Abstract
Early life adversity (ELA) can lead to poor health later in life. However, there is significant variation in outcomes, with some individuals displaying resilience even in the face of adversity. Using longitudinal data collected from free-ranging rhesus macaques between birth and 3 years, we examined whether individual variation in vigilance for threat, an early emerging attentional bias, can account for variation in long-term outcomes between individuals reared in similar environments. We found that ELA and vigilance during infancy interact to predict physiological dysregulation in Sympathetic Nervous System (SNS) and Hypothalamic-Pituitary-Adrenal (HPA) stress responses during juvenility. During high stress periods, High ELA juveniles with high vigilance exhibit less asymmetry than High ELA juveniles with low vigilance. This suggests that although increased vigilance is viewed as a negative consequence of ELA, it might also be a mechanism by which vulnerable individuals proactively buffer themselves from negative outcomes in unstable or threatening environments.
Keywords: Early life adversity, cognitive bias, cortisol, salivary α-amylase, asymmetry, rhesus macaque
Introduction
Early life adversity (ELA), such as parental abuse or neglect, is often associated with poor mental and physical health later in life (e.g., Harlow, Dodsworth, & Harlow, 1965; Nelson, Fox, & Zeanah, 2014). However, whereas many individuals exposed to ELA develop poor health outcomes, others display relative resilience to adversity later in life. A variety of genetic and environmental factors have been proposed to explain why some individuals flourish when others in comparable situations falter (e.g., Belsky & Pluess, 2009; Caspi & Moffit, 2006; Franklin, Saab, & Mansuy, 2012; Hostinar, Cicchetti, & Rogosch, 2014; Parker & Maestripieri, 2011). The role that early-emerging cognitive processes might play in explaining inter-individual variation in vulnerability and resilience to ELA, however, remains poorly understood.
Cognition can help individuals regulate perception of and attention to the world around them, thereby providing them with a potential means by which to cope with adversity. One aspect of cognition that might explain inter-individual variation in resilience is vigilance for threat (hereafter, vigilance), an attentional bias to threat-relevant stimuli, which emerges early in life in both human and nonhuman primates (humans: Farroni, Menon, Rigato, & Johnson, 2007; Grossman, Striano, & Friederici, 2007; rhesus macaques (Macaca mulatta): Mandalaywala, Parker, & Maestripieri, 2014). Vigilance develops in response to environmental input and is often expressed more strongly in individuals previously exposed to ELA (humans: Shackman, Shackman, & Pollak, 2007). Although extreme vigilance is implicated in anxiety disorders (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007), the emergence of vigilance in response to early experiences of adversity can also be a useful adaptation. For example, in adverse environments, active deployment of vigilance might be an effective way of managing exposure to frequent stressors (Frankenhuis & de Weerth, 2013). However, studies have not examined whether variation in vigilance can explain inter-individual variation in developmental outcomes, such that – under condition of adversity – individuals that exhibit greater vigilance show more favorable outcomes than those with less vigilance. In the present study, we adopt a ‘cognition by environment approach’ to assess whether ELA and vigilance for threat during infancy interact to explain inter-individual variation in developmental outcomes in juvenile rhesus macaques, a nonhuman primate model species frequently utilized in research on anxiety and emotion regulation (e.g., Maestripieri et al., 1992; Kalin & Shelton, 2003). Given the many physiological, behavioral, social, and cognitive similarities of rhesus macaques to humans, they are an ideal species in which to investigate how cognition might moderate the effects of adversity to lead to more or less resilient phenotypes later in life.
