Abstract
The lateral habenula (LHb) is a key brain region involved in the pathophysiology of depression. It is activated by stimuli associated with negative experiences and is involved in encoding aversive signals. Hyperactivity of LHb is found in both rodent models of depression and human patients with depression. However, little is known about the underlying molecular mechanisms. Here we show that in LHb neurons, p11, a multifunctional protein implicated in depression, is significantly upregulated by chronic restraint stress. Knockdown of p11 expression in LHb alleviates the stress-induced depression-like behaviors. Moreover, chronic restraint stress induces bursting action potentials in LHb neurons, which are abolished by p11 knockdown. Overexpression of p11 in dopamine D2 receptor-containing LHb neurons of control mice induces depression-like behaviors. These results have identified p11 in LHb as a key molecular determinant regulating negative emotions, which may help to understand the molecular and cellular basis of depression.
Supplementary information
The online version of this article (doi:10.1038/mp.2017.96) contains supplementary material, which is available to authorized users.
Subject terms: Neuroscience, Molecular biology, Physiology
Introduction
Maladaptive molecular and cellular changes in response to aversive stimuli contribute to the manifestation of depression-like symptoms in both animals and humans.1, 2, 3, 4, 5 The lateral habenula (LHb), a nucleus that relays information from the limbic forebrain to midbrain monoamine nuclei, has been identified as a crucial brain region in the regulation of aversion and depression.6, 7, 8, 9, 10, 11 Functional magnetic resonance imaging studies in humans presenting psychiatric disorders have shown that hyperactivity of LHb neurons occurs along with depressive symptoms.9, 12, 13, 14 Furthermore, metabolic and synaptic activities of LHb neurons are enhanced in rodent models of depression, such as those derived from learned helplessness and chronic stress.15, 16, 17
The molecular determinants responsible for the key role of the LHb in depression remain elusive. One possible candidate is p11, a multifunctional protein interacting with 5-HT receptors,18, 19, 20, 21 ion channels,19, 22, 23, 24, 25 enzymes,19, 26, 27 and chromatin remodeling factors,18, 28 which is critically involved in depression-like behaviors and antidepressant actions.18, 20, 28, 29, 30, 31 p11 is enriched in distinct neuronal types, including cholinergic neurons in nucleus accumbens,31 mossy cells and basket cells in dentate gyrus,28 and layer 5 corticostriatal projection neurons.29 Recently, we have found that chronic stress induces the loss of p11 in layer 2/3 prelimbic (PrL) dopamine D2 receptor-expressing (D2+) neurons, which contributes to the diminished PrL glutamatergic synaptic transmission and depression-like behaviors in stressed animals.30 Here we provide evidence that the elevated expression of p11 in LHb neurons after chronic stress is strongly linked to LHb hyperactivation and depression-like behaviors. These results may contribute to understand the key molecular determinant controlling the responses to aversive signals.
Materials and methods
Animals
All procedures involving animals were approved by the Rockefeller University Institutional Animal Care and Use Committee and were in accordance with the National Institutes of Health guidelines. Four transgenic mouse lines were used in this study: p11-EGFP mice,28, 30 D2-Cre mice,30, 31, 32 D2-tdT mice (D2-Cre line crossed with tdTomato line)28, 30 and D1-tdT-D2-eGFP mice (D1-Cre26 crossed with tdTomato line and D2-eGFP mice).30 The mouse breeding methods are in Supplementary Materials and Methods.
Restraint stress and antidepressant treatments
The restraint stress and antidepressant treatments were performed as previously described.28, 30, 33 See methods in Supplementary Materials and Methods for details.
Viruses
The virus production was performed as previously described.30 For optogenetic stimulation, AAV5-CaMKIIa-hChR2 (H134R)-EYFP-WPRE-pA (ChR2) and AAV5-CaMKIIa-EYFP (eYFP) viruses were purchased from UNC Vector Core, Chapel Hill, NC, USA, University of North Carolina. See methods in Supplementary Materials and Methods for details.
Stereotaxic surgery and optogenetic stimulation
The stereotaxic surgery was performed as previously described.30 Briefly, 10-week-old male mice were anesthetized with ketamine and xylazine, and stereotaxically injected with AAV5-CaMKIIa-hChR2 (H134R)-EYFP-WPRE-pA (ChR2), AAV5-CaMKIIa-EYFP (eYFP), Lenti-GFP-shRNA (sh-GFP), Lenti-p11-GFP-shRNA (sh-p11), AAV2-EF1a-DIO-eYFP-WPRE-hGH (AAV-DIO-eYFP) and AAV2-EF1a-DIO-p11-WPRE-hGH (AAV-DIO-p11) into the medial part of the LHb (anteroposterior, −1.92 mm; mediolateral, ±0.4 mm; and dorsoventral, −2.67 mm from bregma). For optogenetic stimulation of LHb neurons, after virus injection, a 200 μm-core-diameter optic fiber (Doric Lenses, Quebec, QC, Canada) was implanted into the injection site. After 3 weeks, depression-like behavioral tests were performed with 473 nm laser light (10 mW, 60 Hz, 5 ms, Agilent Technologies, California, CA, USA).11 See methods in Supplementary Materials and Methods for details.
