Abstract
The lacrimal gland plays a pivotal role in keeping the ocular surface lubricated, and protecting it from environmental exposure and insult. Dysfunction of the lacrimal gland results in deficiency of the aqueous component of the tear film, which can cause dryness of the ocular surface, also known as the aqueous-deficient dry eye disease. Left untreated, this disease can lead to significant morbidity, including frequent eye infections, corneal ulcerations and vision loss. Current therapies do not treat the underlying deficiency of the lacrimal gland, but merely provide symptomatic relief. To develop more sustainable and physiological therapies, such as in vivo lacrimal gland regeneration or bioengineered lacrimal gland implants, a thorough understanding of lacrimal gland development at the molecular level is of paramount importance. Based on the structural and functional similarities between rodent and human eye development, extensive studies have been undertaken to investigate the signaling and transcriptional mechanisms of lacrimal gland development using mouse as a model system. In this review, we describe the current understanding of the extrinsic signaling interactions and the intrinsic transcriptional network governing lacrimal gland morphogenesis, as well as recent advances in the field of regenerative medicine aimed at treating dry eye disease.
Keywords: Lacrimal gland, dry eye, FGF, BMP, stem cell, regeneration
Overview of the lacrimal gland
The lacrimal gland is a tubulo-acinar exocrine gland that produces the aqueous component of the tear film, including water, electrolytes and proteins (Zoukhri, 2010). Critical for ocular health and quality vision, the tear film forms a smooth refractive layer over the cornea, while lubricating the cornea and conjunctiva, supporting ocular surface metabolism, and flushing away dirt and noxious stimuli. This film is composed of three layers: 1) the outermost lipid layer secreted by Meibomian glands that prevents evaporation of tears, 2) the middle aqueous layer produced by the lacrimal gland that accounts for over 90% of tear volume, and 3) the innermost mucous layer produced by goblet cells of the conjunctiva that anchors the tear film to the ocular surface (Johnson and Murphy, 2004). By releasing immunoglobulins into the tears, the lacrimal gland also functions as a secretory immune system to protect the ocular surface against infection (Holly and Lemp, 1977).
In humans, the primary lacrimal gland is located within the upper temporal orbit, emptying its secretions into an anastomosed duct system that delivers the fluid to the ocular surface. The outflow component of the lacrimal system lies at the nasal side of the eye, where puncta located on the upper and lower lids drain fluid into canaliculi leading to the nasolacrimal sac and nose (Figure 1) (Walcott, 1998). In rodents, however, the lacrimal gland is comprised of two lobular structures: one intra-orbital and the other extra-orbital. The primary lacrimal gland is the extra-orbital lobe, located just beneath the ear and connected to the eye via a long duct that joins the intra-orbital lobe just prior to reaching the eye (Figure 2) (Dartt, 2009). The lacrimal gland epithelium is composed of three major cell types: acinar, ductal and myoepithelial cells. The primary secretory apparatus is composed of acinar cells, which make up to 80% of the gland. The luminal sides of the acinar cells are connected to the secretory ducts lined by cuboidal duct cells, which constitute 10–12% of the lacrimal gland cell population and contribute to 30% of the lacrimal gland fluid secretions (Mircheff, 1989). Myoepithelial cells surround the basal side of both acinar and ductal cells. Their function is to apply pressure to the secretory cells to expel the fluid into the duct (Dartt, 2009). Besides these three main cell types, the lacrimal gland stroma also contains fibroblasts that produce collagens, and mast cells that secrete histamines and matrix proteins into the interstitial spaces (Walcott, 1998). The vasculature of the lacrimal gland also brings in plasma cells, lymphocytes, dendritic cells and macrophages, which provide immune protection to the ocular surface (Allansmith et al., 1976; Dartt, 2009). The function of the lacrimal system is controlled by sensory afferent nerves from the cornea and conjunctiva, coupled with parasympathetic and sympathetic efferent nerves innervating the lacrimal gland. These nerves ensure an optimum volume and quality of tear secretion in response to environmental stress (Dartt, 2009).
Figure 1. Schematic of Lacrimal gland functional unit.
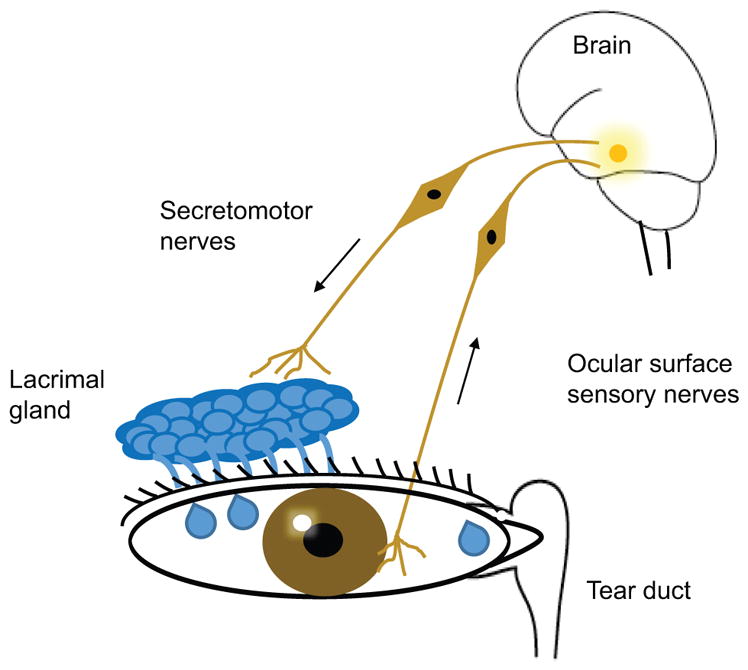
The lacrimal gland functional unit is comprised of a) the lacrimal gland, b) Sensory afferent nerves from the cornea and conjunctiva, c) motor efferent nerves originating from the central nervous system which innervate lacrimal gland, d) the excretory tear duct for drainage of the excess fluid. Impairment in any components of lacrimal gland function unit can destabilize the tear film and cause the dry eye disease.
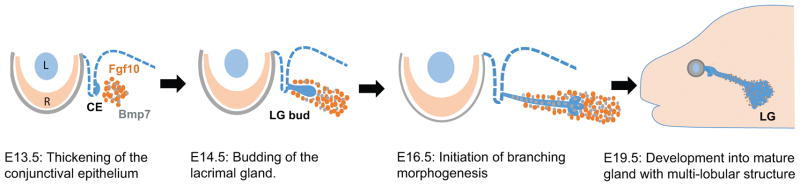
Figure 2. Lacrimal gland development in mouse.
Transverse sections of mouse embryos at different stages are shown. Lacrimal gland development begins with thickening of the CE at E13.5 induced by Fgf10 from the surrounding mesenchyme. These epithelial cells further grow and elongate into a bud from E14.5 through E15.5. Branching of LG initiates at E16.5 under the additional influence of BMP7 signaling, eventually forming a multi-lobular tubulo-acinar structure at E19.5. Lacrimal gland continues to develop even during post-natal stages to become a mature gland capable of regulated tear secretion in adults. L: lens, R: Retina, CE: conjunctival epithelium, LG: lacrimal gland.
Impairment of lacrimal gland function can result in the debilitating condition known as aqueous-deficient dry eye disease, which can progress to corneal ulceration and vision loss if left untreated. The most significant risk factor for dry eye disease is aging, associated with structural and functional changes in the lacrimal gland characterized by atrophied acini, duct obstruction, lymphocytic infiltration and decreased protein secretion (Rocha et al., 2008). Lacrimal gland dysfunction can also arise from inflammation triggered by the dry environment, auto-immune attack as in Sjogren’s syndrome and rheumatoid arthritis, side effects of chemo and radiation therapies, as well as congenital defects (Javadi and Feizi, 2011; He et al., 2013; Park et al., 2015). Several treatments exist, including punctal occlusion to reduce tear drainage, anti-inflammatory drugs such as topical cyclosporine and lifitegrast, and most commonly artificial tears and gels (Babic et al., 2010). However, each of these clinical interventions are primarily palliative, and are not aimed at curing the underlying lacrimal gland deficiency. To this end, regeneration of the damaged lacrimal gland or replacement by bioengineered implants can potentially provide long lasting and physiological cures for dry eye disease. Ensuring the success of these approaches will require a thorough understanding of the molecular mechanism of lacrimal gland development and regeneration. In this review, we will focus on signaling pathways and transcription factors that have been shown to regulate lacrimal gland development in animal models and discuss emerging regenerative therapies that may ultimately provide more sustainable treatment for the dry eye disease.
The molecular mechanism of lacrimal gland development
Signaling interactions: the epithelium-mesenchyme interaction
The lacrimal gland forms as a result of interactions between the conjunctival epithelium and the periocular mesenchyme. In humans, it begins as a thickening of the epithelium at the superior conjunctival fornix, which subsequently invades the underlying mesenchyme to form a highly branched gland (de la Cuadra-Blanco et al., 2003). This is recapitulated in mouse as the budding of the conjunctival epithelium at the temporal side of the eye at the E13.5 stage (Figure 2) (Makarenkova et al., 2000). This tubular bud elongates dorsally toward the ear, accompanied by condensation of the surrounding mesenchyme (Dean et al., 2004). This process can occur independently of retina and lens development, as the lacrimal gland bud develops even in mouse mutants lacking the eyeball (Swindell et al., 2008). Starting at E16.5, the lacrimal gland bud branches out to form a complex intra-orbital and extra-orbital multi-lobular structure, eventually becoming composed of a system of acini, ducts, myoepithelial cells, nerves, plasma cells and connective tissues.