Although there are a variety of developmental outcomes affected by ELA, we focus on the regulation of stress physiology given its established links to health outcomes. Negative mental and physical health outcomes in response to ELA may arise as a consequence of changes to the function and regulation of the stress response, especially in the Sympathetic Nervous System (SNS) and Hypothalamic-Pituitary-Adrenal (HPA) axis (McEwen, 2000). In response to a stressor, activation of the SNS, through release of catecholamines, should lead to prompt initiation of a “fight or flight” response, while activation of the HPA axis, through release of glucocorticoids, should lead to a metabolic response that mobilizes energy stored in tissues and prepares the body for future resource scarcity (Sapolsky, Romero, & Munck, 2000). In an optimally functioning system, the SNS and HPA axis should both exhibit well-regulated responses to a stressor, characterized by an acute response followed by a swift return to baseline (Chrousos & Gold, 1992). However, individuals exposed to ELA often exhibit a dysregulated phenotype characterized by a blunted acute response (i.e., an inability to mount an appropriate stress response) due in part to chronically elevated basal concentrations of stress hormones such as catecholamines (SNS) or cortisol (HPA) (in nonhuman primates: Sanchez, 2006; Sanchez et al., 2010). Although the SNS and HPA play different roles in the stress response system, recent research suggests that coordination between these two systems might be particularly important and thus the notion of dysregulation has been applied to the interaction of stress response systems. These recent studies specifically focus on the extent of asymmetry between these systems (e.g., relatively greater reactivity in one system than the other), with greater asymmetry reflecting greater cross-system dysregulation (reviewed in Kreher, Powers, & Granger, 2012). In humans, research using methods from salivary bioscience, which allow the measurement of correlates of SNS (salivary α-amylase) and HPA axis (salivary cortisol) activity non-invasively, have found that this asymmetry has functional consequences. Asymmetry has been associated with anxiety disorders (e.g., Reeves, Fisher, Newman, & Granger, 2016; Schmacher, Kirschbaum, Frydrich, & Strohle, 2013) and a suite of behavioral problems (e.g., externalizing behavior: Gordis, Granger, Susman, & Trickett, 2008). Asymmetry in SNS and HPA responses to stress has been shown to emerge in response to ELA in both humans (Gordis, Granger, Susman, & Trickett, 2006) and nonhuman primates (e.g., rhesus macaques: Petrullo, Mandalaywala, Maestripieri, Parker, & Higham, 2016). However, the extent of asymmetry in response to ELA is highly variable, suggesting that additional factors are important in determining the extent to which an individual’s physiological stress systems are affected by early adversity. Here we hypothesized that vigilance for threat during infancy would moderate the effects of ELA on later physiological dysregulation in rhesus macaques. Specifically, we tested the prediction that increased vigilance during infancy promotes resilience later in life by attenuating the negative effects of adversity on SNS-HPA asymmetry in juvenility.
Methods
The study was conducted on Cayo Santiago, a small Puerto Rican island with a population of approximately 1,500 rhesus macaques. Subjects were 20 free-ranging rhesus macaques (13 male, 7 female) for whom longitudinal behavioral and physiological data were collected during the first 3 years of life as part of a larger study.
Behavioral data
Using continuous focal animal sampling in which each individual was observed twice weekly, behavioral data were collected across the first 12 weeks of life for 11.8 ± 0.05 hours/infant (mean ± SEM). We quantified ELA through the frequency of maternal rejection and abuse, two behaviors frequently used in studies of infant maltreatment in macaques (e.g., Maestripieri & Carroll, 1998). Maternal rejection occurred any time the mother prevented contact or access to the nipple by holding or pushing the infant away from her body, and maternal abuse occurred any time the mother physically maltreated her infant (e.g., hitting, shoving, dragging, throwing). We converted data to mean hourly rates and averaged abuse and rejection behaviors together to create a single overall frequency of maltreatment for each subject. Subjects were assigned to a high, moderate, or low ELA category based on their relative rate of ELA from a three-way split in maternal maltreatment frequencies (as in Mandalaywala, 2014). As in our previous study (Petrullo et al., 2016), we found that Low ELA individuals exhibit SNS:HPA ratios that differ from Moderate and High ELA individuals, therefore we combined Moderate and High ELA categories into one High ELA category (Low ELA n = 9; High ELA n = 11).
Putting agonistic interaction data from each subject’s mother into a winner-loser matrix in MatMan (de Vries, Netto, & Hanegraff, 1993), we generated linear dominance hierarchies. Following 10 000 iterations, significant linear hierarchies were produced (linearity test using Landau’s linearity index corrected for unknown relationships, p =. 0.03). Each mother/infant dyad was categorized as high (n = 6), middle (n = 8), or low (n = 6) ranking by dividing mothers equally into the top, middle, or bottom third of the hierarchy, respectively.
Cognitive data
Vigilance data were collected as part of a previous study (Mandalaywala et al., 2014). At 8.5 ± 0.08 months old (mean ± SEM) subjects were simultaneously shown two validated stimuli (Bethell et al., 2012a): stimuli were color photographs (8.25 in. long x 11.75 in. high) of an unfamiliar male displaying either an open-mouth threat or a non-emotional expression (see Mandalaywala et al., 2014 for example stimuli), and subjects responses were then videorecorded for 5 sec using a handheld camcorder (Canon FS20). Subjects were approached while sitting calmly and away from the group, and a trial was initiated by setting up the cardboard apparatus less than 2.5 m in front of the subject. Stimuli were initially covered with colored blinders, and after the subjects attention was captured and oriented toward the center of the apparatus (equidistant from both blinders) both blinders were removed simultaneously. Observers blind to experimental aims and condition coded video data frame by frame (30 frames/second), counting the number of frames spent looking at each stimuli separately, and converting to ms. To quantify vigilance, we subtracted the time the subject spent looking at the neutral stimulus from the time the subject spent looking at the threatening stimulus; a positive value indicated more time looking at the threatening stimulus, and a negative value more time looking at the neutral stimulus. All videos were coded by an additional coder with high inter-observer reliability (Cohen’s κ = .82).