Behavioral assessments
Behavioral studies including tail suspension test (TST), forced swim test, sucrose preference test and social interaction test, novelty suppressed feeding test, fecal boli test and two-way active avoidance test were performed to examine the depression-like phenotypes, and locomotor activity. Details are included in Supplementary Materials and Methods.
Immunohistochemistry
Immunostaining was carried out using the standard free-floating method as previously described.30 Detailed description of antibody preparation, antigen retrieval, image acquisition and quantification is presented in Supplementary Materials and Methods.
Western blot analysis and quantitative reverse transcription-PCR
All biochemical analysis were performed as previously described.30 See Supplementary Materials and Methods for details.
Electrophysiological recordings
Whole-cell patch-clamp recording technique was used to measure spontaneous action potentials and synaptic currents in LHb neurons. See Supplementary Materials and Methods for details.
Statistics
Two-sample comparisons were performed using the Student’s t-test, while multiple comparisons were made using one-way analysis of variance followed by a Newman–Keuls post hoc test or two-way analysis of variance by a Bonferroni post hoc test. PRISM software (GraphPad Software, La Jolla, CA, USA) was used to perform statistical analyses. All data are presented as means±s.e.m.
Results
Effects of chronic stress and p11 expression on the activity of LHb neurons
To investigate the functional role of p11 in LHb, we examined p11 expression in a mouse model of depression, in which mice were exposed to chronic restraint stress (RST, 2 h per day, 14 days).28, 30, 33 Immunofluorescence studies of the anatomical distribution of p11 in habenula with anti-GFP antibody using p11 promoter-driven EGFP (p11-EGFP) mice indicated that p11 was present in the LHb, especially in the medial part of the LHb, but not in the medial habenula (Figure 1a). Western blotting results revealed that the protein level of c-fos, a marker of cell activity, was significantly elevated in Hb from chronically stressed mice (Figures 1b and c). Double immunofluorescence staining with GFP and c-fos antibodies in p11-EGFP mice showed that stressed mice exhibited a significantly increased p11 (green) expression in LHb, which was co-localized with the c-fos (red) expression (Figures 1d and e). These data indicate that the LHb neurons activated by chronic stress have elevated p11 expression.
Figure 1.
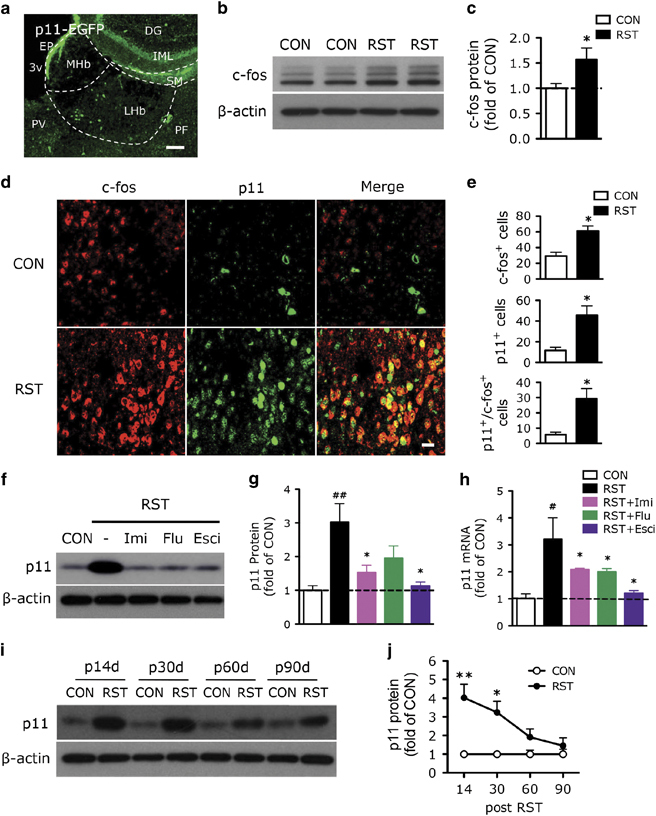
Effects of chronic stress and p11 expression on the activity of LHb neurons. (a) Immunofluorescence image illustrating p11-positive cells (EGFP+, green) in habenula (Hb) of p11-EGFP mice. Scale bar, 100 μm. DG, dentate gyrus; EP, ependymal cells; IML, inner molecular layer of DG; LHb, lateral habenula; MHb, medial habenula; PF, parafascicular thalamic nucleus; PV, paraventricular thalamic nucleus; SM, stria medullaris; 3v, third ventricle. (b, c) Western blot analysis (b) and quantification (c) showing the expression of c-fos in control (CON) and chronically restraint stressed (RST, 2 h per day, 14 days) mice (n=6 per group). *P<0.05, two-tailed t-test. (d, e) Immunofluorescence image (d) and quantification (e) illustrating c-fos-positive cells (red), p11-positive cells (EGFP+, green) and c-fos+/p11+ cells in LHb from control (CON) versus restraint stressed (RST) p11-EGFP mice. Scale bar, 20 μm. (n=6, CON; n=12, RST). *P<0.05, two-tailed t-test. (f–h) Western blots and quantification of p11 protein (f, g) and quantitative PCR of p11 mRNA (h) in LHb from CON and RST mice with or without antidepressant treatments (f and g, n=10, CON; n=12 for each group of RST, RST+imipramine (Imi), RST+fluoxetine (Flu) and RST+escitalopram (Esci); h, n=6 per group). #P<0.05, ##P<0.01, compared to CON; *P<0.05 and **P<0.01, compared to RST, one-way analysis of variance (ANOVA). (i, j) Western blots (i) and quantification (j) showing the expression of p11 in control versus stressed mice at 14, 30, 60 and 90 days after stress cessation (n=6 per group). *P<0.05, **P<0.01, two-way ANOVA. Data presented as means±s.e.m.
