Abstract
Background
Prenatal alcohol exposure can affect neurodevelopment, but few studies have examined associations with autism spectrum disorder (ASD).
Methods
We assessed the association between maternal alcohol use and ASD in the Study to Explore Early Development, a multi-site case-control study of children born September 2003 – August 2006 in the U.S. Regression analyses included 684 children with research clinician-confirmed ASD, 869 children with non-ASD developmental delays or disorders (DDs) and 962 controls ascertained from the general population (POP). Maternal alcohol exposure during each month from three months prior to conception until delivery was assessed by self-report.
Results
Mothers of POP children were more likely to report any prenatal alcohol use than mothers of children with ASD or DD. In trimester one, 21.2% of mothers of POP children reported alcohol use compared to 18.1% and 18.2% of mothers of children with ASD or DD, respectively (adjusted OR for ASD versus POP 0.8, 95% confidence interval 0.6, 1.1). During preconception and the first month of pregnancy, 1–2 drinks on average per week was inversely associated with ASD risk.
Conclusions
These results do not support an adverse association between low-level alcohol exposure and ASD, though these findings were based retrospective self-reported alcohol use. Unmeasured confounding or exposure misclassification may explain inverse associations with 1–2 drinks per week. Pregnant or potentially pregnant women should continue to follow recommendations to avoid alcohol use because of other known effects on infant health and neurodevelopment.
Keywords: alcohol, autism, prenatal, exposure
Autism spectrum disorder (ASD) is a group of neurodevelopmental conditions characterized by social impairments and repetitive or stereotypic behaviors.1 ASD is genetically heritable, though some recent studies implicate environmental factors in a substantial percentage of ASD risk.2, 3 While the specific environmental factors that influence ASD risk remain poorly understood, exposures during the prenatal period are likely important.
Alcohol use during pregnancy merits investigation as a possible ASD risk factor, given studies linking high prenatal alcohol exposure with neurodevelopmental effects, such as decreased intellectual ability, hyperactivity, motor skill impairments, and learning difficulties.4 In addition, two case reports5, 6 and a few small studies7–11 suggest possible co-morbidity between fetal alcohol spectrum disorder (FASD) and/or alcohol abuse during pregnancy and ASD or autism-like symptoms. We found only one epidemiologic study specifically examining alcohol intake and ASD, which observed no association between alcohol and either ASD or infantile autism.12
Here we examined the association between maternally-reported alcohol use during the preconception and prenatal periods and ASD in the Study to Explore Early Development (SEED), a large multi-site case-control study. We evaluated exposure during different time periods throughout the pregnancy because alcohol may have different effects depending on the timing of exposure. We examined preconception alcohol use in order to better characterize the background alcohol exposure patterns in the study groups and because reported alcohol use during the month prior to conception may serve as a proxy for alcohol use around the time of conception.
Methods
Study Sample
SEED is a multi-site case-control study with three study groups: children with ASD, children with non-ASD developmental disorders (DD), and children without ASD ascertained from the general population (POP).13 This analysis from the SEED Phase 1 includes eligible children who were born between September 2003 and August 2006 in one of six study catchment areas (specific regions in California, Colorado, Georgia, Maryland, North Carolina, and Pennsylvania). The children were required to live in this same area at study enrollment and to live with a caregiver since 6 months of age who could provide legal consent and was capable of communicating in English (or Spanish at the California and Colorado sites). Children enrolled in SEED were required to be between 30 and 68 months of age at the time of the research clinical assessment (described below).
Children eligible for the POP group were ascertained by randomly sampling vital records. Those eligible for the DD or ASD groups were primarily identified through developmental disability service providers including public school special education programs and clinical sites. Details are published elsewhere.13 Institutional review boards at each study site and the Centers for Disease Control and Prevention approved the SEED project, and caregivers of enrolled participants gave informed consent.
A computer-assisted telephone interview was administered to the child’s caregiver at enrollment (caregiver interview, CGI); which averaged 55 (range 29–68) months after the child’s birth.14 While any knowledgeable caregiver could be the interview respondent, all questions pertaining to the biological mother’s pregnancy history and index pregnancy were only asked if the respondent was the biological mother. Thus, participants were eligible for this analysis only if the biologic mother was the CGI respondent (99% of CGI respondents). Additionally, children with ASD were included in the analysis only if the diagnosis was clinically confirmed as described below.