Saliva collection
Saliva was collected using Salimetrics® Oral Swabs (State College, PA), following previously validated collection and processing protocols (Higham, Vitale, Rivera, Ayala, & Maestripieri, 2010) as part of a previous study (Petrullo et al., 2016). Saliva samples (N = 217) were collected when subjects were 2.89 ± 0.01 years old (mean ± SEM). Samples were collected during periods of “high stress” (≤15 min after a conflict with conspecifics in which the subject was the aggressed individual; n = 90) and “low stress” (after subject rested or had minimal conspecific engagement for ≥30 min; n = 127). As described in Petrullo et al., 2016, saliva samples were collected between 7:00 and 14:00 by presenting a swab, tied to a piece of rope and lightly coated in Tang, to the subject either by draping it over a rock or hanging it on a tree or other structure. The time that subjects placed the swab in their mouth was recorded using a stopwatch, and once a subject became disinterested in the swab it was cut from the rope, placed into a centrifuge tube with a retainer, and placed on ice. Samples were centrifuged after 14:00 and then frozen at −80 °C until analysis.
Assay protocols
As described elsewhere (Petrullo et al., 2016), saliva samples were assayed for cortisol and sAA using commercial enzyme-immuno-assays (Salimetrics), validated for use in rhesus macaques (Higham et al., 2010). For salivary cortisol, high and low inter-assay coefficients of variation (CV) were 2.4% and 3.9%, respectively, and high and low intra-assay CVs were 7% and 4%, respectively. For sAA, high and low inter-assay CVs were 2.5% and 7.2%, and high and low intra-assay CVs were 3.6% and 5.8%. sAA values were flow rate adjusted by multiplying concentrations by saliva volume (mL) deposited on the swab over the time (min) the swab was chewed during collection; sAA values are expressed in U/min. We excluded samples with undetectably low concentrations or insufficient volume to test reliably. One subject was excluded from all analyses due to having a low stress cortisol concentration average more than three times above the inter-quartile range, leaving a final sample size of 19 (Low ELA n = 9; High ELA n = 10), with n= 127 samples from 19 individuals (cortisol), and n = 75 samples from 13 individuals (sAA).
Data Analysis
For each individual separately, we calculated average Low Stress (LS) cortisol and LS sAA concentrations, as well as average High Stress (HS) cortisol and HS sAA concentrations across all samples. To examine individual reactivity within a biological measure, we calculated cortisol reactivity (HS cortisol: LS cortisol, n = 19) and sAA reactivity (HS sAA: LS sAA, n = 13). For cortisol and sAA reactivity, a larger value indicates a properly functioning stress response (e.g., an individual has a low baseline (LS) concentration followed by a robust response to a stressor (HS): see Sanchez, 2006 for a review).
Finally, to utilize a multi-systems approach (e.g., Granger et al., 2008), we additionally calculated LS asymmetry (LS sAA: LS cortisol, n = 13) and HS asymmetry (HS sAA: HS cortisol, n = 13) to determine whether physiological outcomes were affected in an interactive manner. For LS and HS asymmetry, a larger number indicates a larger relative difference between sAA and cortisol concentrations for an individual, reflecting greater asymmetry, whereas a smaller number indicates a smaller relative difference, reflecting greater symmetry. A larger value, and thus greater asymmetry, is indicative of a dysregulated stress response system, where multiple components of the stress response complex do not recognize or respond to stressors in a coordinated manner (e.g., Ali & Pruessner, 2012; Gordis et al., 2006). Lack of coordination between physiological response systems has been implicated in increasing allostatic load, leading to wear and tear on the system (e.g., McEwen, 1998).
Whereas a previous study found an effect of maternal rank on vigilance in a larger sample that included these subjects (Mandalaywala et al., 2014), using General Linear Models (GLMs) with maternal rank (high, moderate, or low) as the predictor we found no effects of maternal rank on vigilance, F(1,16) = 2.10, p = .155, or on any physiological variables (cortisol reactivity: F(1,16) = 0.90, p = .426; sAA reactivity: F(1,10) = 0.26, p = .775; LS asymmetry: F(1,10) = 0.05, p = .953; HS asymmetry: F(1,10) = 0.99, p = .403). A Chi-squared test revealed that maternal rank was also not associated with ELA, X2 (2, N = 19) = 1.32, p =.517; therefore, in this subset of subjects rank was not included in subsequent analyses. As indicated by independent t-tests with subject sex (male, female) as the predictor, there were no sex differences in rates of ELA, t(17) = −1.32, p = .227, nor were there any differences between males and females in the expression of vigilance, t(17) = −.111, p = .914, or in any of the physiological variables (cortisol reactivity: t(17) = −.941, p = .374; sAA reactivity: t(11) = .192, p = .853; LS asymmetry: t(11) = .421, p = .691; HS asymmetry: t(11) = .954, p = .369); therefore, subject sex was not included as a predictor in subsequent models. For the main analyses, we used GLMs including ELA, vigilance, and the interaction of ELA x vigilance as fixed factors, with the dependent variable being one of the above physiological variables (Table 1). Main and interactive effects are presented for completeness, but only interactive effects are interpreted in analyses where significant main and interactive effects are found. All analyses were undertaken in SPSS 21.0; results with a p < .05 will be considered significant and those with a p < .10 will be described as a trend.