The inductive signals to initiate lacrimal gland budding and branching morphogenesis are Fibroblast Growth Factors (FGFs). Fgf10 in particular is expressed in a distinctive domain in the mesenchyme surrounding the epithelial bud and its expression persists throughout lacrimal gland development. By contrast, Fgf7 expression in the mesenchyme is more diffused (Govindarajan et al., 2000; Makarenkova et al., 2000). Both recombinant human FGF10 and FGF7 were able to induce ectopic budding of the lacrimal gland epithelium in explant cultures of the mouse embryonic eye (Makarenkova et al., 2000). Remarkably, ectopic glands can even be induced in the cornea by transgenic expression of either rat Fgf10 or human FGF7 in the lens, but not by other FGFs, underscoring the potency and specificity of the FGF7/10 subfamily of FGFs for lacrimal gland development (Lovicu et al., 1999; Govindarajan et al., 2000). Fgf10 null mice exhibit a complete loss of the epithelial component of the lacrimal gland despite an intact mesenchyme, while Fgf7 knockout mice have normal lacrimal glands, indicating that Fgf10 is the primary driver of lacrimal gland development (Lovicu et al., 1999; Govindarajan et al., 2000; Makarenkova et al., 2000). Further, Fgf10 is haploinsufficient for lacrimal gland development in both mice and humans, in which a heterozygous loss of function mutation can lead to aplasia of the lacrimal and salivary glands (ALSG), a rare disorder characterized by dryness of the eye and mouth (OMIM #180920) (Entesarian et al., 2005). A more severe congenital disorder called Lacrimo-auriculo-dento-digital (LADD) syndrome affecting lacrimal and salivary glands, ears, teeth and distal limbs has been associated with missense mutations in FGF10 (OMIM #149730) (Rohmann et al., 2006). The majority of LADD mutations result in the disruption of FGF10 protein stability or its capacity to interact with its receptor, but missense mutations affecting secretion and nuclear localization of FGF10 have also been identified (Shams et al., 2007; Mikolajczak et al., 2016). These FGF10 mutations are thought to exert a dominant-negative effect instead of simple loss-of-function, which may explain why more organs are affected in LADD syndrome than in ALSG syndrome (Rohmann et al., 2006). These phenotypes highlight the pivotal role of FGF10/Fgf10 in multi-organ development, but also raise the interesting question of why lacrimal gland development is particularly sensitive to their gene dosage.
In addition to the precise control of Fgf10 at the transcriptional level, the concentration of Fgf10 protein in the periocular mesenchyme is also under exquisite regulation by proteoglycans within the extracellular matrix (ECM) (Figure 3) (Balasubramanian and Zhang, 2016). Previous work from our lab has shown that glycosaminoglycans (GAGs) attached to proteoglycans in the periocular mesenchyme restricts the diffusion of Fgf10 during lacrimal gland development (Qu et al., 2012). Mesenchyme-specific knockouts of the proteoglycan biosynthetic enzyme UDP-Glucose 6-Dehydrogenase (Ugdh) cause excessive diffusion of Fgf10 that is found to disrupt lacrimal gland budding. Interestingly, the lacrimal gland defect can also be produced by mesenchymal specific deletion of heparan sulfate (proteoglycan) modification enzymes N-deacetylase/N-sulfotransferase (Ndst1/2), but not by 2-O-sulfotransferases (Hs2st) and 6-O-sulfotransferases (Hs6st1/2), suggesting that N-sulfation of heparan sulfates is essential for regulating Fgf10 compartmentalization (Qu et al., 2012). Consistent with this model, mutating the key residues of FGF10 that interact with heparan sulfates also resulted in an increased diffusion range of FGF10 in the ECM (Makarenkova et al., 2009). The mutant FGF10 was found to behave like FGF7, which has a lower affinity for heparan sulfates and preferentially promotes lacrimal gland branching instead of elongation.
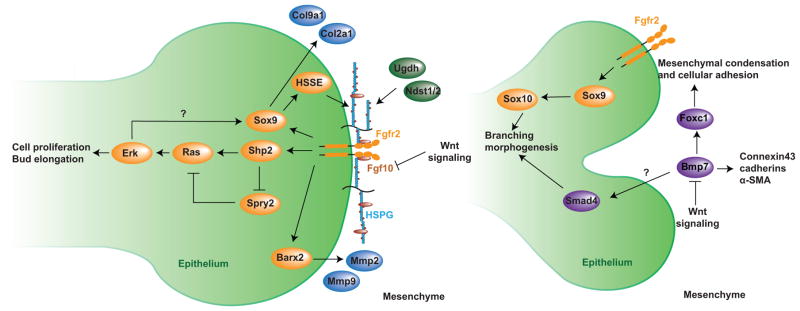
Figure 3. Summary of signaling interactions during lacrimal gland morphogenesis.
(Left) Fgf10 forms a heparan sulfates (HS)-dependent gradient in the periocular mesenchyme, inducing lacrimal gland budding by binding to both Fgfr2b and HS in the epithelium. This activates Shp2, which inhibits the Ras signaling repressor Spry2 and promotes Ras-Erk cascade to stimulate cell proliferation, survival and bud elongation. With transcription factors Sox9 and Barx2, FGF signaling also stimulates expressions of HS synthesizing enzymes (HSSE) and metalloproteinases to remodel the ECM, forming a positive feedback loop to enhance FGF signaling activity. (Right) Bmp7 signaling mediated by Foxc1 is important for mesenchymal condensation during branching morphogenesis. Both FGF and BMP signaling are counterbalanced by canonical Wnt signaling in the mesenchyme. In addition, Smad4-mediated BMP signaling and Sox9-Sox10 cascade also directly regulates the epithelial elongation.
FGF signaling has been shown to cooperate with the transcription factor Barx2 in the lacrimal gland epithelium to regulate the expression of two matrix metalloproteinases (MMP2 and MMP9) involved in ECM remodeling and are secreted into the mesenchyme to promote the release of Fgf10 from proteoglycans (Tsau et al., 2011). This presents a positive feedback mechanism to modulate the Fgf10 concentration ahead of the invading epithelial bud. One question that remains unresolved is whether the control of Fgf10 diffusion by proteoglycans generates a chemokine gradient to spatially guide lacrimal gland development. Answering this question is hampered by the lack of a sensitive assay to determine the endogenous concentration of Fgf10 in the periocular mesenchyme. In this regard, it is worth noting that although endogenous Fgf10 is also expressed in a localized fashion in the embryonic lung, a recent study showed that it can be functionally substituted by ubiquitous expression of Fgf10 during branching morphogenesis (Volckaert et al., 2013). This raises the possibility that an Fgf10 gradient may not be an absolute requirement for the budding and branching of glandular organs.
Biochemical studies have determined that the specific receptor for Fgf10 is Fgf receptor 2(III)b (Fgfr2b), which is expressed in the lacrimal gland epithelium (Makarenkova et al., 2000; Zhang et al., 2006). Indeed, both epithelial ablation of Fgfr2 in vivo, as well as ex vivo knock down of Fgfr2b, disrupts lacrimal gland development (Makarenkova et al., 2000; Pan et al., 2008). Interestingly, LADD syndrome can be caused by either FGFR2 or FGFR3 missense mutations, both of which are assumed to be hypomorphic given that affected patients do not exhibit the typical gain-of-function FGF signaling phenotypes (Rohmann et al., 2006). This observation is in apparent conflict with both the low affinity of Fgfr3 toward Fgf10 in vitro, and the lack of a lacrimal gland defect in Fgfr3 knockout mice (AG and XZ, unpublished results). A functional study is needed to resolve the nature of the LADD-associated FGFR3 mutations. It is also interesting to note that heterozygous ablation of Fgfr2c, which is not the canonical receptor for Fgf10, results in secondary branching defects in the lung, kidney and lacrimal gland (Hajihosseini et al., 2001). Since the Fgfr2 heterozygous null mouse lacks an overt phenotype, it is believed that the Fgfr2c mutant is a gain-of-function allele resulting from an alternative splicing event in the Fgfr2 locus, leading to ectopic expression and activation of Fgfr2b in the mesenchyme. Although the Fgfr2c mutant lacrimal gland retains a mesenchymal sac without Fgf10 expression, it remains to be determined how aberrant Fgfr2b signaling can be activated without Fgf10 in the mesenchyme.
The assembly of an FGF signaling complex on the cell surface requires heparan sulfates as co-receptors (Figure 3). Interestingly, the lacrimal gland bud specifically expresses Ndst1 enzyme in the tip cells, but not in the follower cells that form its stalk (Pan et al., 2008). Ablation of Ndst1 in the epithelium not only disrupts N-sulfation of heparan sulfates, but also abrogates lacrimal gland budding. Similarly, 2-O and 6-O sulfation of heparan sulfates each contribute to Fgf10-induced signaling given that the deletions of Hs6st and Hs2st in the lacrimal gland epithelium resulted in either stunted growth or no bud formation (Qu et al., 2011). Indeed, using a FGF ligand and carbohydrate engagement assay (LACE), we showed that recombinant Fgf10/Fgfr2b proteins were able to form a tight binding complex on the lacrimal gland bud in situ, which was disrupted in heparan sulfate N- or O-sulfation mutants (Pan et al., 2008; Qu et al., 2011). On the other hand, these modifications of heparan sulfates are also under control of FGF signaling, as epithelial ablation of Fgfr2 abolished N-sulfation of heparan sulfates (Pan et al., 2008). This positive feedback mechanism is mediated by Shp2, a non-receptor tyrosine phosphatase that transmits FGF signaling to the Ras-MAPK pathway, partly by inhibiting the negative Ras signaling regulator Sprouty2 (Pan et al., 2010). The key targets of this FGF signaling cascade are likely to be the transcription factors Sox9 and Sox10, which have been shown to regulate the expression of heparan sulfate 3-O-sulfotransferases in an FGF-signaling-dependent manner (Chen et al., 2014b).