Next, we investigated the impact of antidepressant treatment on the stress-induced regulation of p11 expression in LHb. Western blotting and quantitative PCR revealed that the level of p11 protein and mRNA in LHb were significantly increased in chronically stressed mice, which were reversed by 2-week treatment with three distinct antidepressants; imipramine (tricylic class of antidepressant), fluoxetine (selective serotonin reuptake inhibitor) and escitalopram (Figures 1f–h).
We further examined how long the stress-induced upregulation of p11 in LHb was sustained. At 14 and 30 days post stress, the level of p11 in LHb was significantly higher than that in control animals, but at 60 and 90 days post stress, the level of p11 in LHb had returned to the control level (Figures 1i and j), which was consistent with the time frame of depression-like behaviors in these stressed animals.30
To confirm that depressive behaviors can be induced by direct activation of LHb via optogenetic stimulation, we injected AAV-channelrhodopsin (ChR2; AAV-CaMKIIa-ChR2-eYFP) into LHb. Three weeks later, we optically stimulated ChR2-expressing axons in LHb, and performed the two-way active avoidance test and the TST for aversion, helplessness and hopelessness behaviors. ChR2-injected mice with optogenetic stimulation (ChR2+ON) exhibited a significantly decreased avoidance response and increased latency to escape the shock in active avoidance test compared to AAV-eYFP-injected mice (eYFP+ON and eYFP+OFF) or unstimulated ChR2-injected mice (ChR2+OFF) (Supplementary Figures S1a and b). ChR2+ON mice also had an increased immobility in TST (Supplementary Figure S1c), while locomotor activity was not altered (Supplementary Figure S1d). These data suggest that LHb activation induces depression-like behaviors, consistent with prior findings.10
Next, we tested the role of p11 in depression-like behaviors induced by direct activation of LHb. Western blotting results revealed that the protein level of c-fos was upregulated in optically stimulated ChR2-injected mice (Supplementary Figures S1e and f), compared to the three control groups. Immunofluorescence staining using c-fos, ChR2 and p11 antibodies revealed that ~98% of ChR2-expressing LHb neurons were c-fos-positive (c-fos+), and ~73% of p11-expressing neurons were c-fos+/ChR2+ (Supplementary Figure S1g), suggesting that most of the p11-expressing LHb neurons are activated by optogenetic stimulation.
Knockdown of p11 in LHb ameliorates stress-induced depression-like behavior
To determine whether the stress-induced upregulation of p11 in LHb is responsible for depression-like behaviors, we knocked down p11 expression by injecting p11 shRNA (sh-p11) lentivirus into LHb (Figure 2a). The p11 knockdown efficiency in vivo was demonstrated by the significantly diminished p11 expression in LHb from mice with viral expression of p11 shRNA (CON+sh-p11), compared to those injected with GFP-shRNA (CON+sh-GFP) or non-injected (CON). In stressed mice with viral injection of p11 shRNA (RST+sh-p11), the stress-induced p11 upregulation was potently suppressed, compared to those either injected with GFP-shRNA (RST+sh-GFP) or non-injected (RST) (Figures 2b and c).
Figure 2.
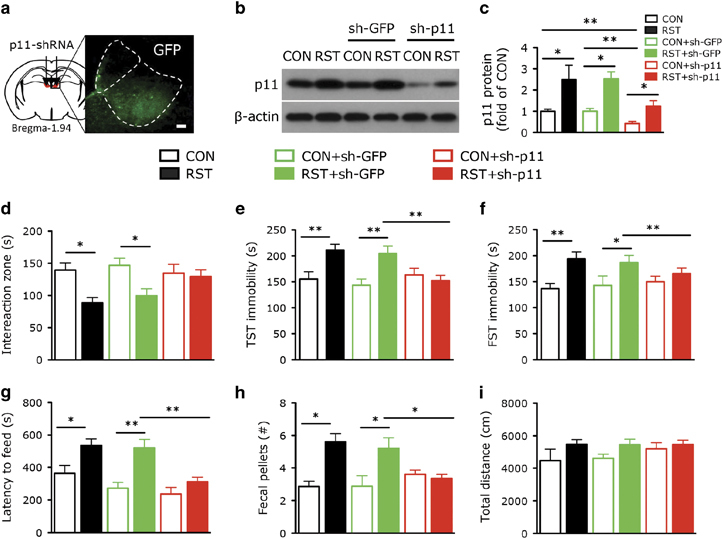
Knockdown of p11 in lateral habenula (LHb) ameliorates stress-induced depression-like behavior. (a) Immunofluorescence image illustrating the expression of viral transgene (GFP) after injecting the GFP-tagged p11 shRNA (sh-p11) lentivirus into LHb. Scale bar, 100 μm. (b, c) Western blots (b) and quantification (c) showing the expression of p11 in LHb from control (CON) and stressed (RST) mice with or without lentiviral injection of GFP-shRNA (sh-GFP) or p11 shRNA (sh-p11) (n=7 per group). (d–i) Depression-like behavior measured by (d) the time spent in the interaction zone in SIT, (e) immobility time in tail suspension test (TST) and (f) forced swim test (FST) in the six groups. (g, h) Anxiety-like behavior measured by (g) the latency to feed in novelty suppressed feeding test and (h) excretion of fecal pellet in fecal boli test in the six groups. (i) Locomotor activity measured by the average total distance traveled by the six groups (n=14, CON and RST; n=8, CON+sh-GFP and RST+sh-GFP; n=11, CON+sh-p11; n=18, RST+sh-p11). *P<0.05, **P<0.01, one-way analysis of variance. Data presented as means±s.e.m.