Table 1.
Results of GLMs for High Stress (HS) and Low Stress (LS) samples.
| LS cortisol | LS sAA | HS cortisol | HS sAA | HS cort: LS cortisol (Cortisol reactivity) | HS sAA: LS sAA (sAA reactivity) | LS sAA: LS cortisol (LS Asymmetry) | HS sAA: HS cortisol (HS Asymmetry) | |
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| ELA | n.s. |
F(1,9) = 5.09 p = 0.050 |
F(1,15) = 8.89 p = 0.009 |
n.s. |
F(1,15) = 10.04 p = 0.003 |
n.s. | n.s. | n.s. |
| Vigilance | n.s. |
F(1,9) = 4.90 p = 0.054 |
n.s. | n.s. | n.s. |
F(1,9) = 10.34 p = 0.011 |
n.s. | n.s. |
| ELA x Vigilance | n.s. | n.s. |
F(1,15) = 7.42 p = 0.016 |
F(1,9) = 3.78 p = 0.084 |
F(1,15) = 10.17 p = 0.006 |
F(1,9) = 7.22 p = 0.025 |
n.s. |
F(1,9) = 10.46 p = 0.010 |
Results
Low Stress cortisol, High Stress cortisol, and cortisol reactivity
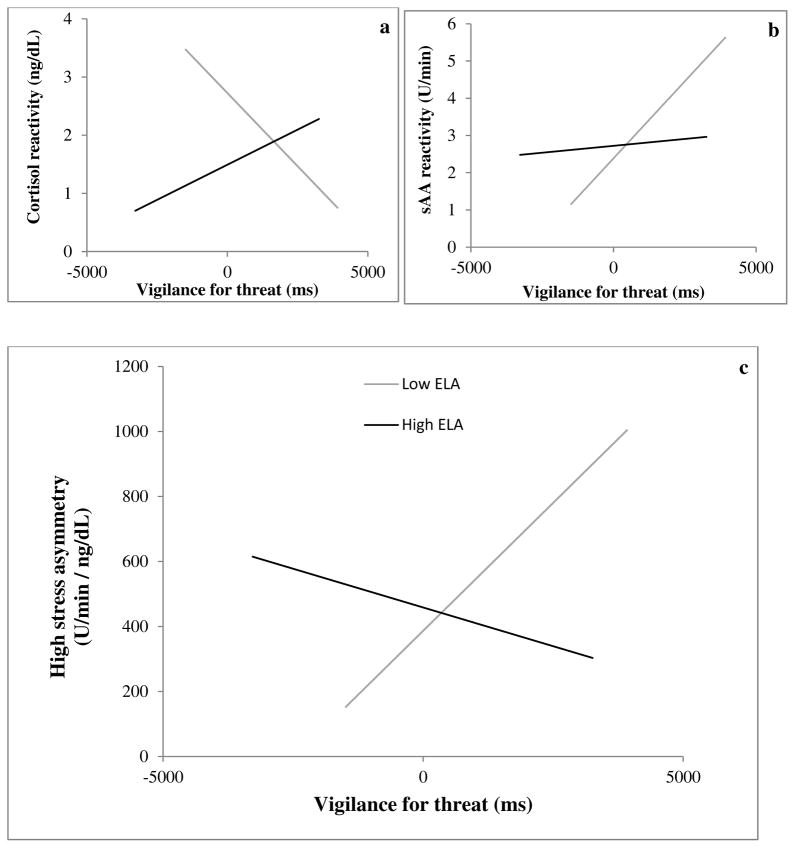
We found no significant effect of ELA, vigilance, or ELA x vigilance on cortisol concentration during LS. However, during HS, ELA, F(1,15) = 8.89, p = .009, and ELA x vigilance, F(1,15) = 7.42, p = .016, predicted cortisol concentrations. Within Low ELA subjects, greater vigilance was associated with lower cortisol concentrations. For High ELA subjects, greater vigilance was associated with higher cortisol concentrations. As in the larger dataset used in Petrullo et al. (2016), ELA predicted cortisol reactivity, with Low ELA subjects exhibiting greater cortisol reactivity than High ELA subjects, F(1,15) = 10.04, p = .003. Additionally, ELA x vigilance predicted cortisol reactivity, F(1,15) = 10.17, p = .006 (Fig. 1a). Within Low ELA subjects, greater vigilance was associated with lower cortisol reactivity. For High ELA subjects, greater vigilance was associated with higher cortisol reactivity.