While Fgf10 is expressed exclusively in the lacrimal gland mesenchyme to guide budding and branching of the epithelium, another growth factor, Bmp7, displays a more complex and dynamic expression pattern during lacrimal gland development. Initially expressed in the periocular mesenchyme surrounding the epithelial bud, Bmp7 is later present in both the epithelial and mesenchymal compartments of the lacrimal gland (Dean et al., 2004). The primary target of Bmp7 signaling, however, appears to be the lacrimal gland mesenchyme. Exposing isolated lacrimal gland epithelium to Bmp7 does not affect budding induced by Fgf10, but, in a mesenchymal culture, Bmp7 resulted in increased cellular proliferation and aggregation marked by expression of connexin43, cadherins and α-smooth muscle actin (α-SMA) (Dean et al., 2004). In contrast, defective condensation of the periocular mesenchyme was found in Bmp7 null mice, which also exhibited smaller glands with misplaced buds and reduced branching. It is thought that the condensation and proliferation of mesenchymal cells induced by BMP signaling is critical for proper branching morphogenesis of the lacrimal gland epithelium. In support of this concept, it was shown that transcription factor Foxc1 was dispensable in the lacrimal gland epithelium, but its loss in the mesenchyme prevented BMP signaling from inducing cellular condensation (Mattiske et al., 2006). As a result, Foxc1 null mice exhibited reduced lacrimal gland size with fewer terminal buds, reminiscent of the Bmp7 null phenotype. In contrast to Bmp7, Bmp4 is found to suppress Fgf10-induced growth and elongation of the lacrimal gland bud in an isolated epithelial culture, suggesting that BMP signaling may also play a direct role in the lacrimal gland epithelium (Dean et al., 2004). BMP signaling is mediated by phosphorylated Smad1/5/8 proteins, which form a complex with Smad4 to activate the downstream transcriptional events. Indeed, a recent study showed that epithelial deletion of Smad4 resulted in smaller lacrimal glands with fewer branches and acini (Liu and Lin, 2014). Interestingly, the lacrimal gland in Smad4 mutants accumulates pigments after birth and is eventually replaced by adipose tissue. These studies suggest that BMP signaling in both the epithelium and the mesenchyme are critical for lacrimal gland development.
Canonical Wnt signaling has been shown to interact with both FGF and BMP signaling to modulate lacrimal gland branching morphogenesis. Activation of the canonical Wnt signaling pathway prevents degradation of β-catenin in the cytoplasm, which is thereby translocated into the nucleus to bind Tcf/Lef transcription factors to induce gene expression. Transcripts of several Wnts (both canonical and non-canonical) are present in the lacrimal gland during development (Dean et al., 2005). Inhibition of Wnt signaling by knocking down β-catenin with morpholinos in lacrimal gland explants leads to increased branching and cell proliferation, and an up-regulation of Fgf10 in the mesenchyme. Activation of Wnt signaling by Wnt3a or LiCl treatment, on the other hand, reduces proliferation of both the epithelial and mesenchymal components of the lacrimal gland with a concurrent reduction in the number of branches. Wnt signaling also suppresses the Bmp7-induced increase of cell proliferation in the lacrimal gland mesenchyme. Thus, Wnt signaling regulates branching morphogenesis by counterbalancing the effects of Fgf10 and Bmp7 (Dean et al., 2005).
Maturation and homeostasis of the lacrimal gland also require Notch signaling, which acts through nuclear translocation of the Notch Intracellular Domain (NICD). NICD subsequently interacts with the recombination signal binding protein for immunoglobin Jk region (RBP-Jk), Histone acyl transferases, and Mastermind-like transcriptional co-activator (Maml), to activate transcription of target genes. Postnatal knockout of Notch1 in the ocular surface causes lacrimal gland degeneration with infiltration of monocytic cells, resulting in a marked reduction in tear volume (Zhang et al., 2013). Maml-mediated Notch signaling is also responsible for maintaining the conjunctival epithelial identity and goblet cell differentiation. This is achieved by augmenting the expression of Klf4/5 transcription factors to control Muc5a expression. Indeed, lacrimal gland in mice containing a Klf5 deletion in the epithelium exhibit excessive inflammation and disorganization of the lacrimal acini (Kenchegowda et al., 2011). More recently, Notch signaling has also been proposed to regulate branching morphogenesis by suppressing cleft-formation (Dvoriantchikova et al., 2017). These studies suggest that Notch signaling contributes to both the development and function of the lacrimal gland.
In summary, after the critical role of Fgf10 in lacrimal gland development was discovered less than two decades ago, it is now appreciated that FGF signaling must interact with other pathways, including BMP, Wnt and Notch, to regulate lacrimal gland budding and branching morphogenesis. Additionally, recent high throughput gene expression analysis has also implicated IGF, TGFβ and Hippo signaling in the human lacrimal gland (Aakalu et al., 2017). In spite of these important findings, our understanding of essential molecular details remains rudimentary. For example, the lacrimal gland epithelum requires an inductive signal from the periocular mesenchyme, but how the mesenchyme itself is specified and whether the signaling is reciprocal in nature is not clear. There are also many unanswered questions regarding the mechanisms of FGF signaling itself. For example, why is Fgf10 particularly potent in inducing ectopic ocular gland, while other Fgfs, such as Fgf1, lack such activity (Robinson et al., 1995; Lovicu et al., 1999; Govindarajan et al., 2000)? This can not be entirely explained by the specificity of Fgf10 for Fgfr2b, because Fgf1 is also capable of activating the same receptor. Downstream of the Fgf receptor, there are multiple intracelullar pathways, including those mediated by Ras-MAPK, PI3K-AKT and PLC-PKC. The specific roles of each, and their downstream targets, are not yet well understood, and require further research.
Transcriptional network in lacrimal gland development
While signaling pathways transduce critical guidance information for lacrimal gland morphogenesis, transcription factors are the ultimate downstream interpreters and executors of the developmental program. The paired-domain transcription factor Pax6 is considered the master regulator of eye development (Gehring and Niimi, 1999). Its expression precedes the budding of the lacrimal gland in the fornix of the conjunctival epithelium and continues in the lacrimal gland epithelium throughout development. A loss of function mutation in even a single allele of Pax6 results in severe impairment in mouse lacrimal gland development, suggesting that Pax6 serves as a competence factor in the epithelium (Makarenkova et al., 2000). In fact, detailed characterizations of Pax6 enhancers have led to the development of the Le-Cre transgene, which can act as both a Cre deletor and reporter in the lacrimal gland epithelium, greatly facilitating the genetic analysis of its development (Ashery-Padan et al., 2000; Pan et al., 2008). Surprisingly, lacrimal gland defects have not been reported in human aniridia (OMIM 106210), a congenital disorder caused by heterozygous mutations in PAX6. Instead, patients with otofaciocervical syndrome-2 carrying homozygous PAX1 mutations (OMIM 615560) display lacrimal duct abnormalities, a phenotype shared with the closely related otofaciocervical syndrome-1 (OMIM 601653) that harbors mutations in the EYA1 gene (Pohl et al., 2013). On the other hand, branchiootorenal syndrome-1 (BOR1, OMIM #113650) caused by heterozygous EYA1 mutations and branchiootic syndrome-3 (OMIM #608389) caused by SIX1 heterodeficiency display many overlapping phenotypic traits including lacrimal gland stenosis, suggesting that these two genes may act in the same genetic cascade. Indeed, Six1 is found in both the duct and acini of the mouse lacrimal gland, and Six1 knockout embryos exhibit small lacrimal glands with poor duct elongation and reduced branching (Laclef et al., 2003). From Drosophila to mammals, Pax, Six and Eya genes have been shown to form a conserved transcriptional network in organogenesis, interactions that may conceivably still occur in lacrimal gland development.
Whereas Pax/Six/Eya genes likely serve as competence factors for lacrimal gland development, additional transcription factors are required to specify the identity of the epithelium. TP63 is a transcription factor that is important for a variety of epithelial structures (Yang et al., 1999). Mutations in this gene abolish lacrimal gland development in mice, and cause Limb-mammary syndrome (OMIM 603543) associated with lacrimal-duct atresia and obstructed lacrimal puncta in humans (van Bokhoven et al., 2001). Otx1 is a homeodomain transcription factor expressed in the conjunctival epithelium and Otx1 knockout mice fail to develop lacrimal glands (Acampora et al., 1996). In addition, loss of epithelial expression of Runx1 results in a delay in embryonic lacrimal gland development characterized by reduced branching and a smaller lacrimal bud at E16.5. It is likely that Runx1 is compensated in part by Runx2 and Runx3, which are also expressed during the time of lacrimal gland development (Voronov et al., 2013).
Certain transcription factors may regulate development of both the epithelial and mesenchymal compartments of the lacrimal gland. The majority of patients carrying heterozygous SOX10 mutations (Waardenburg syndrome, OMIM 611584 and 613266) have either a hypoplastic lacrimal gland or are lacking one entirely, underscoring the requirement of SOX10 for lacrimal gland genesis (Elmaleh-Berges et al., 2013). This finding is further supported by the finding that lacrimal gland defects are present in mice harboring a conditional deletion of Sox10 in the epithelium (Chen et al., 2014b). However, since Sox10 is also expressed by migratory neural crest cells that eventually form the periocular mesenchyme, Sox10 may also indirectly regulate lacrimal gland induction by controlling neural crest migration and differentiation. Similarly, the TFAP2a mutations carried by Branchiooculofacial syndrome patients cause lacrimal duct obstruction (OMIM #113620). Given that Ap2a in the mouse is expressed in both the neural crest and surface ectoderm, we predict that human TFAP2a function may be required by both the mesenchyme and epithelium of the lacrimal gland.
