual and mechanical perturbations (Bertenthal et al., 1997; Forssberg & Hirschfeld, 1992). Here, we tested flexibility in a new arena—adapting sitting posture to changes in the support surface. Specifically, this was the first study to test infants on a non-horizontal surface. Our adjustable slope task challenged balance in two ways: keeping balance while the slope was moving and adapting posture to the new degree of slant while the slope was stationary. Each small increase in slant “ramped up” the challenge by requiring infants to cope with increasingly precarious and unfamiliar positions.
Infants demonstrated impressive behavioral flexibility. Indeed, half of the sample kept balance on forward moving slopes of 32° to 42° and half kept balance on backward moving slopes of 18° to 36° (see Figure 3). Most infants accomplished this remarkable feat with directionally-specific adaptations: They increased their trunk-thigh angles on forward stationary slopes and decreased their trunk-thigh angles on backward stationary slopes. It is important to highlight that directionally specific leaning was deliberate and not the result of passive forces pushing the body into the appropriate configuration or the visco-elastic properties of the body holding the torso upright. Older children and adults, for example, have tight hamstrings. As a consequence, the tight muscles on the back of the legs keep the body from falling forward without requiring sitters to generate muscle force (Winter, Patla, Prince, Ishac, & Gielo-Perczak, 1998)—think of how difficult it is for most adults to put nose to knees in a “forward bend.” Infants, however, have extremely loose hamstrings, so they must work to keep their body from falling nose to knees (pre-sitters often fall by flopping forward into positions unattainable by most adults). So, as destabilizing forces pulled infants’ torsos forward on the forward slopes, they had to work to keep their backs upright. Leaning forward on backward slopes was also not easy for infants. Although infants can easily put nose to knees on a horizontal surface, on backward slopes, gravity pulled their torsos backward (indeed they always fell by tumbling heels over head instead of the other way around). Thus, infants had to recruit abdominal muscles to lean forward and minimize their trunk-thigh angle on backward slopes.
Most impressive was individual infants’ systematic response to each incremental change in slant. In principle, infants could have maintained their baseline posture until the destabilizing forces became so great that they were forced to adjust their trunk-thigh angle, causing their pattern of responding to resemble a step function or series of step functions. But they did not. As shown in Figure 4, postural adjustments were immediate and incremental. Most infants readjusted their resting posture with each increase in slant by slightly increasing the angle of their backward or forward leaning. The required precision is remarkable: To do this, infants had to use visual and proprioceptive information to perceive the new slant of the support surface and the change in forces acting on their torso, and then adapt accordingly. Moreover, infants with more systematic changes in trunk-thigh angle (more optimal delta values) achieved steeper sitable slopes in both directions, indicating that flexibility and function are intimately related. These findings reveal that early sitting skills are far more flexible and adaptive than previously acknowledged.
What Develops
So what do infants learn as they learn to sit? Clearly, infants are not learning fixed solutions to particular motor problems, such as “maintain my trunk-thigh angle at 85°” or “keep my torso vertical to gravity.” Indeed, fixed solutions for posture would be maladaptive in a world of continuous flux. At every point in sitting development, novelty is the rule, not the exception. To our surprise, all of the infants demonstrated flexibility, regardless of their sitting experience or age. Even infants with less than two weeks of sitting experience maintained balance on slopes (see, for example, infants #13 and #19 in the top and bottom panels of Figure 4 who both achieved relatively large steepest sitable slopes and relatively large delta values). This finding suggests that infants acquire behavioral flexibility as they learn to sit. That is, adaptive responding to balance disruptions and adjustments of posture to suit current conditions is integral to the acquisition of independent sitting, not a later-acquired ability. On a horizontal surface, infants maintain balance with a slightly hunched posture. But on a sloping surface, they adjust their posture to the direction and degree of slant—even if it means increasing trunk-thigh angle to 119° as in infant #15 in the forward direction or decreasing trunk-thigh angle to 39° as in infant #06 for the backward direction.
Put another way, when pre-sitting infants practice sitting over and over on the floor, the couch, their caregiver’s knee, and so on, all the while engaged in various tasks of looking and interacting with objects and people, these experiences across a variety of surfaces and tasks, ensure the acquisition of behavioral flexibility. Experience with varied body-environment relations may teach infants to find appropriate solutions for the current situation while also shoring up constituents common to all sitting postures. For example, sitting on the floor and on the couch involve different demands on balance control. Shaking a rattle and turning to watch an event involve different constraints on balance. However, all of these situations strengthen infants’ torso and increase coordination to counteract destabilizing forces, thus allowing infants to adapt posture for a novel situation such as sitting on slopes.