Figure 1.
Model predicted plots (with best-fitting regression lines of the fixed predicted values from GLM for Low and High ELA subjects separately) showing the relationship between vigilance and: (a) cortisol reactivity (HS cort:LS cort); (b) sAA reactivity (HS sAA:LS sAA); and (c) HS asymmetry (HS sAA:HS cort).
Low Stress sAA, High Stress sAA, and sAA reactivity
We found trends for ELA, F(1,9) = 5.09, p = .050, and vigilance, F(1,9) = 4.90, p = .054, to affect sAA concentrations during LS, and there was no significant effect of their interaction. During LS periods, High ELA subjects exhibited marginally lower sAA concentrations than Low ELA subjects. Additionally, subjects with greater vigilance exhibited marginally lower LS sAA concentrations. During HS, we found no significant main effects of ELA or vigilance, but did see a trend in which the interaction of ELA x vigilance predicted HS sAA concentrations, F(1,9) = 3.78, p = .084. Within Low ELA subjects, greater vigilance was associated with a slight tendency to have higher sAA concentrations. For High ELA subjects, greater vigilance was associated with a slight tendency toward lower sAA concentrations.
While we found no main effect of ELA on sAA reactivity, we did find that both vigilance, F(1,9) = 10.34, p = .011, and ELA x vigilance, F(1,9) = 7.22, p = .025, predicted sAA reactivity (Fig. 1b), in the opposite direction to that found for cortisol reactivity. Among Low ELA subjects, greater vigilance was associated with greater sAA reactivity; among High ELA subjects, greater vigilance was associated with less sAA reactivity.
Low Stress asymmetry
Neither ELA, vigilance, nor their interaction predicted asymmetry during LS.
High Stress asymmetry
During HS, there were no significant main effects of ELA or vigilance, but ELA x vigilance significantly predicted asymmetry, F(1,9) = 10.46, p = .010. Within Low ELA subjects, greater vigilance was associated with greater asymmetry, whereas for High ELA subjects, greater vigilance was associated with less asymmetry (Fig. 1c).
Discussion
Among rhesus macaques, ELA and vigilance for threat during infancy predicted asymmetry in SNS-HPA activity in periods of high stress during juvenility. High ELA individuals exhibited blunted cortisol reactivity, a suboptimal physiological profile. However, this effect was moderated by vigilance, such that High ELA individuals with greater vigilance exhibited greater cortisol reactivity. Moreover, High ELA individuals with greater vigilance exhibited less asymmetry in SNS-HPA activity than Low ELA individuals with greater vigilance, lending support to our hypothesis that being vigilant toward threats might be recruited as a cognitive mechanism to attenuate the consequences of ELA.
What exactly is it about vigilance that confers this physiological advantage later in life for those experiencing high ELA? One possibility is that vigilance during infancy is associated with preferable physiological outcomes in juvenility because infants that experienced greater ELA learned earlier how to regulate emotion and arousal in the face of threat (e.g. Bauer, Quas, & Boyce, 2002; Parker & Maestripieri, 2011), giving them the tools early on to cope with adversity across the lifespan. Conversely, High ELA individuals who exhibited relatively weaker vigilance showed significant dysregulation, suggesting that these individuals were ill equipped to deal with subsequent adverse experiences. In line with this explanation, we found that vigilance and ELA were more likely to be associated with subsequent physiological dysregulation during high stress periods, after the individual was exposed to a social stressor. During periods of low stress, stress physiology was relatively unaffected by either vigilance or ELA. This suggests that meaningful differences between groups emerge most strongly when there is a social threat that necessitates a response. Interestingly, even during high stress periods not all aspects of stress physiology were similarly affected by vigilance, adversity, or their interaction. Rather, we found that SNS-HPA asymmetry in response to early life adversity and vigilance was driven by the HPA component of the stress response. This finding is consistent with previous research across species showing that the HPA axis shows attenuation in response to chronic stress over time (Heim & Nemeroff, 2001; Meaney & Szyf, 2005; Sanchez, 2006).