We next investigated the effect of p11 knockdown in the LHb on depression-like behaviors. Compared to the unstressed control groups (CON and CON+sh-GFP), stressed mice injected with or without GFP-shRNA to LHb (RST and RST+sh-GFP) exhibited significantly decreased time in the interaction zone in the social interaction test (Figure 2d), a measurement of social avoidance and withdrawal behavior. RST and RST+sh-GFP mice also had an increased immobility in TST (Figure 2e) and forced swim test (Figure 2f), two measurements of helplessness and hopelessness, as well as anxiety in the novelty suppressed feeding test (Figure 2g) and fecal boli test (Figure 2h). Locomotor activity (Figure 2i) and food consumption (data not shown) were not altered in these mice. Interestingly, stressed mice with LHb expression of p11 shRNA (RST+sh-p11) did not exhibit depression-like behaviors in social interaction test, TST and forced swim test (Figures 2d–f) or anxiety-like behaviors in novelty suppressed feeding test and fecal boli test (Figures 2g and h). No behavioral changes were found in control animals with LHb knockdown of p11 (CON+sh-p11, Figures 2d–i). These data indicate that the stress-induced p11 upregulation in LHb contributes to the manifestation of depression-like behaviors.
Chronic stress-induced hyperexcitability of LHb neurons is abolished by p11 knockdown
To determine the physiological basis underlying the role of LHb p11 in stress-induced depression-like behaviors, we examined the membrane excitability of LHb neurons in control versus stressed animals with or without p11 knockdown. As shown in Figures 3a–c, the resting membrane potentials (~ −45 mV) and the spontaneous action potential frequencies of LHb neurons from stressed animals (RST) were similar to those from control animals (CON), which were not significantly affected by p11 shRNA injection (RST+sh-p11). When cells were hyperpolarized to −65 mV (Figures 3d and e), most of the LHb neurons from control animals (CON) stopped firing APs, while most of the LHb neurons from stressed animals (RST) exhibited the rhythmic bursting of APs. The bursting APs were abolished in stressed animals injected with p11 shRNA (RST+sh-p11), but not with GFP-shRNA (RST+sh-GFP).
Figure 3.
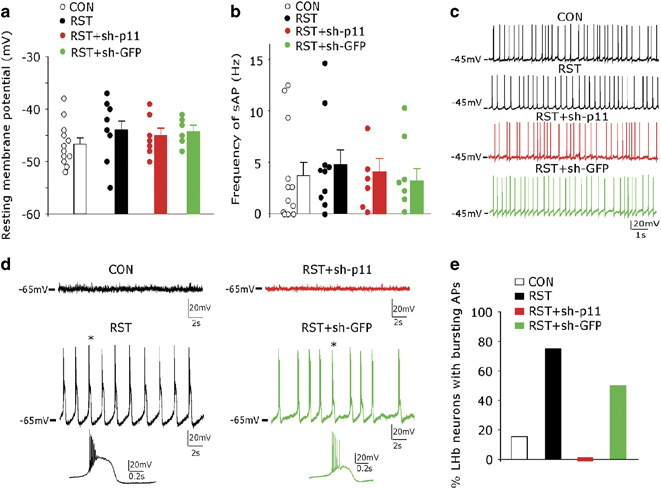
Chronic stress induces burst firing of action potentials in lateral habenula (LHb) neurons, which is abolished by knockdown of p11. (a, b) Scatter plots and bar graphs showing the resting membrane potentials (a) and spontaneous action potential (sAP) frequencies (at −45 mV) (b) in control (CON) and stressed (RST) mice with or without lentiviral injection of p11 shRNA (sh-p11) or GFP-shRNA (sh-GFP) (n=12, CON; n=10, RST; n=7, RST+sh-p11; n=6, RST+sh-GFP). (c) Representative sAP traces in the four groups. (d) Representative traces showing the membrane excitability at the hyperpolarized level (−65 mV) in CON and RST mice with or without lentiviral injection of sh-p11 or sh-GFP. Expanded view of the bursting AP trace at the position denoted by *. (e) Bar graphs showing the percent of LHb neurons exhibiting rhythmic bursting APs (at −65 mV) in the four groups (n=13, CON; n=12, RST; n=7, RST+sh-p11; n=6, RST+sh-G).
