Abstract
In the last few years immunotherapy has become an important cancer treatment modality and while the principles of immunotherapy evolved over many decades, the FDA approvals of sipuleucel-T and ipilimumab began a new wave in immuno-oncology. Despite the current enthusiasm, it is unlikely that any of the immunotherapeutics alone can dramatically change prostate cancer outcomes, but combination strategies are more promising and provide a reason for optimism. Several completed and ongoing studies have shown that the combination of cancer vaccines or checkpoint inhibitors with different immunotherapeutic agents, hormonal therapy (enzalutamide), radiation therapy (radium 223), DNA-damaging agents (olaparib), or chemotherapy (docetaxel) can enhance immune responses and induce more dramatic, long-lasting clinical responses without significant toxicity. The goal of prostate cancer immunotherapy does not have to be complete eradication of advanced disease, but rather the return to an immunologic equilibrium with an indolent disease state. In addition to determining the optimal combination of treatment regimens, efforts are also ongoing to discover biomarkers of immune response. With such concerted efforts, the future of immunotherapy in prostate cancer looks brighter than ever.
Keywords: Prostate cancer, cancer vaccine, checkpoint inhibitor, combination strategies
Introduction
Prostate cancer is an ideal model for therapeutic cancer vaccines, since the prostate is a nonessential organ with multiple tumor-associated antigens as potential targets. In addition, prostate cancer is generally an indolent disease that provides sufficient time for the generation of an antitumor immune response. Although prostate cancer is a known immunogenic disease (1), it can escape the immune system by downregulating human leukocyte antigen class I and thereby render antigen presentation ineffective, by inducing T-cell apoptosis through expression of Fas ligand, by secreting immunosuppressive cytokines such as TGF-β, or by increasing regulatory T cells (Tregs) (2). As in many other cancers, the exact etiology of prostate cancer is still unknown; however, some studies have indicated that inflammation may play a role in its pathogenesis (3). Available treatment options have significantly improved survival for metastatic castration-resistant prostate cancer (mCRPC) patients in the last decade, with 6 new drugs approved since 2010. Along with modern antiandrogen therapies, immunotherapy has the potential to dramatically impact this disease.
Here we review the status of prostate cancer immunotherapy, including cancer vaccines and checkpoint inhibitors, and discuss novel immunotherapy combinations that have progressed furthest in clinical development.
Rationale for the use of immunotherapy in prostate cancer
Sipuleucel-T (Dendreon Corp.), an autologous cellular immunotherapy, was approved by the U.S. Food and Drug Administration in 2010 for treatment of patients with asymptomatic or minimally symptomatic mCRPC. A pivotal phase III clinical trial (IMPACT) randomized 512 mCRPC patients 2:1 to receive sipuleucel-T or placebo. This positive study had a median overall survival (OS) of 25.8 vs. 21.7 months (hazard ratio [HR] = 0.77; P = 0.02), and no significant difference in time to progression (3.7 vs. 3.6 months; HR = 0.95; P = 0.63). The toxicity profile was good, with transient flu-like symptoms and fever as being the most common side effects (4).
To receive this therapeutic cancer vaccine, patients first undergo leukapheresis to obtain autologous peripheral blood mononuclear cells (PBMCs). The PBMCs are activated with a recombinant protein consisting of prostatic acid phosphatase and GM-CSF, then reinfused after 3 days. This process is repeated every 2 weeks for 3 doses. Although the exact mechanism of action of sipuleucel-T is not known, this treatment does not decrease prostate-specific antigen (PSA) levels or tumor size but does prolong OS, likely by affecting tumor growth (5). Interestingly, sipuleucel-T treatment resulted in humoral antigen spread, including development of antibodies to ERAS, KLK2, and KRAS, that was associated with improved OS (6). The Society for Immunotherapy of Cancer Consensus Recommendations indicate that sipuleucel-T vaccine should be considered early in the treatment of mCRPC, since doing so appears to have a greater OS benefit (7).
The immunotherapy revolution gained even greater momentum after several immune checkpoint inhibitors demonstrated durable responses and improved OS in up to 25% of unselected patients with solid tumors (8). Tumors such as melanoma, bladder cancer, and non-small cell lung cancer, among others, are considered “hot” due to their inflamed microenvironment with significant T-cell infiltration, increased programmed death ligand-1(PD-L1) expression, and high neoantigen load (9). Studies have demonstrated that cancers with multiple point mutations, that can serve as neoantigens such as colorectal cancer with high level of microsatellite instability (MSI-H), tend to respond better to immunotherapy (10).However, this is not the case with all tumor types (e.g., kidney cancer) (11) and thus may not fully explain the immune sensitivity of tumors.