Outcome Assessment
All participants completed an ASD screening instrument, the Social Communication Questionnaire (SCQ),15 and a general developmental assessment (Supplemental Figure 1). Because the children were young, a more sensitive SCQ cut-point of 11 was used.13, 16 Children with an SCQ score of ≥11 or a previous ASD diagnosis received a full ASD developmental evaluation that included the Autism Diagnostic Observation Schedule (ADOS)17, 18 and their caregiver was administered the Autism Diagnostic Interview Revised (ADI-R).19, 20 Additionally, children with a suspected ASD at the general developmental assessment also went through the full ASD assessment. Final ASD classification was based on results from the ADOS and ADI-R.16
Children with no previous ASD diagnosis who scored <11 on the SCQ were classified in the POP or DD group depending on ascertainment source. The DD group was recruited from health or educational sources and includes a diverse array of conditions, such as language delays, sensory integration disorders, motor delays, and birth defects, but without symptoms of ASD.21 Children with incomplete ASD assessments or children classified as DD meeting SEED ADOS or ADI-R criteria (i.e., demonstrating ASD symptoms or signs without meeting our case definition) were excluded from this analysis.
Covariate and Exposure Assessment
The CGI was used to collect information on health behaviors during the index pregnancy, sociodemographics, pregnancy history, psychiatric conditions prior to or during the pregnancy, and pre-pregnancy body mass index. Gestational age was primarily obtained from the birth certificate, with medical record data serving as a secondary source if birth certificate data were unavailable. Some maternal health information about psychiatric conditions was also obtained from a self-administered maternal medical history form. The presence of diabetes or hypertension during the preconception and pregnancy period (pre-existing or pregnancy-induced) was based on information from the CGI and medical records.
During the CGI, mothers reported their alcohol use from the period three months before conception until either the child’s date of birth or the end of breastfeeding. An alcoholic beverage was defined as “one beer, one glass of wine, one mixed drink, or one shot of liquor.” Those reporting any alcohol use were asked about drinking in each month and about the average number of drinks per week during that period. Mothers were asked to report times when she had five or more drinks on one occasion, which we classified as episodes of binge-drinking. We created variables for “ever” use of alcohol during each of four periods: three months prior to conception, first trimester, second trimester, and third trimester. We also categorized alcohol exposure by reported average quantity of alcohol consumed per week during each month of the preconception and pregnancy period: no maternal alcohol use, <1 drink per week, 1–2 drinks per week, and 3+ drinks per week. For mothers who reported dose of alcohol use on a trimester level (1.6% of mothers with dose information), we imputed the quantity of alcohol use in each month assuming the quantity was the same throughout all months of the trimester. We created a separate set of exposure variables for binge drinking for trimester periods.
Statistical Analysis
We used multiple logistic regression to model the association between ever versus never reporting alcohol use within each pregnancy period and ASD, with the POP group as the comparison group. We defined the “no exposure” group as those not reporting alcohol use during that particular time period. To examine whether associations were specific to ASD or could be generalized to other neurodevelopmental conditions, we also compared ever versus never drinking in each month of the pregnancy between DD and POP groups. Using logistic regression, we compared odds of ASD and DD for mothers reporting <1 drink per week, 1–2 drinks per week, and 3+ drinks per week to those not reporting any alcohol use during each month of pregnancy. Unexposed was defined as no alcohol use during that month. We report analyses from three months prior to conception through the second month of pregnancy in the main results because too few mothers reported alcohol use beyond month two of pregnancy. Results for subsequent months of pregnancy where there were at least five participants in an exposure outcome group are presented in supplemental tables. We also examined the association between ASD and DD and maternal report of binge drinking episodes in the pre-conception period and first trimester.
We identified potential confounders a priori by considering factors that would be associated with the exposure and the outcome, but not on the causal pathway. We adjusted for the following potential confounders in the analyses: child’s sex, total household income in the year prior to the pregnancy (<$30,000, $30,000–<70,000, $70,000-<110,000, ≥$110,000), self-reported maternal race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic any race, other/multiracial), maternal education at child’s birth (high school diploma or less, some college/trade, bachelor’s degree, advanced degree), maternal parity (0, 1+), at least one maternal psychiatric condition before or during pregnancy (yes, no), maternal smoking during the same month (yes, no), and maternal age at birth (<25, 25–29, 30–34, 35–39, or ≥40 years). Maternal race/ethnicity was considered a confounder because of evidence suggesting potential differences in alcohol use patterns across different sociodemographic groups in the United States22 and known racial differences across the three SEED study groups14. We adjusted for child sex because sex is strongly associated with the outcome. Because we were concerned that other maternal health factors might influence alcohol use during pregnancy, we ran additional models adjusting for maternal body mass index, hypertension, and diabetes and completed a sensitivity analysis restricting the sample to full term children. To further address potential for confounding, we completed an additional sensitivity analysis where we estimated propensity scores for any alcohol use in each trimester period and then weighted the sample by stabilized inverse probability of treatment weights23. In order to see if results differed based on previous pregnancy outcomes or extended time attempting pregnancy, we conducted an additional sensitivity analysis restricting to primiparous women who became pregnant in six months or less. We used multiple imputation with 10 imputations to impute missing covariate information. These analyses were restricted to those participants with complete information on alcohol exposure for relevant time periods.