To test the possibility that the altered excitability of LHb neurons in stressed animals could be caused by changes in synaptic inputs, we examined spontaneous excitatory and inhibitory postsynaptic currents (sEPSC and sIPSC) in LHb neurons. As shown in Supplementary Figures S2a and b, sIPSC frequency, but not amplitude, was significantly reduced in LHb neurons from stressed animals. No changes in sEPSC amplitude or frequency were found (Supplementary Figure S2c). Knockdown of p11 in LHb from stressed animals significantly elevated sIPSC frequency without changing sIPSC amplitude, while injecting the GFP-shRNA was ineffective (Supplementary Figures S3a and b). The amplitude or frequency of sEPSC was not altered by p11 or GFP-shRNA (Supplementary Figure S3c). Taken together, these data suggest that the stress-induced hyperexcitability of LHb neurons may be attributable to the loss of inhibitory inputs, and p11 may normalize LHb neuronal activity via restoring synaptic inhibition.
p11 overexpression in dopamine D2 receptor-containing glutamatergic LHb neurons of control mice induces depression-like behavior
It has been found that LHb D2+ neurons regulate emotional behaviors in response to negative reward by altering LHb neuronal activity.7, 8, 11, 17, 34 Therefore, we sought to determine whether p11 in D2+ LHb neurons was involved in stress-induced depression-like behaviors. Immunofluorescence staining (Supplementary Figure S4; Supplementary Tables S1 and 2) revealed that p11+ neurons in LHb express Ca2+/calmodulin-dependent kinase II (CaMKII) or vesicular GABA transporter, suggesting that they are glutamatergic or GABAergic. In the medial part of LHb, most (~79%) of D2+ neurons, but not D1+ neurons, co-expressed p11 (Figure 4a; Supplementary Figure S5a; Supplementary Table 3), and most (~88%) of p11+/D2+ neurons expressed CaMKII (Figure 4a; Supplementary Table S1), but not vesicular GABA transporter (Supplementary Figure S5; Supplementary Table 2), indicating that they are glutamatergic neurons.
Figure 4.
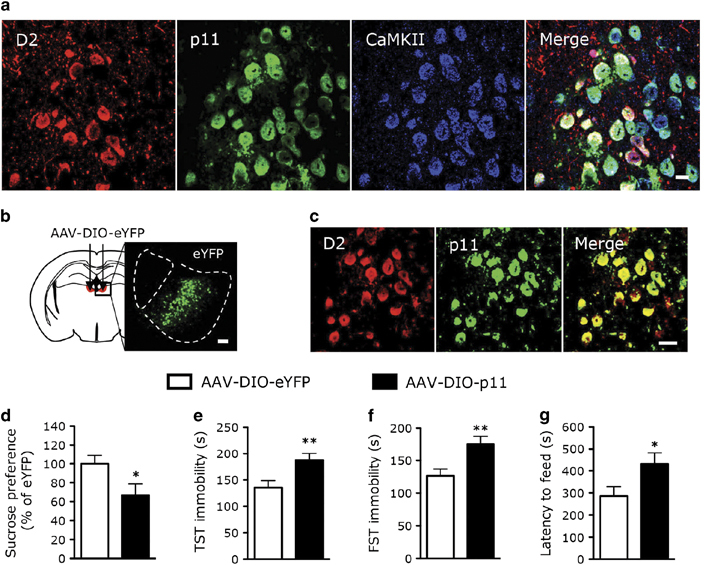
Overexpression of p11 in D2R-containing lateral habenula (LHb) neurons of control animals induces depression-like behavior. (a) Images showing the co-localization of p11 (green) with dopamine D2 receptor (D2; D2-tdT, red) and CaMKII (blue, a marker of glutamatergic neurons) in medial part of the LHb neurons from D2-tdT (D2-Cre × tdTomato) mice. Scale bar, 10 μm. (b) Immunofluorescence image illustrating the localization of D2+ neurons with the viral injection of Cre-dependent eYFP (AAV-DIO-eYFP) into LHb of D2-Cre mice. Scale bar, 100 μm. (c) Immunofluorescence image illustrating the co-localization of Cre-dependent p11 (AAV-DIO-p11) (green) with D2 (red) in LHb neurons. Scale bar, 20 μm. (d–g) Depression-like behaviors as measured by sucrose preference test (d), tail suspension test (TST; e), forced swim test (FST; f) and novelty suppressed feeding test (g) in D2-Cre mice with the viral expression of p11 (AAV-DIO-p11) or eYFP (AAV-DIO-eYFP) in D2+ LHb neurons (n=13, AAV-DIO-eYFP; n=17, AAV-DIO-p11). *P<0.05, **P<0.01, two-tailed t-test. Data presented as means±s.e.m.
To specifically express p11 in D2+ LHb neurons, we injected Cre-dependent viruses into LHb of D2-Cre mice. As shown in Figure 4b, D2-Cre mice injected with AAV-DIO-eYFP exhibited the distribution of D2+ neurons in the medial part of LHb. D2-Cre mice injected with AAV-DIO-p11 exhibited the co-expression of p11 and D2 in LHb (Figure 4c). Depression-like phenotypes emerged in control mice with viral expression of p11 in D2+ LHb neurons, as reflected in sucrose preference test (Figure 4d), TST (Figure 4e), forced swim test (Figure 4f) and novelty suppressed feeding test (Figure 4g) assays. Collectively, these results show that p11 overexpression in D2+ LHb neurons of control animals is sufficient to induce negative emotional behaviors.