Our current knowledge of lacrimal gland transcription factors is largely restricted to those that are active in the epithelium rather than in the mesenchyme. This is in part due to the fact that over 90% of the mature lacrimal gland is epithelial in origin. Mesenchymal condensation is one of the earliest events in lacrimal gland development, but its mechanism and functional significance remain poorly understood. Considering the importance of the mesenchyme in inducing the development of the lacrimal gland epithelium, more efforts should be devoted to understanding its specification and differentiation in the context of lacrimal gland development. In addition, many of the human congenital syndromes mentioned earlier affect the formation of the lacrimal puncta and canaliculi, which are structural components of the lacrimal outflow system rather than being part of the parenchymal ducts existing within the gland itself. In contrast, mouse studies have revealed far more genetic mutations directly disrupting the lacrimal gland itself. It is possible that subtle structural defects in human lacrimal glands, especially those affecting the lacrimal gland duct, are under-recognized because they are both difficult to diagnose and are obscured by the general dry eye symptoms. In contrast, obstruction of the excretory duct is more clinically apparent in its presentation. Further study of lacrimal gland abnormalities in animal models is therefore important to alert and inform clinicians to the potential sources and sites of pathology in various dry eye conditions in humans.
Understanding lacrimal gland regeneration
Various secretory glandular systems including the salivary gland (Takahashi et al., 1998; Takahashi et al., 2004), pancreas (Mansouri, 2012) and mammary gland (Shackleton et al., 2006) have at least a limited ability to self-renew after injury, a process that is mediated by tissue stem cells. Similar to these exocrine glands, the lacrimal gland has also been shown to have significant regenerative potential, raising the possibility that it may be feasible to design therapeutic strategies that take advantage of this feature. Important issues to consider in this context include the molecular cues that trigger regeneration, the signaling pathways involved in the process, the nature and origin of the stem and progenitor cells, and whether these cells can be isolated and expanded in culture.
Recent studies suggest that the mature lacrimal gland can exhibit a robust regeneration program as part of the wound healing process, displaying similar features found in lacrimal gland development. This was first observed in an injury model where the pro-inflammatory cytokine IL1 was injected into the extra-orbital lacrimal gland of adult mice (Zoukhri et al., 2002; Zoukhri et al., 2007). Within 2–3 days of injection, a population of mesenchymal cells marked by the intermediate filament nestin proliferated to repair the injured gland. Interestingly, a subset of nestin-positive cells expressed α-SMA, a myoepithelial cell marker, suggesting a common origin of the acinar and myoepithelial cells involved in the repair process (Zoukhri et al., 2008). This finding is reminiscent of lacrimal gland development wherein common epithelial progenitors give rise to both acinar and myoepithelial cells. The injury and repair process was accompanied by an initial increase and subsequent decline of BMP signaling as indicated by changes in phospho-SMAD1/5/8 levels (Zoukhri et al., 2008). There was also concomitant up-regulation of Runx genes, which, as mentioned previously, are known to regulate lacrimal gland development(Voronov et al., 2013). These studies highlight a conservation of developmental pathways during regeneration/repair.
A critical question to consider is the identity of the stem or progenitor cells responsible for lacrimal gland regeneration. Various studies have suggested that they could be of either mesenchymal, ductal, myoepithelial or acinar origin. The nestin-positive cells isolated from IL1-injected lacrimal glands can be expanded in vitro to form adipocytes with at least some of these cells expressing the mesenchymal stem cell (MSC) markers vimentin, ABCG2, and Sca1 (You et al., 2011). However, transcription factor Snail1, which is involved in the epithelial-mesenchymal transition (EMT), is also induced in the duct epithelial cells upon injury, suggesting that the duct cells may be a source of MSCs during repair (You et al., 2012). In this regard, it is interesting to note that KRT15 is present throughout the lacrimal gland epithelium during embryonic development, but its expression in the adult lacrimal gland becomes restricted to a subset of basal cells surrounding the intercalated duct, a pattern resembling the stem cell niche reported in mammary and salivary glands (Hirayama et al., 2016). On the other hand, an immature stem-like population expressing the putative stem cell markers nestin, Mushahi1 and ABCG2 has been identified in the myoepithelium from the uninjured rat lacrimal gland. These cells can be expanded in culture and differentiated into multiple lineages, suggesting a myoepithelial origin for the intrinsic progenitor cells (Shatos et al., 2012). Single cells isolated from lacrimal gland epithelial cell cultures have also been shown to possess sphere-forming capabilities characteristic of stem cells, but these cells did not express the myoepithelial marker α-SMA (Kobayashi et al., 2012). Using cell surface markers (c-kit+Epcam−CD31−CD45−Sca1−), epithelial progenitor cells have been purified by FACS and shown to form organoids that differentiate into ductal and secretory cells in a 3D culture system (Gromova et al., 2017). Finally, presumptive stem cells expressing the pluripotency markers Sox2, Nanog, and Klf4 can be isolated from the adult mouse lacrimal gland, passaged multiple times in culture, and induced to express markers of all three germ layers (Ackermann et al., 2015). In the human lacrimal gland, stem-cell like cells positive for ABCG2, ALDH and c-kit have also been reported to form spheres in non-adherent cultures.
Taken together, the emerging consensus is that the adult lacrimal gland harbors endogenous stem or progenitor cells, but their identity and location remains controversial. Because the existing studies are limited to in vitro cultures and putative stem cell markers, they may not accurately characterize lacrimal gland stem cells in vivo. We suggest that studies of lacrimal gland regeneration would benefit from the genetic approaches that have propelled the studies of lacrimal gland development. We have previously used the Le-Cre driver to trace the lineage of the Pax6-expressing cells during lacrimal gland development, showing that they specifically reside in the lacrimal gland epithelium in new born mice (Pan et al., 2008). With the increasing repertoire of inducible Cre lines, similar lineage tracing techniques should be readily applicable in resolving the location and nature of the lacrimal gland stem cells.
Current advances in regenerative therapy for the dry eye disease
Given the lack of curative treatments for dry eye disease, regenerative medicine has emerged as a promising approach to provide more permanent and sustainable treatment options. For the treatment of dry eye disease, our understanding of developmental biology, stem cell biology, and the regenerative capacity of the lacrimal gland is of critical importance for advancing this field of medicine. Several studies have already indicated that resident stem or progenitor cells in the mature lacrimal gland can turn on the same signaling pathways and transcription factors used in embryonic development to drive proliferation and differentiation in the adult gland. Currently, two main strategies are being developed for lacrimal gland repair and regeneration: i) capitalizing on the intrinsic regenerative capacity of the lacrimal gland, and ii) developing bioengineered lacrimal glands for tissue replacement. Both strategies will require a detailed knowledge and understanding of lacrimal gland development and remodeling.
Promoting the intrinsic repair and regeneration of the lacrimal gland
Despite the uncertainty regarding the stem/progenitor cells in the adult lacrimal gland, there are significant efforts to develop pharmacological approaches that promote the intrinsic repair process of the gland. It has been shown that inflammation-induced injury causes apoptosis and autophagy, which then trigger the intrinsic repair process (Zoukhri, 2010). This has led to the idea that activating apoptosis through TNF-α agonists can potentially initiate in vivo regeneration of the injured lacrimal gland. In fact, the TNF-α-induced factor TSG-6 has been identified as the main cytokine responsible for the regenerative and immunomodulatory effects of mesenchymal stem cells in different tissues (Dietrich et al., 2016). Recently, topical administration of TSG-6 has been shown to improve tear production and ameliorate ocular surface defects in a mouse model of dry eye (Lee et al., 2015a). Similarly, injection of platelet-rich activated plasma into an adjacent region of the lacrimal gland has been reported to be safe and effective in promoting tear production in Sjogren’s syndrome patients, an effect that may be due to growth factors and cytokines present in the plasma (Avila, 2014). In this regard, it is notable that exogenous FGF2 has been shown to stimulate the repair of atrophic salivary glands in rats, and administration of BMP7 can preserve renal tubular function after acute and chronic kidney injury (Okazaki et al., 2000; Simic and Vukicevic, 2005). With the increasing understanding of FGF and BMP pathways in lacrimal gland development, their ligands and agonists can potentially be exploited to initiate or accelerate repair.
In addition to drug-based therapy, direct cell transplantation has also been explored. Mesenchymal stem cells (MSCs) derived from different sources, including bone marrow, adipose tissue, and glandular organs, are multipotent, non-immunogenic, and anti-inflammatory (Dietrich et al., 2016). These properties are particularly attractive in conditions such as Sjogren’s syndrome, in which inflammation causes a significant decline in the functional capacity and regenerative potential of lacrimal gland progenitor cells (Umazume et al., 2015). There are several lines of evidence demonstrating the beneficial effects of administering MSC-like cells into the lacrimal gland. In a dry eye mouse model, periorbital injection of human MSCs led to a localized reduction in inflammatory cytokines and improved tear secretion (Lee et al., 2015b). Topical administration of MSCs in a rat model of the dry eye disease also improved tear volume and tear film stability (Beyazyildiz et al., 2014). Recently, progenitor cells of the epithelial lineage from adult mouse lacrimal glands were successfully engrafted into the ductal and acinar compartments of chronically diseased lacrimal gland in TSP−/− mice, a model of Sjogren’s syndrome. This procedure partially restored the structural integrity of the lacrimal gland and improved tear secretion (Gromova et al., 2017). These results suggest that cell-based therapy is a promising approach for treating the aqueous-deficient dry eye diseases.