Unlike Low ELA individuals with weaker vigilance, which showed little dysregulation, Low ELA individuals with greater vigilance exhibited dysregulated stress profiles, characterized by decreased cortisol reactivity and increased SNS:HPA asymmetry. In humans, having a hyperactive vigilance for threat in relatively stable and predictable environments is associated with greater physiological asymmetry (e.g., Reeves et al., 2016; Schmacher et al., 2013), and as such, is also implicated in the etiology of anxiety disorders (e.g., Bar-Haim et al., 2007). Similarly, it remains an open question precisely why certain individuals exposed to adversity developed greater vigilance in the first place, whereas others exposed to adversity developed less, as robust attention to threat should be equally useful among any individuals exposed to adversity. To understand better why individuals sometimes develop vigilance profiles that are mismatched to their experience, we can look to studies exploring the genetic basis for cognitive phenotypes. Previous work on the genetic basis of vigilance has shown that genetic factors shape an individual’s propensity to express vigilance (e.g., Fox, Zougkou, Ridgewell, & Garner, 2011; Perez-Edgar et al., 2010), and genetic factors might be similarly likely to influence the very development of a vigilant phenotype as well. By integrating an individual’s genotype into our studies on the development of cognition in response to particular environments, we can better understand when, how, and why individuals develop cognitive and behavioral phenotypes that are best suited to their environment.
Implicit in the above proposed adaptive explanations is the assumption that the early environment is predictive of the future environment, such that individuals who encountered adversity early in life are also likely to encounter adversity later (in line with theories such as the Predictive Adaptive Response hypothesis: see Bateson, Gluckman, & Hanson, 2014). Given that we did not collect data on the environment during the juvenile period, we cannot explicitly test this assumption, and future studies should examine how environmental continuity or change across time influences the effects of vigilance (for a similar approach, see Frankenhuis & Del Giudice, 2012; Schmidt, 2011). Similarly, the adaptive account assumes some level of stability in vigilance across development. Although intra-individual variation in the expression of vigilance across time has not been well studied (but see Schehner & Bar-Haim, 2016), this particular cognitive bias displays hints of flexibility. Studies across multiple species, including humans, and both captive and free-ranging rhesus macaques, show short-term modification of vigilance in response to stressful situations (bumblebees: Bateson, Desire, Guardside, & Wright, 2011; chickens: Salmeto et al., 2011; humans: Mogg, Bradley, & Hallowell, 1994; Wald, Lubin, Holoshitz, & Muller, 2011; captive rhesus macaques: Bethell et al., 2012b; free-ranging rhesus macaques: Mandalaywala, 2014). However, whether individuals can more permanently alter the expression of vigilance in response to changes in the environment remains to be determined.
Nonetheless, our results suggest that among individuals with High ELA, increased vigilance during infancy serves as an adaptive response that results in comparatively less physiological dysregulation later in life. Moreover, by adopting a more comprehensive developmental approach, integrating measures of cognition, behavior, physiology, and environmental context, we can also begin to clarify the developmental timeline and the causal direction of these relations. In the correlational results presented here, it is not possible to infer a causal direction; therefore it is possible that developmental differences in SNS-HPA activity during infancy led to inter-individual differences in vigilance rather than the other way round. Future studies incorporating multi-system measures of stress physiology earlier in life are necessary to better understand these complex interactions. Even with these limitations, our results show that early life experience and cognitive processes interact to shape physiological development, helping to explain inter-individual variation in long-term outcomes and illustrating the utility of the cognition by environment approach.
Acknowledgments
We thank the staff of the Caribbean Primate Research Center, as well as Emily Bethell, Auberi Courchay, Sean Coyne, Keiran Mandalaywala, Eliot Monaco, and Greg Ruber for assistance with data collection and coding.
Funding Statement. Research was supported by National Institutes of Health grant R01-HD067175 to DM and KJP, and grant P40 OD012217-25 from the National Center for Research Resources and the Office of Research Infrastructure Programs of NIH to the CPRC of the University of Puerto Rico. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Footnotes
Ethics Statement. This research was approved by the Institutional Animal Care and Use Committees of New York University, University of Chicago, Stanford University, and University of Puerto Rico.
Author contributions. DM and KJP designed the longitudinal study, TMM developed the cognitive development component, collected cognitive and behavioral data, and carried out statistical analyses with JPH. LAP collected and analyzed endocrine data under the supervision of JPH. TMM and JPH drafted the manuscript while DM, KJP, and LAP provided revisions.