What would be the alternative to acquiring behavioral flexibility while learning to sit? Of course, it is always a possibility that improvements in behavioral flexibility arise solely through neural-muscular maturation (e.g., Gesell, 1946; Hadders-Algra, Brogren, & Forssberg, 1996; Hirschfeld & Forssberg, 1994; McGraw, 1945). However, we found no evidence to support this view. Age at testing, a stand-in for maturation, did not predict infants’ success at sitting on slopes or postural adjustments in either direction.
Another possibility is that infants could acquire new motor skills such as sitting, reaching, crawling, and walking for the standard, simplified situations in which they are typically tested in the laboratory, and then flexibility is acquired later to embellish the basic skill. On this account, infants could first learn to sit on a standard, rigid, horizontal surface; they could first learn to reach for stationary targets offered at midline; they could first learn to crawl and walk while traveling in straight lines on flat ground. Then, with the basics under their belt, infants could gradually add on variations (different support surfaces, toys at varied locations, omnidirectional steps and curved paths, and so on). The current findings argue against this standard-before-flexibility hypothesis. Similarly, recent findings indicate that infants take omnidirectional steps along curved paths from their very first week of walking and that such flexible, situation-specific gait patterns continue unabated across the first 10 months of walking, arguing that flexibility is integral to learning to walk (Lee, Cole, Golenia, & Adolph, in press).
Regardless of whether flexibility is part and parcel of basic skill acquisition, a long history of research shows that experience is critical for improvements in flexibility (Adolph & Robinson, 2013, 2015). Indeed, dozens of studies show that sitting skill improves with experience. More experienced sitters are better able to distinguish possible from impossible actions (Adolph, 2000; Yonas & Hartman, 1993). They show more vertical trunk posture (Saavedra et al., 2012), a narrower sway region (Harbourne & Stergiou, 2003; Kyvelidou et al., 2009), more coordinated reaching actions (Rachwani et al., 2013; Rochat & Goubet, 1995), increased ability to turn and look around the room (Bertenthal & von Hofsten, 1998), and so on. Eventually, children are so adept at keeping balance in a sitting posture that they can ride a sled down a steep, snowy hill and balance on a swing and seesaw. Accordingly, the current study also provided evidence that sitting experience is related to improvements in flexibility. Infants with more sitting experience achieved steeper sitable slopes and had larger delta values for the forward direction.
Different Demands of Forward and Backward Slopes
The differences between forward and backward directions present a puzzling set of findings. Infants displayed more consistent changes in trunk-thigh angles (larger delta values and smaller differences between actual and optimal regression lines) on backward stationary slopes compared to forward, and delta values predicted the steepest sitable slopes in both directions. But infants were less successful keeping balance on backward moving slopes nonetheless. Moreover, sitting experience predicted infants’ steepest sitable slopes and delta values only for the forward direction. How might we reconcile these seemingly discrepant findings?
We expected the backward direction to be more costly and challenging than forward due to the front-back asymmetry in the base of support. So, it was no surprise that infants achieved considerably smaller sitable slopes in the backward direction. Similarly, previous work shows that older children must expend intense muscle effort to sit on a stationary 15° backward slope compared with sitting on a horizontal or forward slope (Hadders-Algra et al., 2007). Adults increase muscle activity when tilting backward in a sitting posture compared to vertical sitting (Munoz & Rougier, 2011).
The unexpected finding was that infants showed larger deltas in the backward direction compared with forward. Most infants (17/22) displayed deltas greater than 1.0 in the backward direction (see bottom right panel of Figure 4). These deltas suggest that infants could perceive changes in slant and could make adjustments in posture. So why did they do so less on the forward slopes? One possibility is that such dramatic postural adjustments were not required to keep balance on forward slopes, so infants chose the path of least resistance. That is, infants responded with less efficient postural adjustments in the forward direction that did the job and required less effort. Similarly, when responding to unexpected disruptions to balance induced with sudden 4° tilts in a horizontal surface (the support surface quickly rotated down and then up to induce forward tilts or up then down to induce backward tilts), infants showed immediate, directionally-specific muscle responses in their trunk for backward tilts, but not for forward (Forssberg & Hirschfeld, 1992; Hirschfeld & Forssberg, 1994). The researchers speculated that when the torso is disrupted backward, the outer edge of the base of support is much closer, which requires rapid responses to prevent falling. But when the torso is disrupted in the forward direction, the large base of support provided by the legs provides more leeway for balance control. An alternative possibility is that infants adapted to the forward slope by using other compensatory strategies that were not being captured with our measure of trunk-thigh angle, like leaning their head back instead of the trunk, or increasing muscle activity. Or perhaps the two directions provided different access to visual information for slant. More sophisticated technology for tracking infants’ movements (e.g., high-speed motion tracking) and eye gaze (head-mounted eye tracking) could investigate these possibilities.