Discussion
The LHb has emerged as a key brain region for the study of aversive behaviors and the pathophysiology of depression.6, 7, 9, 11, 15, 35 LHb neurons, especially in the medial part of the LHb, are activated by aversive emotional cues including stress, disappointment, fear, aggression or anticipation of a negative reward in monkeys, as well as rodents.7, 15, 35, 36 Increased activation of the LHb nucleus leads to the inhibition of the connected serotonergic, noradrenergic, dopaminergic systems and stimulation of the hypothalamic–pituitary–adrenal axis.14, 37, 38, 39 Consistently, depression-like symptoms in neuropsychiatric disorders occur along with hyperactivity of LHb neurons in humans.9, 12, 14 Furthermore, in animal models of depression, synaptic activity, spike output and metabolic activity of LHb neurons were enhanced,15, 16, 38 which is in contrast with the decreased activity in prefrontal cortex (PFC),30, 39, 40 and ventral tagmental area.17
In this study, using complementary molecular, electrophysiological and behavioral approaches, we have identified the stress-induced upregulation of p11 in the LHb neurons as a key molecular mechanism underlying the role of LHb in depression-like behaviors. Knockdown of p11 in LHb neurons ameliorated depressive phenotypes and LHb hyperexcitability in stressed animals. Moreover, overexpression of p11 in LHb neurons of control animals induced depression-like behaviors.
p11 is present in various neuronal circuits in distinct neuronal types, such as cholinergic neurons in nucleus accumbence,31 mossy cells and basket cells in dentate gyrus,28 and layer 5 corticostriatal projection neurons,29 involved in emotional control.20, 28, 29, 30, 31 Lower levels of p11 mRNA and protein have been found in the brains of depressed humans and suicide subjects.18, 41 p11 knockout mice exhibit depression-like behavior,18, 30, 31 and p11-overexpressing mice show antidepressant-like behaviors.18, 20 Recently, we have found that chronic stress induces the downregulation of p11 expression in layer 2/3 PrL cortical neurons and the attenuation of PrL glutamatergic synaptic transmission, which are causally linked to depressive symptoms in stressed animals.30 LHb relays information from the limbic forebrain regions, including PrL, to multiple monoamine centers.16, 37 Reciprocal interaction between LHb and medial PFC (mPFC) via dopaminergic neurons in ventral tagmental area has been found to regulate emotional and cognitive function in rodent, monkeys and humans.6, 42, 43, 44 LHb neurons synapse primarily on dopamine neurons in the ventral tagmental area projecting to the mPFC.6 Pharmacological and optogenetic manipulation of the LHb activity could alter dopaminergic regulation of mPFC neuronal activity, which controls executive processes.6, 43, 45 In stressed animals, the decreased p11 expression and diminished synaptic output of PrL neurons, as well as the increased p11 expression and elevated excitability of LHb neurons, may cooperatively contribute to the emergence of depressive phenotypes.
In both LHb and PrL, increased p11 levels are associated with increased neuronal excitability. It will be important to identify the underlying molecular mechanisms. Our data indicate that p11 upregulation in the LHb-stressed animals may interfere with synaptic inhibition, leading to disinhibition and hyperactivation of the LHb neurons. In addition to the stress-induced upregulation of p11 in LHb, increased βCaMKII expression and PP2A activity in the LHb have been found to act as powerful regulators of the LHb neuronal activity and depression-like phenotypes in stressed animals,16, 46 suggesting that parallel cellular processes converge in the LHb to dictate the response to aversive signals.
The LHb has two major types of neurons, a majority of glutamatergic and a minority of GABAergic,37 both of which express p11. Dopamine signals from the ventral tagmental area innervate the whole LHb, influencing LHb neuronal activity and LHb-dependent functions.6, 10, 47 D2 receptors are functionally expressed in the LHb, especially medial part of the LHb, which regulate aversive behaviors in response to negative rewards.48, 49 Interestingly, we have found that overexpression of p11 in this subpopulation of LHb neurons is sufficient to induce depressive phenotypes in control animals, confirming that p11 in D2+ LHb neurons is an important molecular and cellular determinant for depression.
Finally, it will be important to elucidate the molecular basis for the opposing action of p11 in LHb where it is a depressant agent versus p11 in other brain regions examined where it has an antidepressant action, including the cholinergic neurons in nucleus accumbence,31 mossy cells and basket cells in dentate gyrus,28 layer 5 corticostriatal projection neurons29 and D2+ glutamatergic neurons in layer 2/3 PrL cortex.30
Supplementary information
Acknowledgements
We are grateful to Drs Angus C Nairn, Marc Flajolet, Yotam Sagi and Lucian Medrihan for their helpful advice and discussion; to Dr Jodi Gresack for setting up the two-way active avoidance test; and to Drs Jung-Hyuck Ahn and Yong Kim for generating the AAV construct. We thank Drs Daesoo Kim and Karl Deisseroth for helpful advice on optogenetic stimulation. We thank the Rockefeller University Comparative Bioscience Center, Transgenics Services Laboratory. This work was supported by NIDA grant P01DA010044 (PG), NIMH grant P50MH090963 (PG), DOD/USAMRAA grant W81XWH-09-1-0402 (PG), the JPB Foundation (PG), the Fisher Center For Alzheimer’s Research Foundation (PG), the Leon Black Family Foundation (PG), NIH grants R01 MH108842 (ZY) and R01 DA037618 (ZY), and the donation from E. F. Trachtman (ZY).