References
- Ali N, Pruessner JC. The salivary alpha amylase over cortisol ratio as a marker to assess dysrgulations of the stress systems. Physiology and Behavior. 2012;106:65–72. doi: 10.1016/j.physbeh.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: A metaanalytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Bateson M, Desire S, Guardside SR, Wright GA. Agitated honeybees exhibit pessimistic cognitive biases. Current Biology. 2011;21:1070–1073. doi: 10.1016/j.cub.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson P, Gluckman P, Hanson M. The biology of developmental plasticity and the Predictive Adaptive Response hypothesis. The Journal of Physiology. 2014;592:2357–2368. doi: 10.1113/jphysiol.2014.271460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer AM, Quas JA, Boyce WT. Association between physiological reactivity and children’s behavior: Advantages of a multisystem approach. Journal of Developmental Behavioral Pediatrics. 2002;23:102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Bethell EJ, Holmes A, MacLarnon A, Semple S. Emotion mediates social attention in a non-human primate. PLoS ONE. 2012a;7:e44387. doi: 10.1371/journal.pone.0044387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethell EJ, Holmes A, MacLarnon A, Semple S. Cognitive bias in a non-human primate: husbandry procedures influence cognitive indicators of psychological wellbeing in captive rhesus macaques. Animal Welfare. 2012b;21:185–195. doi: 10.7120/09627286.21.2.185. [DOI] [Google Scholar]
- Caspi A, Moffit TE. Gene-environment interactions in psychiatry: Joining forces with neuroscience. Nature Reviews Neuroscience. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders: Overview of physical and behavioral homeostasis. Journal of the American Medical Association. 1992;267:1244–1252. [PubMed] [Google Scholar]
- de Vries H, Netto WJ, Hanegraff PLH. MatMan: A program for the analysis of sociometric matrices and behavioural transition matrices. Behaviour. 1993;125:157–175. [Google Scholar]
- Farroni T, Menon E, Rigato S, Johnson MH. The perception of facial expressions in newborns. European Journal of Developmental Psychology. 2007;4:2–13. doi: 10.1080/17405620601046832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E, Zougkou K, Ridgewell A, Garner K. The serotonin transporter gene alters sensitivity to attention bias modification: Evidence for a plasticity gene. Biological Psychiatry. 2011;70:1049–1054. doi: 10.1016/j.biopsych.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenhuis WE, Del Giudice M. When do adaptive developmental mechanisms yield maladaptive outcomes? Developmental Psychology. 2012;48:628–642. doi: 10.1037/a0025629. [DOI] [PubMed] [Google Scholar]
- Frankenhuis WE, de Weerth C. Does early-life exposure to stress shape or impair cognition? Current Directions in Psychology. 2013;22:407–412. [Google Scholar]
- Franklin TB, Saab BJ, Mansuy IM. Neural mechanisms of stress resilience and vulnerability. Neuron Review. 2012;75:747–761. doi: 10.1016/j.neuron.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Salivary alpha amylase-cortisol asymmetry in maltreated youth. Hormones & Behavior. 2008;53:96–103. doi: 10.1016/j.yhbeh.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Asymmetry between salivary cortisol and α-amylase reactivity to stress: Relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006;31:976–987. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, El-Sheikh MO, Gordis EB, Stroud LR. Salivary α-amylase in biobehavioral research. Annals of the New York Academy of Sciences. 2007;1098:122–144. doi: 10.1196/annals.1384.008. [DOI] [PubMed] [Google Scholar]
- Grossman T, Striano T, Friederici AD. Developmental changes in infant’s processing of happy and angry facial expressions: A neurobehavioral study. Brain and Cognition. 2007;64:30–41. doi: 10.1016/j.bandc.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Harlow HF, Dodsworth RO, Harlow MK. Total social isolation in monkeys. Proceedings of the National Academy of Sciences of the United States of America. 1965;54:90–97. doi: 10.1073/pnas.54.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Nemeroff C. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biological Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Higham JP, Vitale AB, Rivera AM, Ayala JE, Maestripieri D. Measuring salivary analytes from free-ranging monkeys. Physiology and Behavior. 2010;101:601–607. doi: 10.1016/j.physbeh.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Cicchetti D, Rogosch FA. Oxytocin Receptor Gene (OXTR) polymorphism, perceived social support, and psychological symptoms in maltreated adolescents. Developmental Psychopathology. 2014;26:465–477. doi: 10.1017/S0954579414000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin N, Shelton SE. Nonhuman primate models to study anxiety, emotion regulation, and psychopathology. Annals of the New York Academy of Sciences. 2003;1008:189–200. doi: 10.1196/annals.1301.021. [DOI] [PubMed] [Google Scholar]