Results
Of 3,899 mother-child pairs ever enrolled in SEED, we excluded siblings (n=130) and children with developmental disabilities who showed some ASD characteristics but did not reach the full research criteria for ASD or did not complete the full ASD evaluation (n=755). We also excluded children without for whom the caregiver did not start the CGI (n=431) and for whom the biological mother was not the CGI respondent (n=24). After these restrictions, the sample eligible for the analysis included 686 ASD, 892 DD and 981 POP children. The mothers of these children were more likely to be white, non-Hispanic race, have higher education levels or age 30–39 years than mothers of children ineligible for the analysis (n=1340) (data not shown). Analyses were restricted to participants with information on alcohol exposure for the relevant time periods (684 ASD, 869 DD, 962 POP for the ever/never alcohol use analyses and 680 ASD, 865 DD, 957 POP for analyses with average amounts of alcohol use).
As in the total enrolled sample,14 mothers of children with ASD and DD were more likely to be Hispanic ethnicity or minority race, and to have less education or a lower household income, than mothers of the POP children (Table 1). Mothers of children with ASD or DD were also more likely to report smoking during the preconception and pregnancy period, as well as a history of at least one psychiatric condition. Children in the ASD and DD groups were more likely to be male than the children in the POP group consistent with known sex distributions.
Table 1.
Characteristics by study group in the Study to Explore Early Development (SEED)
| Sample Characteristics | ASDa
|
DDb
|
POPc
|
|---|---|---|---|
| N=686 | N=892 | N=981 | |
| Maternal Race/Ethnicityd | |||
| Non-Hispanic white | 56.3 | 61.3 | 70.3 |
| Non-Hispanic black | 19.9 | 16.7 | 13.0 |
| Hispanic | 12.0 | 13.3 | 8.4 |
| All other/multi-racial | 11.7 | 8.7 | 8.2 |
| Maternal Education at Deliverye | |||
| High school diploma or less | 19.8 | 22.3 | 13.4 |
| Some college/trade | 29.1 | 22.3 | 22.7 |
| Bachelor’s degree | 31.6 | 33.4 | 36.4 |
| Advanced degree | 19.5 | 21.9 | 27.4 |
| Maternal Parityf | |||
| 0 | 47.4 | 39.4 | 45.4 |
| 1+ | 52.6 | 60.6 | 54.6 |
| Maternal Psychiatric Conditions | |||
| No | 64.7 | 71.1 | 77.7 |
| Yes | 35.3 | 28.9 | 22.3 |
| Maternal Smoking Any Monthg | |||
| No | 85.5 | 88.1 | 90.8 |
| Yes | 14.5 | 11.9 | 9.2 |
| Maternal Ageh | |||
| <25 years | 12.7 | 13.3 | 11.5 |
| 25–29 years | 25.1 | 21.7 | 21.6 |
| 30–34 years | 34.0 | 34.6 | 37.3 |
| 35–39 years | 21.9 | 23.9 | 25.7 |
| ≥40 years | 6.3 | 6.4 | 3.9 |
| Child’s Sex | |||
| Female | 18.1 | 36.7 | 46.3 |
| Male | 81.9 | 63.3 | 53.7 |
| Total Household Income Year Prior to Pregnancy ($)i | |||
| <30,000 | 25.1 | 22.9 | 16.0 |
| 30,000–<70,000 | 31.0 | 30.5 | 28.4 |
| 70,000–<110,000 | 26.3 | 28.2 | 32.2 |
| ≥110,000 | 17.7 | 18.4 | 23.4 |
ASD=autism spectrum disorder,
DD=non-ASD developmental delay or disorder,
POP=non-ASD population controls,
n=28 missing maternal race/ethnicity,
n=17 missing maternal education,
n=21 missing maternal parity,
n=40 missing smoking in any month (three months preconception and the pregnancy),
n=1 missing maternal age,
n=118 missing pregnancy household income
As expected, the percentage of women reporting any alcohol use decreased from the preconception period through trimester two in all three study groups (Figure 1). There was an uptick in any alcohol use in trimester three that was more prominent among the POP group. Among mothers who reported any alcohol use, the largest proportion reported <1 drink per week on average (Figure 2).
Figure 1.