PowerPoint slides
Competing interests
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Change history
10/21/2019
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
- 1.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 2.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 3.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN. Mechanisms of stress in the brain. Nat Neurosci. 2015;18:1353–1363. doi: 10.1038/nn.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 6.Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nat Neurosci. 2009;12:77–84. doi: 10.1038/nn.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sartorius A, Kiening KL, Kirsch P, von Gall CC, Haberkorn U, Unterberg AW. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010;67:e9–e11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 10.Shabel SJ, Proulx CD, Trias A, Murphy RT, Malinow R. Input to the lateral habenula from the basal ganglia is excitatory, aversive, and suppressed by serotonin. Neuron. 2012;74:475–481. doi: 10.1016/j.neuron.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci. 2012;15:1105–1107. doi: 10.1038/nn.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- 13.Mayberg HS. Targeted electrode-based modulation of neural circuits for depression. J Clin Invest. 2009;119:717–725. doi: 10.1172/JCI38454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sartorius A, Henn FA. Deep brain stimulation of the lateral habenula in treatment resistant major depression. Med Hypotheses. 2007;69:1305–1308. doi: 10.1016/j.mehy.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 15.Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470:535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li K, Zhou T, Liao L, Yang Z, Wong C, Henn F. betaCaMKII in lateral habenula mediates core symptoms of depression. Science. 2013;341:1016–1020. doi: 10.1126/science.1240729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shumake J, Edwards E, Gonzalez-Lima F. Opposite metabolic changes in the habenula and ventral tegmental area of a genetic model of helpless behavior. Brain Res. 2003;963:274–281. doi: 10.1016/S0006-8993(02)04048-9. [DOI] [PubMed] [Google Scholar]
- 18.Svenningsson P, Chergui K, Rachleff I, Flajolet M, Zhang X, El Yacoubi M. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science. 2006;311:77–80. doi: 10.1126/science.1117571. [DOI] [PubMed] [Google Scholar]
- 19.Svenningsson P, Greengard P. p11 (S100A10)—an inducible adaptor protein that modulates neuronal functions. Curr Opin Pharmacol. 2007;7:27–32. doi: 10.1016/j.coph.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Svenningsson P, Kim Y, Warner-Schmidt J, Oh YS, Greengard P. p11 and its role in depression and therapeutic responses to antidepressants. Nat Rev Neurosci. 2013;14:673–680. doi: 10.1038/nrn3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warner-Schmidt JL, Flajolet M, Maller A, Chen EY, Qi H, Svenningsson P. Role of p11 in cellular and behavioral effects of 5-HT4 receptor stimulation. J Neurosci. 2009;29:1937–1946. doi: 10.1523/JNEUROSCI.5343-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donier E, Rugiero F, Okuse K, Wood JN. Annexin II light chain p11 promotes functional expression of acid-sensing ion channel ASIC1a. J Biol Chem. 2005;280:38666–38672. doi: 10.1074/jbc.M505981200. [DOI] [PubMed] [Google Scholar]
- 23.Girard C, Tinel N, Terrenoire C, Romey G, Lazdunski M, Borsotto M. p11, an annexin II subunit, an auxiliary protein associated with the background K+ channel, TASK-1. EMBO J. 2002;21:4439–4448. doi: 10.1093/emboj/cdf469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okuse K, Malik-Hall M, Baker MD, Poon WY, Kong H, Chao MV. Annexin II light chain regulates sensory neuron-specific sodium channel expression. Nature. 2002;417:653–656. doi: 10.1038/nature00781. [DOI] [PubMed] [Google Scholar]
- 25.van de Graaf SF, Hoenderop JG, Gkika D, Lamers D, Prenen J, Rescher U. Functional expression of the epithelial Ca(2+) channels (TRPV5 and TRPV6) requires association of the S100A10-annexin 2 complex. EMBO J. 2003;22:1478–1487. doi: 10.1093/emboj/cdg162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruse M, Lambert A, Robinson N, Ryan D, Shon KJ, Eckert RL. S100A7, S100A10, and S100A11 are transglutaminase substrates. Biochemistry. 2001;40:3167–3173. doi: 10.1021/bi0019747. [DOI] [PubMed] [Google Scholar]
- 27.Wu T, Angus CW, Yao XL, Logun C, Shelhamer JH. P11, a unique member of the S100 family of calcium-binding proteins, interacts with and inhibits the activity of the 85-kDa cytosolic phospholipase A2. J Biol Chem. 1997;272:17145–17153. doi: 10.1074/jbc.272.27.17145. [DOI] [PubMed] [Google Scholar]