Why did sitting experience predict steepest sitable slopes and delta values in the forward direction, but not backward? Perhaps the answer is that the backward slopes put all infants on a level playing field where disruptions to balance were immediate and dire. But the latitude provided by forward slopes allowed infants to decide whether to use a more or less efficient strategy of postural adjustments. Regardless, a rule of thumb for motor development is that experience leads to more efficient responses for basic motor skills such as sitting, reaching, crawling, and walking (Adolph, Cole, & Vereijken, 2015).
Conclusions: Learning to Sit
Learning to sit entails upright balance control. But sitting is much more than merely maintaining posture. Adaptive, functional sitting requires behavioral flexibility. By observing infants’ balance control on a non-horizontal, sloping surface that allowed for continuous and individualized gradations of difficulty, we discovered that infants acquire impressive flexibility—the ability to adapt their posture to novel variations in the support surface—as they learn to sit.
Acknowledgments
This research was supported by National Institute of Health and Human Development Grant R37-HD33486 to Karen E. Adolph. We gratefully acknowledge the infants and parents who participated. We thank Lisa Hurwitz and the members of the NYU Infant Action Lab for assistance collecting and coding data.
Footnotes
Portions of this work were presented at the 2013 International Society of Developmental Psychobiology, San Diego, CA and the 2016 International Congress of Infant Studies, New Orleans, LA.
The authors declare no financial conflicts of interest.
References
- Adolph KE. Specificity of learning: Why infants fall over a veritable cliff. Psychological Science. 2000;11:290–295. doi: 10.1111/1467-9280.00258. [DOI] [PubMed] [Google Scholar]
- Adolph KE, Avolio AM. Walking infants adapt locomotion to changing body dimensions. Journal of Experimental Psychology: Human Perception and Performance. 2000;26:1148–1166. doi: 10.1037//0096-1523.26.3.1148. [DOI] [PubMed] [Google Scholar]
- Adolph KE, Cole WG, Vereijken B. Intra-individual variability in the development of motor skills in childhood. In: Diehl M, Hooker K, Sliwinski M, editors. Handbook of intra-individual variability across the lifespan. New York: Routledge/Taylor & Francis Group; 2015. pp. 59–83. [Google Scholar]
- Adolph KE, Robinson SR. The road to walking: What learning to walk tells us about development. In: Zelazo P, editor. Oxford handbook of developmental psychology. New York: Oxford University Press; 2013. pp. 403–443. [Google Scholar]
- Adolph KE, Robinson SR. Motor development. In: Liben L, Muller U, editors. Handbook of child psychology and developmental science. 7. Vol. 2. New York: Wiley; 2015. pp. 114–157. Cognitive Processes. [Google Scholar]
- Adolph KE, Vereijken B, Shrout PE. What changes in infant walking and why. Child Development. 2003;74:474–497. doi: 10.1111/1467-8624.7402011. [DOI] [PubMed] [Google Scholar]
- Assaiante C, Amblard B. An ontogenetic model for the sensorimotor organization of balance control in humans. Human Movement Science. 1995;14:13–43. [Google Scholar]
- Barela JA, Godoi D, Freitas PB, Polastri PF. Visual information and body sway coupling in infants during sitting acquisition. Infant Behavior and Development. 2000;23:285–297. [Google Scholar]
- Bertenthal BI, Rose JL, Bai DL. Perception-action coupling in the development of visual control of posture. Journal of Experimental Psychology: Human Perception and Performance. 1997;23:1631–1643. doi: 10.1037//0096-1523.23.6.1631. [DOI] [PubMed] [Google Scholar]
- Bertenthal BI, von Hofsten C. Eye, head and trunk control: The foundation for manual development. Neuroscience and Biobehavioral Review. 1998;22:515–520. doi: 10.1016/s0149-7634(97)00038-9. [DOI] [PubMed] [Google Scholar]
- Butler P, Saavedra S, Sofranac M, Jarvis S, Woollacott MH. Refinement, reliability and validity of the segmental assessment of trunk control (SATCo) Pediatric Physical Therapy. 2010;22:246–257. doi: 10.1097/PEP.0b013e3181e69490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cignetti F, Kyvelidou A, Harbourne RT, Stergiou N. Anterior-posterior and medial-lateral control of sway in infants during sitting acquisition does not become adult-like. Gait and Posture. 2011;33:88–92. doi: 10.1016/j.gaitpost.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis DJ, Hansen L, Luun M, Loberg R, Woollacott MH, Saavedra S, Bencke J. Measuring postural sway in sitting: A new segmental approach. Journal of Motor Behavior. 2015;47:427–435. doi: 10.1080/00222895.2014.1003782. [DOI] [PubMed] [Google Scholar]