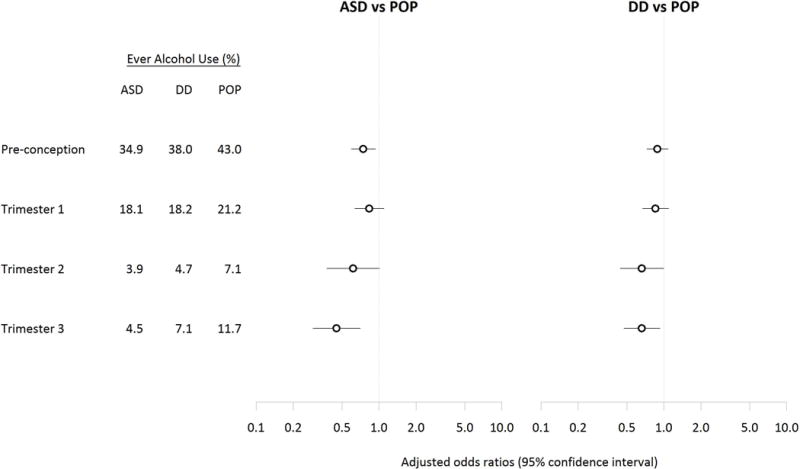
Adjusteda odds ratios and 95% confidence intervals for ASD and DD relative to POP comparing maternal alcohol use ever versus never in each trimester of the pregnancy (N=684 ASD, 869 DD, 962 POP)b.
aAnalyses adjusted for: child’s sex, total household income in the year prior to the pregnancy, self-reported maternal race/ethnicity, maternal education at delivery, maternal parity, at least one maternal psychiatric condition, maternal smoking in any month during preconception and pregnancy, and maternal age at birth.
bIncluded 684 children with ASD (autism spectrum disorder), 869 children with DD (other developmental disabilities), and 962 POP (general population control) children with complete information ever/never alcohol exposure in the four time periods. Figure 2. Adjusteda odds ratios and 95% confidence intervals for ASD and DD relative to POP comparing maternal drinking of an average of <1 drink per week, 1–2 drinks per week, and 3+ drinks per week to no alcohol use in each month during the preconception and early pregnancy period (N=680 ASD, 865 DD, 957 POP) b.
Figure 2.
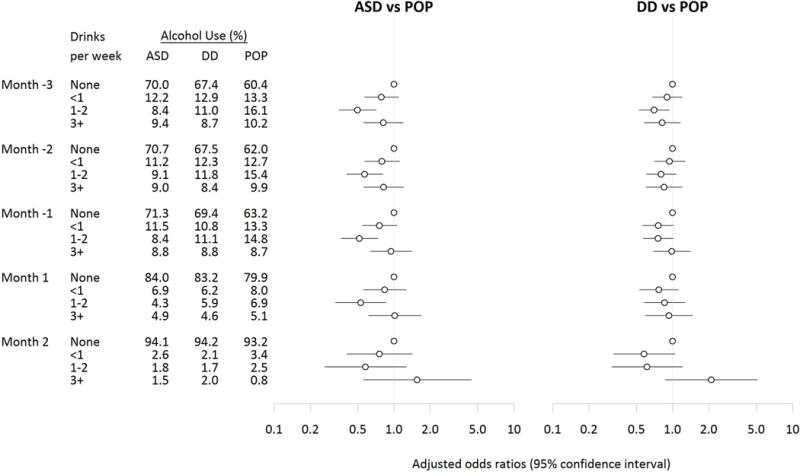
Adjusteda odds ratios and 95% confidence intervals for ASD and DD relative to POP comparing maternal drinking of an average of <1 drink per week, 1–2 drinks per week, and 3+ drinks per week to no alcohol use in each month during the preconception and early pregnancy period (N=680 ASD, 865 DD, 957 POP) b.
aAnalyses adjusted for: child’s sex, total household income in the year prior to the pregnancy, self-reported maternal race/ethnicity, maternal education at delivery, maternal parity, at least one maternal psychiatric condition, maternal smoking in any month during preconception and pregnancy, and maternal age at birth.
bIncluded 680 children with ASD (autism spectrum disorder), 865 children with DD (other developmental disabilities), and 957 POP (general population control) children with information on alcohol dose in the preconception months and the first two months of pregnancy.
Among mothers of the POP children, non-Hispanic white women were more likely to report alcohol use during the three months preconception and during pregnancy than women in other race-ethnicity categories (Supplemental Table 1). Higher education and income were also associated with alcohol use during the preconception period and throughout pregnancy. Smoking and primiparity were associated with any alcohol use during the preconception period and trimester one, but not later in pregnancy. Maternal alcohol use across the different pregnancy time periods was correlated as illustrated in Supplemental Table 2. For example, 79.4% of mothers reporting alcohol use in trimester one also reported alcohol use in the three months preconception.