- 28.Oh YS, Gao P, Lee KW, Ceglia I, Seo JS, Zhang X. SMARCA3, a chromatin-remodeling factor, is required for p11-dependent antidepressant action. Cell. 2013;152:831–843. doi: 10.1016/j.cell.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt EF, Warner-Schmidt JL, Otopalik BG, Pickett SB, Greengard P, Heintz N. Identification of the cortical neurons that mediate antidepressant responses. Cell. 2012;149:1152–1163. doi: 10.1016/j.cell.2012.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seo JS, Wei J, Qin L, Kim Y, Yan Z, Greengard P . Cellular and molecular basis for stress-induced depression. Mol Psychiatry 2016; e-pub ahead of print 26 July 2016. [DOI] [PMC free article] [PubMed]
- 31.Warner-Schmidt JL, Schmidt EF, Marshall JJ, Rubin AJ, Arango-Lievano M, Kaplitt MG. Cholinergic interneurons in the nucleus accumbens regulate depression-like behavior. Proc Natl Acad Sci USA. 2012;109:11360–11365. doi: 10.1073/pnas.1209293109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB. A gene expression atlas of the central nervous system based on bacterial artificial qchromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- 33.Seo JS, Park JY, Choi J, Kim TK, Shin JH, Lee JK. NADPH oxidase mediates depressive behavior induced by chronic stress in mice. J Neurosci. 2012;32:9690–9699. doi: 10.1523/JNEUROSCI.0794-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wirtshafter D, Asin KE, Pitzer MR. Dopamine agonists and stress produce different patterns of Fos-like immunoreactivity in the lateral habenula. Brain Res. 1994;633:21–26. doi: 10.1016/0006-8993(94)91517-2. [DOI] [PubMed] [Google Scholar]
- 35.Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Golden SA, Heshmati M, Flanigan M, Christoffel DJ, Guise K, Pfau ML. Basal forebrain projections to the lateral habenula modulate aggression reward. Nature. 2016;534:688–692. doi: 10.1038/nature18601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Proulx CD, Hikosaka O, Malinow R. Reward processing by the lateral habenula in normal and depressive behaviors. Nat Neurosci. 2014;17:1146–1152. doi: 10.1038/nn.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stamatakis AM, Van Swieten M, Basiri ML, Blair GA, Kantak P, Stuber GD. Lateral hypothalamic area glutamatergic neurons and their projections to the lateral habenula regulate feeding and reward. J Neurosci. 2016;36:302–311. doi: 10.1523/JNEUROSCI.1202-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim SY. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature. 2012;492:428–432. doi: 10.1038/nature11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vadovicova K. Affective and cognitive prefrontal cortex projections to the lateral habenula in humans. Front Hum Neurosci. 2014;8:819. doi: 10.3389/fnhum.2014.00819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anisman H, Du L, Palkovits M, Faludi G, Kovacs GG, Szontagh-Kishazi P. Serotonin receptor subtype and p11 mRNA expression in stress-relevant brain regions of suicide and control subjects. J Psychiatry Neurosci. 2008;33:131–141. [PMC free article] [PubMed] [Google Scholar]
- 42.Kawai T, Yamada H, Sato N, Takada M, Matsumoto M. Roles of the lateral habenula and anterior cingulate cortex in negative outcome monitoring and behavioral adjustment in nonhuman primates. Neuron. 2015;88:792–804. doi: 10.1016/j.neuron.2015.09.030. [DOI] [PubMed] [Google Scholar]
- 43.Lecourtier L, Defrancesco A, Moghaddam B. Differential tonic influence of lateral habenula on prefrontal cortex and nucleus accumbens dopamine release. Eur J Neurosci. 2008;27:1755–1762. doi: 10.1111/j.1460-9568.2008.06130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams ZM, Bush G, Rauch SL, Cosgrove GR, Eskandar EN. Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nat Neurosci. 2004;7:1370–1375. doi: 10.1038/nn1354. [DOI] [PubMed] [Google Scholar]
- 45.Baker PM, Jhou T, Li B, Matsumoto M, Mizumori SJ, Stephenson-Jones M. The lateral habenula circuitry: reward processing and cognitive control. J Neurosci. 2016;36:11482–11488. doi: 10.1523/JNEUROSCI.2350-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lecca S, Pelosi A, Tchenio A, Moutkine I, Lujan R, Herve D. Rescue of GABAB and GIRK function in the lateral habenula by protein phosphatase 2A inhibition ameliorates depression-like phenotypes in mice. Nat Med. 2016;22:254–261. doi: 10.1038/nm.4037. [DOI] [PubMed] [Google Scholar]
- 47.Meye FJ, Soiza-Reilly M, Smit T, Diana MA, Schwarz MK, Mameli M. Shifted pallidal co-release of GABA and glutamate in habenula drives cocaine withdrawal and relapse. Nat Neurosci. 2016;19:1019–1024. doi: 10.1038/nn.4334. [DOI] [PubMed] [Google Scholar]
- 48.Aizawa H, Kobayashi M, Tanaka S, Fukai T, Okamoto H. Molecular characterization of the subnuclei in rat habenula. J Comp Neurol. 2012;520:4051–4066. doi: 10.1002/cne.23167. [DOI] [PubMed] [Google Scholar]
- 49.Jhou TC, Good CH, Rowley CS, Xu SP, Wang H, Burnham NW. Cocaine drives aversive conditioning via delayed activation of dopamine-responsive habenular and midbrain pathways. J Neurosci. 2013;33:7501–7512. doi: 10.1523/JNEUROSCI.3634-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