Developing bioengineered lacrimal gland for transplantation
In the case of extensive lacrimal gland damage, direct transplantation of a donor or bioengineered lacrimal gland may be the only viable approach. However, when considering these forms of therapy, graft rejection and scant supply of donors are frequent issues that arise (Abouna, 2008). A recent study has evaluated the feasibility of porcine lacrimal gland as a potential xenograft candidate for transplantation, identifying significant similarities in morphology, anatomical location and connection to vascular supply. Before being a more commonly accepted route of therapy, however, key challenges, which include immune rejection and differences in tear composition, will need to be resolved (Henker et al., 2013). For a bioengineered lacrimal gland, advances in stem cell biology and biomedical engineering have made it increasingly promising to consider generating replacement tissues in vitro. Its ultimate success, however, requires advances in two essential areas- i) creating an abundant source of lacrimal gland cells with intact secretory function, and ii) a biocompatible scaffold to provide the appropriate microenvironment and structural support for proper histogenesis.
Sources of lacrimal gland cells
As a proof of principal, lacrimal gland germs have recently been reconstituted by combining mouse embryonic epithelial and mesenchymal cells in a gel matrix system (Hirayama et al., 2013). After engraftment into adult mice lacking the extra-orbital gland, these bioengineered germs grew and successfully restored lacrimation. Although these findings are highly promising, the use of embryonic tissue in a clinical setting is presently a grey area. Alternatively, the presence of adult lacrimal stem or progenitor cells may could be a feasible approach to reconstituting the lacrimal gland, if graft immune compatibility can be ensured.
The use of autologous induced pluripotent stem (IPS) cells can theoretically provide an unlimited supply of lacrimal gland cells without immunological complications. By providing appropriate growth factors and substrates that closely mimic the relevant environment during embryonic development, IPS cells have been successfully coaxed to differentiate into numerous cell types, including those constituting glandular organs such as the liver and pancreas (Pagliuca et al., 2014; Ishikawa et al., 2015; Pellegrini et al., 2015; Toyoda et al., 2015). A similar strategy to that used for human embryonic stem cells (hESCs) (Lee et al., 2007; Dincer et al., 2013; Leung et al., 2013) can be applied to IPS cells to derive lacrimal gland tissues. Based on data derived from the study of lacrimal gland development, we suggest that IPS cells can be induced to differentiate into 1) ocular ectodermal cells that will form precursors of the lacrimal gland epithelium, and 2) neural crest cells that will form the periocular lacrimal gland mesenchyme. Signaling molecules such as FGF10 and BMP7 can be used to promote branching morphogenesis of the epithelial cells and condensation of the mesenchymal cells, respectively. These processes can be monitored by the expression of PAX6 and OTX1 for the epithelium, and FOXC1 for the mesenchyme. Alternatively, direct conversion of the IPS cells could potentially be achieved by the forced expression of transcription factors that play important roles in lacrimal gland development. Indeed, it has been recently reported that overexpression of PAX6, FOXC1 and SIX1 induce hESCs to take on morphological and gene expression profiles that resemble the lacrimal gland epithelium (Hirayama et al., 2017). Although many technical hurdles remain, this study suggests that IPS cells may ultimately be programmable to differentiate into lacrimal gland cells.
The next challenge in establishing a reliable source of lacrimal gland cells is to expand them in culture and validate their cellular characteristics. Studies on primary acinar cells have shown that serum-free medium that contains an appropriate mixture of hormonal and growth factor supplements can preserve the cells’ differentiated morphology and secretory capacity in the presence of matrigel. Nonetheless, it should be noted that reduced cell proliferation was observed (Oliver et al., 1987; Hann et al., 1989; Hann et al., 1991). To optimize culture conditions, lacrimal gland epithelial cells from newborn mice were tested for growth factor responsiveness. Both epidermal growth factor (EGF) and hepatocyte growth factor (HGF) were found to promote cell viability and proliferation. Counter-intuitively, however, FGF10 failed to produce any effect (Ueda et al., 2009). Another study, using rabbit lacrimal gland, showed that a combination of EGF, dihydrotestosterone (DHT) and matrigel was able to induce massive proliferation of acinar cells in serum-free Hepato Stim™ Medium (HSM) (Schonthal et al., 2000). These studies highlight the importance of medium formulations and substrate conditions for expanding and preserving functional lacrimal gland cells.
Building the functional lacrimal gland
To produce lacrimal gland germ for surgical implantation, lacrimal gland cells need to be seeded on an appropriate substrate that supports their structure and function. Human amniotic membranes have been shown to promote rabbit lacrimal gland cells to form acini-like structures, but their secretory response diminished over time, possibly as a result of structural impairments contained within the membranes (Schrader et al., 2007). Studies have also described the reconstitution of mouse, rat, rabbit and human lacrimal gland epithelial cells on Matrigel to form cell aggregates, displaying the morphology and secretory function characteristic of lacrimal gland acini (Meneray et al., 1994; Yoshino et al., 1995; Vanaken et al., 1998; Schechter et al., 2002; Ueda et al., 2009; Tiwari et al., 2012). However, because it is derived from mouse sarcoma tissue, there are obvious safety concerns associated with the use of Matrigel in a clinical setting (Kibbey, 1994). Several polymer-based substrates have also been tested or their acting as a scaffold in lacrimal gland morphogenesis with varying degrees of success being achieved (Long et al., 2006; Selvam et al., 2007a; Selvam et al., 2007b; Selvam et al., 2009). Long-term functional studies will be needed to evaluate the efficacy and stability of the engineered tissues.
Human lacrimal gland is organized into a three dimensional structure that is well adapted for its secretory function. To more closely mimic this configuration, lacrimal gland acinar cells from rabbit have been cultured in a microgravity bioreactor to form lacrimal spheroids. However, following a two week period, continuous cell death was frequently displayed (Schrader et al., 2009). The same group recently described a non-immunogenic decellularized tissue matrix derived from pig lacrimal gland, which allows lacrimal gland epithelial cells seeded on this scaffold to maintain acinar-like structures and secretory ability for up to 30 days (Spaniol et al., 2015). In a similar effort, rabbit lacrimal gland progenitor cells derived from a sphere-forming culture have been shown to differentiate and display secretory function in either a decellularized lacrimal gland matrix or 3-D collagen gel (Lin et al., 2016). There are still challenges regarding optimization of the decellularization technique, the seeding process, the long-term culturing conditions, and functional validation. It is notable, however, that a lacrimal outflow duct constructed using acellular bovine dermal matrix has been successfully transplanted into patients to relieve obstructive signs and symptoms (Chen et al., 2014a). Through providing effective mechanical strength, the appropriate micro-environment for lacrimal gland cells, and the necessary structural support for vascular, nerve and ductal supply, the decellularized matrix promises to be an ideal scaffold for bioengineered lacrimal gland.
Summary and future research
Aqueous-deficient dry eye disease is a major health challenge that lacks effective treatment. Although lacrimal gland transplantation is a potentially promising course of treatment, it is plagued by immunological complications and donor shortage. Development of fully functional bioengineered lacrimal glands in vitro is a viable approach to repair damaged lacrimal glands, while stimulation of the intrinsic regenerative potential of the lacrimal gland also holds great promise. Both therapeutic strategies require a deeper understanding of development and regeneration of the gland. From the development perspective, we still have several unanswered questions: 1) how do the common epithelial progenitor cells gives rise to the acinar, ductal and myoepithelial cells, 2) how is the lacrimal gland mesenchyme specified from the neural crest cells, and 3) what factors are required for adult lacrimal gland homeostasis? Additional work is also needed to reveal the signaling cascades that underlie gland morphogenesis, and the transcriptional network that ultimately determines the tissue identity and cell lineage for repair and regeneration. Those studies will pave the way to realizing the full potential of regenerative medicine to treat dry eye disease.
Table 1.
Transcription factors implicated during lacrimal gland development and maintenance.
| Genes | Functional relevance during Lacrimal gland development | |
|---|---|---|
|
| ||
| Mice | Phenotype in humans | |
| Pax6 | Budding, competence factor | Not reported |
| Pax1 | Not studied | Lacrimal duct stenosis |
| Eya1 | Not studied | Lacrimal gland aplasia, duct stenosis |
| Six1 | Branching morphogenesis | Lacrimal duct stenosis |
| Otx1 | Budding | Not reported |
| p63 | Budding | Lacrimal-duct atresia, obstructed lacrimal puncta |
| Runx1–3 | Branching morphogenesis | Not reported |
| Klf5 | Preservation of glandular function during post-natal stages | Not reported |
| Sox10 | Growth and branching morphogenesis | Hypo-plastic or no lacrimal gland |
| Ap2α | Not studied | Lacrimal duct obstruction |
Key Findings.
Abnormalities of the lacrimal gland underlie aqueous-deficient dry eye disease.
FGF signaling is the key regulator of lacrimal gland morphogenesis.
The adult lacrimal gland harbors intrinsic stem or progenitor cells.
Developmental mechanisms can guide efforts in lacrimal gland regeneration.
Acknowledgments
The work was supported by NIH grant EY018868 to XZ. XZ is supported by Jules and Doris Stein Research to Prevent Blindness Professorship. AG is a recipient of STARR fellowship
The authors thank Dr. Steven Brooks and Michael Bouaziz for critical reading of the manuscript and members of the Zhang lab for discussions. The work was supported by NIH grant EY018868 to XZ. XZ is supported by Jules and Doris Stein Research to Prevent Blindness Professorship. AG is a recipient of STARR fellowship.