Mothers of children with ASD or DD were less likely than those of POP controls to report any alcohol use throughout the preconception and pregnancy periods (Figure 1, Supplemental Table 3). The ORs and 95% CIs were similar for crude and adjusted analyses (Supplemental Table 3). In the preconception period, any alcohol use was less likely among mothers of the ASD group relative to POP group (adjusted OR (OR) 0.7, 95% confidence interval (CI) 0.6, 0.9). Any maternal alcohol use during trimesters one and two was also inversely associated with ASD, though results were imprecise. The inverse association was stronger between any maternal alcohol use and ASD in trimester three (OR 0.4, 95% CI 0.3, 0.7). Similar patterns, but more modest inverse associations (especially in the pre-conception period and later in pregnancy) were observed comparing the DD group to the POP group. Most mothers reporting alcohol use in trimesters two and three reported on average <1 drink per week during these time periods (Supplemental Table 4). Adjustment for diabetes, hypertension and BMI did not alter results (Supplemental Table 5). Results were also similar in sensitivity analyses restricted to full term children (Supplemental Table 5) and when stabilized using inverse probability of treatment weights (data not shown). The results of the sensitivity analysis restricted to primiparous women who became pregnant in six months or less were similar to the main findings (Supplemental Table 6).
Examining monthly alcohol use and dose, average alcohol use of 1–2 drinks/week was inversely associated with risk of ASD for each month in the preconception period through month one of pregnancy and with risk of DD for the third month prior to conception (Figure 2, Supplemental Table 4). There was no association (positive or negative) between average alcohol use of 3+ drinks/week and either ASD or DD in the preconception months or pregnancy month one. However, in pregnancy month two, the odds ratios were elevated for the associations between maternal alcohol use of 3+ drinks/week and ASD (OR 1.6, 95% CI 0.6, 4.4), and DD (OR=2.1, 95% CI 0.9, 5.1), though estimates were imprecise. Binge drinking episodes in the pre-conception period and first trimester was not associated with ASD or DD (Supplemental Table 7).
Comment
Principal findings
In this large case-control study in a diverse population, we found no positive associations between low levels of maternal alcohol use and ASD or DD and some evidence of an inverse association, particularly in the preconception period and third trimester. There was no association between 3+ drinks per week reported on average and ASD or DD in the preconception period or first month of the pregnancy. Estimates for the associations between ASD and DD with 3+ drinks per week in the second month of pregnancy were elevated, but imprecise.
The published literature on maternal alcohol use and ASD is limited, though case reports and small studies report co-morbidity between FASD and/or alcohol abuse during pregnancy and autism-like symptoms.5–11 One previous large-scale epidemiologic study using prospectively-collected data from the Danish National Birth Cohort12 found no association between prenatal alcohol use and either ASD or infantile autism, a finding similar to ours. The authors attributed their finding of an inverse association between one binge-drinking episode during pregnancy and ASD to either confounding or chance. Our study contributes a diverse sample from the United States and clinically confirmed ASD case classification, which complements the evidence produced from the prospective registry-based study. While limited epidemiologic evidence does not support an association between prenatal alcohol use and risk of ASD, numerous reasons exist for cautioning women against alcohol use during the pregnancy period, including animal studies suggesting that low-to-moderate doses of alcohol adversely affect neurodevelopment.24, 25 Human studies have been inconsistent.26
Interpretation
We suspect that the observed inverse associations are the consequence of underlying bias that we describe below. An alternative interpretation may be that moderate alcohol use triggers an anti-inflammatory response. Moderate alcohol use has been linked to reduced C-reactive protein, a marker of inflammation,27, 28 and maternal inflammation is suspected as a possible mechanism in ASD etiology.29, 30
There are several potential non-causal explanations for the observed inverse associations between light alcohol use during pregnancy and ASD. First, though we adjusted for available socio-demographic confounders, residual or unmeasured confounding is a possibility. Mothers with higher education were more likely to report alcohol use and be mothers of children in the POP group. Thus, the observed inverse association may result from residual confounding from other factors related to education that we did not ascertain.
We did not have genetic information. Polymorphisms in alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) have been linked to alcohol metabolism and alcohol use patterns. A rare variant of ADH1B gene has been associated with alcohol use patterns during pregnancy31 and the ADH gene cluster has been associated with autism.32 These early reports of genetic variants linked to both reduced alcohol consumption and elevated ASD risk would support the observed inverse association.