Prostate cancer is at the other end of the spectrum: it is a “cold” tumor with minimal T cell infiltrates and very limited response to single-agent checkpoint inhibition, as demonstrated in recent studies (Table 1). The efficacy of ipilimumab (Bristol-Myers Squibb), an anti-CTLA-4 monoclonal antibody, in mCRPC was evaluated in 2 large, placebo-controlled, randomized phase III clinical trials which administered ipilimumab before (12) or after docetaxel chemotherapy (13). In these studies, ipilimumab prolonged progression-free survival (PFS) and PSA responses in a subset of mCRPC patients. However, ipilimumab has no defined role in the management of mCRPC since both studies failed to meet their primary endpoint of improved OS.
Table 1.
Clinical studies of checkpoint inhibitors in prostate cancer
| Drug/reference | Phase/disease/(n) | Dose | Results |
|---|---|---|---|
| Ipilimumab (post-chemo) (13) | Phase III, mCRPC (799) | 8 Gy EBRT to one bone lesion followed by ipilimumab 10 mg/kg or placebo for 4 doses, then maintenance every 3 months | Median OS 11.2 vs.10.0 months (HR = 0.85; P = 0.053) |
| Ipilimumab (chemo-naïve) (12) | Phase III, mCRPC (799) | Ipilimumab 10 mg/kg or placebo every 3 weeks for 4 doses, then maintenance every 3 months | Median OS 28.7 vs. 29.7 months (HR = 1.11; P = 0.3667) |
| Nivolumab (14) | Phase I, mCRPC (17) | Nivolumab 0.1–10 mg/kg i.v. every 2 weeks | No objective responses, one patient sustained > 50% PSA decline |
| Pembrolizumab (15) | Phase I, mCRPC (23) | Pembrolizumab 10 mg/kg every 2 weeks up to 24 months | 3 patients with confirmed PR (ORR 13%) and 9 with SD (39%) |
| Tremelimumab (54) | Phase I, BCR (11) | Tremelimumab with high-dose bicalutamide | 3 patients with prolonged PSA doubling time |
EBRT: external beam radiation therapy; HR: hazard ratio; mCRPC: metastatic castration-resistant prostate cancer; ORR: overall response rate; OS: overall survival; PR: partial response; PSA: prostate-specific antigen; SD: stable disease; BCR: biochemical recurrence (PSA-only disease)
A phase I study of nivolumab (Bristol-Myers Squibb), an anti-PD-1 monoclonal antibody, enrolled, among others, 17 mCRPC patients. No objective responses were observed, although one patient had a sustained PSA decline > 50% (14). KEYNOTE-028, a multicohort phase Ib study of pembrolizumab (Merck), another anti-PD-1 monoclonal antibody, in patients with advanced solid tumors, enrolled 23 patients with mCRPC. Of these, 3 (13%) had a confirmed partial response (PR) and 9 (39%) had stable disease (15). The lack of responses in both studies were possibly due to the fact that responses may correlate with PD-L1 expression, which is minimal in prostate cancer (16).
Pritchard et al. recently reported that 12% of mCRPC patients have MSI and mismatch repair gene mutations (MSH2 or MSH6) (17), while other case series have reported a somewhat lower incidence of MSI in prostate cancer (2%–12%) (18). It is possible that mCRPC patients with MSI may respond better to single-agent checkpoint inhibition, and that is currently being prospectively evaluated (NCT02966587).
Experimental prostate cancer vaccines
PROSTVAC® (rilimogene galvacirepvac)
PROSTVAC (Bavarian Nordic A/S) is an off-the-shelf prostate cancer vaccine that consists of a recombinant vaccinia vector prime followed by multiple boosts with a recombinant fowlpox vector, plus transgenes for PSA and 3 costimulatory molecules (B7.1, ICAM-1, and LFA-3, known as TRICOM) (19). A phase II trial that randomized 122 mCRPC patients to receive PROSTVAC vs. placebo (2:1) demonstrated an improvement in median OS of 8.5 months and a 44% reduction in death rate (20). Revised data confirmed a survival advantage of 26.2 vs. 16.3 months (HR = 0.499; P = 0.0019) (21). The majority of patients (59/104) had increased PSA-specific T-cell responses, and within a subset of patients, 68% demonstrated evidence of antigen spreading 4 weeks after treatment (19). PROSTVAC is currently being evaluated in a phase III clinical trial targeting asymptomatic or minimally symptomatic chemotherapy-naïve mCRPC patients (PROSPECT; NCT01322490). This study randomized 1297 patients to 3 arms: PROSTVAC plus GM-CSF, PROSTVAC plus GM-CSF placebo, and double placebo. Enrollment is completed and results are anticipated as early as the end of 2017. PROSTVAC is also being evaluated in multiple phase II trials as a single agent (Table 1) or as part of combination therapy.