References
- Aakalu VK, Parameswaran S, Maienschein-Cline M, Bahroos N, Shah D, Ali M, Krishnakumar S. Human Lacrimal Gland Gene Expression. PLoS One. 2017;12:e0169346. doi: 10.1371/journal.pone.0169346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abouna GM. Organ shortage crisis: problems and possible solutions. Transplant Proc. 2008;40:34–38. doi: 10.1016/j.transproceed.2007.11.067. [DOI] [PubMed] [Google Scholar]
- Acampora D, Mazan S, Avantaggiato V, Barone P, Tuorto F, Lallemand Y, Brulet P, Simeone A. Epilepsy and brain abnormalities in mice lacking the Otx1 gene. Nat Genet. 1996;14:218–222. doi: 10.1038/ng1096-218. [DOI] [PubMed] [Google Scholar]
- Ackermann P, Hetz S, Dieckow J, Schicht M, Richter A, Kruse C, Schroeder IS, Jung M, Paulsen FP. Isolation and Investigation of Presumptive Murine Lacrimal Gland Stem Cells. Invest Ophthalmol Vis Sci. 2015;56:4350–4363. doi: 10.1167/iovs.15-16475. [DOI] [PubMed] [Google Scholar]
- Allansmith MR, Kajiyama G, Abelson MB, Simon MA. Plasma cell content of main and accessory lacrimal glands and conjunctiva. Am J Ophthalmol. 1976;82:819–826. doi: 10.1016/0002-9394(76)90056-8. [DOI] [PubMed] [Google Scholar]
- Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000;14:2701–2711. doi: 10.1101/gad.184000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila MY. Restoration of human lacrimal function following platelet-rich plasma injection. Cornea. 2014;33:18–21. doi: 10.1097/ICO.0000000000000016. [DOI] [PubMed] [Google Scholar]
- Babic GS, Zlatanovic G, Jocic JD, Cekic S, Vujanovic M. Therapeutical approach to dry eye syndrome. Med Pregl. 2010;63:793–800. doi: 10.2298/mpns1012793s. [DOI] [PubMed] [Google Scholar]
- Balasubramanian R, Zhang X. Mechanisms of FGF gradient formation during embryogenesis. Semin Cell Dev Biol. 2016;53:94–100. doi: 10.1016/j.semcdb.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyazyildiz E, Pinarli FA, Beyazyildiz O, Hekimoglu ER, Acar U, Demir MN, Albayrak A, Kaymaz F, Sobaci G, Delibasi T. Efficacy of topical mesenchymal stem cell therapy in the treatment of experimental dry eye syndrome model. Stem Cells Int. 2014;2014:250230. doi: 10.1155/2014/250230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Gong B, Wu Z, Jetton J, Chen R, Qu C. A new method using xenogeneicacellular dermal matrix in the reconstruction of lacrimal drainage. Br J Ophthalmol. 2014a;98:1583–1587. doi: 10.1136/bjophthalmol-2014-304932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Huang J, Liu Y, Dattilo LK, Huh SH, Ornitz D, Beebe DC. FGF signaling activates a Sox9-Sox10 pathway for the formation and branching morphogenesis of mouse ocular glands. Development. 2014b;141:2691–2701. doi: 10.1242/dev.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009;28:155–177. doi: 10.1016/j.preteyeres.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cuadra-Blanco C, Peces-Pena MD, Merida-Velasco JR. Morphogenesis of the human lacrimal gland. J Anat. 2003;203:531–536. doi: 10.1046/j.1469-7580.2003.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C, Ito M, Makarenkova HP, Faber SC, Lang RA. Bmp7 regulates branching morphogenesis of the lacrimal gland by promoting mesenchymal proliferation and condensation. Development. 2004;131:4155–4165. doi: 10.1242/dev.01285. [DOI] [PubMed] [Google Scholar]
- Dean CH, Miller LA, Smith AN, Dufort D, Lang RA, Niswander LA. Canonical Wnt signaling negatively regulates branching morphogenesis of the lung and lacrimal gland. Dev Biol. 2005;286:270–286. doi: 10.1016/j.ydbio.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Dietrich J, Massie I, Roth M, Geerling G, Mertsch S, Schrader S. Development of Causative Treatment Strategies for Lacrimal Gland Insufficiency by Tissue Engineering and Cell Therapy. Part 1: Regeneration of Lacrimal Gland Tissue: Can We Stimulate Lacrimal Gland Renewal In Vivo? Curr Eye Res. 2016;41:1131–1142. doi: 10.3109/02713683.2016.1148741. [DOI] [PubMed] [Google Scholar]
- Dincer Z, Piao J, Niu L, Ganat Y, Kriks S, Zimmer B, Shi SH, Tabar V, Studer L. Specification of functional cranial placode derivatives from human pluripotent stem cells. Cell Rep. 2013;5:1387–1402. doi: 10.1016/j.celrep.2013.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoriantchikova G, Tao W, Pappas S, Gaidosh G, Tse DT, Ivanov D, Pelaez D. Molecular Profiling of the Developing Lacrimal Gland Reveals Putative Role of Notch Signaling in Branching Morphogenesis. Invest Ophthalmol Vis Sci. 2017;58:1098–1109. doi: 10.1167/iovs.16-20315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmaleh-Berges M, Baumann C, Noel-Petroff N, Sekkal A, Couloigner V, Devriendt K, Wilson M, Marlin S, Sebag G, Pingault V. Spectrum of temporal bone abnormalities in patients with Waardenburg syndrome and SOX10 mutations. AJNR Am J Neuroradiol. 2013;34:1257–1263. doi: 10.3174/ajnr.A3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entesarian M, Matsson H, Klar J, Bergendal B, Olson L, Arakaki R, Hayashi Y, Ohuchi H, Falahat B, Bolstad AI, Jonsson R, Wahren-Herlenius M, Dahl N. Mutations in the gene encoding fibroblast growth factor 10 are associated with aplasia of lacrimal and salivary glands. Nat Genet. 2005;37:125–127. doi: 10.1038/ng1507. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Niimi T. Direct regulatory interaction of the eyeless protein with an eye-specific enhancer in sine oculis gene during eye induction in Drosophila. dev. 1999;126:2253. doi: 10.1242/dev.126.10.2253. [DOI] [PubMed] [Google Scholar]
- Govindarajan V, Ito M, Makarenkova HP, Lang RA, Overbeek PA. Endogenous and ectopic gland induction by FGF-10. Dev Biol. 2000;225:188–200. doi: 10.1006/dbio.2000.9812. [DOI] [PubMed] [Google Scholar]
- Gromova A, Voronov DA, Yoshida M, Thotakura S, Meech R, Dartt DA, Makarenkova HP. Lacrimal Gland Repair Using Progenitor Cells. Stem Cells Transl Med. 2017;6:88–98. doi: 10.5966/sctm.2016-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajihosseini MK, Wilson S, De Moerlooze L, Dickson C. A splicing switch and gain-of-function mutation in FgfR2-IIIc hemizygotes causes Apert/Pfeiffer-syndrome-like phenotypes. Proc Natl Acad Sci U S A. 2001;98:3855–3860. doi: 10.1073/pnas.071586898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann LE, Kelleher RS, Sullivan DA. Influence of culture conditions on the androgen control of secretory component production by acinar cells from the rat lacrimal gland. Invest Ophthalmol Vis Sci. 1991;32:2610–2621. [PubMed] [Google Scholar]
- Hann LE, Tatro JB, Sullivan DA. Morphology and function of lacrimal gland acinar cells in primary culture. Invest Ophthalmol Vis Sci. 1989;30:145–158. [PubMed] [Google Scholar]
- He J, Ding Y, Feng M, Guo J, Sun X, Zhao J, Yu D, Li Z. Characteristics of Sjogren’s syndrome in rheumatoid arthritis. Rheumatology (Oxford) 2013;52:1084–1089. doi: 10.1093/rheumatology/kes374. [DOI] [PubMed] [Google Scholar]
- Henker R, Scholz M, Gaffling S, Asano N, Hampel U, Garreis F, Hornegger J, Paulsen F. Morphological features of the porcine lacrimal gland and its compatibility for human lacrimal gland xenografting. PLoS One. 2013;8:e74046. doi: 10.1371/journal.pone.0074046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama M, Ko SBH, Kawakita T, Akiyama T, SK, Goparaju AYNM, Sakota N, Chikazawa-Nohtomi S, Shimmura K, Tsubota Ko MSH. Identification of transcription factors that promote the differentiation of human pluripotent stem cells into lacrimal gland epithelium-like cells. Aging and Mechanisms of Disease. 2017:1. doi: 10.1038/s41514-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama M, Liu Y, Kawakita T, Shimmura S, Tsubota K. Cytokeratin expression in mouse lacrimal gland germ epithelium. Exp Eye Res. 2016;146:54–59. doi: 10.1016/j.exer.2015.11.020. [DOI] [PubMed] [Google Scholar]
- Hirayama M, Ogawa M, Oshima M, Sekine Y, Ishida K, Yamashita K, Ikeda K, Shimmura S, Kawakita T, Tsubota K, Tsuji T. Functional lacrimal gland regeneration by transplantation of a bioengineered organ germ. Nat Commun. 2013;4:2497. doi: 10.1038/ncomms3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly FJ, Lemp MA. Tear physiology and dry eyes. Surv Ophthalmol. 1977;22:69–87. doi: 10.1016/0039-6257(77)90087-x. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Kobayashi M, Yanagi S, Kato C, Takashima R, Kobayashi E, Hagiwara K, Ochiya T. Human induced hepatic lineage-oriented stem cells: autonomous specification of human iPS cells toward hepatocyte-like cells without any exogenous differentiation factors. PLoS One. 2015;10:e0123193. doi: 10.1371/journal.pone.0123193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadi MA, Feizi S. Dry eye syndrome. J Ophthalmic Vis Res. 2011;6:192–198. [PMC free article] [PubMed] [Google Scholar]