- de Graaf-Peters Victorine B, Bakker Hanneke, van Eykern Leo A, Otten Bert, Hadders-Algra Mijna. Postural adjustments and reaching in 4- and 6-month-old infants: An EMG and kinematical study. Experimental Brain Research. 2007;181(4):647–656. doi: 10.1007/s00221-007-0964-6. [DOI] [PubMed] [Google Scholar]
- Deffeyes JE, Harbourne RT, Stuberg WA, Stergiou N. Sensory information utilization and time delays characterize motor developmental pathology in infant sitting postural control. Motor Control. 2011;15:302–317. doi: 10.1123/mcj.15.2.302. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Hirschfeld H. Postural adjustments in sitting humans following external perturbations: muscle activity and kinematics. Experimental Brain Research. 1992;97:515–527. doi: 10.1007/BF00241545. [DOI] [PubMed] [Google Scholar]
- Gesell A. The ontogenesis of infant behavior. In: Carmichael L, editor. Manual of child psychology. New York, NY: John Wiley; 1946. pp. 295–331. [Google Scholar]
- Hadders-Algra M, Brogren E, Forssberg H. Training affects the development of postural adjustments in sitting infants. Journal of Physiology. 1996;493:289–298. doi: 10.1113/jphysiol.1996.sp021383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadders-Algra M, van der Heide JC, Fock JM, Stremmelaar E, van Eykern LA, Otten B. Effect of seat surface inclination on postural control during reaching in preterm children with cerebral palsy. Physical Therapy. 2007;87:861–871. doi: 10.2522/ptj.20060330. [DOI] [PubMed] [Google Scholar]
- Harbourne RT, Stergiou N. Nonlinear analysis of the development of sitting postural control. Developmental Psychobiology. 2003;42:368–377. doi: 10.1002/dev.10110. [DOI] [PubMed] [Google Scholar]
- Hedberg Asa, Carlberg Eva Brogren, Forssberg Hans, Hadders-Algra Mijna. Development of postural adjustments in sitting position during the first half year of life. Developmental Medicine and Child Neurology. 2005;47:312–320. doi: 10.1017/s0012162205000605. [DOI] [PubMed] [Google Scholar]
- Hedrick TL. Software techniques for two- and three-dimensional kinematic measurements of biological and biomimetic systems. Bioinspiration and Biomimetics. 2008;3:1–6. doi: 10.1088/1748-3182/3/3/034001. [DOI] [PubMed] [Google Scholar]
- Hirschfeld H, Forssberg H. Epigenetic development of postural responses for sitting during infancy. Experimental Brain Research. 1994;97:528–540. doi: 10.1007/BF00241546. [DOI] [PubMed] [Google Scholar]
- Hopkins B, Ronnqvist L. Facilitating postural control: Effects on the reaching behavior of 6-month-old infants. Developmental Psychobiology. 2002;40:168–182. doi: 10.1002/dev.10021. [DOI] [PubMed] [Google Scholar]
- Johnson CP, Blasco PA. Infant growth and development. Pediatric Review. 1997;18:224–242. doi: 10.1542/pir.18-7-224. [DOI] [PubMed] [Google Scholar]
- Kaminski TR, Bock C, Gentile AM. The coordination between trunk and arm motion during pointing movements. Experimental Brain Research. 1995;106:457–466. doi: 10.1007/BF00231068. [DOI] [PubMed] [Google Scholar]
- Karasik LB, Tamis-LeMonda CS, Adolph KE, Bornstein MH. Places and postures: A cross-cultural comparison of sitting in 5-month-olds. Journal of Cross-Cultural Psychology. 2015;46:1023–1038. doi: 10.1177/0022022115593803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korakakis V, Sideris V, Giakas G. Sitting bodily configuration: A study investigating the intra-tester reliability of positioning subjects into a predetermined sitting posture. Manual Therapy. 2014;19:197–202. doi: 10.1016/j.math.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Kyvelidou A, Harbourne RT, Willett WL, Stergiou N. Sitting postural control in infants with typical development, motor delay, or cerebral palsy. Pediatric Physical Therapy. 2013;25:46–51. doi: 10.1097/PEP.0b013e318277f157. [DOI] [PubMed] [Google Scholar]