DCVAC/PCa
DCVAC/PCa (SOTIO a.s.) is therapeutic cancer vaccine made of mature dendritic cells (DCs) exposed to killed human prostate cancer cells (LNCaP). In a phase I/II clinical trial in mCRPC, patients (n = 25) were given DCVAC/PCa concurrently with docetaxel chemotherapy. The vaccine was well-tolerated, with no serious vaccine-related adverse events (AEs) reported. The median OS was 19.0 months, which was better than predicted survival using the Halabi nomogram (11.8 months). None of the immunologic parameters, such as decreased Tregs and increased CD8+ T cells and PSA-specific CD8+ cells, significantly correlated with OS (22). Based on the results of this study, a randomized phase III clinical trial is currently evaluating DCVAC/PCa in combination with concurrent docetaxel (VIABLE; NCT02111577). The study was initiated in May 2014 and will enroll 1200 patients. Results are pending.
ProstAtak® (aglatimagene besadenovec)
ProstAtak (Advantagene Inc.), an adenoviral vector encoding thymidine kinase (oncolytic virus), can cause cancer cell death when activated by oral valacyclovir. A phase I study of ProstAtak enrolled 10 patients with newly diagnosed prostate cancer: 7 with high-risk, 1 with intermediate-risk, and 2 with low-risk disease. Nine patients were treated surgically 7.3 to 15.7 weeks after vector injection. After a median follow-up of 11.3 years, 3 patients developed biochemical recurrence and none of them developed metastases. Treatment was safe and well-tolerated (23).
ProstAtak is currently being tested in a randomized (2:1), placebo-controlled phase III trial in patients with localized disease who are candidates for curative external beam radiation therapy (EBRT) (NCT01436968). Another ongoing phase II/III trial is testing ProstAtak in patients undergoing active surveillance (NCT02768363).
The success of the first cancer vaccine has intensified efforts to develop novel vaccines for prostate cancer, and several are currently in clinical development. Main categories include DNA vaccines, antigen-loaded DCs, and viral vectors targeting several tumor-associated antigens such as prostate stem cell antigen, PSA, and prostate-specific membrane antigen (PSMA), among others. Vaccines currently being evaluated in phase II and III studies are listed in Table 2. The most promising future immunotherapy strategies for prostate cancer are combinations of cancer vaccines with other treatment modalities.
Table 2.
Experimental therapeutic prostate cancer vaccines currently in phase II and III clinical trials
| Vaccine | Type | Phase | Disease stage | NCT |
|---|---|---|---|---|
| DCVac | Dendritic-cell vaccine | III | mCRPC | NCT02111577 |
| PROSTVAC | Poxvirus-based vaccine | III II II II |
mCRPC Adjuvant therapy BCR Active surveillance |
NCT01322490 NCT02772562 NCT02649439 NCT02326805 |
| ProstAtack | Oncolytic virus | III | Curative EBRT Active surveillance |
NCT01436968 NCT02768363 |
| ME TARP | Autologous dendritic-cell vaccine targeting TARP | II | BCR | NCT02362451 |
| DC1 | Alpha-type-1-polarized dendritic cells with apoptotic allogeneic tumor (LNCap) | II | BCR | NCT00970203 |
| GX301 | 4 human telomerase reverse transcriptase (hTERT) peptides and 2 adjuvants | II | mCRPC | NCT02293707 |
| mDC/pDC | Tumor peptide-loaded dendritic cells (myeloid, plasmacytoid, and their combination) | II | mCRPC | NCT02692976 |
| ADXS31-142 | Live-attenuated strain of Listeria monocytogenes encoding PSA fused to a fragment of the immunostimulant listeriolysin O protein | I/II | mCRPC | NCT02325557 |
| DC vaccine | Autologous dendritic cells with mRNA from primary prostate cancer tissue, hTERT, and survivin | I/II | Adjuvant, high risk of PSA relapse | NCT01197625 |
| Ad5-SGE-REIC/Dkk-3 | Recombinant adenovirus designed to increase intracellular production of REIC protein | I/II | Localized prostate cancer | NCT01931046 |
EBRT: external beam radiation therapy; mCRPC: metastatic castration-resistant prostate cancer; BCR: biochemical recurrence (PSA-only disease)
Adoptive cell therapy
Adoptive cell therapy, that isolates and expands autologous or allogeneic tumor-reactive lymphocytes. It has demonstrated activity in melanoma using tumor-infiltrating lymphocytes (TILs) and in hematologic malignancies, melanoma, and synovial sarcoma using chimeric antigen receptor (CAR) T cells (24). An ongoing study is testing CAR-T cells that target PSMA (NCT01140373). Preliminary results show that 2 of the first 3 patients enrolled had stable disease for > 6 months with no reported AEs (25).