Finally, women with health concerns, atypical pregnancies, previous complicated pregnancies, or a previous child with a developmental disability may avoid alcohol preconception and during pregnancy, while healthier women may be more likely to occasionally use alcohol. Since ASD has been linked to some maternal health conditions, such as obesity33 and diabetes,34 health differences between the study groups could be another potential explanation for the inverse association. We observed no differences in results when adjusting for maternal hypertension, diabetes, and BMI, or when restricting to term births, yet we could not account for all associated health factors in our analysis.
We had limited power to evaluate the timing and dose of alcohol exposure. While early pregnancy exposure can produce facial features consistent with Fetal Alcohol Syndrome (FAS), more subtle behavioral effects of alcohol may arise from exposures throughout pregnancy.35 Alcohol exposure was correlated across pregnancy limiting our ability to tease out specific windows of susceptibility. By examining pre-conception alcohol exposure, we aimed to better understand background patterns of alcohol use in our study sample. Though our interpretation of the pre-conception results is complicated by correlation with other time periods, alcohol use pre-conception was inversely association with ASD. This further supports the idea that bias may be driving the inverse association.
Limitations of the data
In addition to the potential for residual confounding discussed above, inferences may also be limited by retrospective self-report of alcohol two to five years after birth of the child. Women may struggle to remember alcohol use over this time frame. The known harms and social biases surrounding alcohol use during pregnancy may also dissuade women from reporting use, and may differ among mothers of children with developmental concerns compared to mothers of controls. The proportion of women reporting alcohol use in SEED was in the range of that reported for the past 30 days in the Behavioral Risk Factor Surveillance System (10.2% and 53.6% for pregnant and non-pregnant women, respectively).22 While SEED mothers reported the average number of drinks per week during each month, we do not have peak alcohol use during pregnancy or the types of alcohol consumed. Beer, wine, and liquor may be associated with distinctive patterns of maternal characteristics. We were unable to adequately assess dose because most women reported light alcohol use.
Finally, selection bias may be a concern. We could not make contact with numerous families initially targeted for participation in SEED; thus, we cannot broadly distinguish non-response from ineligibility. For a subset of SEED sites, data were available to assess non-response. This analysis indicated that maternal age, education, and race-ethnicity were key factors associated with non-response, but that other non-demographic factors were not (unpublished data). Our analyses adjusted for these three factors, but we cannot directly assess whether reported maternal alcohol use was associated with study participation, overall or by study group. If present, such a bias could contribute to the inverse associations observed.
Strengths of the study
Our analyses are strengthened by the use of data from a large multi-site investigation with diverse study participants, detailed information on alcohol use and covariates, and rigorous ASD confirmation using gold standard clinical assessments.16 We also compared patterns among those with ASD to those in a non-ASD development disability group, as well as in relation to a population control group. Similar proportions of mothers in the ASD and DD groups reported alcohol use, indicating that inverse associations were not specific to ASD.
Conclusions
In this large multi-site case-control study with a diverse sample and comprehensive ASD clinical assessment, we did not find evidence that relatively low alcohol use just before or during pregnancy was associated with increased risk of ASD, despite much prior evidence linking high alcohol exposure with adverse neurodevelopmental outcomes. We suspect the observed inverse association between 1–2 drinks on average per week and ASD is likely non-causal, resulting from unmeasured confounding or potentially from biases in sample selection or recall. We were unable to thoroughly examine alcohol dose because so few women in our study reported alcohol use with much frequency during the pregnancy period, but encourage future work on this topic. The effects of alcohol on the developing fetus may differ based on the type, amount and timing of alcohol use, as well as genetic, dietary, and other maternal characteristics, which we did not examine. Given this heterogeneity and other known negative effects of alcohol use during pregnancy, we encourage women to continue to heed current national recommendations to avoid alcohol use during pregnancy.
Supplementary Material
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the California Department of Public Health.
ABS was supported by a training grant from the National Institute of Environmental Health Sciences (T32ES007018). The Study to Explore Early Development is funded by the Centers for Disease Control and Prevention: Cooperative Agreement Number U10DD000180, Colorado Department of Public Health and Environment, and U10DD000750, University of Colorado Denver; Cooperative Agreement Number U10DD000181, Kaiser Foundation Research Institute (CA); Cooperative Agreement Number U10DD000182, University of Pennsylvania; Cooperative Agreement Number U10DD000183, Johns Hopkins University; Cooperative Agreement Number U10DD000184, University of North Carolina at Chapel Hill; and Cooperative Agreement Number U10DD000498, Michigan State University. We acknowledge Yinge Qian for contributions to data cleaning.