- Kyvelidou A, Stuberg WA, Harbourne RT, Deffeyes JE, Blanke D, Stergiou N. Development of upper body coordination during sitting in typically developing infants. Pediatric Research. 2009;65:553–558. doi: 10.1203/PDR.0b013e31819d9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DK, Cole WG, Golenia L, Adolph KE. The cost of simplifying complex developmental phenomena: A new perspective on learning to walk. Developmental Science. doi: 10.1111/desc.12615. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martorell R, Onis M, Martines J, Black M, Onyango A, Dewey KG. WHO motor development study: Windows of achievement for six gross motor development milestones. Acta Paediatrica. 2006;95(S450):86–95. doi: 10.1111/j.1651-2227.2006.tb02379.x. [DOI] [PubMed] [Google Scholar]
- Massion J, Alexandrov A, Frolov A. Why and how are posture and movement coordinated? Progress in Brain Research. 2004;143:13–27. doi: 10.1016/S0079-6123(03)43002-1. [DOI] [PubMed] [Google Scholar]
- McGraw MB. The neuromuscular maturation of the human infant. New York, NY: Columbia University Press; 1945. [Google Scholar]
- Munoz F, Rougier PR. Estimation of center of gravity movements in sitting posture. Journal of Biomechanics. 2011;44:1771–1775. doi: 10.1016/j.jbiomech.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Nashner LM, Shupert CL, Horak FB. Head-trunk movement coordination in the standing posture. Progress in Brain Research. 1988;76:243–251. doi: 10.1016/s0079-6123(08)64511-2. [DOI] [PubMed] [Google Scholar]
- Piper MC, Darrah J. Motor assessment of the developing infant. Philadelphia, PA: WB Saunders; 1994. [Google Scholar]
- Rachwani J, Santamaria V, Saavedra SL, Wood S, Porter F, Woollacott MH. Segmental trunk control acquisition and reaching in typically developing infants. Experimental Brain Research. 2013;228:131–139. doi: 10.1007/s00221-013-3544-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachwani J, Santamaria V, Saavedra S, Woollacott MH. The development of trunk control and its relation to reaching in infancy: A longitudinal study. Frontiers in Human Neuroscience. 2015;9:1–12. doi: 10.3389/fnhum.2015.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochat P, Goubet N. Development of sitting and reaching in 5- to 6-month-old infants. Infant Behavior and Development. 1995;18:53–68. [Google Scholar]
- Rochat P, Goubet N, Senders SJ. To reach or not to reach? Perception of body effectivities by young infants. Infant and Child Development. 1999;8:129–148. [Google Scholar]
- Saavedra SL, van Donkelaar P, Woollacott MH. Learning about gravity: Segmental assessment of upright control as infants develop independent sitting. Journal of Neurophysiology. 2012;108:2215–2229. doi: 10.1152/jn.01193.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmuckler MA. Perception-action coupling in infancy. In: Savelsbergh GJP, editor. The development of coordination in infancy. Amsterdam: Elsevier Science; 1993. pp. 137–173. [Google Scholar]
- van Balen LC, Dijkstra LJ, Hadders-Algra M. Development of postural adjustments during reaching in typically developing infants from 4 to 18 months. Experimental Brain Research. 2012;220:109–119. doi: 10.1007/s00221-012-3121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnhoven TMA, de Onis M, Onyango AW, Wang T, Bjoerneboe GA, Bhandari N, Rashidi B. Assessment of gross motor development in the WHO Multicentre Growth Reference Study. Food and Nutrition Bulletin. 2004;25:S37–S45. doi: 10.1177/15648265040251S105. [DOI] [PubMed] [Google Scholar]
- Winter DA, Mackinnon CD, Ruder GK, Wieman C. An integrated EMG/biomechanical model of upper-body balance and posture during human gait. Progress in Brain Research. 1993;97:359–367. doi: 10.1016/s0079-6123(08)62295-5. [DOI] [PubMed] [Google Scholar]
- Winter DA, Patla AE, Prince F, Ishac M, Gielo-Perczak K. Stiffness control of balance in quiet standing. Journal of Neurophysiology. 1998;80:1211–1221. doi: 10.1152/jn.1998.80.3.1211. [DOI] [PubMed] [Google Scholar]
- Woollacott MH, Debu B, Mowatt M. Neuromuscular control of posture in the infant and child: Is vision dominant? Journal of Motor Behavior. 1987;19:167–186. doi: 10.1080/00222895.1987.10735406. [DOI] [PubMed] [Google Scholar]
- Yonas A, Hartman B. Perceiving the affordance of contact in four- and five-month-old infants. Child Development. 1993;64:298–308. doi: 10.1111/j.1467-8624.1993.tb02911.x. [DOI] [PubMed] [Google Scholar]