Combination therapies
The ideal immunotherapy should: 1) activate effector cytotoxic T cells against specific antigens within the tumor and expand additional T-cell clones that can migrate to the tumor and kill targeted cells, and 2) assist effector cells by neutralizing local immunosuppressive mechanisms responsible for immune escape (PD-1/PD-L1, indoleamine 2,3-dioxygenase, Tregs, etc.) (Figure 1) (26). Observed responses to single-agent checkpoint inhibitors or therapeutic vaccines in prostate cancer have been minimal to modest, and those agents may not be optimal if used as monotherapy.
Figure 1. Requirements for effective immunotherapy.
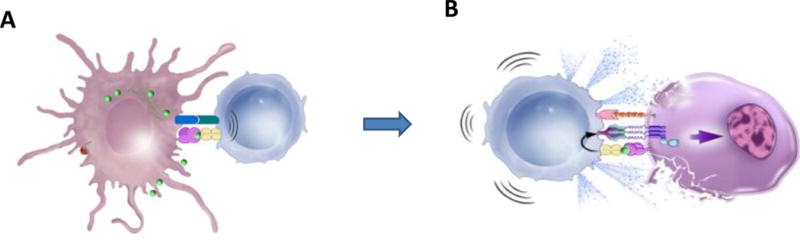
A) Generation of immune response.
Antigen-presenting cells process targeted antigens (green) and present them to T cells with major histocompatibility complexes (MHC) on their surface, along with costimulatory molecules. This complex stimulates T cells to become cytotoxic CD8+ T cells.
B) Functional effector cells within the tumor.
Activated cytotoxic T cells recognize targeted antigens on the surface of cancer cells and release cytotoxins such as perforin and granzymes, triggering caspases and apoptosis. In addition, activated T cells also express Fas ligands that bind to Fas receptors on tumor cells, also inducing apoptosis.
A promising new approach to prostate cancer immunotherapy involves efforts to make prostate cancer more T cell inflamed, since the majority of prostate cancer are not. There are several ways to cause inflammation within prostate tumors and recruiting more effector T cells into the tumor : 1) hormone therapy (can increase inflammatory infiltrates and PD-L1 expression), 2) chemotherapy (killing of cancer cells and releasing cancer antigens), 3) radiation therapy (increasing inflammation and immunomodulatory cytokines), 4) PARP inhibitors (damaging DNA), 5) adoptive cell transfer (generating new T-cells) or 6) combining two checkpoint inhibitors or combinations of a cancer vaccine and an checkpoint inhibitor (immunogenic intensification) (27). These treatment modalities could enhance immune cell response and with concurrent blockade of inhibitory pathways within the tumor microenvironment, could achieve optimal antitumor effects (9).
The timing of treatment is another crucial factor in optimizing immunotherapies for prostate cancer. The ideal timing for prostate cancer immunotherapy is in the neoadjuvant or adjuvant setting or after biochemical recurrence (PSA-only disease) when tumor burden is minimal and immunosuppressive cells and cytokines are at their lowest levels (2). On the other end of the spectrum, mCRPC patients have large tumor volume, multiple immunosuppressive cytokines, and limited time to wait for an immune response to become clinically effective. Studies evaluating immunotherapeutic agents or combinations in the neoadjuvant setting would allow for detailed exploration of the impact of these agents on the tumor microenvironment.
Identifying the the optimal timing and sequence of combination strategies is crucial and have the potential to significantly change outcomes in prostate cancer. Combination of different treatment modalities and immunotherapy is safe and many are currently being studied, with preliminary evidence showing promising activity (Table 3).
Table 3.
Combination strategies for prostate cancer
| Combination | Rationale | Clinical trials |
|---|---|---|
| Radiation + vaccine or checkpoint inhibitor | Cells death => antigen release. Increased inflammation and secretion of immunomodulatory cytokines. |
Radium-223 + sipuleucel-T Radium-223 + pembrolizumab Radium-223 + atezolizumab |
| Chemotherapy + vaccine or checkpoint inhibitor | Reduced tumor burden. Increased Fas expression. Antigen cascade. |
Docetaxel + PROSTVAC Docetaxel + DCVAC/PCa Docetaxel + pembrolizumab |
| Hormone therapy (antiandrogens) + cancer vaccine or checkpoint inhibitor | Reduced tumor burden. Increased production of naive T cells and CD4+ effector T cells. Increased PD-L1 expression (enzalutamide). |
Enzalutamide + PROSTVAC Enzalutamide + pembrolizumab |
| Checkpoint inhibitor + cancer vaccine or another checkpoint inhibitor | Activation of different T-cell population. Increased inflammation. |
Listeria-based vaccine + pembrolizumab |
| PARP inhibitor + checkpoint inhibitor | DNA damage => antigen release. Increased inflammation. |
Olaparib + pembrolizumab Olaparib + durvalumab |
Hormone therapy and immunotherapy (vaccine or checkpoint inhibitor)
Androgen-deprivation therapy affects the immune system by inducing thymic regeneration leading to increased production of naive T cells (28), decreasing CD4+ T-cell tolerance (29), and increasing CD4+ effector T cells (30). The synergistic effect of castration and immunotherapy has been evaluated in multiple clinical trials (31).