- Kreher DA, Powers SI, Granger DA. The relationship between cortisol, salivary alpha-amylase, and cognitive bias in young women. Behavioral Neuroscience. 2012;126:157–166. doi: 10.1037/a0026654. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Carroll KA. Risk factors for infant abuse and neglect in rhesus monkeys. Psychological Science. 1998;9:143–145. [Google Scholar]
- Maestripieri D, Martel FL, Nevison CM, Simpson MJA, Keverne EB. Anxiety in rhesus monkey infants in relation to interactions with their mother and other social companions. Developmental Psychobiology. 1992;24:571–581. doi: 10.1002/dev.420240805. [DOI] [PubMed] [Google Scholar]
- Mandalaywala TM. Doctoral dissertation. 2014. Effects of early life experience on infant rhesus macaque cognition and stress physiology. Retrieved from ProQuest. (Accession No. 3627855) [Google Scholar]
- Mandalaywala TM, Parker KJ, Maestripieri D. Early experience affects the strength of vigilance for threat in rhesus monkey infants. Psychological Science. 2014;25:1893–1902. doi: 10.1177/0956797614544175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Allostasis and allostatic load: Implications for Neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–123. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Maternal care as a model for experience-dependent chromatin plasticity? Trends in Neuroscience. 2005;28:456–463. doi: 10.1016/j.tins.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradle BP, Hallowell N. Attentional bias to threat: Roles of trait anxiety, stressful events, and awareness. The Quarterly Journal of Experimental Psychology Section A: Human Experimental Psychology. 1994;47:841–864. doi: 10.1080/14640749408401099. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Fox NA, Zeanah CH. Romania’s Abandoned Children: Deprivation, Brain Development, and the Struggle for Recovery. Cambridge, MA: Harvard University Press; 2014. [Google Scholar]
- Parker KJ, Maestripieri D. Identifying key features of early stressful experiences that produce stress vulnerability and resilience in primates. Neuroscience and Biobehavioral Reviews. 2011;35:1466–1483. doi: 10.1016/j.neubiorev.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, Bar-Haim Y, McDermott JM, Gorodetsky E, Hodgkinson CA, Goldman D, Ernst M, Pine DS, Fox NA. Variations in the serotonin transporter gene are linked to attention bias patterns to positive and negative emotion faces. Biological Psychology. 2010;83:269–271. doi: 10.1016/j.biopsycho.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrullo LA, Mandalaywala TM, Parker KJ, Maestripieri D, Higham JP. Effects of early life adversity on cortisol/salivary alpha-amylase asymmetry in free-ranging juvenile rhesus macaques. Hormones and Behavior. 2016;86:78–84. doi: 10.1016/j.yhbeh.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves JW, Fisher AJ, Newman MG, Granger DA. Sympathetic and hypothalamic-pituitary-adrenal asymmetry in generalized anxiety disorder. Psychophysiology. 2016;53:951–957. doi: 10.1111/psyp.12634. [DOI] [PubMed] [Google Scholar]
- Salmeto AL, Hymel KA, Carpenter EC, Brilot BO, Bateson M, Sufka KJ. Cognitive bias in the chick anxiety-depression model. Brain Research. 2011;1373:124–130. doi: 10.1016/j.brainres.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Sanchez MM. The impact of early adverse care on HPA axis development: nonhuman primate models. Hormones and Behavior. 2006;50:623–631. doi: 10.1016/j.yhbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, McCormack K, Grand AP, Fulks R, Graff A, Maestripieri D. Effects of sex and early maternal abuse on adrenocorticotropin hormone and cortisol responses to the corticotropin-releasing hormone challenge during the first 3 years of life in group-living rhesus monkeys. Development & Psychopathology. 2010;22:45–53. doi: 10.1017/S0954579409990253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids in- fluence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55, e89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schehner T, Bar-Haim Y. Threat monitoring and attention-bias modification in anxiety and stress-related disorders. Current Directions in Psychological Science. 2016;25:431–437. [Google Scholar]
- Schmidt MV. Animal models for depression and the mismatch hypothesis of disease. Psychoneuroendocrinology. 2011;36:330–338. doi: 10.1016/j.psyneuen.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Schumacher S, Kirschbaum C, Fydrich T, Strohle A. Is salivary alpha-amylase an indicator of autonomic nervous system dysregulations in mental disorders?—A review of preliminary findings and the interactions with cortisol. Psychoneuroendocrinology. 2013;38:729–743. doi: 10.1016/j.psyneuen.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Shackman JE, Shackman AJ, Pollak SD. Physical abuse amplifies attention to threat and increases anxiety in children. Emotion. 2007;7:838–852. doi: 10.1037/1528-3542.7.4.838. [DOI] [PubMed] [Google Scholar]
- Wald I, Lubin G, Holoshitz Y, Muller D. Battlefield-like stress following simulated combat and suppression of attention bias to threat. Psychological Medicine. 2011;41:699–707. doi: 10.1017/S0033291710002308. [DOI] [PubMed] [Google Scholar]