- Johnson ME, Murphy PJ. Changes in the tear film and ocular surface from dry eye syndrome. Prog Retin Eye Res. 2004;23:449–474. doi: 10.1016/j.preteyeres.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Kenchegowda D, Swamynathan S, Gupta D, Wan H, Whitsett J, Swamynathan SK. Conditional disruption of mouse Klf5 results in defective eyelids with malformed meibomian glands, abnormal cornea and loss of conjunctival goblet cells. Dev Biol. 2011;356:5–18. doi: 10.1016/j.ydbio.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibbey MC. Maintenance of the EHS sarcoma and Matrigel preparation. Journal of Tissue Culture methods. 1994;16:227. [Google Scholar]
- Kobayashi S, Kawakita T, Kawashima M, Okada N, Mishima K, Saito I, Ito M, Shimmura S, Tsubota K. Characterization of cultivated murine lacrimal gland epithelial cells. Mol Vis. 2012;18:1271–1277. [PMC free article] [PubMed] [Google Scholar]
- Laclef C, Souil E, Demignon J, Maire P. Thymus, kidney and craniofacial abnormalities in Six 1 deficient mice. Mech Dev. 2003;120:669–679. doi: 10.1016/s0925-4773(03)00065-0. [DOI] [PubMed] [Google Scholar]
- Lee G, Kim H, Elkabetz Y, Al Shamy G, Panagiotakos G, Barberi T, Tabar V, Studer L. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol. 2007;25:1468–1475. doi: 10.1038/nbt1365. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Kim DH, Ryu JS, Ko AY, Ko JH, Kim MK, Wee WR, Khwarg SI, Oh JY. Topical TSG-6 Administration Protects the Ocular Surface in Two Mouse Models of Inflammation-Related Dry Eye. Invest Ophthalmol Vis Sci. 2015a;56:5175–5181. doi: 10.1167/iovs.14-16307. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Ko AY, Ko JH, Lee HJ, Kim MK, Wee WR, Khwarg SI, Oh JY. Mesenchymal stem/stromal cells protect the ocular surface by suppressing inflammation in an experimental dry eye. Mol Ther. 2015b;23:139–146. doi: 10.1038/mt.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AW, Kent Morest D, Li JY. Differential BMP signaling controls formation and differentiation of multipotent preplacodal ectoderm progenitors from human embryonic stem cells. Dev Biol. 2013;379:208–220. doi: 10.1016/j.ydbio.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Sun G, He H, Botsford B, Li M, Elisseeff JH, Yiu SC. Three-Dimensional Culture of Functional Adult Rabbit Lacrimal Gland Epithelial Cells on Decellularized Scaffold. Tissue Eng Part A. 2016;22:65–74. doi: 10.1089/ten.TEA.2015.0286. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lin D. Necessity of Smad4 for the normal development of the mouse lacrimal gland. Jpn J Ophthalmol. 2014;58:298–306. doi: 10.1007/s10384-014-0307-7. [DOI] [PubMed] [Google Scholar]
- Long L, Liu Z, Wang T, Deng X, Yang K, Li L, Zhao C. Polyethersulfone dead-end tube as a scaffold for artificial lacrimal glands in vitro. J Biomed Mater Res B Appl Biomater. 2006;78:409–416. doi: 10.1002/jbm.b.30502. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, Kao WW, Overbeek PA. Ectopic gland induction by lens-specific expression of keratinocyte growth factor (FGF-7) in transgenic mice. Mech Dev. 1999;88:43–53. doi: 10.1016/s0925-4773(99)00169-0. [DOI] [PubMed] [Google Scholar]
- Makarenkova HP, Hoffman MP, Beenken A, Eliseenkova AV, Meech R, Tsau C, Patel VN, Lang RA, Mohammadi M. Differential interactions of FGFs with heparan sulfate control gradient formation and branching morphogenesis. Sci Signal. 2009;2:ra55. doi: 10.1126/scisignal.2000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarenkova HP, Ito M, Govindarajan V, Faber SC, Sun L, McMahon G, Overbeek PA, Lang RA. FGF10 is an inducer and Pax6 a competence factor for lacrimal gland development. Development. 2000;127:2563–2572. doi: 10.1242/dev.127.12.2563. [DOI] [PubMed] [Google Scholar]
- Mansouri A. Development and regeneration in the endocrine pancreas. ISRN Endocrinol. 2012;2012:640956. doi: 10.5402/2012/640956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiske D, Sommer P, Kidson SH, Hogan BL. The role of the forkhead transcription factor, Foxc1, in the development of the mouse lacrimal gland. Dev Dyn. 2006;235:1074–1080. doi: 10.1002/dvdy.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneray MA, Fields TY, Bromberg BB, Moses RL. Morphology and physiologic responsiveness of cultured rabbit lacrimal acini. Invest Ophthalmol Vis Sci. 1994;35:4144–4158. [PubMed] [Google Scholar]
- Mikolajczak M, Goodman T, Hajihosseini MK. Interrogation of a Lacrimo-auriculo-dento-digital syndrome protein reveals novel modes of Fibroblast growth factor 10 (FGF10) function. Biochem J. 2016 doi: 10.1042/BCJ20160441. [DOI] [PubMed] [Google Scholar]
- Mircheff AK. Lacrimal fluid and electrolyte secretion: a review. Curr Eye Res. 1989;8:607–617. doi: 10.3109/02713688908995761. [DOI] [PubMed] [Google Scholar]
- Okazaki Y, Kagami H, Hattori T, Hishida S, Shigetomi T, Ueda M. Acceleration of rat salivary gland tissue repair by basic fibroblast growth factor. Arch Oral Biol. 2000;45:911–919. doi: 10.1016/s0003-9969(00)00035-2. [DOI] [PubMed] [Google Scholar]
- Oliver C, Waters JF, Tolbert CL, Kleinman HK. Growth of exocrine acinar cells on a reconstituted basement membrane gel. In Vitro Cell Dev Biol. 1987;23:465–473. doi: 10.1007/BF02628416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliuca FW, Millman JR, Gurtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA. Generation of functional human pancreatic beta cells in vitro. Cell. 2014;159:428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Carbe C, Powers A, Feng GS, Zhang X. Sprouty2-modulated Kras signaling rescues Shp2 deficiency during lens and lacrimal gland development. Development. 2010;137:1085–1093. doi: 10.1242/dev.042820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Carbe C, Powers A, Zhang EE, Esko JD, Grobe K, Feng GS, Zhang X. Bud specific N-sulfation of heparan sulfate regulates Shp2-dependent FGF signaling during lacrimal gland induction. Development. 2008;135:301–310. doi: 10.1242/dev.014829. [DOI] [PubMed] [Google Scholar]
- Park YS, Gauna AE, Cha S. Mouse Models of Primary Sjogren’s Syndrome. Curr Pharm Des. 2015;21:2350–2364. doi: 10.2174/1381612821666150316120024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini S, Ungaro F, Mercalli A, Melzi R, Sebastiani G, Dotta F, Broccoli V, Piemonti L, Sordi V. Human induced pluripotent stem cells differentiate into insulin-producing cells able to engraft in vivo. Acta Diabetol. 2015;52:1025–1035. doi: 10.1007/s00592-015-0726-z. [DOI] [PubMed] [Google Scholar]
- Pohl E, Aykut A, Beleggia F, Karaca E, Durmaz B, Keupp K, Arslan E, Palamar M, Yigit G, Ozkinay F, Wollnik B. A hypofunctional PAX1 mutation causes autosomal recessively inherited otofaciocervical syndrome. Hum Genet. 2013;132:1311–1320. doi: 10.1007/s00439-013-1337-9. [DOI] [PubMed] [Google Scholar]
- Qu X, Carbe C, Tao C, Powers A, Lawrence R, van Kuppevelt TH, Cardoso WV, Grobe K, Esko JD, Zhang X. Lacrimal Gland Development and Fgf10-Fgfr2b Signaling Are Controlled by 2-O- and 6-O-sulfated Heparan Sulfate. J Biol Chem. 2011;286:14435–14444. doi: 10.1074/jbc.M111.225003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Pan Y, Carbe C, Powers A, Grobe K, Zhang X. Glycosaminoglycan-dependent restriction of FGF diffusion is necessary for lacrimal gland development. Development. 2012;139:2730–2739. doi: 10.1242/dev.079236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ML, Overbeek PA, Verran DJ, Grizzle WE, Stockard CR, Friesel R, Maciag T, Thompson JA. Extracellular FGF-1 acts as a lens differentiation factor in transgenic mice. Development. 1995;121:505–514. doi: 10.1242/dev.121.2.505. [DOI] [PubMed] [Google Scholar]
- Rocha EM, Alves M, Rios JD, Dartt DA. The aging lacrimal gland: changes in structure and function. Ocul Surf. 2008;6:162–174. doi: 10.1016/s1542-0124(12)70177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohmann E, Brunner HG, Kayserili H, Uyguner O, Nurnberg G, Lew ED, Dobbie A, Eswarakumar VP, Uzumcu A, Ulubil-Emeroglu M, Leroy JG, Li Y, Becker C, Lehnerdt K, Cremers CW, Yuksel-Apak M, Nurnberg P, Kubisch C, Schlessinger J, van Bokhoven H, Wollnik B. Mutations in different components of FGF signaling in LADD syndrome. Nat Genet. 2006;38:414–417. doi: 10.1038/ng1757. [DOI] [PubMed] [Google Scholar]
- Schechter J, Stevenson D, Chang D, Chang N, Pidgeon M, Nakamura T, Okamoto CT, Trousdale MD, Mircheff AK. Growth of purified lacrimal acinar cells in Matrigel raft cultures. Exp Eye Res. 2002;74:349–360. doi: 10.1006/exer.2001.1158. [DOI] [PubMed] [Google Scholar]
- Schonthal AH, Warren DW, Stevenson D, Schecter JE, Azzarolo AM, Mircheff AK, Trousdale MD. Proliferation of lacrimal gland acinar cells in primary culture. Stimulation by extracellular matrix, EGF, and DHT. Exp Eye Res. 2000;70:639–649. doi: 10.1006/exer.2000.0824. [DOI] [PubMed] [Google Scholar]