Antiandrogens such as enzalutamide (Astellas Pharma/Medivation) also induce immunogenic modulation (32). Enzalutamide is currently being tested in combination with PROSTVAC in mCRPC (NCT01867333) and in biochemical recurrence (NCT01875250). Interestingly, mCRPC patients who progressed on enzalutamide were shown to have increased expression of PD-1 in circulating immune cells (33). Graff et al. reported a case series of 10 mCRPC patients enrolled in a phase II trial of pembrolizumab after progression on enzalutamide. Three out of 10 patients had rapid PSA declines and 2 had PRs, including one patient with MSI (34). KEYNOTE-365 is currently investigating pembrolizumab combination therapies in mCRPC, including pembrolizumab + olaparib, pembrolizumab + docetaxel + prednisone, and pembrolizumab + enzalutamide (NCT02861573).
Chemotherapy and immunotherapy (vaccine)
Many chemotherapies, such as docetaxel and gemcitabine, have a positive impact on the immune system. Preclinical studies have shown that docetaxel can increase antigen presentation and Fas expression, activate an antigen cascade (35), modulate the tumor microenvironment, and improve vaccine efficacy (36). Several ongoing studies are evaluating the combination of docetaxel and vaccine, including PROSTVAC + docetaxel in castration-sensitive prostate cancer (NCT02649855) and docetaxel + DCVAC/PCa (VIABLE; NCT02111577) in mCRPC.
Radiation (EBRT or radiopharmaceuticals) and immunotherapy (vaccine or checkpoint inhibitors)
Radiation can impact the immune system by damaging DNA, increasing expression of MHC class I, Fas, and ICAM-1, and by increasing cytokines such as TNF-α and IL-6. The goal is to modify the phenotype of cancer cells, making it easier for immune cells to recognize and kill them (37).
A phase II study of 153samarium-EDTMP (Lantheus Holding), a radiopharmaceutical, plus PROSTVAC randomized 21 post-docetaxel mCRPC patients to receive the combination and 18 to receive 153samarium-EDTMP alone. The median PFS was 3.7 months for the combination vs. 1.7 months for 153samarium-EDTMP alone (HR = 0.51; P = 0.041), with no difference in median OS. No patients in the 153samarium-EDTMP-alone arm had a PSA decline, while 4/21 (19%) patients in the combination arm had a PSA decline ≥ 30% (38).
Radium-223 (Bayer Pharma), a novel radiopharmaceutical, has demonstrated improved OS in mCRPC (39). Preclinical data indicate that it also has immunomodulatory effects (40). An ongoing phase I study is evaluating the combination of radium-223 and atezolizumab (Genentech) in mCRPC (NCT02814669), while another is evaluating the combination of radium-223 and sipuleucel-T (NCT02463799).
PARP inhibitors and checkpoint inhibitors
A recent report suggested that 11.8% of mCRPC patients have germline mutations in genes mediating DNA-repair processes, a rate higher than previously anticipated (41). Preclinical studies using a BRCA-1-deficient ovarian cancer model demonstrated that combining a CTLA-4 antibody with a PARP inhibitor had a synergistic effect, resulting in immune-mediated tumor killing and improved survival (42). In the TOPARP-A trial, 50 mCRPC patients previously treated with docetaxel were given olaparib, a PARP inhibitor. Sixteen patients (33%) showed a response, and 12 of them had response lasting > 6 months. Interestingly, 16 patients were also found to have mutations in DNA-repair genes, and 14 of these 16 patients (88%) showed a response (43).
Preliminary results of a phase II study of durvalumab (AstraZeneca), a PD-L1 antibody, plus olaparib (AstraZeneca) were recently reported (44). Overall, 8/10 patients showed declines in PSA, 5 of which were > 50%. The combination was well-tolerated and showed activity in an unselected population, with a median PFS of 7.8 months. Responses were observed in all patient subgroups, regardless of the number of prior lines of therapy, including those without mutations in DNA-repair pathways. This ongoing trial has an accrual goal of 25 patients (NCT02484404). Preclinical studies have shown that PARP1 inhibitors can suppress androgen receptor activity and tumor growth even in the absence of BRCA mutations or DNA-damaging agents (45). While we don’t know the mechanism of the activity seen, the double-strand DNA breaks caused by PARP inhibitors could lead to STING pathway upregulation. Immunogenic modulation or neoantigen formation may also explain observed activity in mCRPC.