Footnotes
DR. ALISON B SINGER (Orcid ID : 0000-0002-8907-6620)
DR. CHRISTINA CORDERO (Orcid ID : 0000-0003-1032-5303)
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease and Control and Prevention.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 2.Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Archives of General Psychiatry. 2011;68:1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A. The familial risk of autism. JAMA. 2014;311:1770–1777. doi: 10.1001/jama.2014.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattson SN, Riley EP. A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcoholism: Clinical and Experimental Research. 1998;22:279–294. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- 5.Harris SR, MacKay LL, Osborn JA. Autistic behaviors in offspring of mothers abusing alcohol and other drugs: a series of case reports. Alcoholism: Clinical and Experimental Research. 1995;19:660–665. doi: 10.1111/j.1530-0277.1995.tb01564.x. [DOI] [PubMed] [Google Scholar]
- 6.Nanson JL. Autism in fetal alcohol syndrome: a report of six cases. Alcoholism: Clinical and Experimental Research. 1992;16:558–565. doi: 10.1111/j.1530-0277.1992.tb01417.x. [DOI] [PubMed] [Google Scholar]
- 7.Aronson M, Hagberg B, Gillberg C. Attention deficits and autistic spectrum problems in children exposed to alcohol during gestation: a follow-up study. Developmental Medicine and Child Neurology. 1997;39:583–587. doi: 10.1111/j.1469-8749.1997.tb07493.x. [DOI] [PubMed] [Google Scholar]
- 8.Bishop S, Gahagan S, Lord C. Re-examining the core features of autism: a comparison of autism spectrum disorder and fetal alcohol spectrum disorder. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2007;48:1111–1121. doi: 10.1111/j.1469-7610.2007.01782.x. [DOI] [PubMed] [Google Scholar]
- 9.Stevens SA, Nash K, Koren G, Rovet J. Autism characteristics in children with fetal alcohol spectrum disorders. Child Neuropsychology. 2013;19:579–587. doi: 10.1080/09297049.2012.727791. [DOI] [PubMed] [Google Scholar]
- 10.Mukherjee R, Layton M, Yacoub E, Turk J. Autism and autistic traits in people exposed to heavy prenatal alcohol: data from a clinical series of 21 individuals and nested case control study. Advances in Mental Health and Intellectual Disabilities. 2011;5:42–49. [Google Scholar]
- 11.Landgren M, Svensson L, Stromland K, Andersson Gronlund M. Prenatal alcohol exposure and neurodevelopmental disorders in children adopted from eastern Europe. Pediatrics. 2010;125:e1178–1185. doi: 10.1542/peds.2009-0712. [DOI] [PubMed] [Google Scholar]
- 12.Eliasen M, Tolstrup JS, Nybo Andersen AM, Gronbaek M, Olsen J, Strandberg-Larsen K. Prenatal alcohol exposure and autistic spectrum disorders–a population-based prospective study of 80,552 children and their mothers. International Journal of Epidemiology. 2010;39:1074–1081. doi: 10.1093/ije/dyq056. [DOI] [PubMed] [Google Scholar]
- 13.Schendel DE, Diguiseppi C, Croen LA, Fallin MD, Reed PL, Schieve LA, et al. The Study to Explore Early Development (SEED): a multisite epidemiologic study of autism by the Centers for Autism and Developmental Disabilities Research and Epidemiology (CADDRE) network. Journal of Autism and Developmental Disorders. 2012;42:2121–2140. doi: 10.1007/s10803-012-1461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiGuiseppi CG, Daniels JL, Fallin DM, Rosenberg SA, Schieve LA, Thomas KC, et al. Demographic profile of families and children in the Study to Explore Early Development (SEED): Case-control study of autism spectrum disorder. Disability and Health Journal. 2016;9:544–551. doi: 10.1016/j.dhjo.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rutter M, Bailey A, Lord C. SCQ: Social Communication Questionnaire. Los Angeles, CA: Western Psychological Services; 2003a. [Google Scholar]
- 16.Wiggins LD, Reynolds A, Rice CE, Moody EJ, Bernal P, Blaskey L, et al. Using standardized diagnostic instruments to classify children with autism in the study to explore early development. Journal of Autism and Developmental Disorders. 2015;45:1271–1280. doi: 10.1007/s10803-014-2287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lord C, Rutter M, DiLavore PC, Risi S. Autism diagnositic observation schedule. Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- 18.Gotham K, Risi S, Pickles A, Lord C. The Autism Diagnostic Observation Schedule: revised algorithms for improved diagnostic validity. Journal of Autism and Developmental Disorders. 2007;37:613–627. doi: 10.1007/s10803-006-0280-1. [DOI] [PubMed] [Google Scholar]