- Schrader S, Kremling C, Klinger M, Laqua H, Geerling G. Cultivation of lacrimal gland acinar cells in a microgravity environment. Br J Ophthalmol. 2009;93:1121–1125. doi: 10.1136/bjo.2008.137927. [DOI] [PubMed] [Google Scholar]
- Schrader S, Wedel T, Kremling C, Laqua H, Geerling G. Amniotic membrane as a carrier for lacrimal gland acinar cells. Graefes Arch Clin Exp Ophthalmol. 2007;245:1699–1704. doi: 10.1007/s00417-007-0612-7. [DOI] [PubMed] [Google Scholar]
- Selvam S, Chang WV, Nakamura T, Samant DM, Thomas PB, Trousdale MD, Mircheff AK, Schechter JE, Yiu SC. Microporous poly(L-lactic acid) membranes fabricated by polyethylene glycol solvent-cast/particulate leaching technique. Tissue Eng Part C Methods. 2009;15:463–474. doi: 10.1089/ten.tec.2008.0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvam S, Thomas PB, Gukasyan HJ, Yu AS, Stevenson D, Trousdale MD, Mircheff AK, Schechter JE, Smith RE, Yiu SC. Transepithelial bioelectrical properties of rabbit acinar cell monolayers on polyester membrane scaffolds. Am J Physiol Cell Physiol. 2007a;293:C1412–1419. doi: 10.1152/ajpcell.00200.2007. [DOI] [PubMed] [Google Scholar]
- Selvam S, Thomas PB, Trousdale MD, Stevenson D, Schechter JE, Mircheff AK, Jacob JT, Smith RE, Yiu SC. Tissue-engineered tear secretory system: functional lacrimal gland acinar cells cultured on matrix protein-coated substrata. J Biomed Mater Res B Appl Biomater. 2007b;80:192–200. doi: 10.1002/jbm.b.30584. [DOI] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- Shams I, Rohmann E, Eswarakumar VP, Lew ED, Yuzawa S, Wollnik B, Schlessinger J, Lax I. Lacrimo-auriculo-dento-digital syndrome is caused by reduced activity of the fibroblast growth factor 10 (FGF10)-FGF receptor 2 signaling pathway. Mol Cell Biol. 2007;27:6903–6912. doi: 10.1128/MCB.00544-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatos MA, Haugaard-Kedstrom L, Hodges RR, Dartt DA. Isolation and characterization of progenitor cells in uninjured, adult rat lacrimal gland. Invest Ophthalmol Vis Sci. 2012;53:2749–2759. doi: 10.1167/iovs.11-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simic P, Vukicevic S. Bone morphogenetic proteins in development and homeostasis of kidney. Cytokine Growth Factor Rev. 2005;16:299–308. doi: 10.1016/j.cytogfr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Spaniol K, Metzger M, Roth M, Greve B, Mertsch S, Geerling G, Schrader S. Engineering of a Secretory Active Three-Dimensional Lacrimal Gland Construct on the Basis of Decellularized Lacrimal Gland Tissue. Tissue Eng Part A. 2015;21:2605–2617. doi: 10.1089/ten.TEA.2014.0694. [DOI] [PubMed] [Google Scholar]
- Swindell EC, Liu C, Shah R, Smith AN, Lang RA, Jamrich M. Eye formation in the absence of retina. Dev Biol. 2008;322:56–64. doi: 10.1016/j.ydbio.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Schoch E, Walker NI. Origin of acinar cell regeneration after atrophy of the rat parotid induced by duct obstruction. Int J Exp Pathol. 1998;79:293–301. doi: 10.1046/j.1365-2613.1998.710405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Shinzato K, Domon T, Yamamoto T, Wakita M. Mitotic proliferation of myoepithelial cells during regeneration of atrophied rat submandibular glands after duct ligation. J Oral Pathol Med. 2004;33:430–434. doi: 10.1111/j.1600-0714.2004.00234.x. [DOI] [PubMed] [Google Scholar]
- Tiwari S, Ali MJ, Balla MM, Naik MN, Honavar SG, Reddy VA, Vemuganti GK. Establishing human lacrimal gland cultures with secretory function. PLoS One. 2012;7:e29458. doi: 10.1371/journal.pone.0029458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda T, Mae S, Tanaka H, Kondo Y, Funato M, Hosokawa Y, Sudo T, Kawaguchi Y, Osafune K. Cell aggregation optimizes the differentiation of human ESCs and iPSCs into pancreatic bud-like progenitor cells. Stem Cell Res. 2015;14:185–197. doi: 10.1016/j.scr.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Tsau C, Ito M, Gromova A, Hoffman MP, Meech R, Makarenkova HP. Barx2 and Fgf10 regulate ocular glands branching morphogenesis by controlling extracellular matrix remodeling. Development. 2011;138:3307–3317. doi: 10.1242/dev.066241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Karasawa Y, Satoh Y, Nishikawa S, Imaki J, Ito M. Purification and characterization of mouse lacrimal gland epithelial cells and reconstruction of an acinarlike structure in three-dimensional culture. Invest Ophthalmol Vis Sci. 2009;50:1978–1987. doi: 10.1167/iovs.08-2503. [DOI] [PubMed] [Google Scholar]
- Umazume T, Thomas WM, Campbell S, Aluri H, Thotakura S, Zoukhri D, Makarenkova HP. Lacrimal Gland Inflammation Deregulates Extracellular Matrix Remodeling and Alters Molecular Signature of Epithelial Stem/Progenitor Cells. Invest Ophthalmol Vis Sci. 2015;56:8392–8402. doi: 10.1167/iovs.15-17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bokhoven H, Hamel BC, Bamshad M, Sangiorgi E, Gurrieri F, Duijf PH, Vanmolkot KR, van Beusekom E, van Beersum SE, Celli J, Merkx GF, Tenconi R, Fryns JP, Verloes A, Newbury-Ecob RA, Raas-Rotschild A, Majewski F, Beemer FA, Janecke A, Chitayat D, Crisponi G, Kayserili H, Yates JR, Neri G, Brunner HG. p63 Gene mutations in eec syndrome, limb-mammary syndrome, and isolated split hand-split foot malformation suggest a genotype-phenotype correlation. Am J Hum Genet. 2001;69:481–492. doi: 10.1086/323123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanaken H, Vercaeren I, Claessens F, De Vos R, Dewolf-Peeters C, Vaerman JP, Heyns W, Rombauts W, Peeters B. Primary rat lacrimal cells undergo acinar-like morphogenesis on reconstituted basement membrane and express secretory component under androgen stimulation. Exp Cell Res. 1998;238:377–388. doi: 10.1006/excr.1997.3856. [DOI] [PubMed] [Google Scholar]
- Volckaert T, Campbell A, Dill E, Li C, Minoo P, De Langhe S. Localized Fgf10 expression is not required for lung branching morphogenesis but prevents differentiation of epithelial progenitors. Development. 2013;140:3731–3742. doi: 10.1242/dev.096560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronov D, Gromova A, Liu D, Zoukhri D, Medvinsky A, Meech R, Makarenkova HP. Transcription factors Runx1 to 3 are expressed in the lacrimal gland epithelium and are involved in regulation of gland morphogenesis and regeneration. Invest Ophthalmol Vis Sci. 2013;54:3115–3125. doi: 10.1167/iovs.13-11791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcott B. The Lacrimal Gland and Its Veil of Tears. News Physiol Sci. 1998;13:97–103. doi: 10.1152/physiologyonline.1998.13.2.97. [DOI] [PubMed] [Google Scholar]
- Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C, McKeon F. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- Yoshino K, Tseng SC, Pflugfelder SC. Substrate modulation of morphology, growth, and tear protein production by cultured human lacrimal gland epithelial cells. Exp Cell Res. 1995;220:138–151. doi: 10.1006/excr.1995.1300. [DOI] [PubMed] [Google Scholar]
- You S, Avidan O, Tariq A, Ahluwalia I, Stark PC, Kublin CL, Zoukhri D. Role of epithelial-mesenchymal transition in repair of the lacrimal gland after experimentally induced injury. Invest Ophthalmol Vis Sci. 2012;53:126–135. doi: 10.1167/iovs.11-7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You S, Kublin CL, Avidan O, Miyasaki D, Zoukhri D. Isolation and propagation of mesenchymal stem cells from the lacrimal gland. Invest Ophthalmol Vis Sci. 2011;52:2087–2094. doi: 10.1167/iovs.10-5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lam O, Nguyen MT, Ng G, Pear WS, Ai W, Wang IJ, Kao WW, Liu CY. Mastermind-like transcriptional co-activator-mediated Notch signaling is indispensable for maintaining conjunctival epithelial identity. Development. 2013;140:594–605. doi: 10.1242/dev.082842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoukhri D. Mechanisms involved in injury and repair of the murine lacrimal gland: role of programmed cell death and mesenchymal stem cells. Ocul Surf. 2010;8:60–69. doi: 10.1016/s1542-0124(12)70070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoukhri D, Fix A, Alroy J, Kublin CL. Mechanisms of murine lacrimal gland repair after experimentally induced inflammation. Invest Ophthalmol Vis Sci. 2008;49:4399–4406. doi: 10.1167/iovs.08-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoukhri D, Hodges RR, Byon D, Kublin CL. Role of proinflammatory cytokines in the impaired lacrimation associated with autoimmune xerophthalmia. Invest Ophthalmol Vis Sci. 2002;43:1429–1436. [PMC free article] [PubMed] [Google Scholar]
- Zoukhri D, Macari E, Kublin CL. A single injection of interleukin-1 induces reversible aqueous-tear deficiency, lacrimal gland inflammation, and acinar and ductal cell proliferation. Exp Eye Res. 2007;84:894–904. doi: 10.1016/j.exer.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]