Vaccines and checkpoint inhibitors (immunogenic intensification)
Preclinical studies have suggested that different therapeutic cancer vaccine platforms can activate different T-cell populations, even if they target the same antigens (46), and that the combination of cancer vaccines and checkpoint inhibitors has synergistic effects (47, 48). Ideally, cancer vaccines should activate immune cells and direct them to the tumor (49), where they can increase lymphocyte infiltration and drive increased PD-L1 expression within the tumor microenvironment. One of the major concerns with this approach has been the possibility of increased toxicity; however, preliminary data suggest that this combination is no more toxic than a single-agent checkpoint inhibitor (50). Consequently, a vaccine plus a PD-1 inhibitor may be less toxic than a vaccine plus a CTLA-4 inhibitor.
A phase I trial evaluated the combination of GVAX (Aduro Biotech), a whole tumor-cell vaccine, and ipilimumab in mCRPC. Seven of 28 patients had > 50% PSA declines, while one patient had a complete response (51). Another study treated 30 patients, 24 of whom were chemotherapy-naïve, with PROSTVAC and ipilimumab. Six of the 30 patients had PSA declines > 50%. The median OS was 34.4 months, and 2-year OS was 73%, which was better than historical controls (52).
A phase I/II study of a Listeria-vector vaccine (Advaxis, Inc.) plus pembrolizumab is currently accruing patients with mCRPC (NCT02325557). Another phase II study will evaluate the combination of PROSTVAC, ipilimumab, and nivolumab in prostate cancer patients prior to curative surgery (NCT02933255).
The most interesting combination strategies combine cancer vaccines and checkpoint inhibitors or 2 different checkpoint inhibitors plus vaccines. Our group recently reported a phase I trial of ipilimumab in combination with PROSTVAC in 30 mCRPC patients. Among chemotherapy-naïve patients, 14 (58%) did have PSA declines. Median OS was 34.4 months, with a median Halabi-predicted survival of 17.2 months (53), suggesting a treatment effect with a favorable safety profile. An ongoing phase I study of PROSTVAC in combination with nivolumab and/or ipilimumab in men with prostate cancer (NCT02933255) prior curative surgery will evaluate the impact of this immunologic combination on the tumor microenvironment, focusing on immune cell infiltration as the primary endpoint.
Conclusions
While prostate cancer appears to have been left out of the ongoing immunotherapeutic revolution currently underway in medical oncology, that perspective may be nearsighted. As a phase III study with the therapeutic cancer vaccine sipuleucel-T has demonstrated, and early data from trials of checkpoint inhibitors suggest, prostate cancer can be amenable to immunotherapeutic strategies, which will likely involve multiple immune-based platforms. Combinations of therapies that can change the “cold” prostate cancer tumor microenvironment to immunologically “hot” by driving T cells to the tumor may be one way to optimize immunotherapy in prostate cancer. Existing conventional therapies such as chemotherapies, antiandrogens, and radiopharmaceuticals have demonstrated pro-immune effects and may become part of future immune-based platforms. Many clinical trials are evaluating immunotherapy combinations, some of them earlier on in the disease process. Results of these studies will shape the future of prostate cancer immunotherapy.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Drake CG. Prostate cancer as a model for tumour immunotherapy. Nat Rev Immunol. 2010;10:580–93. doi: 10.1038/nri2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drake CG, Jaffee E, Pardoll DM, De Marzo AM, Platz EA, Sutcliffe S, et al. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 3.De Marzo AM, Platz EA, Sutcliffe S, Xu J, Gronberg H, Drake CG, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256–69. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 5.Stein WD, Gulley JL, Schlom J, Madan RA, Dahut W, Figg WD, et al. Tumor regression and growth rates determined in five intramural NCI prostate cancer trials: the growth rate constant as an indicator of therapeutic efficacy. Clin Cancer Res. 2011;17:907–17. doi: 10.1158/1078-0432.CCR-10-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drake CG. The potential role of antigen spread in immunotherapy for prostate cancer. Clin Adv Hematol Oncol. 2014;12:332–4. [PMC free article] [PubMed] [Google Scholar]
- 7.McNeel DG, Bander NH, Beer TM, Drake CG, Fong L, Harrelson S, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of prostate carcinoma. J Immunother Cancer. 2016;4:92. doi: 10.1186/s40425-016-0198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahoney KM, Atkins MB, Gajewski TF. Prognostic and predictive markers for the new immunotherapies. Oncology (Williston Park) 2014;28(Suppl 3):39–48. [PubMed] [Google Scholar]