- 19.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 20.Rutter M, Le Couteur A, Lord C. ADI-R: The Autism Diagnotic Interview-Revised. Los Angeles, CA: Western Psychological Services; 2003b. [Google Scholar]
- 21.Wiggins LD, Levy SE, Daniels J, Schieve L, Croen LA, DiGuiseppi C, et al. Autism spectrum disorder symptoms among children enrolled in the Study to Explore Early Development (SEED) Journal of Autism and Developmental Disorders. 2015;45:3183–3194. doi: 10.1007/s10803-015-2476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan CH, Denny CH, Cheal NE, Sniezek JE, Kanny D. Alcohol use and binge drinking among women of childbearing age – United States, 2011–2013. MMWR: Morbidity and Mortality Weekly Report. 2015;64:1042–1046. doi: 10.15585/mmwr.mm6437a3. [DOI] [PubMed] [Google Scholar]
- 23.Brookhart MA, Wyss R, Layton JB, Sturmer T. Propensity score methods for confounding control in nonexperimental research. Circulation: Cardiovascular Quality and Outcomes. 2013;6:604–611. doi: 10.1161/CIRCOUTCOMES.113.000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cullen CL, Burne TH, Lavidis NA, Moritz KM. Low dose prenatal ethanol exposure induces anxiety-like behaviour and alters dendritic morphology in the basolateral amygdala of rat offspring. PloS One. 2013;8:e54924. doi: 10.1371/journal.pone.0054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider ML, Moore CF, Kraemer GW. Moderate alcohol during pregnancy: learning and behavior in adolescent rhesus monkeys. Alcoholism: Clinical and Experimental Research. 2001;25:1383–1392. [PubMed] [Google Scholar]
- 26.Flak AL, Su S, Bertrand J, Denny CH, Kesmodel US, Cogswell ME. The association of mild, moderate, and binge prenatal alcohol exposure and child neuropsychological outcomes: a meta-analysis. Alcoholism: Clinical and Experimental Research. 2014;38:214–226. doi: 10.1111/acer.12214. [DOI] [PubMed] [Google Scholar]
- 27.Albert MA, Glynn RJ, Ridker PM. Alcohol consumption and plasma concentration of C-reactive protein. Circulation. 2003;107:443–447. doi: 10.1161/01.cir.0000045669.16499.ec. [DOI] [PubMed] [Google Scholar]
- 28.Imhof A, Froehlich M, Brenner H, Boeing H, Pepys MB, Koenig W. Effect of alcohol consumption on systemic markers of inflammation. Lancet. 2001;357:763–767. doi: 10.1016/S0140-6736(00)04170-2. [DOI] [PubMed] [Google Scholar]
- 29.Abdallah MW, Larsen N, Grove J, Norgaard-Pedersen B, Thorsen P, Mortensen EL, et al. Amniotic fluid inflammatory cytokines: potential markers of immunologic dysfunction in autism spectrum disorders. World Journal of Biological Psychiatry. 2013;14:528–538. doi: 10.3109/15622975.2011.639803. [DOI] [PubMed] [Google Scholar]
- 30.Goines PE, Croen LA, Braunschweig D, Yoshida CK, Grether J, Hansen R, et al. Increased midgestational IFN-gamma, IL-4 and IL-5 in women bearing a child with autism: A case-control study. Molecular Autism. 2011;2:13. doi: 10.1186/2040-2392-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuccolo L, Fitz-Simon N, Gray R, Ring SM, Sayal K, Smith GD, et al. A non-synonymous variant in ADH1B is strongly associated with prenatal alcohol use in a European sample of pregnant women. Human Molecular Genetics. 2009;18:4457–4466. doi: 10.1093/hmg/ddp388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zuo L, Wang K, Zhang XY, Pan X, Wang G, Tan Y, et al. Association between common alcohol dehydrogenase gene (ADH) variants and schizophrenia and autism. Human Genetics. 2013;132:735–743. doi: 10.1007/s00439-013-1277-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li M, Fallin MD, Riley A, Landa R, Walker SO, Silverstein M, et al. The Association of Maternal Obesity and Diabetes With Autism and Other Developmental Disabilities. Pediatrics. 2016;137:e20152206. doi: 10.1542/peds.2015-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu G, Jing J, Bowers K, Liu B, Bao W. Maternal diabetes and the risk of autism spectrum disorders in the offspring: a systematic review and meta-analysis. Journal of Autism and Developmental Disorders. 2014;44:766–775. doi: 10.1007/s10803-013-1928-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coles C. Critical periods for prenatal alcohol exposure: evidence from animal and human studies. Alcohol Research and Health. 1994;18:22. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