- 9.Gajewski TF. The Next Hurdle in Cancer Immunotherapy: Overcoming the Non-T-Cell-Inflamed Tumor Microenvironment. Semin Oncol. 2015;42:663–71. doi: 10.1053/j.seminoncol.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–8. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174–9. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J Clin Oncol. 2017;35:40–7. doi: 10.1200/JCO.2016.69.1584. [DOI] [PubMed] [Google Scholar]
- 13.Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–12. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen A, Massard C, Ott P, Haas N, Ejadi S, Wallmark J, et al. Pembrolizumab for patients with advanced prostate adenocarcinoma: Preliminary results from the KEYNOTE-028 study. Ann Oncol. 2016;27(suppl 6):725PD. doi: 10.1093/annonc/mdy232. [DOI] [PubMed] [Google Scholar]
- 16.Martin AM, Nirschl TR, Nirschl CJ, Francica BJ, Kochel CM, van Bokhoven A, et al. Paucity of PD-L1 expression in prostate cancer: innate and adaptive immune resistance. Prostate Cancer Prostatic Dis. 2015;18:325–32. doi: 10.1038/pcan.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pritchard CC, Morrissey C, Kumar A, Zhang X, Smith C, Coleman I, et al. Complex MSH2 and MSH6 mutations in hypermutated microsatellite unstable advanced prostate cancer. Nat Commun. 2014;5:4988. doi: 10.1038/ncomms5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Wang J, Fraig MM, Metcalf J, Turner WR, Bissada NK, et al. Defects of DNA mismatch repair in human prostate cancer. Cancer Res. 2001;61:4112–21. [PubMed] [Google Scholar]
- 19.Gulley JL, Madan RA, Tsang KY, Jochems C, Marte JL, Farsaci B, et al. Immune impact induced by PROSTVAC (PSA-TRICOM), a therapeutic vaccine for prostate cancer. Cancer Immunol Res. 2014;2:133–41. doi: 10.1158/2326-6066.CIR-13-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kantoff PW, Gulley JL, Pico-Navarro C, Podrazil M, Horvath R, Becht E, et al. Revised Overall Survival Analysis of a Phase II, Randomized, Double-Blind, Controlled Study of PROSTVAC in Men With Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol. 2017;35:124–5. doi: 10.1200/JCO.2016.69.7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Podrazil M, Horvath R, Becht E, Rozkova D, Bilkova P, Sochorova K, et al. Phase I/II clinical trial of dendritic-cell based immunotherapy (DCVAC/PCa) combined with chemotherapy in patients with metastatic, castration-resistant prostate cancer. Oncotarget. 2015;6:18192–205. doi: 10.18632/oncotarget.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rojas-Martinez A, Manzanera AG, Sukin SW, Esteban-Maria J, Gonzalez-Guerrero JF, Gomez-Guerra L, et al. Intraprostatic distribution and long-term follow-up after AdV-tk immunotherapy as neoadjuvant to surgery in patients with prostate cancer. Cancer Gene Ther. 2013;20:642–9. doi: 10.1038/cgt.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hinrichs CS, Rosenberg SA. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol Rev. 2014;257:56–71. doi: 10.1111/imr.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slovin SF, Wang X, Borquez-Ojeda O, Stefanski J, Olszewska M, Taylor C, et al. Targeting castration resistant prostate cancer (CRPC) with autologous PSMA-directed CAR+ T cells. J Clin Oncol. 2012;30(suppl 15) abstr TPS4700-TPS. [Google Scholar]
- 26.Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. 2016;14:73. doi: 10.1186/s12916-016-0623-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Sullivan Coyne G, Madan RA, Gulley JL, Sutherland JS, Goldberg GL, Hammett MV, et al. Nivolumab: promising survival signal coupled with limited toxicity raises expectations. J Clin Oncol. 2014;32:986–8. doi: 10.1200/JCO.2013.54.5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutherland JS, Goldberg GL, Hammett MV, Uldrich AP, Berzins SP, Heng TS, et al. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol. 2005;175:2741–53. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- 29.Drake CG, Doody AD, Mihalyo MA, Huang CT, Kelleher E, Ravi S, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7:239–49. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mercader M, Bodner BK, Moser MT, Kwon PS, Park ES, Manecke RG, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A. 2001;98:14565–70. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arlen PM, Gulley JL, Todd N, Lieberman R, Steinberg SM, Morin S, et al. Antiandrogen, vaccine and combination therapy in patients with nonmetastatic hormone refractory prostate cancer. J Urol. 2005;174:539–46. doi: 10.1097/01.ju.0000165159.33772.5b. [DOI] [PubMed] [Google Scholar]
- 32.Ardiani A, Farsaci B, Rogers CJ, Protter A, Guo Z, King TH, et al. Combination therapy with a second-generation androgen receptor antagonist and a metastasis vaccine improves survival in a spontaneous prostate cancer model. Clin Cancer Res. 2013;19:6205–18. doi: 10.1158/1078-0432.CCR-13-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bishop JL, Sio A, Angeles A, Roberts ME, Azad AA, Chi KN, et al. PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget. 2015;6:234–42. doi: 10.18632/oncotarget.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graff JN, Alumkal JJ, Drake CG, Thomas GV, Redmond WL, Farhad M, et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget. 2016;7:52810–7. doi: 10.18632/oncotarget.10547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodge JW, Garnett CT, Farsaci B, Palena C, Tsang KY, Ferrone S, et al. Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic T lymphocytes and is distinct from immunogenic cell death. Int J Cancer. 2013;133:624–36. doi: 10.1002/ijc.28070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 2010;16:4583–94. doi: 10.1158/1078-0432.CCR-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finkelstein SE, Salenius S, Mantz CA, Shore ND, Fernandez EB, Shulman J, et al. Combining immunotherapy and radiation for prostate cancer. Clin Genitourin Cancer. 2015;13:1–9. doi: 10.1016/j.clgc.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Heery CR, Madan RA, Stein MN, Stadler WM, Di Paola RS, Rauckhorst M, et al. Samarium-153-EDTMP (Quadramet(R)) with or without vaccine in metastatic castration-resistant prostate cancer: A randomized Phase 2 trial. Oncotarget. 2016;7:69014–23. doi: 10.18632/oncotarget.10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fossa SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 40.Malamas AS, Gameiro SR, Knudson KM, Hodge JW. Sublethal exposure to alpha radiation (223Ra dichloride) enhances various carcinomas’ sensitivity to lysis by antigen-specific cytotoxic T lymphocytes through calreticulin-mediated immunogenic modulation. Oncotarget. 2016;7:86937–47. doi: 10.18632/oncotarget.13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pritchard CC, Mateo J, Walsh MF, De Sarkar N, Abida W, Beltran H, et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N Engl J Med. 2016;375:443–53. doi: 10.1056/NEJMoa1603144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Higuchi T, Flies DB, Marjon NA, Mantia-Smaldone G, Ronner L, Gimotty PA, et al. CTLA-4 Blockade Synergizes Therapeutically with PARP Inhibition in BRCA1-Deficient Ovarian Cancer. Cancer Immunol Res. 2015;3:1257–68. doi: 10.1158/2326-6066.CIR-15-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N Engl J Med. 2015;373:1697–708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karzai F, Madan RA, Owens H, Hankin A, Couvillon A, Houston N, et al. A phase II study of the anti-programmed death ligand-1 antibody durvalumab (D; MEDI4736) in combination with PARP inhibitor, olaparib (O), in metastatic castration-resistant prostate cancer (mCRPC) J Clin Oncol. 2017;35(suppl 6S) abstr 162. [Google Scholar]
- 45.Schiewer MJ, Knudsen KE. Transcriptional roles of PARP1 in cancer. Mol Cancer Res. 2014;12:1069–80. doi: 10.1158/1541-7786.MCR-13-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boehm AL, Higgins J, Franzusoff A, Schlom J, Hodge JW. Concurrent vaccination with two distinct vaccine platforms targeting the same antigen generates phenotypically and functionally distinct T-cell populations. Cancer Immunol Immunother. 2010;59:397–408. doi: 10.1007/s00262-009-0759-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fu J, Malm IJ, Kadayakkara DK, Levitsky H, Pardoll D, Kim YJ. Preclinical evidence that PD1 blockade cooperates with cancer vaccine TEGVAX to elicit regression of established tumors. Cancer Res. 2014;74:4042–52. doi: 10.1158/0008-5472.CAN-13-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soares KC, Rucki AA, Wu AA, Olino K, Xiao Q, Chai Y, et al. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J Immunother. 2015;38:1–11. doi: 10.1097/CJI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fong L, Carroll P, Weinberg V, Chan S, Lewis J, Corman J, et al. Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol. 2016;39:98–106. doi: 10.1097/COC.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van den Eertwegh AJ, Versluis J, van den Berg HP, Santegoets SJ, van Moorselaar RJ, van der Sluis TM, et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:509–17. doi: 10.1016/S1470-2045(12)70007-4. [DOI] [PubMed] [Google Scholar]
- 52.Madan RA, Mohebtash M, Arlen PM, Vergati M, Rauckhorst M, Steinberg SM, et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:501–8. doi: 10.1016/S1470-2045(12)70006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Madan RA, Mohebtash M, Arlen PM, Vergati M, Rauckhorst M, Steinberg SM, et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:501–8. doi: 10.1016/S1470-2045(12)70006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McNeel DG, Smith HA, Eickhoff JC, Lang JM, Staab MJ, Wilding G, et al. Phase I trial of tremelimumab in combination with short-term androgen deprivation in patients with PSA-recurrent prostate cancer. Cancer Immunol Immunother. 2012;61:1137–47. doi: 10.1007/s00262-011-1193-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


